Supplemental Digital Content is available in the text.
Keywords: cardiovascular outcomes, diabetic kidney disease, LEADER, liraglutide, type 2 diabetes mellitus
Abstract
Background:
LEADER trial (Liraglutide Effect and Action in Diabetes: Evaluation of CV Outcome Results) results demonstrated cardiovascular benefits for patients with type 2 diabetes mellitus at high cardiovascular risk on standard of care randomized to liraglutide versus placebo. The effect of glucagon-like peptide-1 receptor agonist liraglutide on cardiovascular events and all-cause mortality in patients with type 2 diabetes mellitus and chronic kidney disease is unknown. Liraglutide’s treatment effects in patients with and without kidney disease were analyzed post hoc.
Methods:
Patients were randomized (1:1) to liraglutide or placebo, both in addition to standard of care. These analyses assessed outcomes stratified by baseline estimated glomerular filtration rate (eGFR; <60 versus ≥60 mL/min/1.73 m2) and baseline albuminuria. The primary outcome (composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke) and secondary outcomes, including all-cause mortality and individual components of the primary composite outcome, were analyzed using Cox regression.
Results:
Overall, 2158 and 7182 patients had baseline eGFR <60 or ≥60 mL/min/1.73 m2, respectively. In patients with eGFR <60 mL/min/1.73 m2, risk reduction for the primary composite cardiovascular outcome with liraglutide was greater (hazard ratio [HR], 0.69; 95% CI, 0.57–0.85) versus those with eGFR ≥60 mL/min/1.73 m2 (HR, 0.94; 95% CI, 0.83–1.07; interaction P=0.01). There was no consistent effect modification with liraglutide across finer eGFR subgroups (interaction P=0.13) and when analyzing eGFR as a continuous variable (interaction P=0.61). Risk reductions in those with eGFR <60 versus ≥60 mL/min/1.73 m2 were as follows: for nonfatal myocardial infarction, HR, 0.74; 95% CI, 0.55–0.99 versus HR, 0.93; 95% CI, 0.77–1.13; for nonfatal stroke, HR, 0.51; 95% CI, 0.33–0.80 versus HR, 1.07; 95% CI, 0.84–1.37; for cardiovascular death, HR, 0.67; 95% CI, 0.50–0.90 versus HR, 0.84; 95% CI, 0.67–1.05; for all-cause mortality, HR, 0.74; 95% CI, 0.60–0.92 versus HR, 0.90; 95% CI, 0.75–1.07. Risk reduction for the primary composite cardiovascular outcome was not different for those with versus without baseline albuminuria (HR, 0.83; 95% CI, 0.71–0.97; and HR, 0.92; 95% CI, 0.79–1.07, respectively; interaction P=0.36).
Conclusions:
Liraglutide added to standard of care reduced the risk for major cardiovascular events and all-cause mortality in patients with type 2 diabetes mellitus and chronic kidney disease. These results appear to apply across the chronic kidney disease spectrum enrolled.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov/. Unique identifier: NCT01179048.
Clinical Perspective.
What Is New?
In this post hoc subgroup analysis of the LEADER trial (Liraglutide Effect and Action in Diabetes: Evaluation of CV Outcome Results), liraglutide reduced the risk of major adverse cardiovascular events and all-cause mortality compared with placebo in patients with chronic kidney disease (CKD) defined as estimated glomerular filtration rate <60 mL/min/1.73 m2.
Liraglutide also reduced the risk of major adverse cardiovascular events in patients with albuminuria, defined as urinary albumin-to-creatinine ratio >30 mg/g (micro- and macroalbuminuria).
The overall risk of adverse events did not differ between liraglutide- and placebo-treated patients either with or without CKD in the LEADER trial.
What Are the Clinical Implications?
Liraglutide may be considered as a therapeutic option for patients with type 2 diabetes mellitus with or at high risk for atherosclerotic cardiovascular disease who also have CKD.
The present results demonstrate that the results of the LEADER trial on cardiovascular efficacy and safety of liraglutide apply to patients with CKD, which is clinically important given that those patients with type 2 diabetes mellitus and CKD have a high cardiovascular risk burden and few options for antihyperglycemic therapies.
Type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD) are closely associated. Diabetic kidney disease is the most common cause of CKD and affects ≈40% of patients with T2DM.1 Patients with diabetic kidney disease are at substantially increased risk of cardiovascular (CV) morbidity and mortality,1 and most die from CV disease before reaching end-stage kidney disease or requiring renal replacement therapy.2,3 Low estimated glomerular filtration rate (eGFR) or presence of albuminuria independently predict CV outcomes such as CV death, myocardial infarction (MI), and stroke in patients with T2DM.4–7 Current standard CV treatment for these patients broadly aligns with current guidelines for patients with normal kidney function. However, a beneficial effect shown in patients with normal kidney function does not necessarily apply to patients with CKD, for example, as has been seen with statins in patients with advanced CKD.8–10
In previous landmark trials, intensive glycemic control limited the risk of microvascular complications of T2DM11 but did not demonstrate CV benefits.12 Results from recent CV outcome trials with basal insulin13,14 and dipeptidyl peptidase-4 inhibitors15–17 ensured CV safety, whereas CV benefits were reported for glucagon-like peptide-1 receptor agonists18,19 and sodium-glucose cotransporter-2 (SGLT-2) inhibitors,20,21 thus suggesting CV-protective effects at least partly independent of glycemic control.
There are no dedicated controlled trials that have examined interventions or medications to lower the excessive CV risk of CKD in association with T2DM. Subgroup analyses of the recent CV outcome trials in T2DM with dipeptidyl peptidase-4 inhibitors15–17,22–24 and SGLT-2 inhibitors20,21,25 showed no different effects of the antihyperglycemic medications on major CV outcomes in those with and without CKD. Many antihyperglycemic medications exhibit altered pharmacokinetics and pharmacodynamics when kidney function is reduced,26 which may potentially affect safety and efficacy. Furthermore, the dose of many of those medications needs to be reduced in the presence of CKD, which may affect their impact on CV disease. The metabolism and elimination of liraglutide is mostly unaffected with decreasing kidney function, and it has been approved for use in patients with severe renal impairment without dose adjustment.27
The LEADER trial (Liraglutide Effect and Action in Diabetes: Evaluation of CV Outcome Results) reported a reduced risk of CV outcomes, all-cause death, and renal outcomes for patients with T2DM on standard of care treated with liraglutide compared with placebo.18,28 As such, liraglutide is now approved to reduce the risk of major adverse CV events in adults with T2DM and established CV disease.27
The aim of these analyses is to further examine the effect of liraglutide versus placebo on CV and safety outcomes in subgroups of patients with eGFR <60 mL/min/1.73 m2 versus ≥60 mL/min/1.73 m2 and those with micro- and macroalbuminuria versus those without albuminuria. We hypothesized that the CV benefit would remain evident in those subgroups with CKD.
Methods
Design
The data analyzed for this publication are available from the corresponding author on reasonable request. The trial design and methods have been published previously.29 LEADER was a multicenter, double-blind, placebo-controlled trial performed at 410 sites in 32 countries. Patients with T2DM and a high risk of CV disease were randomized 1:1 to liraglutide or placebo, both in addition to standard of care.18,29
Patients
Patients with T2DM and a glycohemoglobin (HbA1c) level ≥7.0%, with no upper limit, were eligible if they were either drug-naive or treated with oral antihyperglycemic agents or insulin (human neutral protamine Hagedorn, long-acting analogue, or premixed only). LEADER was designed to recruit a subgroup of ≥660 patients with an eGFR <60 mL/min/1.73 m2: ≈220 patients with severe renal impairment (eGFR <30 mL/min/1.73 m2) and ≥440 patients with moderate renal impairment (CKD stage 3; eGFR 30–60 mL/min/1.73 m2).28
Other major inclusion criteria were either (1) ≥50 years of age with ≥1 CV comorbidity, including prior MI or stroke; prior coronary, carotid, or peripheral arterial revascularization or presence of >50% stenosis; documented coronary heart disease; CKD ≥stage 3; or chronic heart failure New York Heart Association class II–III; or (2) ≥60 years of age with ≥1 more CV risk factor, including micro- or macroalbuminuria, hypertension and left ventricular hypertrophy, left ventricular systolic or diastolic dysfunction, or an ankle-brachial index <0.9. Complete inclusion and exclusion criteria were published in the original trial report.29
CKD Subgroups
CKD subgroups in these analyses were prespecified based on baseline eGFR (<60 and ≥60 mL/min/1.73 m2) and baseline albuminuria (without albuminuria and micro- and macroalbuminuria [<30 mg/g versus ≥30 mg/g creatinine]) measured at randomization. In addition to the main comparisons between baseline eGFR subgroups and baseline albuminuria subgroups, a comparison for the primary composite CV outcome and expanded composite CV outcome was conducted between a subgroup of patients of particular high renal risk, namely, with both low eGFR and micro- and macroalbuminuria versus a subgroup of patients who had ≥1 of an eGFR ≥60 mL/min/1.73 m2 or normoalbuminuria. We also analyzed eGFR as a continuous variable using a restricted cubic splines model.
Study Procedures
After a 2-week placebo run-in to establish injection adherence, patients were randomized in a blinded manner 1:1 to 1.8 mg (or maximum tolerated dose) of liraglutide or matching placebo once daily, in addition to standard of care. For patients with suboptimal glucose control after randomization, dose increase or initiation of any antihyperglycemic agents (except glucagon-like peptide-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, or pramlintide) was permitted, and investigators were directed to treat all patients to standard of care according to guidelines.18
Outcomes
The primary outcome was the time from randomization to first occurrence of a composite CV outcome consisting of CV death, nonfatal (including silent) MI, or nonfatal stroke. Secondary time-to-event outcomes included an expanded composite CV outcome (CV death, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina pectoris or heart failure), CV death, all strokes (fatal and nonfatal), all MIs (fatal and nonfatal), and all-cause death. Other secondary outcomes included change in HbA1c, body weight, blood pressure, and low-density lipoprotein cholesterol from baseline to 36 months. CV events were adjudicated in a blinded manner by board-certified cardiologist experts via an external contract research organization (ICON). Serious adverse events and predefined medical events of special interest (MESIs) were reported by the investigators or captured via prespecified searches among all adverse events and laboratory results. A MESI was a predefined event of scientific and medical concern, which can be serious or nonserious, and not necessarily with a causal relationship with the trial product.18
Statistical Analysis
The statistical analysis plan for LEADER has been published previously.28,29 The required sample size was based on the primary composite CV outcome. All randomized patients were included and contributed to each analysis from the time of randomization until first occurrence of the analyzed outcome, death, or end of follow-up, whichever came first. Between-group comparisons of effectiveness and safety laboratory assessments were performed at 36 months, the last trial visit at which such assessments were made at the same time point for the entire population. All the analyses were performed at the nominal α level of 0.05 without correction for multiple hypothesis testing. Time-to-event analyses applied a Cox proportional hazards model with treatment as a covariate. For each outcome, the interaction between treatment group and CKD subgroup was evaluated in a model adjusting for baseline covariates, including sex, region, age, diabetes mellitus duration, race, ethnicity, CV risk (established disease versus risk factors only), antihyperglycemic therapy, body mass index, and HbA1c. An additional analysis of the primary outcome considered continuous eGFR as a restricted cubic spline function, and a test of interaction between this term and treatment group was performed.
Ethics
The trial was approved by institutional review boards, and all patients provided written informed consent. The trial was conducted in accordance with the Declaration of Helsinki.
Results
Patients
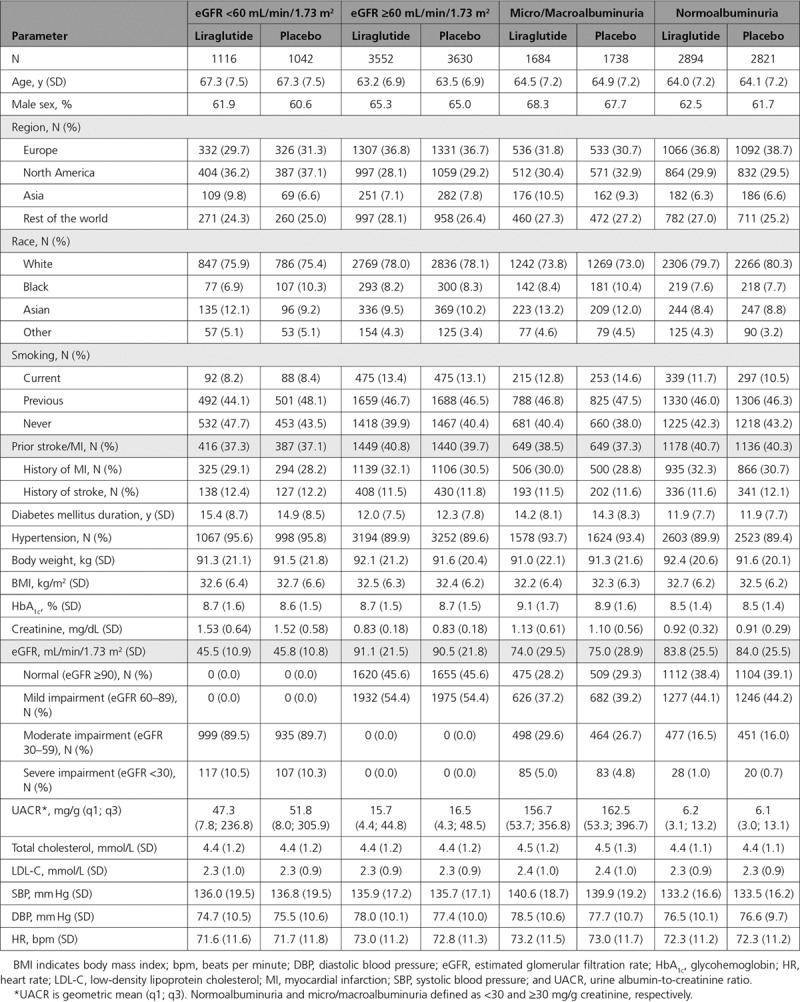
The disposition of trial participants has been published previously.18 Patient characteristics and demographics at baseline by eGFR and albuminuria status are shown in Table 1. Mean eGFR in patients with baseline eGFR <60 and ≥60 mL/min/1.73 m2 (n=2158 and n=7182, respectively) was 45.7±10.9 and 90.8±21.6 mL/min/1.73 m2, respectively, with no differences between treatment groups (Table 1). Those with eGFR <60 mL/min/1.73 m2 generally had a slightly higher usage of antihypertensive, diuretic, and lipid-lowering medications as well as higher prior insulin use compared with those with eGFR ≥60 mL/min/1.73 m2 (Table I in the online-only Data Supplement).
Table 1.
Patient Baseline Characteristics and Demographics by eGFR and Albuminuria Status
Primary Composite CV Outcome: Time to First Major Adverse CV Events
The primary composite CV outcome (1302 events in total) occurred in a lower proportion of patients taking liraglutide than placebo (15.4% versus 21.4%, respectively, in those with eGFR <60 mL/min/1.73 m2, and 12.3% versus 13.0%, respectively, in those with eGFR ≥60 mL/min/1.73 m2). For the primary composite CV outcome, the risk reduction observed with liraglutide was greater in the subgroup of patients with eGFR <60 mL/min/1.73 m2 (hazard ratio [HR], 0.69; 95% CI, 0.57–0.85) than in those with eGFR ≥60 mL/min/1.73 m2 (HR, 0.94; 95% CI, 0.83–1.07; interaction P=0.01) (Figure 1). However, examining treatment differences in finer eGFR subgroups of 15 mL/min/1.73 m2 intervals revealed no consistent difference in the treatment effect of liraglutide on the primary composite CV outcome dependent on eGFR (interaction P=0.13) (Figure 2). There was also no interaction of eGFR at baseline with the treatment effect when eGFR was used as a continuous variable (Figure I in the online-only Data Supplement). Irrespective of treatment, the primary composite CV outcome was more likely in patients with eGFR <60 mL/min/1.73 m2 compared with eGFR ≥60 mL/min/1.73 m2 (P<0.0001).
Figure 1.
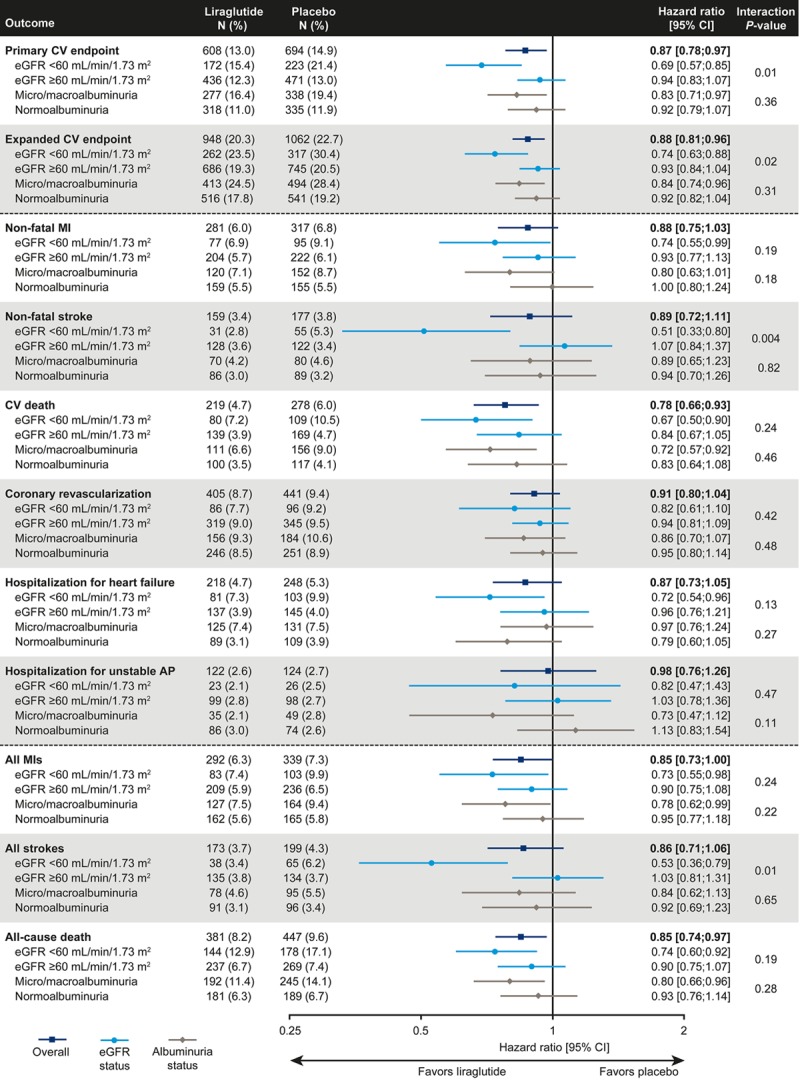
Hazard ratios between treatment groups for primary and key secondary outcomes by baseline eGFR and albuminuria groups. Time-to-event analyses applied a Cox proportional-hazards model with treatment as a covariate. Primary composite CV outcome was a composite of nonfatal stroke, nonfatal MI, or CV death. The expanded composite CV outcome was the same as the primary composite CV outcome plus coronary revascularization or hospitalization for unstable AP or heart failure. AP indicates angina pectoris; CV, cardiovascular; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; N, number of patients with an event; and %, proportion of patients with an event.
Figure 2.
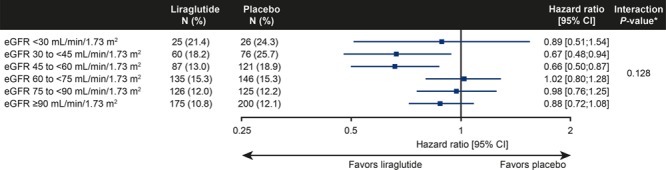
Hazard ratios between treatment groups for the primary composite CV outcome by baseline eGFR subgroups in all patients. Time to first event is analyzed using Cox proportional hazards model with treatment, subgroup, and the interaction between treatment and subgroup as factors. *P value is from the test statistic for testing the interaction between treatment and baseline eGFR-MDRD group. The primary composite CV outcome includes CV death, nonfatal myocardial infarction, or nonfatal stroke. CV indicates cardiovascular; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; and N (%), number of patients with an event (proportion of patients within the subgroup with an event).
For micro- and macroalbuminuria and normoalbuminuria subgroups, the corresponding HRs for primary and key secondary outcomes by baseline eGFR and time to primary composite CV outcome in patients according to baseline eGFR were 0.83 (95% CI, 0.71–0.97) and 0.92 (95% CI, 0.79–1.07; interaction P=0.36), respectively (Figure 1). Further analyses illustrating the effect of liraglutide versus placebo in finer subgroups of eGFR and albuminuria and the time course for development of CV events can be found in Figures II through VI in the online-only Data Supplement. A sensitivity analysis of the primary composite CV outcome, taking into account adjustment for baseline HbA1c, body weight, systolic blood pressure, and renin-angiotensin system inhibitors, revealed similar results to the unadjusted results presented in this study (data not shown).
Individual Components of the Primary Composite CV Outcome
The effects of liraglutide versus placebo were consistent across the individual components of the primary CV outcome. In the subgroup of patients with eGFR <60 mL/min/1.73 m2, liraglutide reduced the risk of nonfatal MI compared with placebo (HR, 0.74; 95% CI, 0.55–0.99) compared with those with eGFR ≥60 mL/min/1.73 m2 (HR, 0.93; 95% CI, 0.77–1.13; interaction P=0.19) (Figure 1). A similar pattern was observed for nonfatal stroke (HR, 0.51; 95% CI, 0.33–0.80 and HR, 1.07; 95% CI, 0.84–1.37; interaction P=0.004) and CV death (HR, 0.67; 95% CI, 0.50–0.90 and HR, 0.84; 95% CI, 0.67–1.05; interaction P=0.24) for patients with eGFR <60 mL/min/1.73 m2 and ≥60 mL/min/1.73 m2, respectively (Figure 1). The risk of nonfatal MI, nonfatal stroke, and CV death was reduced with liraglutide compared with placebo in both the micro- and macroalbuminuria and normoalbuminuria subgroups (Figure 1).
Other Secondary Outcomes
All-cause death occurred less frequently with liraglutide versus placebo, and that difference was numerically greater in those with eGFR <60 mL/min (12.9% versus 17.1%; HR, 0.74; 95% CI, 0.60–0.92) than in those with eGFR >60 mL/min (6.7% versus 7.4%; HR, 0.90; 95% CI, 0.75–1.07; interaction P=0.19). Similarly, a greater benefit of liraglutide in those with low eGFR (Figure 1) was observed, particularly for the expanded composite CV outcome (interaction P=0.02), for all strokes (interaction P=0.01), and for all MIs (interaction P=0.24).
Subgroups of Patients With Low eGFR and Micro- and Macroalbuminuria
In the high-renal-risk subgroup of patients with eGFR <60 mL/min/1.73 m2 and micro- and macroalbuminuria (n=1130), liraglutide reduced the risk of the primary composite CV outcome compared with placebo (HR, 0.63; 95% CI, 0.49–0.82); a more modest risk reduction was seen for those with either eGFR ≥60 mL/min/1.73 m2 or normoalbuminuria (n=8007) (HR, 0.93; 95% CI, 0.82–1.05; interaction P=0.008).
Clinical Parameters
Declines in HbA1c and body weight were consistently greater with liraglutide versus placebo for all eGFR and albuminuria subgroups. There was evidence of interaction between treatment and baseline eGFR for body weight (interaction P=0.01), with greater weight loss associated with liraglutide treatment among patients with eGFR <60 mL/min/1.73 m2 (estimated treatment difference, −2.92 kg; 95% CI, −3.51 to −2.33 compared with −2.07 kg; 95% CI, −2.38 to −1.76 for those with eGFR ≥60 mL/min/1.73 m2). Systolic blood pressure and low-density lipoprotein cholesterol changes were slight and varied according to baseline eGFR and albuminuria status. Patients with eGFR ≥60 mL/min/1.73 m2 achieved numerically greater reductions in systolic blood pressure than those with eGFR <60 mL/min/1.73 m2 (interaction P=0.12) (Figure 3).
Figure 3.
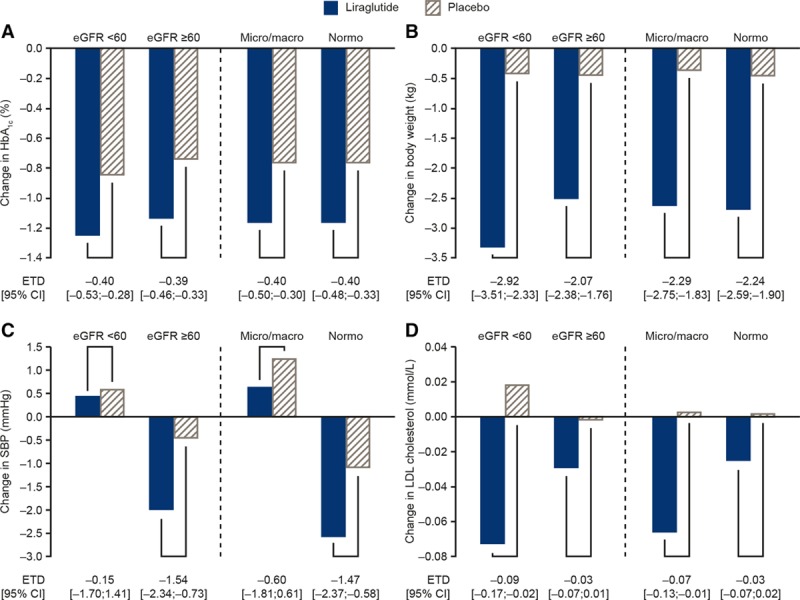
Change in HbA1c (A), body weight (B), SBP (C), and LDL cholesterol (D) at 36 months. Interaction P between treatment and eGFR/albuminuria status at baseline was not significant, except for eGFR and weight (P=0.012). Changes are presented at 36 months (the last visit when all participants had their annual analysis of all laboratory results). eGFR indicates estimated glomerular filtration rate; ETD, estimated treatment difference; HbA1c, glycohemoglobin; LDL, low-density lipoprotein; Micro/macro, microalbuminuria/macroalbuminuria; Normo, normalbuminuria; and SBP, systolic blood pressure.
Adverse Events
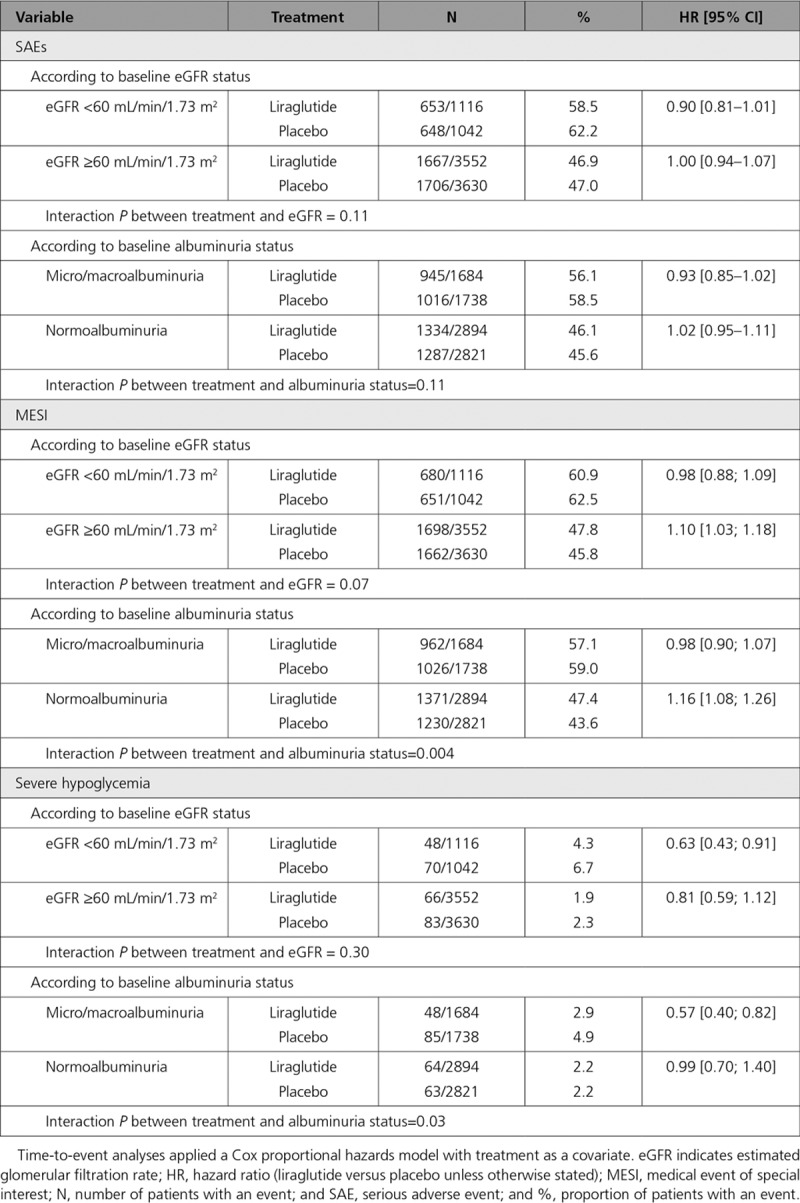
As expected, serious adverse events, MESIs, and severe hypoglycemia were more common among patients with eGFR <60 mL/min/1.73 m2 compared with ≥60 mL/min/1.73 m2. In general, however, the proportion of patients with serious adverse events and MESIs was not increased with liraglutide treatment compared with placebo, although fewer MESIs were reported with placebo compared with liraglutide among those with eGFR ≥60 mL/min/1.73 m2 and among patients with normoalbuminuria. Severe hypoglycemia was more frequent with placebo compared with liraglutide in those with eGFR <60 mL/min/1.73 m2 than ≥60 mL/min/1.73 m2 (HR, 0.63; 95% CI, 0.43–0.91 versus HR, 0.81; 95% CI, 0.59–1.12), and the relative benefit of liraglutide over placebo (less hypoglycemia with liraglutide) was greater in the eGFR <60 mL/min/1.73 m2 subgroup (Table 2).
Table 2.
Safety of Liraglutide by eGFR and Albuminuria Status
Discussion
These analyses demonstrate that, in subgroups of patients with T2D, high CV risk, and CKD, including those with low eGFR and/or elevated albuminuria, liraglutide reduced the risk of major adverse CV events at least as effectively as in subgroups without such risks. These findings are important given that evidence consistently shows that patients with diabetes and low eGFR or elevated albuminuria experience substantially greater CV morbidity and mortality than those with diabetes mellitus and normal eGFR and albuminuria.5–7,30,31 In addition, the armamentarium of antihyperglycemic medications is restricted in those with CKD.
The benefit of liraglutide in LEADER participants with CKD was evident for the primary composite CV outcome (MI, stroke, and CV death) and also for the expanded composite CV outcome (plus coronary revascularization and hospitalization for unstable angina or heart failure). For all components of the primary and expanded composite CV outcome, and notably for all-cause death, the estimated treatment effect of liraglutide was numerically larger in those with eGFR <60 mL/min/1.73 m2. When we subdivided eGFR and albuminuria subgroups more finely, we could not detect increasing benefits of liraglutide versus placebo with advancing CKD (see Figure 2 and Figures II and III in the online-only Data Supplement). Those observations, however, are hampered by reduced statistical power, particularly for the subgroup with eGFR <30 mL/min/1.73 m2, which included only 224 patients. Similarly, when analyzing eGFR as a continuous variable, no significant effect modification with liraglutide versus placebo on outcomes was observed. Again, those analyses are limited by the power of the interaction test and complexity of spline function (Figure I in the online-only Data Supplement).
Because eGFR <60 mL/min/1.73 m2 was defined in the inclusion criteria of LEADER as equivalent to established CV disease29 and the latter subgroup achieved increased CV benefit compared with the subgroup with high CV risk,18 we also analyzed here subgroups with or without prior history of MI or stroke (Figures IV and V in the online-only Data Supplement). The benefit of liraglutide versus placebo in those with eGFR <60 mL/min/1.73 m2 appeared to be numerically greater (compared with those who had eGFR ≥60 mL/min/1.73 m2) in patients with and without prior MI or stroke (Figure IV in the online-only Data Supplement).
There were no differences in the effectiveness of liraglutide in those with and without albuminuria. This was true for the primary composite CV outcome, as well as for the expanded composite CV outcome, all MIs and all-cause death (interaction P not significant for all outcomes). This pattern was not substantially altered when subgroups of micro- and macroalbuminuria were considered separately or when subgroups defined by both eGFR and albuminuria categories were analyzed (Figures II and III in the online-only Data Supplement).
The current analyses revealed no new safety issues with liraglutide treatment in patients with CKD. There were no indications that occurrence of serious adverse events was greater with liraglutide than with placebo in either renal subgroup. It is already known that liraglutide is associated with a lower risk of hypoglycemia compared with placebo with uneven addition of other antihyperglycemic medications. That difference, in absolute terms, appeared to increase with lower eGFR, which is consistent with previous results.32
Liraglutide had pronounced lowering effects on HbA1c and weight and slight lowering effects on systolic blood pressure and low-density lipoprotein cholesterol. Those effects did not appear to be different in those with or without low eGFR or micro- and macroalbuminuria, whereas the modest blood pressure–lowering effect of liraglutide may be confined to those with normal eGFR and normoalbuminuria. Liraglutide is not eliminated by the kidney, and its pharmacokinetics appear to be unchanged with decreasing eGFR.33 Therefore, enhanced availability of the medication in CKD is unlikely to play a role.
Post hoc data from the EMPA-REG OUTCOME trial (Empagliflozin Cardiovascular Outcomeb Event Trial in Type 2 Diabetes Mellitus Patients) reported on effects of the SGLT-2 inhibitor empagliflozin versus placebo in subgroups with low eGFR (30–60 mL/min/1.73 m2).34 Empagliflozin was as effective on CV outcomes and all-cause death in those with as without diabetic kidney disease. Similar findings of the SGLT-2 inhibitor canagliflozin have recently been reported in a subpopulation with baseline eGFR between 30 and 60 mL/min/1.73 m2.21 Empagliflozin is not indicated for use in patients with an eGFR <45 mL/min/1.73 m2,35 and canagliflozin is not recommended to be initiated in patients with an eGFR <45 mL/min/1.73 m236 because their antihyperglycemic action is blunted with low eGFR. However, the latter restrictions may change in due course.
Given the proven CV benefit of liraglutide,27,37 updated guidelines such as those from the American Diabetes Association38 and recent commentaries suggest that liraglutide should be considered as a preferred second-line option for the treatment of T2DM, particularly in patients who have established CV disease or are at high risk of developing CV disease.39 The aim is to achieve glycemic targets as well as improve CV outcomes. Some authors also recommend that patients with T2DM and stage 3 CKD are particularly suited to liraglutide therapy.39 Our findings do not contradict this idea and suggest that liraglutide may be beneficial in patients with CKD. The latter is clinically important because patients with diabetes mellitus and reduced eGFR have a high CV burden but a substantially restricted list of antihyperglycemic agents because of increased risk of adverse events (eg, metformin), less metabolic efficacy (eg, SGLT-2 inhibitors), or pharmacokinetics (eg, some sulfonylureas).
Some limitations must be considered when interpreting the current post hoc subgroup analyses. Such analyses can only be hypothesis-forming, and those hypotheses need to be examined in dedicated trials. LEADER was powered to analyze the primary composite CV outcome in all patients. Thus, testing multiple secondary CV outcomes in a subgroup analysis may be susceptible to both a lack of power and issues related to multiple comparisons; as such, significant results may be chance findings.
In conclusion, these analyses show that in the context of comprehensive standard of care, liraglutide treatment of patients with T2DM and CKD at high CV risk leads to a reduction in diverse CV outcomes and all-cause mortality, in line with the overall results of the trial. The benefits are evident in those with and without CKD. The risk of adverse events observed with liraglutide compared with placebo did not differ across those with or without renal risks.
Acknowledgments
Part of these data have been published in the form of an abstract for the American Heart Association (2017) 90th Scientific Session (Circulation 2017;136(Suppl 1):A15035). Medical writing and submission support were provided by Nathan Ley of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk. J.F.E.M. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors had access to the final study results. J.F.E.M., B.J.v.S., and T.I. drafted this manuscript, which was revised and approved by all authors, who also assume responsibility for its content. We also thank the participants, investigators, trial site staff, and leadership, employees, and contractors of the sponsor who were involved in the conduct of the trial.
Sources of Funding
This study was funded by Novo Nordisk.
Disclosures
J.F.E.M. reports receipt of speaker honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Braun, Eli Lilly & Co, Fresenius, Gambro, Medice, Novo Nordisk, Relypsa, and Roche; research support from Boehringer Ingelheim, the Canadian Institutes Health Research, Celgene, the European Union, Novo Nordisk, Roche, and Sandoz; and consultation fees from AstraZeneca, Bayer, Celgene, Eli Lilly & Co, Fresenius, Lanthio Pharma, Novo Nordisk, Relypsa, Sanifit, and Vifor Pharma. V.F. reports receipt of consultation fees from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co, Janssen, Intarcia Therapeutics Takeda, Novo Nordisk, Pamlab, and Sanofi; speaker honoraria from AstraZeneca, Sanofi, and Takeda; and research grants from Asahi, Abbott, Bayer, Eli Lilly & Co, Endo Barrier, Gilead Sciences, and Novo Nordisk. O.M. reports attendance at advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi; grants paid to institution as a study physician by AstraZeneca/Bristol-Myers Squibb; receipt of research grant support through Hadassah Hebrew University Hospital from Novo Nordisk; and speaker honoraria from AstraZeneca/Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly & Co, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Sanofi. I.R. reports attendance at advisory boards for AstraZeneca/Bristol-Myers Squibb, Eli Lilly & Co, Labstyle Innovations Ltd, Medscape LLC, Merck Sharp & Dohme, Novo Nordisk, Orgenesis, Sanofi, and SmartZyme Innovation Ltd; receipt of consultation fees from AstraZeneca/Bristol-Myers Squibb, FuturRx Gili Medical, Insuline Medical, and Kamada Ltd,; speaker honoraria from AstraZeneca/Bristol-Myers Squibb, Eli Lilly & Co, Johnson & Johnson, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi, and Teva Pharmaceutical Industries; and being a stock/shareholder in GlucoMe Ltd, Insuline Medical, Labstyle Innovations Ltd, SmartZyme Innovation Ltd, and Orgenesis. B.G., T.I., and B.J.v.S. report being full-time employees of Novo Nordisk. N.R.P. reports receipt of speaker and advisory board honoraria from AstraZeneca (concerning diabetes mellitus), Novo Nordisk, Servier, and Takeda; and research grants for his research group (relating to T2DM) from the British Heart Foundation, Diabetes UK, Julius Clinical, and the National Institute for Health Research Efficacy and Mechanism Evaluation.
Supplementary Material
Footnotes
Sources of Funding, see page 2917
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.118.036418.
References
- 1.Nasri H. On the occasion of the world diabetes day 2013; diabetes education and prevention; a nephrology point of view. J Renal Inj Prev. 2013;2:31–32. doi: 10.12861/jrip.2013.11. doi: 10.12861/jrip.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson S, James M, Wiebe N, Hemmelgarn B, Manns B, Klarenbach S, Tonelli M Alberta Kidney Disease Network. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26:2504–2511. doi: 10.1681/ASN.2014070714. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT, Jr Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Macmahon S, Chalmers J ADVANCE Collaborative Group. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–1821. doi: 10.1681/ASN.2008121270. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T Chronic Kidney Disease Prognosis Consortium. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality: a collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 6.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S HOPE Study Investigators. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 8.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 10.Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 11.Zoungas S, Arima H, Gerstein HC, Holman RR, Woodward M, Reaven P, Hayward RA, Craven T, Coleman RL, Chalmers J Collaborators on Trials of Lowering Glucose (CONTROL) group. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:431–437. doi: 10.1016/S2213-8587(17)30104-3. doi: 10.1016/S2213-8587(17)30104-3. [DOI] [PubMed] [Google Scholar]
- 12.Turnbull FM, Abraira C, Anderson RJ, Byington RP, Chalmers JP, Duckworth WC, Evans GW, Gerstein HC, Holman RR, Moritz TE, Neal BC, Ninomiya T, Patel AA, Paul SK, Travert F, Woodward M. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298. doi: 10.1007/s00125-009-1470-0. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- 13.Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 14.Marso SP, McGuire DK, Zinman B, Poulter NR, Emerson SS, Pieber TR, Pratley RE, Haahr PM, Lange M, Brown-Frandsen K, Moses A, Skibsted S, Kvist K, Buse JB DEVOTE Study Group. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723–732. doi: 10.1056/NEJMoa1615692. doi: 10.1056/NEJMoa1615692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 16.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 17.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 18.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 20.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 21.Neal B, Perkovic V, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 22.Engel SS, Suryawanshi S, Stevens SR, Josse RG, Cornel JH, Jakuboniene N, Riefflin A, Tankova T, Wainstein J, Peterson ED, Holman RR TECOS Study Group. Safety of sitagliptin in patients with type 2 diabetes and chronic kidney disease: outcomes from TECOS. Diabetes Obes Metab. 2017;19:1587–1593. doi: 10.1111/dom.12983. doi: 10.1111/dom.12983. [DOI] [PubMed] [Google Scholar]
- 23.Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, Steg PG, Davidson JA, Nicolau JC, Corbalan R, Hirshberg B, Frederich R, Im K, Umez-Eronini AA, He P, McGuire DK, Leiter LA, Raz I, Scirica BM SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR-TIMI 53 Trial. Diabetes Care. 2015;38:696–705. doi: 10.2337/dc14-1850. doi: 10.2337/dc14-1850. [DOI] [PubMed] [Google Scholar]
- 24.Scirica BM, Mosenzon O, Bhatt DL, Udell JA, Steg PG, McGuire DK, Im K, Kanevsky E, Stahre C, Sjöstrand M, Raz I, Braunwald E. Cardiovascular outcomes according to urinary albumin and kidney disease in patients with type 2 diabetes at high cardiovascular risk: observations from the SAVOR-TIMI 53 trial. JAMA Cardiol. 2018;3:155–163. doi: 10.1001/jamacardio.2017.4228. doi: 10.1001/jamacardio.2017.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B EMPA-REG OUTCOME Investigators. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation. 2018;137:119–129. doi: 10.1161/CIRCULATIONAHA.117.028268. doi: 10.1161/CIRCULATIONAHA.117.028268. [DOI] [PubMed] [Google Scholar]
- 26.Arnouts P, Bolignano D, Nistor I, Bilo H, Gnudi L, Heaf J, van Biesen W. Glucose-lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transplant. 2014;29:1284–1300. doi: 10.1093/ndt/gft462. doi: 10.1093/ndt/gft462. [DOI] [PubMed] [Google Scholar]
- 27.FDA. Victoza US prescribing information. August 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf. Accessed March 14, 2018.
- 28.Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, Tornøe K, Zinman B, Buse JB LEADER Steering Committee and Investigators. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839–848. doi: 10.1056/NEJMoa1616011. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 29.Marso SP, Poulter NR, Nissen SE, Nauck MA, Zinman B, Daniels GH, Pocock S, Steinberg WM, Bergenstal RM, Mann JF, Ravn LS, Frandsen KB, Moses AC, Buse JB. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166:823–30.e5. doi: 10.1016/j.ahj.2013.07.012. doi: 10.1016/j.ahj.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Carrero JJ, Grams ME, Sang Y, Ärnlöv J, Gasparini A, Matsushita K, Qureshi AR, Evans M, Barany P, Lindholm B, Ballew SH, Levey AS, Gansevoort RT, Elinder CG, Coresh J. Albuminuria changes are associated with subsequent risk of end-stage renal disease and mortality. Kidney Int. 2017;91:244–251. doi: 10.1016/j.kint.2016.09.037. doi: 10.1016/j.kint.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drury PL, Ting R, Zannino D, Ehnholm C, Flack J, Whiting M, Fassett R, Ansquer JC, Dixon P, Davis TM, Pardy C, Colman P, Keech A. Estimated glomerular filtration rate and albuminuria are independent predictors of cardiovascular events and death in type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetologia. 2011;54:32–43. doi: 10.1007/s00125-010-1854-1. doi: 10.1007/s00125-010-1854-1. [DOI] [PubMed] [Google Scholar]
- 32.Davies MJ, Bain SC, Atkin SL, Rossing P, Scott D, Shamkhalova MS, Bosch-Traberg H, Syrén A, Umpierrez GE. Efficacy and safety of Liraglutide Versus Placebo as Add-on to Glucose-Lowering Therapy in Patients With Type 2 Diabetes and Moderate Renal Impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care. 2016;39:222–230. doi: 10.2337/dc14-2883. doi: 10.2337/dc14-2883. [DOI] [PubMed] [Google Scholar]
- 33.Jacobsen LV, Hindsberger C, Robson R, Zdravkovic M. Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br J Clin Pharmacol. 2009;68:898–905. doi: 10.1111/j.1365-2125.2009.03536.x. doi: 10.1111/j.1365-2125.2009.03536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 35.FDA. Jardiance (empagliflozin) prescribing information. 2015. http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf. Accessed March 14, 2018.
- 36.FDA. Invokana (canagliflozin) prescribing information. 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204042s000lbl.pdf. Accessed March 14, 2018.
- 37.EMA. Victoza summary of opinion (post authorisation). 2017. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001026/human_med_001137.jsp&mid=WC0b01ac058001d124. Accessed March 14, 2018.
- 38.ADA. 9. Cardiovascular disease and risk management: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl)(1):S86–S104. doi: 10.2337/dc18-S009. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 39.Standl E, Schnell O, McGuire DK, Ceriello A, Ryden L. Integration of recent evidence into management of patients with atherosclerotic cardiovascular disease and type 2 diabetes. Lancet Diabetes Endocrinol. 2017;5:391–402. doi: 10.1016/S2213-8587(17)30033-5. doi: 10.1016/S2213-8587(17)30033–5. [DOI] [PubMed] [Google Scholar]


