Abstract
A prospective, randomized, three-arm, evaluator blinded study to demonstrate the feasibility of a telerehabilitation (TR) program in individuals with ambulatory deficits secondary to Multiple Sclerosis (MS) and evaluate its efficacy when compared to conventional on-site physical therapy (PT) was completed. Thirty participants were evaluated at baseline and randomized to one of three groups with intervention lasting 8 weeks: Group 1 (control)- customized unsupervised home-based exercise program (HEP) 5 days a week; Group 2 (TR)- remote PT supervised via audio/visual real-time telecommunication twice weekly; Group 3 (PT)- in-person PT at the medical facility twice weekly. Outcomes included patient reported outcomes (PROs) obtained through questionnaires, and measurements of gait and balance performed with bedside tests and a computerized system. Functional gait assessment improved from baseline in all three groups. There were no significant differences between the TR and the conventional PT groups for a variety of outcome measures. TR is a feasible method to perform PT in persons with MS and has comparable efficacy to conventional in-person PT as measured by patient reported outcomes and objective outcomes of gait and balance.
Keywords: Balance, Gait, Multiple Sclerosis, Telerehabilitation
Gait dysfunction has been identified by persons with multiple sclerosis (MS) as the most concerning limitation (Cameron & Wagner, 2011; Heesen et al., 2008), and is a common manifestation with surveys establishing that 41% have ambulatory deficits and 54% experience imbalance (Larocca, 2011). Decreased postural balance has been suggested as the leading cause of falls in people with MS with 52% of participants reporting a fall in the past 6 months (Finlayson, Peterson, & Cho, 2006). Nilsagard, Lundholm, Denison, and Gunnarsson (2009) reported 63% of studied MS participants fell at least once during a 3-month period with an increase in frequency conditioned by aging and disease progression. The risk or the fear of falling affects these individuals’ participation in social interactions and physical activities and can lead to a negative effect on their physical and emotional independence.
Functional improvement of established physical deficits can be achieved through different interventions to include neurorehabilitation methods such as physical therapy (PT). These treatments aim to reduce existing disabilities and increase functional independence. Optimizing the functional ambulatory status of people with MS could result in improved quality of life, independence, and safety.
PT services are delivered in outpatient settings at hospitals and specialized clinics, or at home through home health services. Access and adherence to specialized PT interventions are limited by a variety of factors such as availability, geographical location, mobility limitations, time constraint, transportation difficulties, health insurance coverage, and financial burden (Petajan & White, 1999; Rio et al., 2005). Addressing these barriers is an important and necessary step in improving patient care in MS.
Telecommunication technology offers the capacity to supervise and direct a PT program remotely through audio and visual real-time communication and is a viable solution to minimize several of the identified barriers to care. Given the variety of factors that impair access to specialized rehabilitation services in MS, designing and implementing a telecommunication PT program would provide a practical, accessible, and effective way to improve function and well-being. In addition, performing the program in the home setting could facilitate adherence, adapt to the real life environment, improve self-reliability, and generate a therapeutic alliance with the caregiver.
Although telerehabilitation (TR) research is still in its early stages, preliminary studies have shown some improvement in balance and postural control in people with MS that underwent a TR program (Gutierrez et al., 2013; Ortiz-Gutierrez et al., 2013). No adverse events have been identified as a consequence of utilizing this intervention (Khan, Amatya, Kesselring, & Galea, 2015). A study by Finkelstein, Lapshin, Castro, Cha, and Provance (2008) implemented a 12 week physical TR program for individuals with MS that resulted in significant improvement of the 25-foot walk, 6-minute walk and the Berg Balance Scale (BBS) tests compared to baselines scores.
METHODS
A proof of concept prospective, randomized, three-arm, evaluator blinded, 8-week pilot study with 30 subjects randomized in a 1:1:1 fashion was conducted. All individuals underwent a baseline medical and PT evaluation by a neurologist with expertise in MS (GP) and a physical therapist with extensive knowledge of this condition (AT) respectively. Participants were assigned to an unsupervised customized exercise program, or to supervised adaptable sessions with the treating physical therapist either through telecommunication or in-person, all lasting eight weeks, resulting in the three study groups: Group 1- unsupervised HEP (control group) five days a week; Group 2- remote PT supervised via audio/visual real-time telecommunication twice weekly (TR group); Group 3- HEP plus in-person PT at the Oklahoma Medical Research Foundation (OMRF) Multiple Sclerosis Center of Excellence PT facility two times weekly (PT group). One patient in the PT group dropped out due to an MS relapse.
The OMRF institutional review board approved the study. The logistics of the telecommunication process were optimized with full compliance with privacy regulations. All participants had to sign an informed consent prior to participating in the study. There were no barriers identified for participation in the TR group.
Outcome variables included clinical assessments of gait and balance and were obtained at baseline and at end of study by a single evaluator (CF-P) who was blinded to the group allocation. Gait and balance variables were measured using the NeuroCom Smart Balance Master (Natus Medical Incorporated, Pleasanton, CA) at baseline and exit visits. This system has demonstrated good utility for evaluating gait and balance in MS (Fjeldstad, Pardo, Bemben, & Bemben, 2011) and other diseases (Burke, Franca, Ferreira de Meneses, Cardoso, & Marques, 2010; Ondo et al., 2000). The device is equipped with a movable visual surround and a dual-plate force platform with capability of rotation in order to measure vertical forces exerted by the participant’s feet on the force plate during testing. Further, the long force plate component evaluates walking and postural control during ambulation.
GAIT MEASURES CONDUCTED
FUNCTIONAL GAIT ASSESSMENT
Functional gait assessment (FGA) is a 10-item evaluation of gait function. Each item ranges from 0 (severe impairment) to 3 (normal). A maximum score of 30 is possible. A 6-meter (20-foot) walkway marked with a 30.48 cm (12 inch) width is required for this test. Tests include gait on level surface, change in gait speed, gait with horizontal head turn, gait with vertical head turn, gait with pivot turn, step over obstacle, gait with narrow base of support, gait with eyes closed, ambulating backwards, and walking a set of steps (Walker et al., 2007).
TIMED 25 FOOT WALK
Timed 25 foot walk (T25FW) is a quantitative mobility and leg function performance test based on a timed 25-walk. The subject is directed to one end of a clearly marked 25-foot course and instructed to walk 25 feet as quickly and safely possible. The time is recorded in seconds from the moment the first foot crosses the 0 foot mark and ends when the lead foot crosses the 25 foot mark. Participants should have a minimum 3-step start so not to begin in an idle state.
WALK ACROSS
Walk across (WA) quantifies characteristics of gait as the patient walks across the length of the force plate using the Neurocom Smart Balance Master. This test characterizes steady gait by having the patient begin three steps behind and continuing beyond the force plate. Parameters measured are average step width (cm) and step length (seconds).
BALANCE MEASURES CONDUCTED
BERG BALANCE SCALE
Berg Balance Scale (BBS) is a widely used clinical functional test of a person’s static and dynamic balance abilities (Berg, Wood-Dauphinee, Williams, & Maki, 1992; Blum & Korner-Bitensky, 2008) designed to measure balance in a clinical setting. Items included are sustained static standing in a given position for a specific time, tandem stance, one-legged stance, and stance with eyes closed. Each item ranges from 0–4, with 0 indicating the lowest level of score and 4 the highest level of score and physical function with a maximum score of 56. A score of <45 indicates a greater risk for falling (Berg et al., 1992).
NEUROCOM SMART BALANCE MASTER TESTS
Neurocom Smart Balance Master tests included: (1) Tandem Walk where the participant walks heel to toe from one end of the force plate to the other in order to measure sway velocity (deg/sec) and sway width (cm); (2) Limits of Stability (LOS) which quantifies the maximum distance the patient can intentionally displace their center of gravity in the four cardinal directions as well as the four diagonal directions, and their ability to maintain stability while in those positions quantified for this study as percentage of directional control; and (3) Sensory Organizational Test (SOT) which objectively identifies any abnormalities of the participant’s use of the three sensory systems that assist in postural control, namely somatosensory, visual and vestibular input through a composite score calculated from evaluations delivering inaccurate information to the participant’s eyes, feet, and joints through sway referencing of the visual surround and the support surface (combination of normal, absent or swayed-reference vision and fixed or sway-referenced support).
PATIENTS REPORTED OUTCOMES (PROS)
SHORT FORM 36
Short Form 36 (SF36) developed by RAND, is a self-report questionnaire widely used to assess generic measures of health-related quality of life and consists of 8 subscales and two summary scores. The subscales include physical functioning, role limitations due to physical problems, bodily pain, general health perceptions, vitality, social functioning, role-limitations due to emotional problems, and mental health. The two summary scores include physical (SF36p) and mental (SF36m) components. It takes approximately 10 minutes to administer, (Fischer et al., 1999; Marrie, Miller, Chelune & Cohen, 2003).
MODIFIED FATIGUE IMPACT SCALE
Modified Fatigue Impact Scale (MFIS) is a self-report questionnaire based on how fatigue impacts an individual’s life. It consists of 21 items and covers fatigue in terms of physical, cognitive, and psychosocial functioning. It takes 5–10 minutes to administer (Fisk et al. 1994).
MS SELF-EFFICACY QUESTIONNAIRE
The MS Self-Efficacy (MSSE) questionnaire is a self-report 14-item instrument to assess a general sense of perceived self-efficacy of coping with living with MS. It takes about 5 minutes to complete (Rigby, Domenech, Thornton, Tedman, & Young, 2003).
ACTIVITIES-SPECIFIC BALANCE CONFIDENCE SCALE
The Activities-specific Balance Confidence Scale (ABC Scale) indicates the level of confidence in performing various activities of ambulation without losing balance or becoming unsteady. Participants rate their confidence on the scale form 0% (no confidence) to 100% (complete confidence) for each of the 16 items that compose the questionnaire (Powell & Myers, 1995).
DISEASE-SPECIFIC MEASURES
EXPANDED DISABILITY STATUS SCALE
Expanded Disability Status Scale (EDSS) is a metric widely used to measure disability in MS. Based on a complete neurological examination, seven different functional systems and ambulation are carefully scored. The EDSS is an ordinal clinical rating scale ranging from 0 (normal neurologic examination) to 10 (death due to MS) in half-point increments. The neurological examination that is needed to make the ratings can take anywhere from 15 minutes to a half-hour and is often administered by a neurologist (Kurtzke, 1983).
STATISTICAL ANALYSES
Statistical analysis was completed using The R Project for Statistical Computing software (The R Foundation, 2016). T- test (two-tailed) was performed on the mean of the differences (after-before) for each variable grouped by treatment type to test for significant differences from 0 with the purpose to determine if each treatment had a statistically significant effect on the considered variable.
Next, false discovery rate (FDR) corrected pair-wise t tests (two-tailed) were performed to test for significant differences amongst the considered variable across treatments to determine if a particular treatment had a statistically significantly different effect on a variable than the other two treatments. With these two analyses it can be determined if (a) a particular treatment makes a significant impact on the considered variable, and (b) is one treatment significantly more impactful on a variable than the other treatments.
RESULTS
DEMOGRAPHICS
The characteristics of the group are as follows: female 69%, mean age 54.7±12.3 years, relapsing remitting MS (RRMS) 60%, secondary progressive MS (SPMS) 23%, and primary progressive MS (PPMS) 17%. The control group consisted of 8 with RRMS and 2 with SPMS. The TR group consisted of 4 RRMS, 3 SPMS and 3 PPMS. The PT group consisted of 5 RRMS, 3 SPMS and 1 PPMS. Mean expanded disability status scale (EDSS) was 4.3±1.1 for the entire cohort (control group 4.4, TR group 4.4, PT group 4.3). Descriptive characteristics distributed by group are found in Table 1.
Table 1.
Demographic Characteristics and Outcome Variables
| Total N=29 | Control n=10 | TR n=10 | PT n=9 | |
|---|---|---|---|---|
| Age mean±SD | 54.7±12.3 | 54.4±10.8 | 55.1±13.9 | 54.7±13.5 |
| Female (n) | 20 | 6 | 7 | 7 |
| EDSSpre | 4.3±1.1 | 4.4±1.1 | 4.4±1.0 | 4.3±1.4 |
| EDSSpost | 4.1±1.2 | 4.4±1.3 | 4.4±1.1 | 4.3±1.5 |
| T25FWpre | 10.0±4.7 | 10.0±5.8 | 10.5±5.2 | 9.5±3.5 |
| T25FWpost | 8.9±3.8 | 9.5±5.5 | 9.2±3.2 | 8.1±2.5 |
| BBSpre | 45.1±7.7 | 46.1±5.9 | 43.5±10.0 | 46.0±7.1 |
| BBSpost | 47.4±6.7 | 48.4±5.6 | 45.6±8.8 | 48.3±5.2 |
| FGApre | 19.0±6.5 | 19.8±6.1 | 17.6±6.5 | 19.8±7.7 |
| FGApost | 22.5±6.3 | 22.7±6.0 | 21.6±7.2 | 23.4±6.5 |
| ABCpre | 52.9±19.1 | 51.6±14.3 | 53.7±23.8 | 53.6±21.5 |
| ABCpost | 57.9±23.8 | 60.8±21.3 | 50.8±28.1 | 62.6±23.7 |
| MSSEpre | 51.0±11.9 | 49.1±8.5 | 50.1±14.7 | 54.1±13.0 |
| MSSEpost | 54.6±12.5 | 54.3±12.3 | 51.8±13.1 | 58.2±13.3 |
| MFISpre | 46.6±15.8 | 46.9±14.1 | 47.3±17.8 | 45.5±18.1 |
| MFISpost | 38.5±16.0 | 41.9±13.1 | 40.8±20.8 | 32.2±13.3 |
| SF-36m-pre | 48.1±10.6 | 52.6±8.6 | 49.3±9.3 | 41.9±12.5 |
| SF-36m-post | 52.4±10.9 | 50.5±12.0 | 53.3±10.3 | 53.8±11.8 |
| SF-36p-pre | 29.1±8.3 | 28.0±6.9 | 32.3±10.0 | 26.7±8.0 |
| SF-36p-post | 37.9±12.5 | 31.2±7.0 | 35.8±7.7 | 48.5±16.7 |
Note. Mean ± SD
GAIT AND BALANCE OUTCOMES
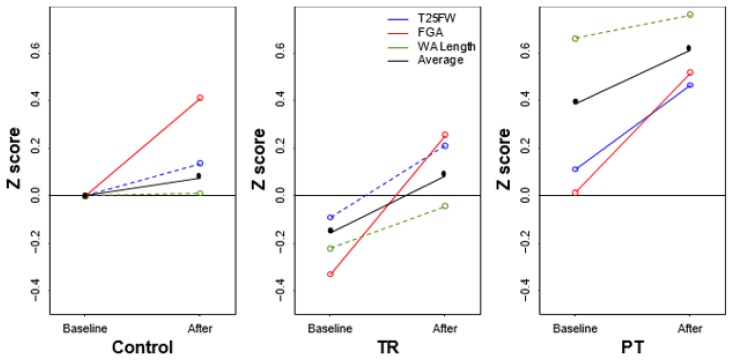
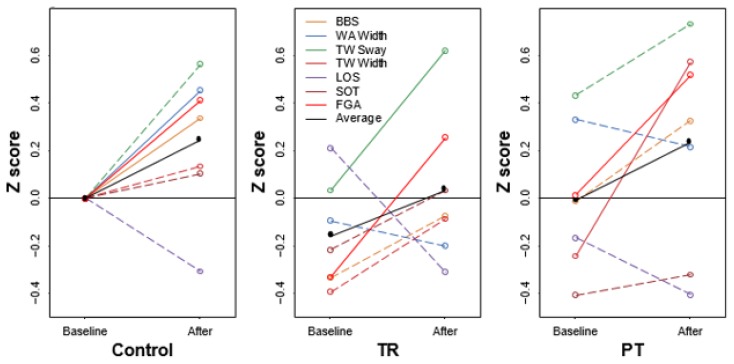
The FGA improved in all three groups from baseline (P<0.05) with no significant differences between the TR and the PT group. Other outcomes that showed improvement from baseline include BBS and WA width for the control group, TW sway for the TR group, and TW width and T25FW for the PT group. Comparison of the mean differences between each pairing of groups yielded equivalent results with no statistical differences (Figures 1 and 2; Tables 2 and 3).
Figure 1.
Gait scores.
Figure 2.
Balance scores.
Table 2.
Gait Variables1
| Gait | mean.control | mean.TR | mean.PT | control.diff.zero | TR.diff.zero | PT.diff.zero | p.TR-PT | p.TR-control | p.PT- |
|---|---|---|---|---|---|---|---|---|---|
| n=10 | n=10 | n=9 | n=10 | n=10 | n=9 | n=10 | n=10 | n=9 | |
| FGA | 2.9000 | 4.0000 | 3.5556 | 0.0002* | 0.0006* | 0.0095* | 0.7308 | 0.7308 | 0.7308 |
| T25FT | −0.5540 | −1.2960 | −1.3478 | 0.0708 | 0.0933 | 0.0255* | 0.9566 | 0.6391 | 0.6391 |
| WA.length | 0.1400 | 3.1680 | −0.2167 | 0.4841 | 0.0855 | 0.5362 | 0.6430 | 0.6430 | 0.9273 |
Table 3.
Balance Variables
| Balance | mean.control | mean.TR | mean.PT | control.diff.zero | TR.diff.zero | PT.diff.zero | p.TR-PT | p.TR-control | p.PT-control |
|---|---|---|---|---|---|---|---|---|---|
| n=10 | n=10 | n=9 | n=10 | n=10 | n=9 | n=10 | n=10 | n=9 | |
| BBS | 2.3000 | 2.1000 | 2.3333 | 0.0255* | 0.0742 | 0.0579 | 0.9850 | 0.9850 | 0.9850 |
| FGA | 2.9000 | 4.0000 | 3.5556 | 0.0002* | 0.0006* | 0.0095* | 0.7308 | 0.7308 | 0.7308 |
| LOS | −5.9630 | −9.9567 | −4.8044 | 0.8964 | 0.8865 | 0.7257 | 0.9014 | 0.9014 | 0.9014 |
| SOT | 1.5000 | 4.0000 | 2.0000 | 0.2263 | 0.1737 | 0.3355 | 0.9212 | 0.9212 | 0.9212 |
| TW.sway | −1.6780 | −1.7360 | −0.7922 | 0.0775 | 0.0132* | 0.2486 | 0.7879 | 0.9658 | 0.7879 |
| TW.width | −0.8820 | −2.0960 | −5.3733 | 0.2573 | 0.0707 | 0.0199* | 0.2484 | 0.5918 | 0.1843 |
| WA.width | −2.1260 | 0.5470 | 0.3644 | 0.0154* | 0.7405 | 0.5837 | 0.9111 | 0.2040 | 0.2040 |
PATIENT REPORTED OUTCOMES
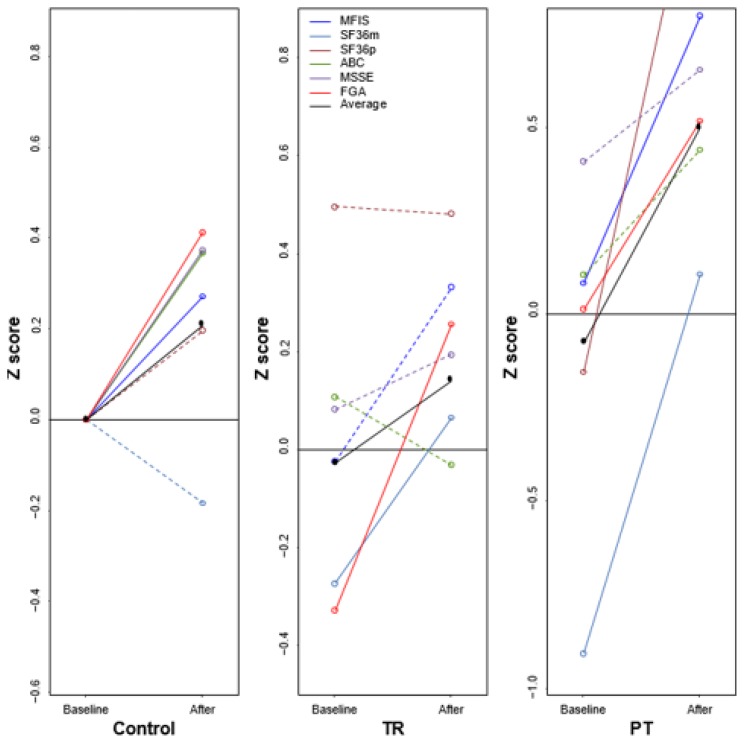
The control group demonstrated significant improvement (p<0.05) in the ABC Scale, FGA, and MSSE from baseline. The TR group showed significant improvement (p<0.05) for the SF36m. The PT group had significant improvement (p<0.05) for MFIS, SF36m, and SF36p. Comparing the mean difference scores pairwise between treatment groups it was found that SF36m significantly improved in the PT group compared to the control group (p=0.0047 FDR corrected) and SF36p for PT group was significantly improved compared to the control and TR groups (p=0.0090 FDR corrected) (Figure 3 and Table 4).
Figure 3.
Patient reported outcomes.
Table 4.
Patient Reported Outcomes
| PROs | mean.control | mean.TR | mean.PT | control.diff.zero | TR.diff.zero | PT.diff.zero | p.TR-PT | p.TR-control | p.PT-control |
|---|---|---|---|---|---|---|---|---|---|
| n=10 | n=10 | n=9 | n=10 | n=10 | n=9 | n=10 | n=10 | n=9 | |
| ABC Scale | 9.2000 | −2.9000 | 9.0000 | 0.0279* | 0.6004 | 0.1351 | 0.4757 | 0.4757 | 0.9865 |
| FGA | 2.9000 | 4.0000 | 3.5556 | 0.0002* | 0.0006* | 0.0095* | 0.7308 | 0.7308 | 0.7308 |
| MFIS | −5.0000 | −6.5000 | −13.3333 | 0.0835 | 0.0530 | 0.0175* | 0.3762 | 0.7932 | 0.3762 |
| MSSE | 5.2000 | 1.7000 | 4.1111 | 0.0372* | 0.3223 | 0.0618 | 0.7965 | 0.7965 | 0.7965 |
| SF.36.mental | −2.0500 | 2.9667 | 11.8333 | 0.8389 | 0.0438* | 0.0118* | 0.0546 | 0.2112 | 0.0047* |
| SF.36.physical | 3.2000 | 4.1556 | 21.7875 | 0.0849 | 0.0762 | 0.0073* | 0.009* | 0.8643 | 0.0090* |
For Tables 2, 3 and 4, the first three columns are the difference between the mean after treatment score minus the mean before treatment score. Columns 4–6 are the p-values for the t-tests to determine if columns 1–3 values are significantly different than 0. Columns 7–9 are the p-values (FDR corrected) for the two sample paired t-tests comparing the mean differences between each pairing of groups.
DISCUSSION
MS can result in significant physical dysfunction, with motor impairment and decreased mobility ranking among the most common disabling symptoms (Cameron & Wagner, 2011; Heesen et al., 2008; Larocca, 2011). Access to specialized medical care is an important limiting factor in properly controlling the disease process, preventing new manifestations, and achieving maximum functional level once disability ensues. Multiple factors limit access to specialized MS care to include regional availability, geographical distance, level of physical disability, transportation logistics, employment obligations, insurance coverage and financial reasons (Petajan & White, 1999; Rio et al., 2005). Neurorehabilitation efforts directed towards regaining, improving and maintaining motor abilities can address these problems and PT is the cornerstone of such approaches. The benefits of this intervention are well documented in the literature (Giesser 2015; Motl & Pilutti 2012; Sandroff et al., 2012). Access to specialized rehabilitation professionals with knowledge of the complexity of MS is further compromised by the high number of visits that are inherent to the rehabilitation process.
Telemedicine has the capability to overcome many of the previously mentioned barriers to access to health care and provide specialized services to persons with MS. PT is conventionally performed in an individualized setting during in-person encounters between the therapist and the patient as it is traditionally considered a hands-on intervention. The possibility of using telemedicine to provide PT services is attractive and is in need of validation.
TR studies in MS have been limited but have shown encouraging results. Improvement in balance and postural control in people with MS that underwent a TR program was demonstrated when using a virtual reality system (Gutierrez et al., 2013; Ortiz-Gutierrez et al., 2013). Significant improvement in gait speed was achieved with a 12-week TR program including people with MS, (Finkelstein, Lapshin, Castro, Cha, & Provance, 2008). An internet-based study comparing TR with hippotherapy showed improvement in static and dynamic balance capacity with both interventions (Frevel & Maurer 2015). Increasing and sustaining physical activity 3 months after intervention was obtained through an internet delivered behavioral intervention in persons with MS, but no significant change in mobility or quality of life was identified (Dluglonski, Moll, Mohr, & Sandroff, 2012). Given methodological features and design characteristics, there is limited evidence to date of the efficacy of TR in improving functional activities and quality of life in adults with MS (Khan, Amatya, Kesselring, & Galea, 2015). Most these interventions, albeit delivered through a telemedicine system, were static during the duration of the trial given lack of direct interaction with a PT during the execution of the physical activity. No adverse events have been identified as a consequence of utilizing these various TR interventions.
In this study, we used a variety of outcomes related to the individual’s perception of health, fatigue, balance, and self-efficacy in addition to objective measures of gait and balance with conventional tests and novel computerized analysis systems with the objective of determining if TR had comparable results with traditional in-person PT. A unique feature was the adaptability of the TR system with modification of the exercise regimen, resembling what is done with conventional PT intervention, as each one of the remote sessions were performed live with direct audio and visual communication with the physical therapist. Furthermore, different from previous studies, the comparator groups included individuals that were undergoing in-person PT. The FGA, a main outcome of gait that assesses ambulation under a variety of conditions, improved from baseline in all three groups, to include the one performing an unsupervised, non-adaptable but customized exercise program at home. This outcome alone argues for the benefit of individualized physical activity and rehabilitation in MS. The remainder of the gait and balance outcomes either improved or remained stable. In comparing TR and PT, all of the post-intervention objective variables of ambulation were equivalent. As it pertains to PROs, the MSSE, which is a measure of self-efficacy, improved for the control group only. We speculate this result may be secondary to personal empowerment after successfully concluding a prolonged, 8-week, exercise program without direct supervision. The SF36 mental health domain improved for the TR and PT groups. Intergroup analysis between TR and PT showed a superior outcome for PT on the SF 36 physical component only.
In general, the results of the intervention with TR were comparable in effect with conventional in-person PT. There were no logistical nor health related impediments for the complete execution of the trial. Only one participant did not complete the study due to unrelated onset of an MS relapse.
Future studies should include a larger cohort with refined outcomes based on the results of this pilot study to categorically demonstrate the large-scale feasibility and effectiveness of TR. Sustained benefits should be explored with new assessments several months following the interventions. Positive results could facilitate implementation of TR as a solution for access to specialized services in remote, rural, or underserved areas, to provide rehabilitation opportunities to individuals with mobility and transportation limitations even within urban areas, and support the need for acceptance of this modality for reimbursement by third party payers.
CONCLUSION
TR offers a feasible intervention for neurorehabilitation in persons with MS and has comparable results with conventional in-person physical therapy when measured by patient reported outcomes and objective measures of gait and balance.
Footnotes
For Tables 2–4: ABC=Activities-specific Balance Confidence Scale; FGA=Functional Gait Assessment; MFIS=Modified Fatigue Impact Scale; MSSE-MS Self-efficacy Questionnaire; SF-36=Short Form-36 Questionnaire, mental and physical subscales. T25FT=Times 25 foot walk; WA=Walk across; TW= Tandem Walk; BBS=Berg Balance Scale; LOS=Limits of Stability; SOT=Sensory Organizational Test; EDSS=Expanded Disability Status Scale.
REFERENCES
- Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: Validation of an instrument. Canadian Journal of Public Health. 1992;83(Suppl 2):S7–11. [PubMed] [Google Scholar]
- Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: A systematic review. Physical Therapy. 2008;88:559–566. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- Burke TN, Franca FJ, Ferreira de Meneses SR, Cardoso VI, Marques AP. Postural control in elderly persons with osteoporosis: Efficacy of an intervention program to improve balance and muscle strength: A randomized controlled trial. American Journal of Physical Medicine & Rehabilitation. 2010;89:549–556. doi: 10.1097/PHM.0b013e3181ddccd2. [DOI] [PubMed] [Google Scholar]
- Cameron MH, Wagner JM. Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment. Current Neurology and Neuroscience Reports. 2011;11:507–515. doi: 10.1007/s11910-011-0214-y. [DOI] [PubMed] [Google Scholar]
- Dlugonski D, Moll RW, Mohr DD, Sandroff BM. Internet-delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: Sustainability and secondary outcomes. Psychology, Health & Medicine. 2012;17:636–51. doi: 10.1080/13548506.2011.652640. [DOI] [PubMed] [Google Scholar]
- Finkelstein J, Lapshin O, Castro H, Cha E, Provance PG. Home-based physical telerehabilitation in patients with multiple sclerosis: A pilot study. Journal of Rehabilitation Research and Development. 2008;45:1361–1373. [PubMed] [Google Scholar]
- Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Archives of Physical Medicine and Rehabilitation. 2006;87:1274–1279. doi: 10.1016/j.apmr.2006.06.002. quiz 1287. [DOI] [PubMed] [Google Scholar]
- Fischer JS, LaRocca NG, Miller DM, Ritvo PG, Andrews H, Paty D. Recent developments in the assessment of quality of life in multiple sclerosis (MS) Multiple Sclerosis. 1999;4:251–259. doi: 10.1177/135245859900500410. [DOI] [PubMed] [Google Scholar]
- Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clinical Infectious Diseases. 1994;1:79–83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- Fjeldstad C, Pardo G, Bemben D, Bemben M. Decreased postural balance in multiple sclerosis patients with low disability. International Journal of Rehabilitation Research. Internationale Zeitschrift fur Rehabilitationsforschung Revue internationale de recherches de readaptation. 2011;34(1):53–58. doi: 10.1097/MRR.0b013e32833d6ccb. [DOI] [PubMed] [Google Scholar]
- Frevel D, Maurer M. Internet-based home training is capable to improve balance in multiple sclerosis: A comparative trial with hippotherapy. European Journal of Physical and Rehabilitation Medicine. 2015;51:23–30. [PubMed] [Google Scholar]
- Giesser B. Exercise in the management of persons with multiple sclerosis. Therapeutic Advances in Neurological Disorders. 2015;8:123–130. doi: 10.1177/1756285615576663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez RO, Galan Del Rio F, Cano de la Cuerda R, Alguacil Diego IM, Gonzalez RA, Page JC. A telerehabilitation program by virtual reality-video games improves balance and postural control in multiple sclerosis patients. NeuroRehabilitation. 2013;33:545–554. doi: 10.3233/NRE-130995. [DOI] [PubMed] [Google Scholar]
- Heesen C, Bohm J, Reich C, Kasper J, Goebel M, Gold SM. Patient perception of bodily functions in multiple sclerosis: Gait and visual function are the most valuable. Multiple Sclerosis. 2008;14:988–991. doi: 10.1177/1352458508088916. [DOI] [PubMed] [Google Scholar]
- Khan F, Amatya B, Kesselring J, Galea M. Telerehabilitation for persons with multiple sclerosis. Cochrane Database of Systematic Reviews. 2015;(4):CD010508. doi: 10.1002/14651858.CD010508.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Larocca NG. Impact of walking impairment in multiple sclerosis: Perspectives of patients and care partners. Patient. 2011;4:189–201. doi: 10.2165/11591150-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Marrie RA, Miller DM, Chelune GJ, Cohen JA. Validity and reliability of the MSQLI in cognitively impaired patients with multiple sclerosis. Multiple Sclerosis. 2003;6:621–626. doi: 10.1191/1352458503ms971oa. [DOI] [PubMed] [Google Scholar]
- Motl R, Pilutti LA. The benefits of exercise training in multiple sclerosis. Nature Reviews Neurology. 2012;8:487–497. doi: 10.1038/nrneurol.2012.136. [DOI] [PubMed] [Google Scholar]
- Nilsagard Y, Lundholm C, Denison E, Gunnarsson LG. Predicting accidental falls in people with multiple sclerosis -- a longitudinal study. Clinical Rehabilitation. 2009;23:259–269. doi: 10.1177/0269215508095087. [DOI] [PubMed] [Google Scholar]
- Ondo W, Warrior D, Overby A, Calmes J, Hendersen N, Olson S, Jankovic J. Computerized posturography analysis of progressive supranuclear palsy: A case-control comparison with Parkinson’s disease and healthy controls. Archives of Neurology. 2000;57:1464–1469. doi: 10.1001/archneur.57.10.1464. [DOI] [PubMed] [Google Scholar]
- Ortiz-Gutierrez R, Cano-de-la-Cuerda R, Galan-del-Rio F, Alguacil-Diego IM, Palacios-Cena D, Miangolarra-Page JC. A telerehabilitation program improves postural control in multiple sclerosis patients: A Spanish preliminary study. International Journal of Environmental Research and Public Health. 2013;10:5697–5710. doi: 10.3390/ijerph10115697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petajan JH, White AT. Recommendations for physical activity in patients with multiple sclerosis. Sports Medicine. 1999;27:179–191. doi: 10.2165/00007256-199927030-00004. [DOI] [PubMed] [Google Scholar]
- Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 1995;50A(1):M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- Rio J, Porcel J, Tellez N, Sanchez-Betancourt A, Tintore M, Arevalo MJ, … Montalban X. Factors related with treatment adherence to interferon beta and glatiramer acetate therapy in multiple sclerosis. Multiple Sclerosis. 2005;11:306–309. doi: 10.1191/1352458505ms1173oa. [DOI] [PubMed] [Google Scholar]
- Rigby SA, Domenech C, Thornton EW, Tedman S, Young CA. Development and validation of a self-efficacy measure for people with multiple sclerosis: The Multiple Sclerosis Self-efficacy Scale. Multiple Sclerosis. 2003;9(1):73–81. doi: 10.1191/1352458503ms870oa. [DOI] [PubMed] [Google Scholar]
- Sandroff B, Dulugonski D, Weikert Y, Suh Y, Balantrapu R, Moti RW. Physical activity and multiple sclerosis: New insights regarding inactivity. Acta Neurologica Scandinavica. 2012;126:256–262. doi: 10.1111/j.1600-0404.2011.01634.x. [DOI] [PubMed] [Google Scholar]
- The R Foundation. R: A language and environment for statistical computing. 2016. Retrieved from https://www.r-project.org/
- Walker ML, Austin AG, Banke GM, Foxx SR, Gaetano L, Gardner LA, … Penn L. Reference group data for the functional gait assessment. Physical Therapy. 2007;87:1468–1477. doi: 10.2522/ptj.20060344. [DOI] [PubMed] [Google Scholar]