Supplemental digital content is available in the text.
Key Words: carcinosarcoma, epithelial-mesenchymal transition, pancreas, pancreatic ductal adenocarcinoma, undifferentiated (anaplastic) carcinoma
Abstract
Objectives
The aim of this study was to identify an association of pancreatic anaplastic carcinoma (APC) with the epithelial-mesenchymal transition (EMT).
Methods
Resected APCs (n = 24) were examined to assess components of APCs, including carcinomatous, transitional, and sarcomatous regions. Analysis was performed based on the immunoreactivity of E-cadherin and 3 EMT-related proteins: Slug (zinc finger protein SNAI2), Twist (Twist-related protein 1), and Zeb1 (zinc finger E-box–binding homeobox 1). Expression score was determined based on staining intensity and stained area of the target cells. Finally, we performed a hierarchical clustering based on the expression pattern of E-cadherin and EMT-related proteins of the sarcomatous component.
Results
The expression score of E-cadherin decreased in the order of sarcomatous > transitional > carcinomatous components (P < 0.01). Although there were significant differences in the immunohistochemical scores of Slug, Twist, and Zeb1 between carcinomatous and transitional components (P < 0.01), the significant difference in immunohistochemical score of Zeb1 between transitional and sarcomatous components was found (P < 0.05). Furthermore, APCs were divided into 2 subgroups based on the expression patterns of E-cadherin and EMT-related proteins (hierarchical clustering analysis). Consequently, these subgroups were distinguished by Twist expression.
Conclusions
Epithelial-mesenchymal transition plays an essential role in the pathogenesis of APC.
Pancreatic cancer often shows a poor prognosis, and it is the fourth leading cause of cancer-related death in the developed world.1 Curative surgical resection is critically important for a good prognosis.2,3 However, only 10% to 20% of patients are candidates for surgical resection at the time of diagnosis.4,5 Pancreatic cancer typically spreads rapidly into the peritoneum and is seldom detected in its early stages, which is a major reason why it is one of the leading causes of cancer death.6 Although it is difficult to precisely predict the aggressive behavior of pancreatic cancer, specific histologic types are well known as predictive factors of tumor prognosis. Anaplastic (undifferentiated) carcinoma (APC) of the pancreas has been regarded as a malignant tumor showing a poorer prognosis.7,8 This tumor has similarities to pleomorphic carcinoma, pleomorphic large cell carcinoma, pleomorphic giant cell carcinoma, spindle cell carcinoma, sarcomatoid carcinoma, and carcinosarcoma.9 Anaplastic (undifferentiated) carcinomas that were first described by Sommer and Meissner10 have been reported to be one of the most malignant tumors in the pancreas and account for 2% to 7% of all pancreatic cancers.7,10,11 Histologically, APCs are biphasic, composed of malignant epithelial and mesenchymal elements,12–14 and they have a highly aggressive character and poor prognosis in humans.7,8
The epithelial-mesenchymal transition (EMT), characterized by changes in cell phenotype from epithelial to mesenchymal morphology, is an important step in the invasion and metastasis of cancer.15,16 Although the EMT plays an important role in embryonic processes, its role in the pathogenesis of cancer is increasingly recognized.16,17 A recent study has identified a specific molecular mechanism underlying EMT.18 According to this theory, 3 or 4 key factors regulate the EMT program. Two of the key factors are the Snail-related zinc-finger transcription factors (Snail and Slug).18 Snail and Slug are thought to contribute to the invasion and metastatic process of cancer cells by promoting the EMT.19 In addition, zinc finger E-box–binding homeobox 1 (Zeb1) binds the regulatory gene sequence of the E-box that is involved in the promoter region of E-cadherin.18 As a result, Zeb1 represses the expression of E-cadherin and promotes the process of EMT by interacting with Snail 1.20,21 Twist is also an important factor that regulates the EMT.18 Twist-related protein 1 (Twist 1) cooperates with Snail1 in the induction of Zeb1 expression.22 These findings suggest that 3 or 4 proteins related to the EMT play crucial roles in the change of epithelial components to mesenchymal components that are characteristic histologic features of APC.
In the present study, we hypothesized that the EMT plays a major role in the pathogenesis of APC. The aim of this study was to identify the association of APC with the progression of the EMT process.
MATERIALS AND METHODS
Patients
We examined 24 APCs that had been retrieved from surgical pathology files of Iwate Medical University Hospital (Morioka, Japan), Tohoku University Hospital (Sendai, Japan), Sendai Medical Center (Sendai, Japan), and Sendai City Medical Center (Sendai, Japan). Histologic diagnosis was performed according to the criteria of the World Health Organization classification.9 The clinicopathologic stage was determined according to the tumor-node-metastasis (TNM) classification of malignant tumors (eighth edition) of the Union for International Cancer Control. In addition, histologic findings were defined by the Japan Pancreas Society classification. Clinicopathologic findings, including median age, sex, location, median diameter of tumor, histologic variants, TNM stage, lymphatic invasion, venous invasion, and nerve invasion are summarized in Table 1. The median follow-up period of patients examined was 26.7 months.
TABLE 1.
Clinicopathologic Characteristics of Patients With APCs
The research protocol was approved by the ethics committees of all institutions: Iwate Medical University (H29-11), Tohoku University Hospital (2017-1-111), Sendai Medical Center (29–33), and Sendai City Medical Center (2017–0022). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Sampling for Histologic Evaluation
All specimens were fixed in 10% neutral-buffered formalin and embedded in paraffin wax. All archival slides of APCs, which were originally prepared from formalin-fixed specimens, were stained with hematoxylin-eosin according to our routine hospital process. For this study, archival slides of all cases were reviewed, and the specimens including the morphologically archetypal areas of APCs, including epithelial and mesenchymal elements, were selected as representative tissue specimens.
Antibodies and Immunohistochemistry
The antibody sources used in this study are shown in Table 2. Paraffin sections were cut (3 μm) and mounted on poly-l-lysine–coated glass slides (Matsunami, Tokyo, Japan). Sections were routinely dewaxed and dehydrated and then subjected to heat-induced epitope retrieval in high pH Target Retrieval Solution (Dako, Carpinteria, Calif). The slides were placed in peroxidase-blocking solution (Dako) to inhibit non–specific-binding activity.
TABLE 2.
Antibodies Used for Immunohistochemical Studies

Immunostaining for E-cadherin and EMT-related proteins, including Slug, Twist and Zeb1, was performed by placing the sections in a microwave oven for 30 minutes for antigen retrieval. After primary antibody treatment, sections were examined using the EnVision HRP detection system (Dako). The antigen-antibody complex was visualized with DAB+ liquid chromogen (Dako) and counterstained with hematoxylin before mounting. Representative histologic findings and the immunoreactivity of E-cadherin and EMT markers in APC are shown in Figure 1.
FIGURE 1.
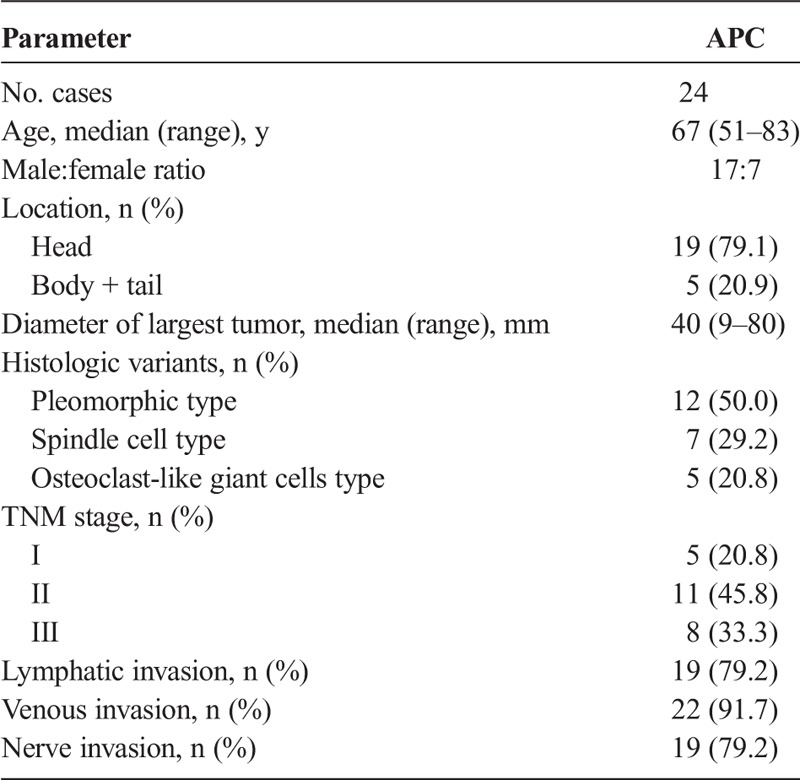
Histologic findings and immunoreactivity of E-cadherin and EMT transcription factors in APCs. Undifferentiated (anaplastic) carcinoma harboring foci of carcinomatous component (A) (original magnification ×40) and spindle cell–type APC showing pleomorphic tumor cells (B). Hematoxylin-eosin–stained sections (original magnification ×100). Immunohistochemistry detected E-cadherin in the cell membrane of carcinomatous component (C) (original magnification ×100). Slug (D), Twist (E), and Zeb1 (F) were positive in the nuclei of sarcomatous components (D–F, original magnification ×200).
Histologic Findings
Each slide was reviewed by 3 of the investigators (K.I., R.Y., and M.O.) using a multihead microscope, and a consensus was reached in all cases. We determined the 3 histologic components in representative hematoxylin-eosin–stained slides as follows: a ductal adenocarcinoma component with well- to moderately differentiated cells (carcinomatous component), a transitional zone containing a ductal adenocarcinoma component and an APC component with sarcomatous features (transitional component), and the APC component with sarcomatous features (sarcomatous component).
Evaluation for Immunohistochemical Examination
Immunoreactivity of the epithelial marker E-cadherin was positive with whole or partial membranous expression. The EMT markers (Slug, Twist, and Zeb1) were considered positive when observed within stained nuclei. The immunoreactivity scores for E-cadherin, Slug, Twist, and Zeb1 were determined by the staining intensity and the frequency of positive cells within each histologic section. The staining intensity was evaluated semiquantitatively as follows: 0 (none), 1 (mild), 2 (moderate), and 3 (intense). The area of the stained cells was divided into 6 levels, where X is the percentage observed: 0 (0% cells), 1 (0% ≤ x ≤ 1% cells), 2 (1% ≤ x ≤ 10% cells), 3 (10% ≤ x ≤ 33% cells), 4 (33% ≤ x ≤ 67% cells), and 5 (x ≥ 67% cells). The total score for each section was then evaluated by adding the intensity and positive percentage scores (score 0–8). Briefly, the immunoreactivity of the 3 target components of the tumor (carcinomatous, transitional, and sarcomatous components that were the same areas in representative hematoxylin-eosin–stained slides) was evaluated in 250 × 250-μm areas at 200× magnification in each case. Figure 2 shows the transitional components in APCs and the immunoreactivity of E-cadherin and EMT-related proteins.
FIGURE 2.
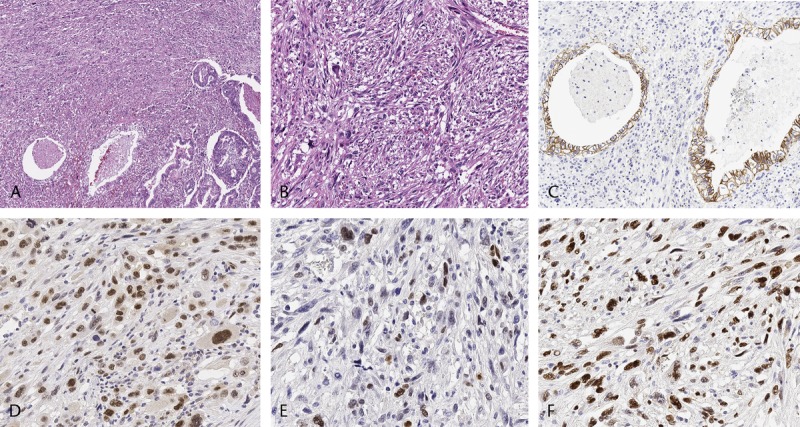
Representative histologic findings and immunoreactivity of E-cadherin and EMT transcription factors in the transitional component of APCs (A–I, original magnification ×400). A–C, D–F, and G–I were observed in the same location. Immunoreactivity of E-cadherin on the cell membrane (B, E, and H). Nuclear expression of Slug (C), Twist (F), and Zeb1 (I) was observed in the transitional component of APCs.
Hierarchical Cluster Analysis
We conducted hierarchical clustering based on the markers' scores to evaluate differences in the expression patterns of E-cadherin and EMT markers (Slug, Twist, and Zeb1) in patients with APC. Hierarchical cluster analysis was performed for clustering of the samples according to the above scoring system (score 0–8) in APC patients to achieve maximal homogeneity for each subgroup and the greatest differences between the groups using open-access clustering software (Cluster 3.0 software; bonsai.hgc.jp/~mdehoon/software/cluster/software.htm). The clustering algorithm was set to centroid linkage clustering, which is the standard hierarchical clustering method used in biological studies.
Statistical Analysis
The analysis was performed using Microsoft Excel (version 2013) (Microsoft, Redmond, Wash) and JMP 13 (SAS Institute Inc, Cary, NC). Statistical significance was evaluated using Fisher exact test, the Kruskal-Wallis test, and the Mann-Whitney U test. To identify predictors of overall survival for patients with APC, univariate and multivariate analyses were conducted according to the Cox proportional hazards model. Patient survival time was calculated from the date of surgery until death (overall survival). P ≤ 0.05 was considered significant.
RESULTS
Comparison of Immunoreactivity Scores of E-cadherin and EMT-Related Proteins Among 3 Target Components in APC (Carcinomatous, Transitional, and Sarcomatous Components)
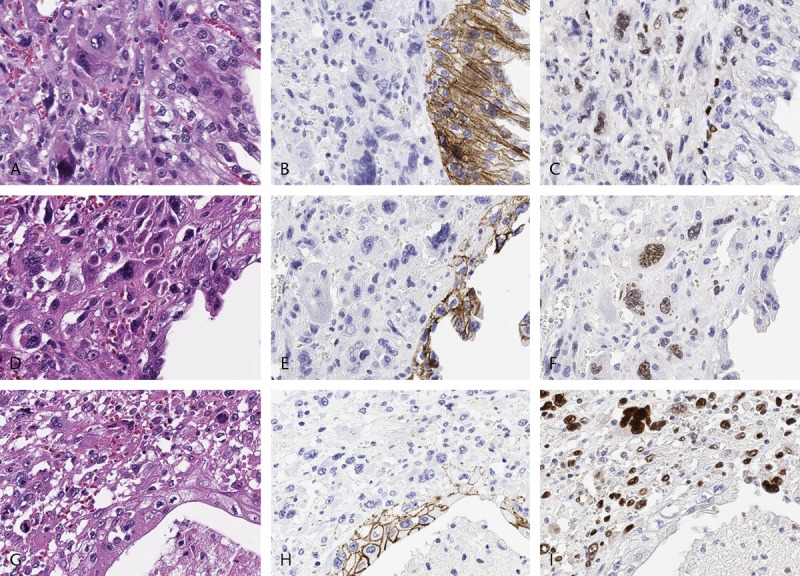
Figure 3 summarizes the immunoreactivities of E-cadherin and EMT-related proteins (Slug, Twist, and Zeb1) in 3 target components of APCs. E-cadherin's immunoreactivity progressively decreased from the carcinomatous to the transitional to the sarcomatous components (P < 0.01) (Fig. 3A). The immunoreactivity scores of Slug were significantly higher in transitional and sarcomatous components than in the carcinomatous component (P < 0.01) (Fig. 3B). The immunoreactivity scores of Twist were higher in transitional and sarcomatous components than in the carcinomatous component (P < 0.05) (Fig. 3C). The immunoreactivity scores of Zeb1 were significantly higher in transitional and sarcomatous components than in the carcinomatous component (P < 0.01) (Fig. 3D). Furthermore, the Zeb1 immunoreactivity score in the sarcomatous component was higher than in the transitional component (P < 0.05) (Fig. 3D).
FIGURE 3.
Comparison of immunoreactivity scores of E-cadherin and EMT transcription factors among 3 target components in APC (carcinomatous, transitional, and sarcomatous components). E-cadherin (A), Slug (B), Twist (C) and Zeb1 (D) are presented. Numbers indicate immunoreactivity score. *P < 0.05 and **P < 0.01.
Hierarchical Cluster Analysis of APCs Based on the Expression Scores of E-cadherin and EMT-Related Proteins
Two distinct immunohistologic subgroups (subgroups 1 and 2) emerged, as shown in Figure 4. We examined differences in clinicopathologic findings between the 2 subgroups in APCs. There were no significant differences in the frequencies of median age, sex, location, median diameter of tumor, histologic variants, TNM stage, lymphatic invasion, venous invasion, or nerve invasion between subgroups 1 and 2 (Supplemental Table 1, http://links.lww.com/MPA/A693).
FIGURE 4.
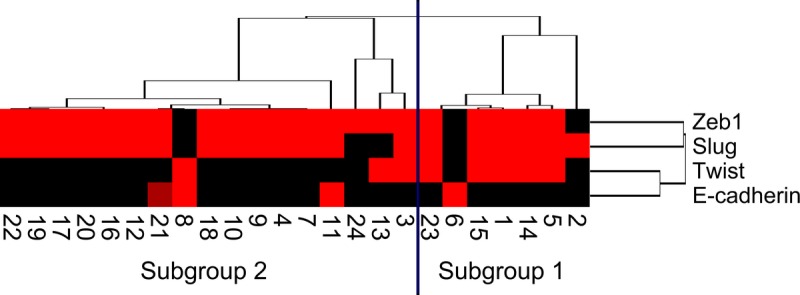
Hierarchical cluster analysis of APCs based on the protein expression patterns of E-cadherin, Slug, Twist, and Zeb1 in the sarcomatous component. Undifferentiated carcinomas were subdivided into 2 subgroups. The vertical line shows the expression of each marker in the tumor cells of the sarcomatous component, and the horizontal lines denote “relatedness” between samples. The colors of the box express the immunoreactivity score (0–8), ranging from black (score 0) to red (score 8). Numbers indicate patient number.
The immunoreactivities of E-cadherin and EMT-related proteins in each subgroup in the sarcomatous components of APC are shown in Figure 5. The immunoreactivity scores of Twist were significantly higher in subgroup 1 than in subgroup 2 (P ≤ 0.05) (Fig. 5C). However, there were no differences between the 2 subgroups in the frequencies of immunoreactivity scores of E-cadherin, Slug, or Zeb1 (Figs. 5A, B, D). Thus, APCs examined in this study were categorized into 2 distinct patterns in the cluster analysis.
FIGURE 5.
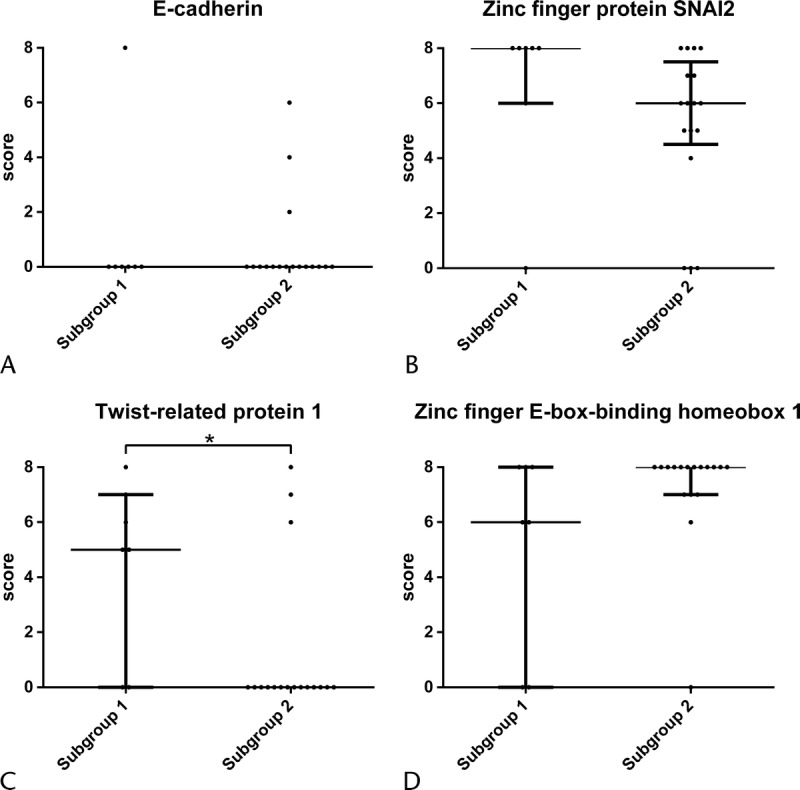
Comparison of immunoreactivity scores of E-cadherin and EMT transcription factors between subgroups 1 and 2 in the sarcomatous component of APC. E-cadherin (A), Slug (B), Twist (C) and Zeb1 (D) are presented. Numbers indicate immunoreactivity score. *P < 0.05.
Association of Each Subgroup With Patient Outcome in APC
Next, we examined the correlations between immunohistologic subgroups (constructed according to hierarchical clustering) and patient outcome as well as clinicopathologic findings. The overall 2-year survival rate in all APC patients was 27.1%. Clinicopathologic factors such as tumor size, histologic variants (presence or absence of osteoclast-like giant cells), TNM stage, lymphatic invasion, venous invasion, nerve invasion, and immunohistologic subgroups were not predictors of overall survival. Subgroup 1 was weakly correlated with poor overall patient survival on univariate analysis (P = 0.05) (Supplemental Table 2, http://links.lww.com/MPA/A693). However, subgroup 1 was not retained as an independent patient prognostic factor for APC on multivariate Cox regression analysis.
DISCUSSION
The transdifferentiation of epithelial cells into mesenchymal cells, known as the EMT, is essential in cancer progression.15,16 The EMT has been molecularly characterized by the down-regulation of E-cadherin. This repression is mediated by the binding of EMT transcription factors such as Slug, Twist, and Zeb1 to E-boxes present in the E-cadherin promoter.17,18 Carcinosarcoma, which corresponds to pancreatic APC, is composed of 3 histologic tissue types, including carcinomatous, transitional, and sarcomatous elements. Carcinosarcoma is a prototype model of EMT, indicating that a transdifferentiation program occurred in the tumor tissue. However, the associated development of APC (carcinosarcoma in pancreatic tumors) with reductions of E-cadherin and expression of EMT-related proteins is not well characterized. We attempted to identify the molecular processes underlying APC in terms of altered expression of Slug, Twist, and Zeb1.
The APC that we examined in this study was divided into 2 components in terms of its pathogenesis. The “carcinomatous component → transitional component” could be considered an early phase, whereas the “transitional component → sarcomatous component” could be regarded as a late phase. This concept is useful to understand the pathogenesis of APC, and it may provide novel insights into the natural history of the disease and the response to treatment and ultimately improve the prognosis.
Key EMT-related regulators that reduce E-cadherin expression include Slug, Twist, and Zeb1.18,20,21 However, E-cadherin is not regulated by all 3 of these transcriptional repressors in tumor cells from different organs.17–20 For example, Twist may act more strongly on the EMT than do Slug and Zeb1. The impact of the 3 proteins on the EMT may vary among different tumors. In the present study, we showed that the transition from the carcinomatous component to the transitional component was closely associated with a reduction of E-cadherin and high expression of Slug, Twist, and Zeb1. This finding suggests that the 3 proteins could reduce expression of E-cadherin during the early histologic changes into intermediate histologic elements (transitional component).
Zeb1 is a nuclear factor that specifically binds to and represses the promoter region of E-cadherin,18 suggesting that reduced expression of E-cadherin could occur in tumor cells.20,21 In the present study, significant differences between sarcomatous, carcinomatous, and transitional components were found in the immunohistochemical scores of Zeb1. In addition, the immunohistochemical score of E-cadherin was significantly lower in the sarcomatous component than in both the carcinomatous and the transitional components. This result suggests that expression of Zeb1 strongly reduces E-cadherin expression in the sarcomatous component compared with the transitional component. Although the prognosis of APC patients is generally worse than those for other types of pancreatic cancer,1,5,7,14 the poor prognosis of APC depends on the sarcomatous component. Therefore, it is possible that the poor prognosis of APC may be associated with the enhanced expression of Zeb1 in the sarcomatous component. This proposition is consistent with the finding that the expression of Zeb1 has a crucial impact on patient survival. In fact, previous studies have shown that the expression of Zeb1 in endometrial cancers, colorectal carcinomas, and prostate cancer is linked to aggressive disease, poor differentiation, development of metastases, and poor clinical prognosis.23–27 We suggest that expression of Zeb1 strongly contributes to the development of APC.
In the present study, we categorized APCs into 2 subgroups that were stratified by their expression pattern of E-cadherin and EMT-related proteins (the EMT phenotype) in the sarcomatous component that may determine the poor prognosis of such patients. In addition, tumors in subgroup 1 were characterized by high expression of Twist, which plays a crucial role in regulating the EMT progression.18,22 Li et al28 showed that Twist expression was an independent factor that predicted a poor prognosis in pancreatic ductal adenocarcinoma. Several studies also have shown that increased expression of Twist was correlated with poor prognosis in breast cancers.29,30 In this study, subgroup 1 was characterized by high Twist expression that was weakly correlated with poor overall patient survival on univariate analysis. However, this association was not retained upon multivariate analysis. A larger study may be needed to identify the association of subgroups stratified by their expression pattern of the EMT-phenotype with patient outcome in APCs.
In conclusion, elevated expression of Slug, Twist, and Zeb1 reduces the levels of E-cadherin in the transition from the carcinomatous component to the transitional component of APCs. In addition, it is likely that increased Zeb1 expression and complete loss of E-cadherin expression are correlated with the progression of APC through their influence on the progression of the EMT. Furthermore, APC patients were classified into 2 subgroups according to the expression patterns of E-cadherin and EMT-related proteins in sarcomatous components. These subgroups were distinguished by Twist expression. Accordingly, inhibiting the expression or function of EMT-inducing transcription factors may lead to new strategies in the treatment of malignancies.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank members of the Department of Molecular Diagnostic Pathology, Iwate Medical University, for their technical assistance.
Footnotes
This work was supported by JSPS KAKENHI grant JP16K08655.
The authors declare no conflict of interest.
Supplemental digital contents are available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.pancreasjournal.com).
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Winter JM, Cameron JL, Campbell KA, et al. 1423 Pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210; discussion 1210–1211. [DOI] [PubMed] [Google Scholar]
- 3.House MG, Gönen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549–1555. [DOI] [PubMed] [Google Scholar]
- 4.Connolly MM, Dawson PJ, Michelassi F, et al. Survival in 1001 patients with carcinoma of the pancreas. Ann Surg. 1987;206:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. [DOI] [PubMed] [Google Scholar]
- 6.Lüttges J, Vogel I, Menke M, et al. The retroperitoneal resection margin and vessel involvement are important factors determining survival after pancreaticoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Virchows Arch. 1998;433:237–242. [DOI] [PubMed] [Google Scholar]
- 7.Clark CJ, Graham RP, Arun JS, et al. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. J Am Coll Surg. 2012;215:627–634. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi K, Nakamura K, Shimizu S, et al. Pleomorphic carcinoma of the pancreas: reappraisal of surgical resection. Am J Gastroenterol. 1998;93:1151–1155. [DOI] [PubMed] [Google Scholar]
- 9.Fukushima N, Hruban RH, Kato Y, et al. Ductal adenocarcinoma variants and mixed neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al., eds. World Health Organization Classification of Tumours of the Digestive Tract. Lyon, France: IARC Press; 2010:292–295. [Google Scholar]
- 10.Sommers SC, Meissner WA. Unusual carcinomas of the pancreas. AMA Arch Pathol. 1954;58:101–111. [PubMed] [Google Scholar]
- 11.Strobel O, Hartwig W, Bergmann F, et al. Anaplastic pancreatic cancer: presentation, surgical management, and outcome. Surgery. 2011;149:200–208. [DOI] [PubMed] [Google Scholar]
- 12.Hansen T, Burg J, Kirkpatrick CJ, et al. Osteoclast-like giant cell tumor of the pancreas with ductal adenocarcinoma: case report with novel data on histogenesis. Pancreas. 2002;25:317–320. [DOI] [PubMed] [Google Scholar]
- 13.Molberg KH, Heffess C, Delgado R, et al. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998;82:1279–1287. [DOI] [PubMed] [Google Scholar]
- 14.Paal E, Thompson LD, Frommelt RA, et al. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann Diagn Pathol. 2001;5:129–140. [DOI] [PubMed] [Google Scholar]
- 15.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel). 1995;154:8–20. [DOI] [PubMed] [Google Scholar]
- 16.Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. [DOI] [PubMed] [Google Scholar]
- 17.Lee JM, Dedhar S, Kalluri R, et al. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. [DOI] [PubMed] [Google Scholar]
- 20.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Lamouille S, Derynck R. TGF-beta–induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dave N, Guaita-Esteruelas S, Gutarra S, et al. Functional cooperation between Snail1 and twist in the regulation of Zeb1 expression during epithelial to mesenchymal transition. J Biol Chem. 2011;286:12024–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spoelstra NS, Manning NG, Higashi Y, et al. The transcription factor ZEB1 is aberrantly expressed in aggressive uterine cancers. Cancer Res. 2006;66:3893–3902. [DOI] [PubMed] [Google Scholar]
- 24.Singh M, Spoelstra NS, Jean A, et al. ZEB1 expression in type I vs type II endometrial cancers: a marker of aggressive disease. Mod Pathol. 2008;21:912–923. [DOI] [PubMed] [Google Scholar]
- 25.Singh AB, Sharma A, Smith JJ, et al. Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells. Gastroenterology. 2011;141:2140–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez-Tilló E, de Barrios O, Siles L, et al. Zeb1 promotes invasiveness of colorectal carcinoma cells through the opposing regulation of uPA and PAI-1. Clin Cancer Res. 2013;19:1071–1082. [DOI] [PubMed] [Google Scholar]
- 27.Figiel S, Vasseur C, Bruyere F, et al. Clinical significance of epithelial-mesenchymal transition markers in prostate cancer. Hum Pathol. 2017;61:26–32. [DOI] [PubMed] [Google Scholar]
- 28.Li K, Xu B, Xu G, et al. CCR7 regulates Twist to induce the epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma. Tumour Biol. 2016;37:419–424. [DOI] [PubMed] [Google Scholar]
- 29.Zhao M, Hu HG, Huang J, et al. Expression and correlation of Twist and gelatinases in breast cancer. Exp Ther Med. 2013;6:97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grzegrzolka J, Biala M, Wojtyra P, et al. Expression of EMT markers SLUG and TWIST in breast cancer. Anticancer Res. 2015;35:3961–3968. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


