Abstract
Objectives
This study was conducted to determine whether essential oils had anti-influenza A/WS/33 virus activity and whether there were specific compounds associated with this activity.
Methods
There were 63 essential oils evaluated for anti-influenza (A/WS/33 virus) activity using a cytopathic effect reduction method. The chemical composition of the anti-influenza essential oils was phytochemically analyzed by gas chromatography-mass spectrometry.
Results
The antiviral assays demonstrated that 11 of the 62 essential oils (100 μg/mL) possessed anti-influenza activity, reducing visible cytopathic effects of influenza A/WS/33 virus activity by > 30%. Furthermore, marjoram, clary sage and anise oils exhibited anti-influenza A/WS/33 virus activity of > 52.8%. However, oseltamivir (the anti-influenza A and B drug), showed cytotoxicity at the same concentration (100 μg/mL) as the essential oils. The chemical composition detected by GC–MS analysis, differed amongst the 3 most potent anti-viral essential oils (marjoram, clary sage and anise oils) except for linalool, which was detected in all 3 essential oils.
Conclusion
This study demonstrated anti-influenza activity in 11 essential oils tested, with marjoram, clary sage and anise essential oils being the most effective at reducing visible cytopathic effects of the A/WS/33 virus. All 3 oils contained linalool, suggesting that this may have anti-influenza activity. Further investigation is needed to characterize the antiviral activity of linalool against influenza A/WS/33 virus.
Keywords: essential oils, influenza, linalool, virus
Introduction
Influenza viruses are enveloped RNA viruses that infect humans and animals, and cause respiratory complications resulting in high morbidity rates [1]. The preferred treatment for influenza infection are neuraminidase inhibitors (oseltamivir and zanamivir) [2]. However, their use has been limited by side-effects, and the emergence of resistant viral strains [3,4].
Essential oils are known to possess multifunctional properties other than their traditional roles, as various biological agents have been shown to demonstrate anti-bacterial, anti-fungal, and anti-inflammatory activities [5,6]. Several studies have documented antiviral activity of essential oils [7–9]. Recent studies have demonstrated that eucalyptus essential oil showed inhibitory effects on adenovirus and mumps virus [10]. A previous study showed that the volatile oil from Cynanchum stauntonii possessed direct inhibitory activity against influenza virus [11].
In this present study, the potential anti-viral properties of 62 essential oils on influenza A/WS/33 virus have been analyzed using the cytopathic effect (CPE) reduction method. Marjoram (Thymus mastichina L.), clary sage (Salvia sclarea L.) and anise (Pimpinella anisum L.) oils showed anti-influenza A/WS/33 activity and were phytochemically examined by gas chromatography-mass spectrometry (GC-MS) analysis, and their chemical compositions analyzed.
Materials and Methods
1. Materials and cell culture
Essential plant oils (n = 62) were purchased from UNIQ F&F Co., Ltd. (Seoul, Korea) and listed in Table 1. The samples were deposited in Seoul National University herbarium. To test the materials, the oils were solubilized in dimethylsulfoxide to give a final concentration of 10 mg/mL, and stored at -20°C until further use.
Table 1.
List of 62 plant essential oils tested for antiviral activity.
| Family | Species | Oil | Part |
|---|---|---|---|
| Alliaceae | Allium sativum L. | Garlic | Root |
| Annonaceae | Cananga odorata Hook. f. et Thomson | Ylang ylang | Flower |
| Apiaceae | Pimpinella anisum L. | Anise | Fruit |
| Apiaceae | Carum carvi L. | Caraway seed | Seed |
| Apiaceae | Coriandrum sativum L. | Coriander | Flower |
| Apiaceae | Coriandrum sativum L. | Coriander herb | Leaf |
| Apiaceae | Foeniculum vulgare Mill. | Fennel | Seed |
| Asteraceae | Artemesia vulgaris L. | Armoise | Whole plant |
| Asteraceae | Tagetes minuta L. | Tagette | Leaf |
| Cupressaceae | Juniperus irginiana L. | Cedarwood | Bark |
| Cupressaceae | Cupressus sempervirens L. | Cypress | Twig |
| Cupressaceae | Juniperus communis L. | Juniperberry | Berry |
| Ericaceae | Gaultheria procumbens L. | Wintergreen | Leaf |
| Fabaceae | Myroxylon balsamum var. pereirae Royle | Balsam peru | Resin |
| Lamiaceae | Ocimum basilicum L. | Basil | Flower |
| Lamiaceae | Nepeta cataria L. | Catnip | Leaf & flower |
| Lamiaceae | Salvia sclarea L. | Clary sage | Flower |
| Lamiaceae | Hyssopus officinalis L. | Hyssop | Leaf |
| Lamiaceae | Thymus mastichina L. | Marjoram | Leaf |
| Lamiaceae | Origanum vulgare L. | Oregano | Leaf |
| Lamiaceae | Pogostemon cablin (Blanco) Benth. | Patchouly | Leaf |
| Lamiaceae | Mentha piperita L. | Peppermint | Flower |
| Lamiaceae | Mentha pulegium L. | Pennyroyal | Leaf |
| Lamiaceae | Rosmarinus officinalis L. | Rosemary | Flower |
| Lamiaceae | Satureja hortensis L. | Savory | Leaf |
| Lamiaceae | Mentha spicata L. | Spearmint | Flower |
| Lamiaceae | Thymus vulgaris L. | Thyme | Leaf |
| Lamiaceae | Thymus vulgaris L. | Thyme white | Leaf |
| Lauraceae | Cinnamomum cassia Bl. | Cassia pure | Bark |
| Lauraceae | Cinnamomum zeylanicum Garc. Ex Blume Nees | Cinnamon bark | Bark |
| Lauraceae | Cinnamomum zeylanicum Blume | Cinnamon leaf | Leaf |
| Lauraceae | Aniba roseadora var. amazonica Ducke | Rosewood | Wood |
| Myrtaceae | Eucalyptus globulus Labill. | Eucalyptus | Leaf |
| Myrtaceae | Pimenta dioica (L.) Merr. | Pimento berry | Flower |
| Myrtaceae | Melaleuca alternifolia (Maid. & Bet.) Cheel | Tea tree | Leaf |
| Oleaceae | Eugenia caryophyllata Thumb. | Clove bud | Bud |
| Oleaceae | Eugenia caryophyllata Thumb. | Clove leaf | Leaf |
| Pinaceae | Pinus sylvestris L. | Pine | Needle |
| Poaceae | Cymbopogon nardus L. | Citronella java | Leaf |
| Poaceae | Pelargonium graveolens L. | Geranium | Flower |
| Poaceae | Cymbopogon martinii Stapf. | Palmarosa | Grass |
| Rutaceae | Citrus bergamia Risso | Bergamot | Peel |
| Rutaceae | Citrus paradisi Macfadyen | Grapefruit | Fruit |
| Rutaceae | Citrus sinensis (L .) Osbeck | Orange | Peel |
| Rutaceae | Citrus limonum L. | Lemon | Peel |
| Rutaceae | Citrus aurantifolia Swing. | Lime | Peel |
| Rutaceae | Citrus reticulata Blanco | Mandarine | Peel |
| Rutaceae | Citrus aurantium L. | Neroli | Flower |
| Santalaceae | Santalum album L. | Sandalwood | Wood |
| Rosaceae | Rosa damascene Mill. | Rose | Flower |
| Asteraceae | Chamomilla recutita (L.) Rauschert | Chamomile blue | Flower |
| Asteraceae | Artemisia dracunculus L. | Estragon | Leaf |
| Poaceae | Cymbopogon citratus (DC) Stapf. | Lemongrass | Whole plant |
| Myrtaceae | Pimenta racemosa (Mill.) J.W.Moore | Bay | Leaf |
| Lauraceae | Litsea cubeba L. | Litsea cubeba | Fruit |
| Clusiaceae | Calophyllum inophyllum L. | Tamanu | Fruit |
| Rutaceae | Zanthoxylum armatum | Xanthoxylum | Seed |
| Myrtaceae | Eucalyptus citriodora | Eucalyptus (citriodora) | Leaf |
| Zingiberaceae | Zingiber officinale Roscoe | Ginger | Rhizome |
The Influenza A/WS/33 virus was provided by ATCC (American Type Culture Collection, Manassas, VA, USA) and propagated in Madin-Darby canine kidney (MDCK) cells at 37°C. MDCK cells were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum and 0.01% antibiotic-antimycotic solution. Antibiotic-antimycotic solution, trypsin- ethylenediamine tetra-acetic acid (EDTA), fetal bovine serum and MEM were supplied by Gibco BRL (Grand Island, NY). Tissue culture plates were purchased from Falcon (BD Biosciences, Franklin Lakes, NJ). Sulforhodamine B (SRB) was purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of reagent grade. Oseltamivir (F. Hofmann-La Roche Ltd, Switzerland) was purchased from a pharmacy in Korea as prescribed by a traditional Korean doctor.
2. Antiviral and cytotoxicity assay
Anti-influenza (A/WS/33 virus) activity of essential oils was determined by the CPE reduction method [12]. There were 2 × 104 MDCK cells/well seeded into a 96-well culture plate in MEM supplemented with trypsin-EDTA containing 0.01% antibiotic–antimycotic solution and incubated at 37°C in 5% CO2 for 24 hours. Thereafter, the medium was aspirated and cells were washed with phosphate buffered saline (PBS). Subsequently, the diluted virus suspension (0.09 mL) containing 50% tissue culture infective dose (TCID50) of the virus, was added to MDCK cells to produce an appropriate CPE within 48 hours after infection. Thereafter, MEM containing essential oils in 100 μg/mL were added to each well. The culture plates were incubated at 37°C in 5% CO2 for 2 days until 50% CPE was achieved. Subsequently, the 96-well plates were washed once with PBS (100 mL). Ice-cold 70% acetone in water (100 mL) was added to each well and incubated for 30 minutes at −20°C. After 70% acetone had evaporated, the plates were dried in an oven at 55°C for 30 minutes. The plate was developed using 0.4% (w/v) SRB in 1% acetic acid solution (100 mL) which was added to each well and left to stand for 30 minutes at room temperature. The excess SRB solution was removed by washing the plates 5 times with 1% acetic acid in water, then the plate was dried at 55°C. Bound SRB was then solubilized with 10 mM unbuffered Tris-base (SigmaAldrich) solution (100 mL). After 30 minutes, the absorbance was read at 524 nm with a VERSAmax microplate reader (Molecular Devices, Palo Alto, CA, USA) with a reference absorbance determined at 650 nm. The percent protection achieved by the test compound in the influenza A/WS/33 virus-infected cells was calculated using the equation below. Oseltamivir was used as the positive control and dimethylsulfoxide as a negative control. The morphology of influenza A/WS/33 virus-infected cells was observed using a light microscope at 32 × 10 magnification (AXIOVERT10; ZEISS, Oberkochen, Germany), and images were recorded.
To evaluate cytotoxicity, MDCK cells were seeded onto a 96-well culture plate at a concentration of 2 × 104 cells per well. The next day, the medium was replaced with medium containing serially diluted compounds. After 2 days of incubation at 37°C in 5% CO2, cytotoxicity was evaluated using the SRB assay. The culture medium was aspirated and cells were washed with PBS. The next step was performed per the antiviral activity assay described above. Results were expressed as the percentage of the controls.
3. Effect of essential oils on morphological changes of influenza virus-induced MDCK cells
The effect of essential oils on influenza virus-induced CPE was observed. Briefly, MDCK cells were seeded onto a 96-well culture plate at a concentration of 2 × 104 cells per well. The next day, the culture medium was removed and the cells were washed with PBS. Diluted virus suspension (0.09 mL) and 0.01 mL of medium supplemented with trypsin-EDTA containing essential oils at 100 μg/mL were added to each well. After incubation at 37°C in 5% CO2 for 2 days, the morphology of cells was observed under the microscope at 32 × 10 magnifications (AXIOVERT10, ZEISS, Germany), and images were recorded.
4. Gas chromatography
Gas chromatography analysis was performed on an Agilent 6890N equipped with a DB-1MS column (30 mm × 0.25 mm i.d., 0.25 μm film thickness, J&W Scientific, Folsom, CA). The oven temperature was programmed as: isothermal at 40°C for 1 minute, then raised to 250°C (6°C/minute) and held at this temperature for 4 minutes. Helium was used as the carrier gas at the rate of 1.5 mL/minute in split mode (50:1 ratio). The constituents of the plant essential oil were identified by comparing their GC retention indices (RI). The RI of the constituents for each plant essential oil were identified by co-injection of essential oil and a mixture of aliphatic hydrocarbons (C8–C20; Sigma-Aldrich, St. Louis, USA). RI was calculated using the equation proposed by van Den Dool and Kratz (1963) [13].
5. Gas chromatography-mass spectrometry
The oils (marjoram, clary sage and anise) were analyzed further on a gas chromatograph (Agilent 6890N)-mass spectrometer (Agilent 5973N MSD) equipped with a DB-5MS column (30 m × 0.25 mm i.d., 0.25 μm film thickness, J & W Scientific, Folsom, CA). The oven temperature was programmed as described previously. Helium was used as the carrier gas at the rate of 1.0 mL/minute. The effluent of the GC column was introduced directly into the source of the MS via a transfer line (250°C). Ionization voltage was 70 eV and the ion source temperature was 230°C. Scan range was 41–450 amu. Compounds were identified by comparison of mass spectra of each peak with those of authentic samples in the NIST MS library.
Results
1. Marjoram, clary sage and anise oils possess anti-influenza A/WS/33 virus activity
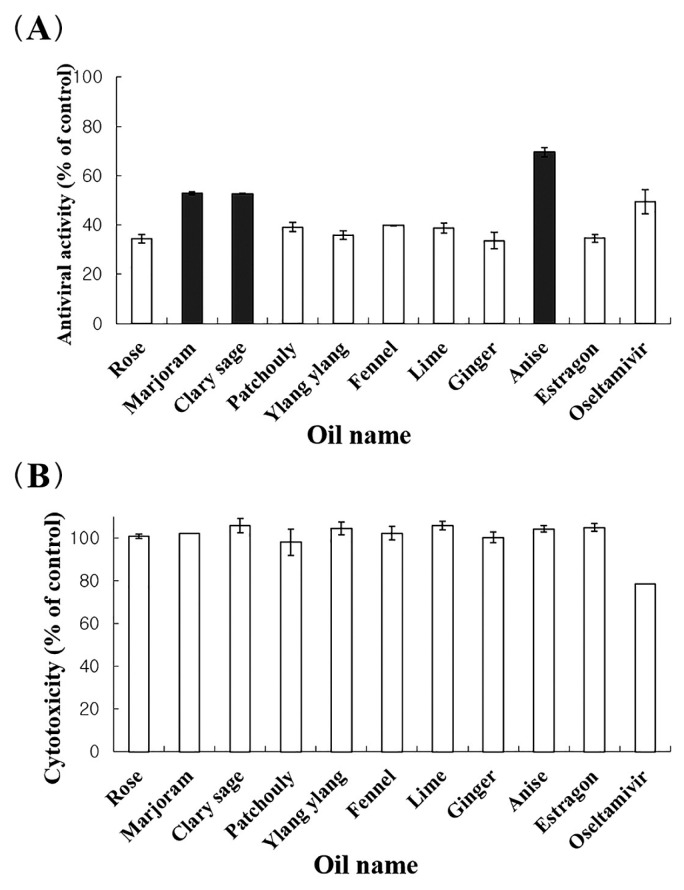
Anti-influenza (A/WS/33 virus) activity of all essential oils were investigated. Eleven essential oils amongst the 62 essential oils tested possessed antiviral activity of > 30% and did not show cytotoxicity at a concentration of 100 μg/mL (Figure 1A). There were 3 oils (marjoram, clary sage and anise oils) that exhibited a higher anti-influenza activity (> 52%) than oseltamivir, with no cytotoxicity at a concentration of 100 μg/mL. However, oseltamivir showed cytotoxicity at this concentration (Figure 1B).
Figure 1.
Antiviral activity of essential oils on influenza A/WS/33 virus.
Virus suspension and media containing essential oil (100 μg/mL) were added to the cells. After incubation 2 days, antiviral activity was investigated using the CPE reduction assay.
Results are presented as the mean percentage values obtained from 3 independent experiments carried out in triplicate ± SD.
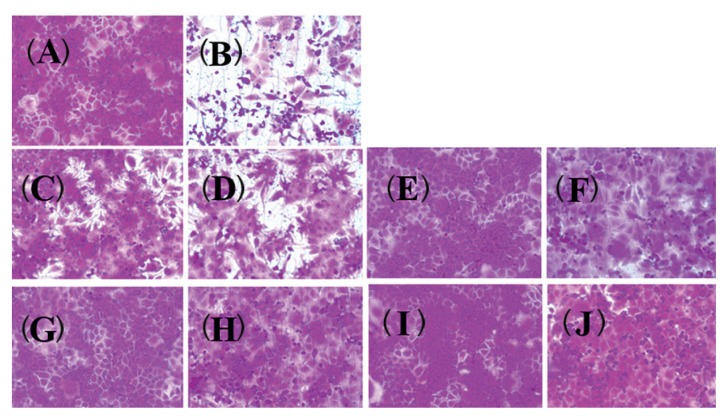
2. Marjoram, clary sage and anise oils reduce anti-influenza A/WS/33 virus-induced morphological changes
The effects of essential oils on influenza A/WS/33 virus-induced CPE were investigated (Figure 2). After a 2-day infection of MDCK cells with influenza A/WS/33 virus, mock cells (Figure 2A) or cells treated with 100 μg/mL essential oils (Figures 2E, 2G and 2I) showed typical morphology. At this concentration (100 μg/mL), there were no signs of cytotoxicity induced by the essential oils. However, oseltamivir was weakly toxic to MDCK cells at concentration of 100 μg/mL (Figure 2C). Infection with influenza A/WS/33 virus, in the absence of essential oils, resulted in a severe CPE (Figure 2B). Addition of the essential oils to the influenza infected MDCK cells, inhibited the formation of a visible CPE (Figures 2F, 2H and 2J). However, the addition of oseltamivir to influenza A/WS/33 virus-infected MDCK cell resulted in a small reduction in CPE (Figure 2D). Thus, the CPE of the virus infection was shown to be prevented by the presence of the essential oils.
Figure 2.
The effect of essential oils on influenza B/Lee/40 virus-induced CPE. The virus-infected cells were treated with essential oils (100 μg/mL). After incubation 2 days, the cells was stained by SRB and the morphology was examined.
Non-infected cells; (B) virus-infected cells without oils; (C) non-infected cells with oseltamivir; (D) virus-infected cells with oseltamivir; (E) non-infected cells with marjoram oil; (F) virus-infected cells with marjoram oil; (G) non-infected cells with clary sage oil; (H) virus-infected cells with clary sage oil; (I) non-infected cells with anise oil; (J) virus-infected cells with anise oil.
CPE = cytopathic effect; SRB = sulforhodamine B.
3. Chemical compositions of the marjoram, clary sage and anise oils
The chemical compositions of marjoram, clary sage and anise essential oils are shown in Tables 2 to 4. A total of 10 compounds were identified in marjoram oil by GC and GC-MS analysis (Table 2). Among the identified compounds, 1,8-cineole (64.61%) was the most abundant compound followed by linalool (15.28%) and β–pinene (5.81%; Table 2). The chemical compositions of clary sage oil showed linalayl acetate (61.16%) to be the highest concentration, followed by linalool (22.06%) and α–terpineol (4.21%; Table 3). The chemical compositions of anise oil were trans-anethole (82.78%), estragole (8.21%) and linalool (2.74%; Table 4).
Table 2.
Chemical composition of marjoram oil.
| No. | Compound | Retention time (min) | Relative composition ratio (%) |
|---|---|---|---|
| 1 | α –pinene | 928 | 4.21 |
| 2 | Sabinene | 962 | 1.82 |
| 3 | β–pinene | 967 | 5.81 |
| 4 | β–myrcene | 981 | 1.02 |
| 5 | p-cymene | 1,010 | 0.94 |
| 6 | 1,8-cineole | 1,018 | 64.61 |
| 7 | Linalool | 1,084 | 15.28 |
| 8 | Terpinen-4-ol * | 1,159 | 1.29 |
| 9 | α–terpineol * | 1,170 | 2.40 |
| 10 | Bornylacetate * | 1,435 | 2.61 |
| Total | 100.0 |
Identified by mass library.
Table 4.
Chemical composition of anise oil.
| No. | Compound | Retention time (min) | Relative composition ratio (%) |
|---|---|---|---|
| 1 | α –pinene | 928 | 0.85 |
| 2 | Limonene | 1,019 | 2.55 |
| 3 | Linalool | 1,084 | 2.74 |
| 4 | Estragole | 1,173 | 8.21 |
| 5 | 4-Allylanisole * | 1,211 | 1.21 |
| 6 | trans-Anethole | 1,259 | 82.78 |
| 7 | Charvicol * | 1,650 | 1.08 |
| Total | 99.41 |
Identified by mass library.
Table 3.
Chemical composition of clary sage oil.
| No. | Compound | Retention time (min) | Relative composition ratio (%) |
|---|---|---|---|
| 1 | β–myrcene | 981 | 1.53 |
| 2 | Linalool | 1,084 | 22.06 |
| 3 | α–Terpineol | 1,170 | 4.21 |
| 4 | Cinnamaldehyde * | 1,234 | 1.81 |
| 5 | Linalayl acetate * | 1,240 | 61.16 |
| 6 | Geranyl acetate | 1,360 | 2.40 |
| Total | 93.17 |
Identified by mass library.
Discussion
Many antiviral compounds have been developed against the influenza virus, the long-term efficacy of which is often limited due to toxicity or the emergence of drug-resistant virus mutants [14]. Hence, new approaches for the control of highly pathogenic influenza viruses must be explored.
Previous studies showed antiviral activity of essential oils against DNA viruses such as herpes simplex virus [7,8]. Ocimum basilicum (OB), also known as sweet basil, showed antiviral activities against DNA viruses (herpes viruses (HSV), adenoviruses (ADV) and hepatitis B virus and RNA viruses (coxsackievirus B1 (CVB1) and enterovirus 71 (EV71). Apigenin, linalool and ursolic acid isolated from crude aqueous and ethanolic extracts of OB, exhibited a broad spectrum of antiviral activity against these viruses [15].
In this work, marjoram, clary sage and anise oils exhibited strong anti-influenza A/WS/33 virus activity. Linalool was a common constituent in the chemical compositions of marjoram, clary sage and anise oils. Therefore, the anti-influenza A/WS/33 activity of marjoram, clary sage and anise oils appeared to be associated with linalool. However, further studies will be required to explore the anti-influenza A/WS/33 virus effects of linalool.
In conclusion, marjoram, clary sage and anise oils showed interesting anti-influenza A/WS/33 activity. A common constituent of the 3 oils was linalool. Therefore, further studies are required to understand whether or not linalool possesses antiviral activity against influenza A/WS/33 virus.
Acknowledgments
This paper was supported (in part) by Research Funds of Kwangju Women’s University in 2018 (KWUI18-035).
Footnotes
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
References
- 1.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56(1):152–79. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garman E, Laver G. Controlling influenza by inhibiting the virus’s neuraminidase. Curr Drug Targets. 2004;5(2):119–36. doi: 10.2174/1389450043490604. [DOI] [PubMed] [Google Scholar]
- 3.Kiso M, Mitamura K, Sakai-Tagawa Y, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364(9436):759–65. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- 4.Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437(7062):1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- 5.Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86(6):985–90. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 6.Meepagala KM, Sturtz G, Wedge DE. Antifungal constituents of the essential oil fraction of Artemisia dracunculus L. var. dracunculus. J Agric Food Chem. 2002;50(24):6989–92. doi: 10.1021/jf020466w. [DOI] [PubMed] [Google Scholar]
- 7.Tragoolpua Y, Jatisatienr A. Anti-herpes Simplex Virus Activities of Eugenia caryophyllus (Spreng). Bullock & S. G. Harrison and Essential Oil, Eugenol Phytother Res. 2007;21(12):1153–8. doi: 10.1002/ptr.2226. [DOI] [PubMed] [Google Scholar]
- 8.Koch C, Reichling J, Schneele J, et al. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomed. 2008;15(1–2):71–8. doi: 10.1016/j.phymed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Schnitzler P, Schuhmachera A, Astania A, et al. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomed. 2008;15(9):734–40. doi: 10.1016/j.phymed.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Cermelli C, Fabio A, Fabio G, et al. Effect of eucalyptus essential oil on respiratory bacteria and viruses. Curr Microbiol. 2008;56(1):89–92. doi: 10.1007/s00284-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 11.Yang ZC, Wang BC, Yang XS, et al. Chemical composition of the volatile oil from Cynanchum stauntonii and its activities of anti-influenza virus. Colloids and Surfaces B: Biointerfaces. 2005;43(3–4):198–202. doi: 10.1016/j.colsurfb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Choi HJ, Kim JH, Lee CH, et al. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81(1):77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Den Dool H, Kratz PD. Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr. 1963;11:463–71. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 14.Hayden FG. Antivirals for influenza: historical perspectives and lessons learned. Antivir Res. 2006;71(1–2):372–8. doi: 10.1016/j.antiviral.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Chiang LC, Ng LT, Cheng PW, et al. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol. 2005;32(10):811–6. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]