Supplemental Digital Content is available in the text.
Keywords: paralysis, myelitis, enterovirus, enterovirus D68, laboratory surveillance
Abstract
Background:
Enterovirus-D68 (EV-D68) is a respiratory virus within the genus Enterovirus and the family of Picornaviridae. Genetically, it is closely related to rhinovirus that replicates in the respiratory tract and causes respiratory disease. Since 2014, EV-D68 has been associated with the neurologic syndrome of acute flaccid myelitis (AFM).
Methods:
In October 2016, questionnaires were sent out to a European network including 66 virologists and clinicians, to develop an inventory of EV-D68–associated AFM cases in Europe. Clinical and virologic information of case patients was requested. In addition, epidemiologic information on EV testing was collected for the period between March and October 2016.
Results:
Twenty-nine cases of EV-D68–associated AFM were identified, from 12 different European countries. Five originated from France, 5 from Scotland and 3 each from Sweden, Norway and Spain. Twenty-six were children (median age 3.8 years), 3 were adults. EV-D68 was detected in respiratory materials (n = 27), feces (n = 8) and/or cerebrospinal fluid (n = 2). Common clinical features were asymmetric flaccid limb weakness, cranial nerve deficits and bulbar symptoms. On magnetic resonance imaging, typical findings were hyperintensity of the central cord and/or brainstem; low motor amplitudes with normal conduction velocities were seen on electromyography. Full clinical recovery was rare (n = 3), and 2 patients died. The epidemiologic data from 16 European laboratories showed that of all EV-D68–positive samples, 99% was detected in a respiratory specimen.
Conclusions:
For 2016, 29 EV-D68–related AFM cases were identified in mostly Western Europe. This is likely an underestimation, because case identification is dependent on awareness among clinicians, adequate viral diagnostics on respiratory samples and the capability of laboratories to type EVs.
Enterovirus-D68 (EV-D68) is a member of the genus Enterovirus, which belongs to the Picornaviridae family. The genus Enterovirus consists of many species, including human EV-A, B, C, D and human rhinovirus A, B, C, which can be further classified in different genotypes. Examples of EV genotypes are coxsackievirus, enterovirus A71, poliovirus and echovirus. These viruses are associated with a range of clinical symptoms, such as myocarditis, hand–foot–mouth disease, acute flaccid paralysis (AFP) and aseptic meningitis. The majority of enteroviruses replicate in the gastrointestinal tract and can be detected in stool samples. EV-D68 and rhinovirus, however, replicate in the upper airways and are best detected in respiratory samples.
Since 2014, EV-D68 has gained interest after causing a large respiratory disease outbreak in North America.1 Symptoms varied in severity from a common cold to respiratory failure requiring mechanical ventilation. Most hospitalized patients were children and severe cases often had underlying pulmonary conditions, such as asthma.2 The concurrent circulation of EV-D68 in Europe was shown by a joint effort of the European Society of Clinical Virology (ESCV) – European Centre for Disease Prevention and Control (ECDC) EV-D68 study group: 16,332 respiratory samples were screened for EV-D68 and 343 (2.1%) were positive.3
During this 2014 epidemic, 120 cases of acute flaccid myelitis (AFM) in children were identified in the United States, with the most common virus detected in upper respiratory tract specimens being EV-D68.4 AFM is a polio-like neurologic condition, characterized by an acute onset of asymmetric multifocal limb weakness with spinal cord lesions evident on magnetic resonance imaging (MRI).5 An epidemiologic link was made between the AFM upsurge and the concurrent EV-D68 outbreak in the United States.4,6 During the same period, 4 AFM patients with respiratory EV-D68 infections were reported in Europe.7–9
In the winter of 2015/2016, 2 AFM cases with concurrent EV-D68 infection were identified in Wales,10 and in July 2016, a severe case of EV-D68–related AFM in a 4-year-old boy was identified in the Netherlands.11 Subsequently, through an e-mail alert to the previously established ESCV-ECDC EV-D68 study group network, more cases of EV-D68–related AFM were rapidly identified. An intense collaboration between virologists and clinicians (ie, pediatric neurologists and infection disease specialists) from across Europe was established. In this article, we present the clinical and virologic data of the 29 cases that were identified through this network, to illustrate the clinical picture and to improve future patient identification.
The 2014 ESCV-ECDC collaborative work3 showed that only limited data were available on the epidemiology of respiratory EV infections. This was especially, but not exclusively, the case for Eastern and Southern European countries, among others because of a lack of diagnostic testing and typing of EVs in respiratory samples. In line with our study in 2014, we collected epidemiologic data for Europe in 2016. We present data on EV and EV-D68 testing and positivity rates, to emphasize the impact of adequate diagnostics, and on notification regulations of AFM in the various European countries.
MATERIALS AND METHODS
Members of the 2014 ESCV-ECDC EV-D68 study group3, mostly virologists, were contacted by the coordinating center (University Medical Center, Groningen, The Netherlands) through an e-mail alert. Additionally, EV reference laboratories in Eastern Europe were informed of the initiative, as they were underrepresented in the study group. Finally, through this network and in reply to scientific presentations or publications on the subject, we contacted clinicians who diagnosed or treated a patient with EV-D68–related AFM. The collaborating centers and clinicians (from this point on referred to as the 2016 EV-D68 AFM Working Group, with 66 members (Supplemental Digital Content 1, http://links.lww.com/INF/D269) were sent a questionnaire by which they were asked to report the number of EV-D68–related AFM cases diagnosed in 2016. For each case, information was inquired regarding age, gender, prodromal phase, neurologic abnormalities (mental status, signs of nuchal rigidity, cranial nerve dysfunction, limb weakness, tendon reflexes and sensory disturbances), virologic diagnostics, neurologic investigations [cerebrospinal fluid (CSF) analysis, MRI, electromyography (EMG)] and clinical follow-up.
Additionally, information on diagnostic EV testing was collected via the questionnaire. For the period between March and October 2016, we requested the number of EV tests performed on all clinical specimens (respiratory, CSF, feces and blood), the number of EV-positive tests and the number of EV-D68–positive tests. Twenty-one laboratories responded, including both diagnostic and reference laboratories. The national reference center of Bulgaria reported that EV detection was performed in their institution, but no further typing was done for non-polio EVs. Furthermore, the data from 3 laboratories that tested less than 100 samples for EV (from Portugal, Czech and Finland) and from 1 laboratory that could not detect EV-D68 with the current techniques (from Estonia) were excluded.
Finally, the notification status of AFM was questioned per country.
Case Definition EV-D68–related AFM
AFM is a specific form of AFP. Its definition was stated in 2015 and adapted since by the Centers for Disease Control and Prevention.5 The 3 key components of the EV-D68–related AFM case definition are: (1) Acute onset of focal limb weakness, (2) MRI showing a spinal cord lesion largely restricted to the grey matter and spanning 1 or more spinal segments and (3) Detection of EV-D68 in a respiratory, fecal, blood or CSF specimen using a validated polymerase chain reaction (PCR) assay for EV-D68, or a validated PCR assay for EVs in general and subsequent sequencing and typing. If MRI is not performed, or findings are normal, and the CSF shows pleocytosis, the patient is considered a “probable” case.
Typing and Phylogenetic Mapping
To compare the viral sequences, the collaborating centers were asked to share the sequencing files of their EV-D68 cases (both of respiratory and AFM cases). Alternatively, samples could be sent to one of the participating laboratories for sequencing. Typing was performed using the standard method described by Nix et al,12 which consists of partial sequencing of the viral protein 1. Phylogenetic analysis was performed using BioNumerics Software version 6.6 (Applied Maths, Sint-Martens-Latem, Belgium).
Ethics Approval
The research ethics committee of the coordinating center confirmed exemption from the Medical Research Involving Human Subjects Act (Decree M17.207412). Local ethics approval and informed consent from participating patients or their parents were obtained according to individual institutional requirements.
RESULTS
EV-D68–related AFM Cases
We received the clinical data from 29 EV-D68–related AFM cases, from 12 different countries. Table 1 shows the clinical data of these cases (more extensive descriptions can be found in the Table, Supplemental Digital Content 2, http://links.lww.com/INF/D270). The distribution of cases over Europe is shown in the (Figure, Supplemental Digital Content 3, http://links.lww.com/INF/D271. Twenty-six children were affected, with a median age of 3.8 years (range 1.6–9.0) and 3 adults were included in this series. Gender was equally distributed. EV-D68 was detected in a respiratory sample of 27 patients, in the feces of 8 patients and in the CSF of 2 patients. Only 1 child was coinfected with another neurotropic virus, EV-A71.
TABLE 1.
Clinical Description of 29 Enterovirus-D68–related Acute Flaccid Myelitis Cases, Europe 2016

Medical history was nonsignificant, except for an adult patient who received an allogeneic hematopoietic stem cell transplantation for Non-Hodgkin B-cell lymphoma 2 years earlier.13 A prodromal phase with fever (n = 24) and/or respiratory symptoms (n = 26) preceded weakness by a median period of 2 days. Weakness was flaccid and usually asymmetric, with decreased or absent reflexes. Upper limbs were more frequently and often more severely affected than lower limbs. Cranial nerve deficits were common (n = 17). Nineteen patients needed ventilatory support. Information on duration of ventilator dependency was scarce, but at least 7 children needed tracheostomy for long-term ventilator support.
CSF analysis frequently showed a moderate pleocytosis (normal value <5 leukocytes/µL), while protein levels were mostly normal or slightly raised (normal value <0.55 g/L, dependent on age and center).
MRI was reported to be abnormal in 25 cases, with hyperintensity in the central grey matter of the cervical and/or thoracic spine in 23 patients and hyperintensity of the dorsal brainstem in 17 patients. Figure 1 shows the typical AFM lesions on MRI for 2 patients. EMG was performed in 11 patients and generally revealed decreased amplitude of compound motor action potentials with normal conduction velocities and absence of conduction blocks, compatible with anterior horn cell disease. Spontaneous muscle fiber activity, either positive sharp waves or fibrillations, was found in the affected muscles when needle EMG was performed after sufficient time (range: 7 days to 3 months).
FIGURE 1.
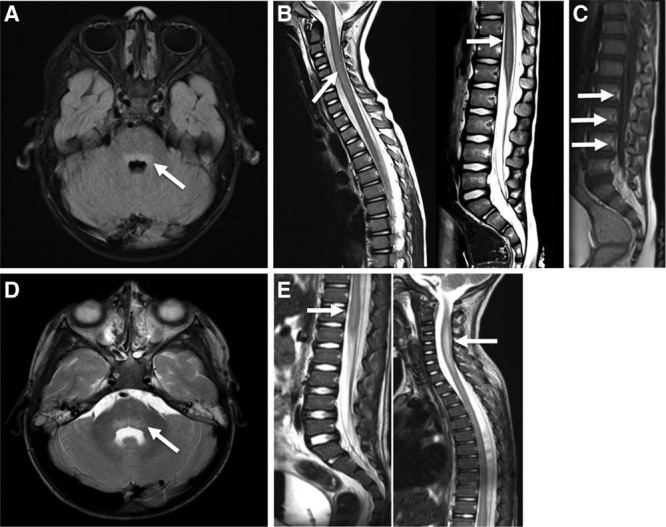
Typical magnetic resonance imaging findings in patients with enterovirus-D68–associated acute flaccid myelitis. Magnetic resonance images of 2 cases (case a: A–C; case b: D–E), dating from the first week after the start of neurologic symptoms, showing typical imaging features of AFM. A + D, Transversal T2-weighted images of the brain show a slight hyperintensity (arrow) in the dorsal pons. B + E, Sagittal T2-weighted images show hyperintensity (arrow) of the central gray matter in the cervical and thoracic regions in both patients. C, A sagittal T1-weighted image with gadolinium shows enhancement (arrows) of the caudal roots.
Most patients were treated with intravenous immunoglobulins (n = 20), steroids (n = 17) or both (n = 15), with typically only partial recovery over time. Follow-up time ranged from 0.5 to 12 months. Two patients, who both showed EV-D68 in the CSF, died. One was a 55-year-old immunocompromised woman, who died of respiratory failure. The second was a 3.5-year-old child, who died of severe ventilation acquired pneumonia with septic shock.
Epidemiology and Diagnostics
From March to October 2016, 21,875 EV tests were reported by 16 European laboratories, as shown in the Table (Supplemental Digital Content 4, http://links.lww.com/INF/D272). This table does not contain the data from EV-D68 AFM cases that were reported by clinicians without epidemiologic data from the diagnostic laboratory, so it has only a partial overlap with Table 1. Of the 21,875 EV tests reported on all clinical specimens, 2111 were EV positive (10%; excluding those EV-positive samples for which no denominator was given). Of the total number of 2381 EV-positive samples, 416 were EV-D68 (17%). Taking a closer look at respiratory samples, 10,226 EV tests were performed, with 987 (10%) EV-positive samples. Of the total amount of 1067 EV-positive samples in respiratory specimens, 414 were EV-D68 positive (39%). Only 1 of 558 EV-positive CSF samples (0.18%) and 1 of 711 EV-positive feces samples (0.14%) was positive for EV-D68 (data not shown in the table).
Sequence data of the viral protein 1 region were available for 6 of 29 AFM patients, and together with many other EV-D68 strains of 2014 and 2016, these were used for sequence analysis. The Figure (Supplemental Digital Content 5, http://links.lww.com/INF/D273) shows the dominance of the B3 clade in 2016, irrespective of respiratory or neurologic symptoms.
Notification Regulations
The following information was obtained regarding notification regulations: AFP/AFM is a reportable disease in all European countries within the scope of polio eradication. Only in Norway, also non-polio AFP/AFM cases are notifiable and the requirement of a respiratory sample for testing was added after the EV-D68 outbreak in 2014. In Germany and France, non-polio AFP/AFM is voluntarily reported. In Norway, Sweden, Ireland, Italy, France and Slovenia, (entero-) (viral) meningitis/encephalitis is reportable. In Denmark, EV meningitis and paralysis are reportable and recently the required specimens for testing were expanded with a respiratory sample.19 For the remaining countries, no clear regulations exist for non-polio AFP/AFM cases.
DISCUSSION
The association between EV-D68 and AFM has become clear since 2014, although causality was not yet proven.4,6,20 The recent publication of a mouse model,21 in which mice that had been inoculated with EV-D68 developed symptoms of myelitis, added important evidence supporting causality. Furthermore, using the Bradford-Hill criteria, 2 groups evaluated both the epidemiologic and biologic evidence linking EV-D68 to AFM.22,23 Several case reports and small case series have been published from the United States, Canada, South America, Australia, Asia and Europe, describing patients with EV-D68–related AFM.7,8,10,11,13–18,24–30 In this article, we presented the first comprehensive EV-D68 AFM case series and an epidemiologic overview for Europe in 2016.
Clinical Manifestations and Treatment
In children, the median age of 3.8 years at onset of AFM was in line with the median age in a Japanese EV-D68–related AFM upsurge in 2015 (4.4 years30), but somewhat lower than the median age of those affected in 2014 in the United States (7.1 years4). If this is a true difference, it would be interesting to investigate if lower serologic protection rates in the 4-year-olds in 2016 could have caused this shift. We included the 3 adult cases in our series to point out that EV-D68–related AFM is not restricted to childhood age.
The clinical presentation of the affected patients in Europe 2016 resembled that of patients from other parts of the world regarding prodromal symptoms and neurologic manifestations, with asymmetric flaccid limb weakness, sometimes accompanied by pain, cranial nerve deficits and bulbar symptoms.26 It may be difficult to distinguish AFM clinically from other neurologic diseases, such as Guillain–Barre syndrome, acute transverse myelitis, Miller Fisher syndrome or acute disseminated encephalomyelitis. Additionally, mild cases can be easily missed. The case definition provides descriptions of specific MRI lesions along the spinal cord. Additionally, in the literature, lesions in the grey matter of the anterior horn and in the brainstem are described, as well as contrast enhancement of nerve roots.31 When MRI is not performed or these specific MRI lesions are not (yet) visible, patients may meet the criteria of a probable case when they show a mild CSF pleocytosis,5,26 as did 1 of our patients. Two patients strictly did not fulfill the criteria of the case definition, as MRI results and CSF analyses were lacking. They were nevertheless included in this study, based on the clinical picture of AFM with respiratory insufficiency and/or bulbar symptoms, and the detection of EV-D68 in respiratory samples.
If feasible in the young child, EMG findings can be of great value in supporting the diagnosis of AFM. Thus far, children with EV-D68–associated AFM generally showed low amplitude compound muscle action potentials, most often with normal conduction velocity, without signs of sensory nerve conduction abnormalities. In a later stage of disease, spontaneous muscle fiber activity can be found in the affected muscles.26,32
Although an attempt was made to capture the features of EV-D68–related AFM in a case definition, it should be emphasized that EV-D68 is not the only virus that can cause AFM. For example, West-Nile virus and other EVs, such as EV-A71, should be considered as causative agents. Neither is AFM the only neurologic disorder that is associated with EV-D68 infection; EV-D68 has been found in patients with rhombencephalitis24 and, in this cases series, 1 child from France was submitted with a Guillain–Barre syndrome and a concurrent EV-D68 infection (data not shown).
Various treatment regimens were prescribed in this case series. It is unfortunately not possible to deduce any positive or negative effects from these data. Similarly, in other series, no therapeutic intervention seemed to have significantly improved outcome. However, with a mouse model, Hixon et al21 showed that EV-D68 immune-sera protected mice from development of paralysis and death when administered before viral challenge. Furthermore, recent data using this mouse model showed a favorable effect of intravenous immunoglobulin administered after infection as well; high-dose corticosteroids, however, had a negative effect on motor function and mortality.33 Because of these findings, treatment protocols with corticosteroids as a first-line treatment may be subject to discussion.
In the literature, full neurologic rehabilitation has occurred only in a minority of patients after a 12- to 18-month period of follow-up, although MRI lesions may disappear.34,35 The 2016 EV-D68 AFM Working Group aims at a standardized follow-up of the European patients beyond 12 months after the onset of illness, to get more insight in the natural course of the disease and to further improve education of patients and parents on the prognosis of EV-D68–related AFM.
In this series, the 2 patients who showed EV-D68 in the CSF did not survive. This may imply more severe disease, but larger studies are needed to evaluate this.
Epidemiology and Diagnostics
Our data showed a wide range of both EV positivity rates and EV-D68 positivity rates between the laboratories. This is likely explained by differences in non-polio EV-surveillance strategies and testing and typing algorithms in Europe, as mapped out by Harvala et al.36 A way to overcome these differences would be the intensification of non-polio EV-surveillance, such as initiated in Denmark and by the European non-polio EV network.19,37
Standard EV diagnostics, as well as poliovirus surveillance, generally relies on testing in feces, as poliovirus and the majority of other EV serotypes can indeed be detected in fecal samples. However, our epidemiologic data show that 99% of EV-D68–positive samples were respiratory specimens. This underlines that EV-D68 has a predominant respiratory tropism and respiratory specimens are required for identification of the virus.9,26,35 The near absence of EV-D68 in fecal samples is in line with a previous study.38 However, in our case series, EV-D68 was more frequently detected in feces and CSF than was expected based on our epidemiologic data. This difference likely reflects the widespread occurrence of EV-D68 respiratory disease, with the virus being present in respiratory specimens, and the rarity of EV-D68–related AFM, with the virus potentially present in multiple compartments, plus a more thorough microbiologic investigation in AFM patients because of disease severity.
Recently, the World Health Organization and the Pan American Health Organization have released an epidemiologic alert to include testing for EV-D68 on respiratory samples in cases of AFP/AFM, both for case management and for surveillance purposes.39 It is important to note that not all respiratory PCR panels include EV as a target. Second, not all molecular tests that target EVs are able to detect EV-D68 or distinguish EV-D68 from rhinoviruses. Communication between clinicians and virologists is therefore essential to optimize diagnostics.
Sequence analysis showed that most of the EV-D68 strains in 2014 clustered with clades A1, A2, B1 and B2.3,40 In 2016, however, nearly all strains belonged to subclade B3 in Europe as well as in the United States.40 The clinical importance of this shift is yet unclear.
This study reveals that crucial information is often not (timely) available, among others by a lack of non-polio AFP/AFM notification regulations, and therefore the overview is by no means complete. By activating the 2016 EV-D68 AFM Working Group network, we were able to identify 29 EV-D68–related AFM cases in Europe in 2016, but these probably represent only the tip of the iceberg. All cases were reported by countries that had also joined in the 2014 initiative. Clearly, these countries were already interested in EV-associated diseases and were therefore more prompted to identify cases when confronted with paralyzed patients. Additional AFM cases that may have been due to EV-D68 but did not have etiology confirmed because of late or absent sampling and testing, likely have been missed.
As EV-D68 has shown a cyclic pattern since 2010,11,29,41 it is conceivable that the virus might reappear in the very near future. As no major changes have occurred in making AFM reportable in Europe, a new outbreak may go largely undetected by the health authorities. In the short term, we might benefit most from an e-mail alert system, by which clinicians and laboratories inform each other on the start of the EV season, the upsurge of rare types and on special EV-associated syndromes, such as AFM.
Supplementary Material
Footnotes
List of the members of 2016 EV-D68 AFM Working Group is provided in Supplemental Digital Content 1, http://links.lww.com/INF/D269.
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Messacar K, Abzug MJ, Dominguez SR. 2014 outbreak of enterovirus D68 in North America. J Med Virol. 2016;88:739–745.. [DOI] [PubMed] [Google Scholar]
- 2.Midgley CM, Watson JT, Nix WA, et al. ; EV-D68 Working Group. Severe respiratory illness associated with a nationwide outbreak of enterovirus D68 in the USA (2014): a descriptive epidemiological investigation. Lancet Respir Med. 2015;3:879–887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poelman R, Schuffenecker I, Van Leer-Buter C, et al. European surveillance for enterovirus D68 during the emerging North-American outbreak in 2014. J Clin Virol. 2015;71:1–9.. [DOI] [PubMed] [Google Scholar]
- 4.Sejvar JJ, Lopez AS, Cortese MM, et al. Acute flaccid myelitis in the United States, August-December 2014: results of Nationwide Surveillance. Clin Infect Dis. 2016;63:737–745.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Acute flaccid myelitis. Case definitions. Available at: https://www.cdc.gov/acute-flaccid-myelitis/hcp/case-definition.html. Accessed January 15th, 2018.
- 6.Aliabadi N, Messacar K, Pastula DM, et al. Enterovirus D68 infection in children with acute flaccid myelitis, Colorado, USA, 2014. Emerg Infect Dis. 2016;22:1387–1394.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pfeiffer HC, Bragstad K, Skram MK, et al. Two cases of acute severe flaccid myelitis associated with enterovirus D68 infection in children, Norway, autumn 2014. Euro Surveill. 2015;20:21062. [DOI] [PubMed] [Google Scholar]
- 8.Lang M, Mirand A, Savy N, et al. Acute flaccid paralysis following enterovirus D68 associated pneumonia, France, 2014. Euro Surveill. 2014;19:20952. [DOI] [PubMed] [Google Scholar]
- 9.Varghese R, Iyer A, Hunter K, et al. Sampling the upper respiratory tract for enteroviral infection is important in the investigation of an acute neurological illness in children. Eur J Paediatr Neurol. 2015;19:494–495.. [DOI] [PubMed] [Google Scholar]
- 10.Williams CJ, Thomas RH, Pickersgill TP, et al. Cluster of atypical adult Guillain-Barre syndrome temporally associated with neurological illness due to EV-D68 in children, South Wales, United Kingdom, October 2015 to January 2016. Euro Surveill. 2016;21:30119. [DOI] [PubMed] [Google Scholar]
- 11.Knoester M, Schölvinck EH, Poelman R, et al. Upsurge of Enterovirus D68, the Netherlands, 2016. Emerg Infect Dis. 2017;23:140–143.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006;44:2698–2704.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giombini E, Rueca M, Barberi W, et al. Enterovirus D68-associated acute flaccid myelitis in immunocompromised woman, Italy. Emerg Infect Dis. 2017;23:1690–1693.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrerizo M, García-Iñiguez JP, Munell F, et al. First cases of severe flaccid paralysis associated with Enterovirus D68 infection in Spain, 2015-2016. Pediatr Infect Dis J. 2017;36:1214–1216.. [DOI] [PubMed] [Google Scholar]
- 15.Dyrdak R, Grabbe M, Hammas B, et al. Outbreak of enterovirus D68 of the new B3 lineage in Stockholm, Sweden, August to September 2016. Euro Surveill. 2016;21:30403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esposito S, Chidini G, Cinnante C, et al. Acute flaccid myelitis associated with enterovirus-D68 infection in an otherwise healthy child. Virol J. 2017;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stacpoole SRL, Molyneux A, Bäumer D. Acute segmental poliomyelitis-like flaccid paralysis in an adult in the UK, associated with enterovirus D68. Pract Neurol. 2017;17:297–301.. [DOI] [PubMed] [Google Scholar]
- 18.Kirolos A, Mark K, Waugh C, et al. Cluster of acute flaccid paralysis in children following enterovirus D68 infection in Scotland. Eur J Public Health. 2017;27(suppl_3):ckx187.697. [Google Scholar]
- 19.Barnadas C, Midgley SE, Skov MN, et al. An enhanced Enterovirus surveillance system allows identification and characterization of rare and emerging respiratory enteroviruses in Denmark, 2015-16. J Clin Virol. 2017;93:40–44.. [DOI] [PubMed] [Google Scholar]
- 20.Messacar K, Schreiner TL, Maloney JA, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet. 2015;385:1662–1671.. [DOI] [PubMed] [Google Scholar]
- 21.Hixon AM, Yu G, Leser JS, et al. A mouse model of paralytic myelitis caused by enterovirus D68. PLoS Pathog. 2017;13:e1006199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyda A, Stelzer-Braid S, Adam D, Chughtai AA, MacIntyre CR. The association between acute flaccid myelitis (AFM) and Enterovirus D68 (EV-D68) - what is the evidence for causation? Euro Surveill. 2018;23:17-00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messacar K, Asturias EJ, Hixon AM, et al. Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality. Lancet Infect Dis. 2018;18:e239–e247.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antona D, Kossorotoff M, Schuffenecker I, et al. Severe paediatric conditions linked with EV-A71 and EV-D68, France, May to October 2016. Euro Surveill. 2016;21:30402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelmann I, Fatoux M, Lazrek M, et al. Enterovirus D68 detection in respiratory specimens: association with severe disease. J Med Virol. 2017;89:1201–1207.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messacar K, Schreiner TL, Van Haren K, et al. Acute flaccid myelitis: a clinical review of US cases 2012-2015. Ann Neurol. 2016;80:326–338.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggieri V, Paz MI, Peretti MG, et al. Enterovirus D68 infection in a cluster of children with acute flaccid myelitis, Buenos Aires, Argentina, 2016. Eur J Paediatr Neurol. 2017;21:884–890.. [DOI] [PubMed] [Google Scholar]
- 28.Crone M, Tellier R, Wei XC, et al. Polio-like illness associated with outbreak of upper respiratory tract infection in children. J Child Neurol. 2016;31:409–414.. [DOI] [PubMed] [Google Scholar]
- 29.Levy A, Roberts J, Lang J, et al. Enterovirus D68 disease and molecular epidemiology in Australia. J Clin Virol. 2015;69:117–121.. [DOI] [PubMed] [Google Scholar]
- 30.Chong PF, Kira R, Mori H, et al. ; Acute Flaccid Myelitis Collaborative Study Investigators. Clinical features of acute flaccid myelitis temporally associated with an Enterovirus D68 outbreak: results of a Nationwide Survey of acute flaccid paralysis in Japan, August-December 2015. Clin Infect Dis. 2018;66:653–664.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maloney JA, Mirsky DM, Messacar K, et al. MRI findings in children with acute flaccid paralysis and cranial nerve dysfunction occurring during the 2014 enterovirus D68 outbreak. AJNR Am J Neuroradiol. 2015;36:245–250.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hovden IA, Pfeiffer HC. Electrodiagnostic findings in acute flaccid myelitis related to enterovirus D68. Muscle Nerve. 2015;52:909–910.. [DOI] [PubMed] [Google Scholar]
- 33.Hixon AM, Clarke P, Tyler KL. Evaluating treatment efficacy in a mouse model of Enterovirus D68-associated paralytic myelitis. J Infect Dis. 2017;216:1245–1253.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin JA, Messacar K, Yang ML, et al. Outcomes of Colorado children with acute flaccid myelitis at 1 year. Neurology. 2017;89:129–137.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yea C, Bitnun A, Robinson J, et al. Longitudinal outcomes in the 2014 acute flaccid paralysis cluster in Canada. J Child Neurol. 2017;32:301–307.. [DOI] [PubMed] [Google Scholar]
- 36.Harvala H, Jasir A, Penttinen P, Pastore Celentano L, Greco D, Broberg E. Surveillance and laboratory detection for non-polio enteroviruses in the European Union/European Economic Area, 2016. Euro Surveill. 2017;22:1600807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harvala H, Broberg E, Benschop K, et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J Clin Virol. 2018;101:11–17.. [DOI] [PubMed] [Google Scholar]
- 38.Van Leer-Buter CC, Poelman R, Borger R, et al. Newly identified Enterovirus C genotypes, identified in the Netherlands through routine sequencing of all Enteroviruses detected in clinical materials from 2008 to 2015. J Clin Microbiol. 2016;54:2306–2314.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan American Health Organization. Epidemiological alert: acute flaccid myelitis associated with enterovirus D68. Available at: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=42783&lang=en. Accessed January 15th, 2018.
- 40.Wang G, Zhuge J, Huang W, et al. Enterovirus D68 subclade B3 strain circulating and causing an outbreak in the United States in 2016. Sci Rep. 2017;7:1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahamat-Langendoen J, Riezebos-Brilman A, Borger R, et al. Upsurge of human enterovirus 68 infections in patients with severe respiratory tract infections. J Clin Virol. 2011;52:103–106.. [DOI] [PubMed] [Google Scholar]


