Abstract
Egg yolk phosvitins, generated through the fragmentation of vitellogenins, are among the most heavily phosphorylated proteins ever described. Despite the early discovery in 1900 that chicken phosvitin is a phosphoprotein and its subsequent employment as an artificial substrate for a number of protein kinases, the identity of the enzyme(s) responsible for its phosphorylation remained a matter of conjecture until present. Here we provide evidence that phosvitin phosphorylation is catalyzed by Fam20C, an atypical protein kinase recently identified as the genuine casein kinase and responsible for the phosphorylation of many other secreted proteins at residues specified by the S-x-E/pS consensus. Such a conclusion is grounded on the following observations: i) the levels of Fam20C and phosphorylated vitellogenin rise in parallel upon treatment of zebrafish with oestrogens; ii) Zebrafish phosvitin is readily phosphorylated upon coexpression in U2OS cells with Fam20C, but not with its catalytically inactive mutant; iii) a peptide reproducing a stretch of 12 serines, which are phosphorylated in chicken phosvitin despite lacking the C terminal priming motif S-x-E, is efficiently phosphorylated by both recombinant and native Fam20C. The last finding expands the repertoire of potential targets of Fam20C to include several proteins known to harbour (p-Ser)n clusters not specified by any known kinase consensus.
Keywords: Fam20C, Vitellogenesis, phosvitin, sphingolipid signaling, phosphoserine stretches
Graphical Abstract
Despite its early discovery in 1900 the kinase(s) responsible for the multiphosphorylation of egg yolk phosvitin remained a mystery until now. Here we provide in vitro and in vivo evidence that the atypical kinase Fam20C, responsible for the generation of many secreted proteins, fulfils all criteria for being considered a genuine phosvitin kinase although many clusters of phosvitin phosphoserines lack its canonical priming consensus SSEE.
Introduction.
In the infancy of studies on protein phosphorylation the few phosphoproteins known at that time were extensively used as artificial substrates to detect protein kinase activities in biological samples. This especially applies to casein, the first phosphoprotein ever described [1] which was instrumental to the early discovery of protein kinase activity in rat tissues [2] paving the road toward the characterization of two pleiotropic members of the kinome, denoted now-a-days by the acronyms CK1 (a family of 7 isoforms in human) and CK2, reminiscent of their “casein kinase” activity. However, it soon became clear that neither CK1 nor CK2 are the genuine casein kinase(s) responsible for the phosphorylation of casein in the Golgi apparatus of lactating mammary gland (“G-CK”). As reviewed in [3], the genuine casein kinase was identified only recently and shown to be identical to Fam20C, an atypical protein kinase [4–6] responsible for the phosphorylation of a plethora of secreted proteins [7] at sites specified by the S-x-E/pS consensus. Such a consensus enables Fam20C to act both as a “priming” and a “primed” kinase able to phosphorylate stretches of several consecutive serines provided they end with the ….S-x-E motif. These are found in several Fam20C protein substrates, notably in casein fractions and osteopontin [8].
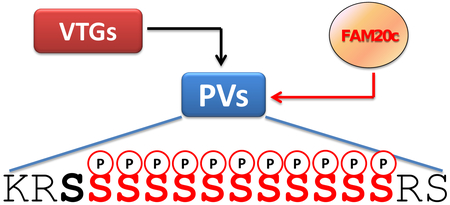
Poly-phosphoserine stretches even longer than those found in casein and osteopontin are present in chicken egg yolk phosvitin, the second phosphoprotein ever described [9], which was often used in early studies on protein phosphorylation to replace casein as an artificial substrate of “casein kinases” e.g. [10–12]. Phosvitin is generated by cleavage of vitellogenin (VTG), which is synthesized in liver and secreted into the oviduct to reach the oocyte and possibly other tissues [13]. Many phosvitins have a huge content of phosphate, which is bound covalently to long stretches of serine residues. Hen phosvitins (generated by vitellogenins I and II) contain long clusters of more than 10 consecutive phosphoserines (Fig. 1). As phosvitin is a secreted protein interchangeable with casein as an artificial substrate of “casein kinases,” it was expectable that its in vivo phosphorylation would also be performed by Fam20C. However, as pointed out elsewhere [14] such an expectation is challenged by the observation that pS-x-E motifs are absent in hen phosvitin, where long pSer clusters are present lacking this consensus at their C-terminal end (see also Fig. 1).
Fig. 1. Aminoacid sequence of chicken and zebrafish phosvitins.
A. Chicken phosvitin 2, showing the phosphorylated residues (highlighted in red) [15]; the sequence encompassing R3S12R3 peptide is underlined. B. Phosvitin derived from Zebrafish VTG1. The sequence shown is >95% identical to those of phosvitins generated from the other Zebrafish VTGs.
This raises the question as to whether an independent, presently still unidentified “phosvitin kinase” exists, or if rows of numerous serines can be recognized and targeted by Fam20C and/or other “casein kinases” (notably CK1 and CK2) even if they lack the consensus for non primed phosphorylation. This in fact would be sufficient to trigger the subsequent hierarchical phosphorylation of other nearby seryl residues.
Here we show that Fam20C, but not CK1 or CK2, can readily phosphorylate a synthetic peptide (R3S12R3) reproducing a fragment of phosvitin 2 harbouring 12 consecutive phosphoserines, and that Zebrafish phosvitin expressed in U2OS cells is also robustly phosphorylated by Fam20C. Furthermore, upon treatment of Zebrafish with oestrogens, a dramatic rise of vitellogenin synthesis is paralleled by an increased activity of Fam20C. These results demonstrate that Fam20C can also act as a genuine phosvitin kinase and discloses new perspectives about the identification of additional targets of this atypical kinase.
Results
The sequence of hen Phosvitin 2, corresponding to residues 1106–1328 of vitellogenin II, is shown in Fig. 1A. Highlighted are 57 p-Ser sites unambiguously localized by MS/MS sequence analyses [15]. These residues don’t account for all the phosphate incorporated into phosvitin 2, but they are sufficient to conclude that, based on the absence of a canonical Fam20C consensus site (S-x-E), none of the identified phosphoserines is expected to be generated by Fam20C. This applies in particular to the stretch of 12 adjacent serines, 11 of which are phosphorylated, that correspond to the sequence 1193–1204, flanked by two and one basic residues at its N-terminal and C-terminal ends, respectively (..SKRSsssssssssssRS…).
To assess the potential of Fam20C and the other “casein kinases” to phosphorylate a stretch of serines lacking the kinase consensus, a peptide was synthesized encompassing residues 1191–1205 of vitellogenin II, with the addition of 3 basic residues at its two termini to give RRR-SSSSSSSSSSSS-RRR (R3S12R3). This modification makes the peptide amenable to the phosphocellulose paper assay to evaluate phosphorylation even if several serines, instead of just one, become phosphorylated through a hierarchical mechanism. At acidic pH, the R3S12R3 peptide will still bear at least one net positive charge, and therefore will bind to phosphocellulose, also when five of its serines become phosphorylated during the kinase reaction.
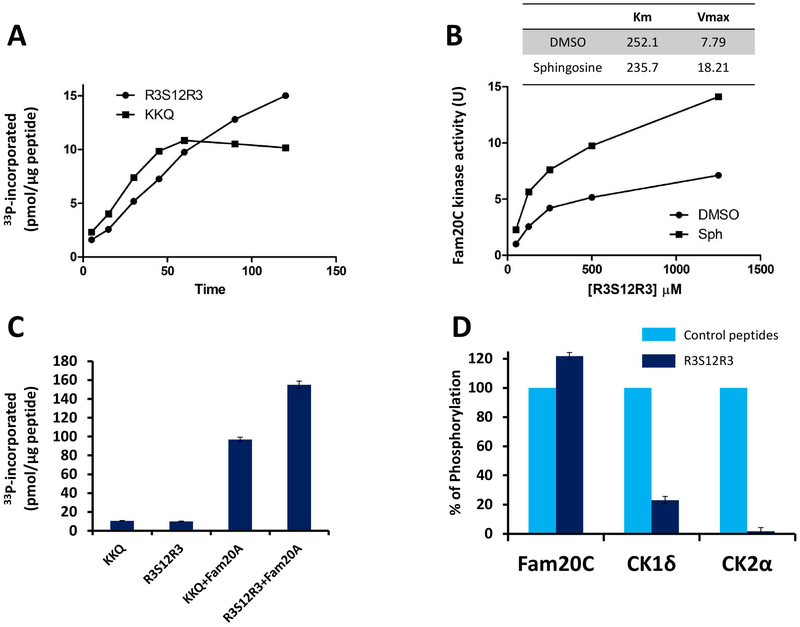
The time course phosphorylation of the R3S12R3 peptide by recombinant Fam20C was compared under identical conditions to that of the specific Fam20C peptide substrate derived from its physiological target β-casein. As shown in Fig. 2A, both peptides were readily phosphorylated, providing the clear-cut demonstration that Fam20C is able to recognize a stretch of serines devoid of its canonical consensus sequence (S-x-E/pS). The two curves however differentiate for their shape which in the case of the R3S12R3 peptide denotes a cooperative effect and does not reach a plateau after 1 h incubation, as in the case of the canonical (KKQ) peptide. This is consistent with the concept that, although the overall phosphorylation stoichiometry remains with both peptides largely below the 1:1 ratio, in the case of R3S12R3 a progressive hierarchical phosphorylation of multiple residues takes place, primed by the initial phosphorylation of a single residue.
Fig. 2. The R3S12R3 peptide derived from chicken phosvitin 2 is readily phosphorylated by Fam20C, but not by CK1 and CK2.
A) Time course phosphorylation of R3S12R3 and β28–40 peptides in the presence of 200μM ATP. B) kinetics of R3S12R3 phosphorylation by Fam20C in the absence or presence of 25μM sphingosine. C) Stimulatory effect of Fam20A added to Fam20C in 20 fold molar excess, in the presence of 200 μM ATP. Incubation time: 60 min. D) R3S12R3 phosphorylation by Fam20C, CK2, and CK1 expressed as per count of the respective canonical peptides (β28–40, CK2 tide and CK1 tide respectively). Reported values are means ± SD of three independent experiments; for details see Materials and Methods.
Fig. 2B shows the kinetics of the R3S12R3 peptide phosphorylated by recombinant Fam20C: as already observed with all Fam20C substrates, either proteins or peptides [16], the phosphorylation efficiency is increased by sphingosine, through a mechanism that implies both a decrease of Km and an increase of Vmax. It should be noted that the kinetic constants are similar to those observed with the canonical peptide substrate, corroborating the concept that rows of several consecutive serines are recognized by Fam20C with an efficiency comparable to that of a canonical site specified by the S-x-E motif (See Fig. 2B). Also to note is the stimulatory effect of Fam20A on the phosphorylation of both the canonical and the R3S12R3 peptide by Fam20C (Fig. 2C). By contrast, as shown in Fig. 2D, CK2 is completely inactive toward the R3S12R3 peptide and CK1 displays toward it a very modest activity, negligible as compared to its specific peptide substrate.
The experiments shown in Fig. 2 were performed in vitro with a construct of recombinant Fam20C. In order to confirm that also native Fam20C is able to phosphorylate (Ser)n stretches devoid of the canonical consensus, we took advantage of MDA cells, recently shown to express endogenous Fam20C in sufficient amounts that its activity can be detected in kinase assays with the specific β-casein derived peptide as substrate [17]. These cells, moreover, are available both as wild type and knocked out with respect to Fam20C by the CRISPR/Cas9 methodology.
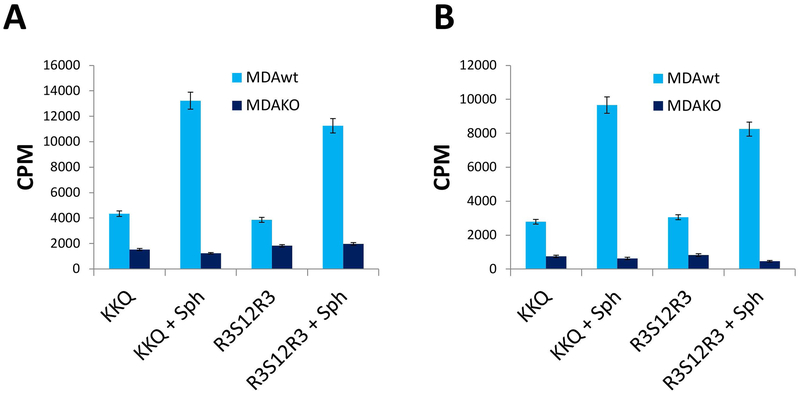
Lysates from these cells were tested for their ability to phosphorylate the R3S12R3 peptide either in the absence or presence of sphingosine, and 500 μM staurosporine was added to minimize the contribution of kinases other that Fam20C, whose peculiar feature is insensitivity to up to 1mM staurosporine[18]. It is expectable in fact that, in the absence of staurosporine, the peptide will be a good substrate for several basophilic kinases, owing to the 6 arginyl residues flanking the 12 Ser stretch.
Under these conditions, as shown in Fig. 3 A and B, the phosphorylation pattern of the R3S12R3 peptide is comparable to that of the specific Fam20C peptide substrate. Indeed it is significantly phosphorylated in both lysate (Fig- 3A) and conditioned medium (Fig 3B) by wt MDA cells but not by MDA cells where Fam20C has been knocked out. This provides the proof of concept that the phosphorylation of the R3S12R3 peptide in the presence of staurosporine, as well as that of the β-casein derived Fam20C peptide substrate, is indeed catalysed by endogenous Fam20C.
Fig. 3. Phosphorylation of the R3S12R3 peptide by endogenous Fam20C.
. Lysate (A) and the conditioned medium (B) of MDA-MB-231cells wt and Fam20C KO were assayed in the presence of 500 μM staurosporine. Activity (cpm: counts per minute) was monitored by comparing R3S12R3 peptide and the specific peptide substrate (β28–40). Reported values are means ± SD of three independent experiments; for details see Materials and Methods.
The experiments above unambiguously demonstrate that, unlike CK1 and CK2, Fam20C is able to readily phosphorylate clusters of adjacent serines lacking its canonical consensus. Its actual implication in the phosphorylation of phosvitin, however, remained a matter of conjecture. To address this question, we have focused on Zebrafish phosvitin, whose sequence, shown in Fig. 1B, is not as rich in phosphoserines as hen phosvitin, but it also contains clusters of up to 6 adjacent phosphoserines devoid of any canonical Fam20C consensus (…S-x-E), which could account for a non-primed phosphorylation capable to prime the hierarchical phosphorylation of additional serines located upstream. Its only canonical site (S-S-E) is in fact localized upstream rather than down-stream of the region rich in (Ser)n clusters and its phosphorylation, therefore, is not expected to prime any further phosphorylation (see Fig. 1B).
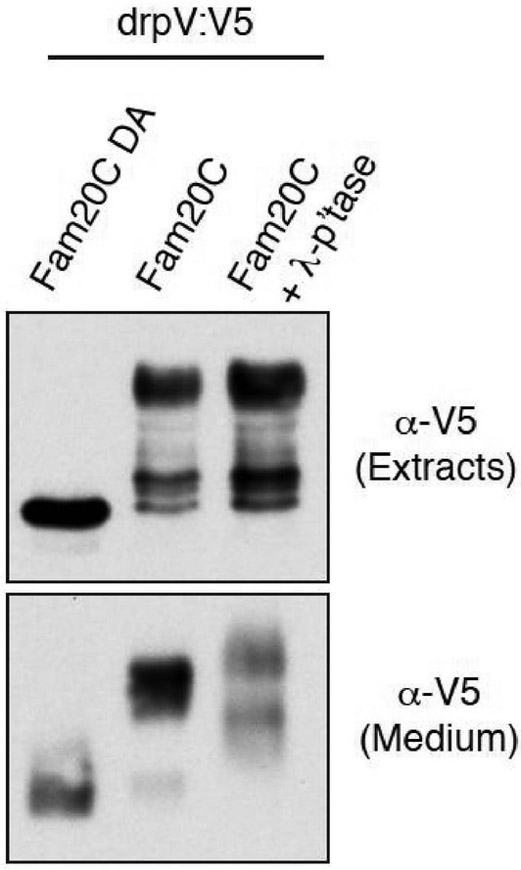
Zebrafish phosvitin engineered to contain an SP co-expressed with Fam20C in U2OS cells was found to be extensively phosphorylated (as judged from the remarkable electrophoretic mobility shift during SDS PAGE giving rise to a ladder of several bands) both in the extracts and conditioned medium, while only the un-phosphorylated band (not upshifted) could be detected in the same cells expressing the catalytically inactive D478A mutant of Fam20C (Fig. 4). While the lowest up-shifted band detected in the extract could represent the derivative mono-phosphorylated at the only seryl residue conforming to the canonical consensus, this clearly cannot apply to the upper bands, with special reference to the main band at the top of the ladder, displaying a striking upshift. It can be concluded therefore that upon expression in U2OS cells zebrafish phosvitin undergoes phosphorylation by co-expressed Fam20C at multiple seryl residues not conforming to its canonical consensus.
Fig.4. Fam20C phosphorylates zebrafish phosvitin.
Protein immunoblotting of V5 immunoprecipitates from conditioned medium of U2-OS cells cotransfected with V5-tagged Danio rerio phosvitin (drpV:V5) and Fam20C WT or catalytic mutant D478A (DA). The total extracts were also analyzed for V5 by immunoblotting. Note that only the immunoprecipitates from conditioned medium were treated with phosphatase.
To identify a functional link between phosvitin and Fam20C, we concentrated on reports showing that vitellogenin (VTG), the precursor of phosvitin in oviparous animals, is a sensitive biomarker of estrogenic pollution [13]. In particular, exposure of zebrafish to low concentrations (50ng/L) of 17α-ethinylestradiol (EE2) are sufficient to promote a dramatic increase in VTG biosynthesis not only in liver, plasma and gonad, but also in many other tissues. We reasoned that, being VTG the precursor of heavily phosphorylated phosvitin, its increased biosynthesis should be paralleled by a concomitant raise of the protein kinase(s) committed to the generation of phosphorylated phosvitin. Therefore, monitoring Fam20C activity in zebrafish samples upon treatment with EE2 could provide the proof of concept that this kinase is implicated in this process.
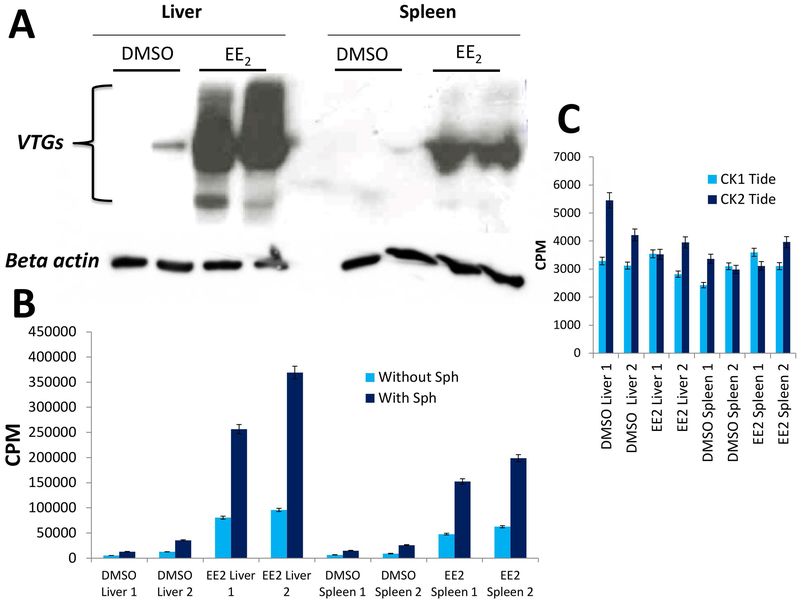
Fig. 5A shows that upon treatment of zebrafish with oestrogens a dramatic rise of VTGs takes place both in liver and spleen, while under normal conditions these proteins are nearly undetectable. As shown in Fig. 5B, such a phenomenon is accompanied by a comparably striking increase of Fam20C activity, as judged from the phosphorylation of its specific peptide substrate. This clearly reflects Fam20C activity since it is unaffected by 0.5 mM staurosporine and is stimulated by sphingosine (Figure 5B), which are two distinctive properties of Fam20C. To note that no significant increase of activity of CK1 and CK2 was observed, as judged by using their specific peptide substrates (Figure 5C). These data disclose a functional link between Fam20C and VTG, consistent with the concept that the former is indeed committed to the generation of phosphorylated phosvitin in zebrafish.
Fig. 5. Simultaneous rise of VTG expression and Fam20C activity upon Zebrafish exposure to 17α-estradiol (EE2).
A) Expression of VTG was detected with a specific antibody in the liver and spleen of two zebrafish adult females (sample 1 and 2). B) Fam20C activity was measured in the same samples with the specific peptide substrate (β28–40) in the presence of 500 μM staurosporine. C) At variance with Fam20C the activities of CK1 and CK2 are not significantly altered upon Zebrafish exposure to EE2. Reported values are means ± SD of three independent experiments; for details see Materials and Methods.
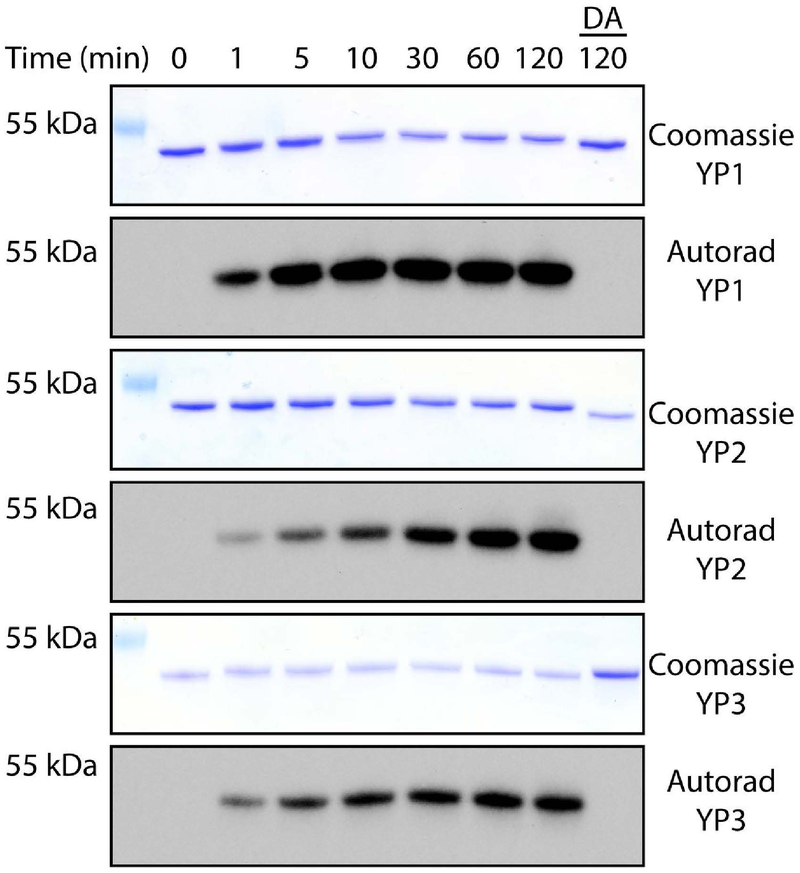
The presence of Fam20C in organisms without mineralized tissues, such as worms and flies, suggests that its ancestral role may indeed be to phosphorylate vitellogenin. In higher dipteran insects, such as Drosophila, the functional analogs of vitellogenin are the yolk proteins 1–3 (YPs) [19]. Drosophila melanogaster YPs are phosphorylated on multiple serine residues that conform to the Fam20C consensus and occur together in phosvitin-like domains [20, 21]. We find that Drosophila YPs are Fam20C substrates in vitro (Fig. 6). Our demonstration that Fam20C phosphorylates both vertebrate and invertebrate phosvitin domains suggests that Fam20C evolved to phosphorylate vitellogenins long before it acquired a role in tissue mineralization.
Fig 6. Phosphorylation of Drosophila yolk proteins by Fam20C.
Time dependent incorporation of 32P from [γ−32P]ATP into yolk proteins (YP) 1, 2 and 3 by Fam20C or the D478A mutant (DA). Reaction products were separated by SDS-PAGE, visualized by Coomassie staining, and radioactivity was detected by autoradiograph.
Discussion
Phosvitin is probably the most heavily phosphorylated protein ever described. The phosphorylation of phosvitin domains and related lipid transport proteins is a widespread, persistent, and evolutionarily ancient modification; likely as old as the common egg-laying animal, which lived in the Precambrian period 500 million years ago [22]. Bird, [23] fish [24] and insect [25], vitellogenin, as well as Drosophila yolk proteins [26] are all phosphorylated within phosvitin-like domains. Despite the conservation and persistence of yolk protein phosphorylation across egg-laying animals, the kinase that performs this phosphorylation has remained unknown.
Because VTG is a secreted protein, its kinase (or kinases) must reside within the lumen of the secretory pathway or outside the cell. Our understanding of extracellular phosphorylation has been incomplete because the kinases that catalyze the phosphorylation of secreted proteins have only recently been identified [14, 27]. The secretory pathway kinases are only distantly related to intracellular kinases, and are classified into two evolutionarily distinct families: The Family with sequence similarity 20C (Fam20C)-family and the VLK-family (reviewed in [27]).
Fam20C phosphorylates protein substrates on serine residues, making it a promising candidate for the “genuine phosvitin kinase.” However, several observations have shed some doubt on this relationship. First, many phosphoserine stretches in phosvitins from different species lack the C-terminal “primary” consensus sequence, S-x-E, that in caseins and in other secreted substrates of Fam20C triggers the progressive phosphorylation of upstream serines, primed by the generation of the “secondary” consensus S-x-pS. Second, the spectrum of human disorders caused by Fam20C mutations implies a crucial role in tissue mineralization [4]. Nonetheless, Fam20C is conserved in organisms without mineralized tissues, such as worms and flies.
Here, we provide evidence that the phosphorylation of phosvitin is catalysed by the same atypical kinase, Fam20C, previously identified as the genuine Golgi apparatus casein kinase (G-CK) responsible for the phosphorylation of many other secreted proteins. Our data show not only that Fam20C is able to phosphorylate phosvitins in vitro and in cells but also that a functional relatedness exists in zebrafish between estrogen induced biosynthesis of VTGs (the precursors of phosvitins) and Fam20C activity.
The mechanism(s) by which Fam20C is upregulated during zebrafish oogenesis remains an open question. Two mechanisms capable to increase Fam20C activity are known, namely association with Fam20A to form hyperactive heterodimers [28] and sphingolipid signalling [16, 17]. In vitro both are able to increase the phosphorylation of the phosvitin derived R3S12R3 peptide by Fam20C (see Fig. 2, panel B and C). In zebrafish the presence of Fam20A in addition to Fam20C [28] suggests that the former mechanism may contribute to the dramatic raise of Fam20C activity promoted by estrogen treatment. The same mechanism however is hardly conceivable in other organisms, like flies, where the functional analogues of phosvitin are also phosphorylated by Fam20C but Fam20A is absent [28]. Additional experimentation will be necessary to shed light on the mechanism(s) promoting up-regulation of Fam20C during oogenesis and, more in general under conditions where an abrupt raise of its activity takes place.
Collectively taken, our data solve a “cold case” dating back to 1900, when hen egg phosvitin was discovered as the second phosphoprotein known at that time [9], and subsequently employed to replace casein as a “foreign” substrate to detect and measure the in vitro activity of “acidophilic” protein kinases. The kinase responsible for the in vivo phosphorylation of egg yolk phosvitins (and/or of their precursors vitellogenins), however, remained a mystery until now.
The apparent paradox that phosphoserine clusters of many phosvitins do not conform to the Fam20C consensus sequence (S-x-E/pS) has been explained by showing that rows of several consecutive serines can be efficiently phosphorylated by Fam20C even though they are devoid of this consensus.
This observation expands the already huge repertoire of potential targets of Fam20C, considering that in the human proteome there are many examples of multi-phosphorylated proteins harbouring stretches of phosphoserines devoid of any consensus recognized by known kinases, and whose mechanism of phosphorylation therefore remains a matter of conjecture [8]. CK1 and CK2, the other two acidophilic protein kinases able to drive hierarchical phosphorylation, fail to phosphorylate clusters of serines devoid of a priming consensus (see Fig. 2D). Although we cannot rule out that other kinase(s) might exist able to catalyse the primary input (the seeding) of phosphate into a stretch of serines, thus triggering the subsequent hierarchical phosphorylation of additional residues, for the time being Fam20C looks as the first choice candidate to perform alone the whole job. This especially applies whenever we are dealing with proteins undergoing secretion at some stages of their life, coming therefore in touch with Fam20C.
Notably, a direct genetic link between Fam20C and phosvitin has yet to be demonstrated. The current animal and cell-based models of Fam20C are limited to placental vertebrates that bear live young, a vastly underrepresented group among animals with Fam20C. This has limited our ability to study the relationship between Fam20C and vitellogenesis. Future work will aim to develop transgenic models of Fam20C deficiency in oviparous species.
Identifying the phosvitin kinase and understanding the functional consequences of these phosphorylation events could have broad biological implications. For example, the apolipoproteins are related to vitellogenin and are also phosphoproteins that undergo receptor-mediated endocytosis. Indeed, PCSK9, ApoA2, ApoB, ApoE, ApoL1, and ApoA5 are all substrates of Fam20C in humans [7]; however, the functional significance of these modifications are not well-understood. Thus, understanding how phosphorylation regulates vitellogenesis could provide important clues to human lipid homeostasis. Determining the phosvitin kinase also has implications for the basic biology of many organisms relevant to human health, such as insect disease vectors, pests, and helminth parasites. In addition to solving the longstanding mystery of the “real phosvitin kinase,” the results of this work will enable the discovery of the regulatory mechanisms of vitellogenesis and other diverse processes that involve VTG and members of the large lipid transport protein family, such as apolipoproteins.
Materials and Methods
Reagents
Sphingosine, staurosporine, 17α-Ethynylestradiol (EE2) and phosphatase Inhibitor Cocktails 2 and 3 were purchased from Sigma-Aldrich and dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich), 100%; The synthetic peptide substrate b(28–40), KKIEKFQSEEQQQ [29] was synthetized by CRIBI Peptide Facility, University of Padova. Recombinant Fam20C-SUMO was purified as described in [4]. Fam20C KO MDA-MB-231 cells were described in [7]. Tissue Extraction Reagent, protease inhibitors, precast SDS-PAGE were purchased from ThermoFisher Scientific, while Immobilon-P membranes was retrieved from Merk Millipore. Anti-Fam20C and vitellogenin antibodies (154740 and 36804, respectively) were purchased from Abcam, while Anti-beta actin from Sigma-Aldrich (A5441). HRP-conjugated secondary antibodies were purchased from Biorad (1706515 and 17065166).
Generation of constructs
cDNAs for Drosophila melanogaster Yp1, Yp2, and Yp3 were purchased from the Drosophila Genomics Resource Center at Indiana University. Open reading frames (excluding the signal peptide) were amplified by PCR and cloned into a modified pET28a bacterial expression vector (ppSUMO) containing an N-terminal 6× His tag, followed by the yeast Sumo (smt3) coding sequence.
Peptide synthesis
The chicken phosvitin 2 fragment RRRSSSSSSSSSSSSRRR-NH2 (R3S12R3), corresponding to VTG-2 sequence 1193–1204 with suitable modifications (see Results) was synthesized by solid phase method using a multiple peptide synthesizer (SyroII, MultiSynTech GmbH) on a Rink Amide MBHA resin (Novabiochem, Bad Soden, Germany). The fluoren-9-ylmethoxycarbonyl (Fmoc) strategy was used throughout the peptide chain assembly, utilizing O-(Benzotriazol-1-yl)-N,N,N’,N’-tetramethyluronium tetrafluoroborate (TBTU) and 3-hydroxytriazolo[4,5-b]pyridine (HOAT) as coupling reagents. The side-chain protected amino acid building blocks used were N-α-Fmoc-Nω-(2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl)-L-arginine, and N-α-Fmoc-O-tert-butyl-L-serine. Cleavage of the peptide was performed by reacting the peptidyl-resins with a mixture containing TFA/TES/H2O (95%, 2,5%, 2,5%) for 2.5 h. Crude peptide were purified by a preparative reverse phase HPLC. Molecular masses of the peptide wereconfirmed by mass spectroscopy on a MALDI TOF-TOF mass spectrometer (model 4800 – Applied Biosystems).
Purification of recombinant proteins
E. coli BL21 (DE3) competent cells were transformed with ppSUMO-YP1, YP2 or YP3, and the cells were grown in Luria–Bertani medium at 37 °C until the A600 reached ∼0.5–0.7. Protein expression was induced by addition of 0.4 mM isopropyl β-d-thiogalactopyranoside overnight at 21 °C. The cells were recovered by centrifugation at 5,000 × g for 10 min, resuspended in lysis buffer (50 mM Tris⋅HCl (pH 8.0), 300 mM NaCl, 1 mM PMSF, 1mM DTT), and lysed using sonication. Lysates were centrifuged at 35,000 × g for 45 min and the 6× His-tagged fusion proteins were purified from the supernatant by Ni2+-NTA-agarose chromatography. The resin was washed extensively with lysis buffer containing 25 mM imidazole, and the fusion proteins were eluted with lysis buffer containing 300 mM imidazole. The proteins were cleaved overnight at 4 °C with 3 μg/mL recombinant 6×-His tagged Ulp1 Sumo protease. The proteins were further purified by gel filtration chromatography using an AKTA Pure FPLC chromatography system (GE Healthcare). Recombinant human Fam20C and Fam20C D478A were purified from insect cells as described in [30]. MBP-tagged Fam20A was prepared as described in [31]
In vitro kinase assays
Unless differently indicated, Fam20C (50 ng), CK2α (15 ng) and CK1δ (20 ng) activity were assayed in a final volume of 40 μL of solution containing 50 mM Tris-HCl pH 7.5, 5mM MnCl2 or MgCl2. The reaction was started by addition of 5 μL of a reaction mixture containing 50 μM [γ−33P] ATP (500–1000 cpm•pmol−1), and 300 μM the synthetic peptide substrates KKIEKFQSEEQQQ (β28–40, FAM20C), RRRADDSDDDDD (CK2-Tide, CK2), RRKHAAIGDDDDAYSITA (CK1-Tide, CK1) and RRRSSSSSSSSSSSSRRR-NH2 (R3S12R3). After 10 min incubation at 37 °C, the reaction was stopped by addition of 5 μL of 0.5 M orthophosphoric acid before spotting 20-μL aliquots on to phosphocellulose filters. Filters were washed in 75 mM phosphoric acid (5–10 mL each) four times then once in methanol and dried before counting with PerkinElmer TriCarb 2810 TR. In the kinetic experiment, Fam20C activity is expressed in U, one unit (U) being defined as the amount of Fam20C that catalyzes the incorporation of 1 pmol phosphate•min−1 into the peptide substrate.
Kinase assays using Drosophila yolk proteins as substrate were performed in 50 mM HEPES pH 7, 10 mM MnCl2, 1 mM [γ−32P] ATP (SA = 100 cpm/pmol), 0.25 mg/mL recombinant substrate and 10 μg/mL Fam20C or Fam20C (D478A). Reactions were incubated at 30°C and terminated at the indicated time points by the addition of 15 mM EDTA, SDS loading buffer and boiling. Reaction products were separated by SDS-PAGE and visualized by Coomassie blue staining. Destained gels were dried and incorporated radioactivity visualized by autoradiography.
Cell culture and Cell lysis
MDA-MB-231 and Fam20C KO MDA-MB-231 cells were maintained in 5% CO2 in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 100 U•mL−1 penicillin, and 100 mM streptomycin, in an atmosphere containing 5% CO2. Cell treatments were performed in six-well plates at about 70–80% confluence in a complete medium. Cells were detached, centrifuged, extensively washed with PBS, and lysed by the addition of ice-cold buffer containing 20 mM Tris–HCl (pH 8), 150 mM NaCl, 1 mM EGTA, 1% Triton X-100 (v/v), protease inhibitor cocktail Complete (Roche Diagnostics, Mannheim, Germany). After 20 min of incubation in ice, the lysate was centrifuged 10 min at 10 000 g at 4 °C.
Cotransfection experiments.
Danio rerio phosvitin (residues 919–1167 of D. rerio vtg1) was cloned into a modified pcDNA vector containing the human interluekin-2 (IL-2) signal sequence and a C-terminal V5-tag using the following primers:
Dr.pV_BamH1_Fwd: AAAACGGATCCACGTCAGAAACATTGAAGATCACAGTGCTG
Dr.pV_Xba1_Rev: AAAATCTAGATGGAATATCATTTCCAAGGAATCTATTCTG
The restriction sites are underlined.
U2OS cells were cultured and cotransfected with C-terminally tagged Fam20C or the D478A mutant and C-termially V5-tagged D. rerio pV essentially as in [4]. Protein immunoblotting, immunoprecipitations and lambda phosphatase treatment were also as described in [4].
Kinase activity determinations in cell lysate and conditioned medium
All the lysate assays were performed as described above for in vitro assay, with 5 mM MnCl2 or MgCl2 and in the absence and presence of 25 μM sphingosine and 500 μM staurosporine, as previously described in [17].
Fish and treatment
Adult fish were kept for seven days in 0.5 liters tanks filled with fish water at neutral pH, with a 14:10 h light:dark cycle at 26 °C, according to standard procedures (http://ZFIN.org). Stock solution of 10 mg/L and 1 mg/L was prepared by dissolving 17α-ethinylestradiol (EE2) in DMSO and fish water. Two groups of adult zebrafish (six females for each condition) were tested with or without EE2 treatment. One group was exposed for 7 days to 50 ng/L (168.7 pmol/L) of EE2, and the control group was exposed to DMSO (10 ml in 400 ml). Treatment was repeated every day and at day 7 post-treatment, fish were euthanized according to standard procedures approved by the Local Ethical Committee at the University of Padova and National Agency (Italian Ministry of Ministry of Health, project n.77 and 78/2013).
Western Blot analysis
Fish liver and spleen samples were manually dissected and immediately lysed in Tissue Extraction Reagent (Thermofisher, Milan, Italy) in the presence of protease inhibitors and phosphatase Inhibitor Cocktails 2 and 3; the lysates were centrifuged at 13,000×g for 30 min at 4 °C. The supernatant was collected and protein concentration was determined by the Bradford method. Extracted proteins were supplemented with 4x sample buffer, heated at 70°C for 10 min and run onto precast SDS-PAGE. Proteins were transferred on Immobilon-P membranes in 25 mM Tris, 192 mM glycine, 20% methanol (v/v), 0.1% SDS. Membranes were incubated overnight with primary antibodies against vitellogenin, FAM20C, beta actin. Primary antibodies (VTG and FAM20C) were used at 1:1000 or 1:5000 (beta-actin) dilution. HRP-conjugated secondary antibodies were used at a 1:3000 dilution. HRP Chemiluminescence signals were detected by Kodak Image Station 4000MMpro (Eastman Kodak, New Haven, CT) and analyzed with the Kodak Image software.
Statistical analysis
All experiments have a minimum of three replicates. Data are presented as means with error bars indicating the SD. Statistical significance of differences between experimental groups was assessed using Student’s t test. Differences in mean were considered significant if P < 0.05.
Acknowledgements
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC, grant IG 18756) to LAP, University of Padova (MORO_SID17_01,) to EM, and Welch Foundation (Grant No I-1911) and NIH (grant R00DK099254) to VST and aT32 training grant to MB (5T32GM007062–44).
Abbreviations.
- DMSO
dimethyl sulfoxide
- Fam20C
family with sequence similarity 20, member C
- G-CK
Golgi-casein kinase or genuine casein kinase
- VTG
vitellogenin; TES, triethylsilane
- TFA
trifluoroacetic acid
- EE2
17α-Ethynylestradiol
References
- 1.Hammarsten O (1883) Zur Frage ob Caseïn ein einheitlicher Stoff sei. Hoppe Seylers. Z Physiol Chem 7, 227–273 [Google Scholar]
- 2.Burnett G & Kennedy EP (1954) The enzymatic phosphorylation of proteins. J Biol Chem 211, 969–980. [PubMed] [Google Scholar]
- 3.Venerando A, Ruzzene M & Pinna LA (2014) Casein kinase: the triple meaning of a misnomer. Biochem J 460, 141–156, doi: 10.1042/BJ20140178BJ20140178 [pii]. [DOI] [PubMed] [Google Scholar]
- 4.Tagliabracci VS, Engel JL, Wen J, Wiley SE, Worby CA, Kinch LN, Xiao J, Grishin NV & Dixon JE (2012) Secreted kinase phosphorylates extracellular proteins that regulate biomineralization. Science 336, 1150–1153, doi: 10.1126/science.1217817science.1217817 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishikawa HO, Xu A, Ogura E, Manning G & Irvine KD (2012) The Raine syndrome protein FAM20C is a Golgi kinase that phosphorylates bio-mineralization proteins. PLoS One 7, e42988, doi: 10.1371/journal.pone.0042988PONE-D-12-16448 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lolli G, Cozza G, Mazzorana M, Tibaldi E, Cesaro L, Donella-Deana A, Meggio F, Venerando A, Franchin C, Sarno S, et al. (2012) Inhibition of protein kinase CK2 by flavonoids and tyrphostins. A structural insight. Biochemistry 51, 6097–6107, doi: 10.1021/bi300531c. [DOI] [PubMed] [Google Scholar]
- 7.Tagliabracci VS, Wiley SE, Guo X, Kinch LN, Durrant E, Wen J, Xiao J, Cui J, Nguyen KB, Engel JL, et al. (2015) A Single Kinase Generates the Majority of the Secreted Phosphoproteome. Cell 161, 1619–1632, doi: 10.1016/j.cell.2015.05.028S0092-8674(15)00578-4 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesaro L & Pinna LA (2015) The generation of phosphoserine stretches in phosphoproteins: mechanism and significance. Mol Biosyst 11, 2666–2679, doi: 10.1039/c5mb00337g. [DOI] [PubMed] [Google Scholar]
- 9.Leven PA & Alsberg C (1900) Zur chemie der paranucleinsaure. Hopper-Seyler’s Z Physiol Chem 31, 543–555. [Google Scholar]
- 10.Rabinowitz M & Lipmann F (1960) Reversible phosphate transfer between yolk phosphoprotein and adenosine triphosphate. J Biol Chem 235, 1043–1050. [PubMed] [Google Scholar]
- 11.Rodnight R & Lavin BE (1964) Phosvitin kinase from brain: activation by ions and subcellular distribution. Biochem J 93, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meggio F & Pinna LA (1988) Phosphorylation of phosvitin by casein kinase-2 provides the evidence that phosphoserines can replace carboxylic amino acids as specificity determinants. Biochim Biophys Acta 971, 227–231, doi: 0167-4889(88)90196-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 13.Zhong L, Yuan L, Rao Y, Li Z, Zhang X, Liao T, Xu Y & Dai H (2014) Distribution of vitellogenin in zebrafish (Danio rerio) tissues for biomarker analysis. Aquat Toxicol 149, 1–7. [DOI] [PubMed] [Google Scholar]
- 14.Tagliabracci VS, Pinna LA & Dixon JE (2013) Secreted protein kinases. Trends Biochem Sci 38, 121–130, doi: 10.1016/j.tibs.2012.11.008S0968-0004(12)00191-0 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czernick D, Liu J, Serge D & Salih E (2013) Topographical distribution of phosphorylation sites of phosvitins by mass spectrometry. J Proteomics 83, 76–98, doi: 10.1016/j.jprot.2013.02.016 S1874-3919(13)00091-2 [pii]. [DOI] [PubMed] [Google Scholar]
- 16.Cozza G, Salvi M, Banerjee S, Tibaldi E, Tagliabracci VS, Dixon JE & Pinna LA (2015) A new role for sphingosine: Up-regulation of Fam20C, the genuine casein kinase that phosphorylates secreted proteins. Biochim Biophys Acta 1854, 1718–1726, doi: 10.1016/j.bbapap.2015.04.023 S1570-9639(15)00132-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 17.Cozza G, Salvi M, Tagliabracci VS & Pinna LA (2017) Fam20C is under the control of sphingolipid signaling in human cell lines. FEBS J 284, 1246–1257, doi: 10.1111/febs.14052. [DOI] [PubMed] [Google Scholar]
- 18.Tibaldi E, Arrigoni G, Brunati AM, James P & Pinna LA (2006) Analysis of a sub-proteome which co-purifies with and is phosphorylated by the Golgi casein kinase. Cell Mol Life Sci 63, 378–389, doi: 10.1007/s00018-005-5506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terpstra P & Ab G (1988) Homology of Drosophila yolk proteins and the triacylglycerol lipase family. J Mol Biol 202, 663–665. [DOI] [PubMed] [Google Scholar]
- 20.Zhai B, Villen J, Beausoleil SA, Mintseris J & Gygi SP (2008) Phosphoproteome analysis of Drosophila melanogaster embryos. J Proteome Res 7, 1675–1682, doi: 10.1021/pr700696a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan MD & Mahowald AP (1982) Phosphorylation of the vitellogenin polypeptides of Drosophila melanogaster. Insect Biochemistry 12, 4, doi: 10.1016/0020-1790(82)90055-5. [DOI] [Google Scholar]
- 22.Byrne BM, Gruber M & Ab G (1989) The evolution of egg yolk proteins. Prog Biophys Mol Biol 53, 33–69. [DOI] [PubMed] [Google Scholar]
- 23.Levene PA & Alsberg C (1900) Zur chemie der paranucleinsaure. Hoppe-Seyler’s Zeitschrift für Physiologische Chemie 31, 13. [Google Scholar]
- 24.Sharrock WJ, Rosenwasser TA, Gould J, Knott J, Hussey D, Gordon JI & Banaszak L (1992) Sequence of lamprey vitellogenin. Implications for the lipovitellin crystal structure. J Mol Biol 226, 903–907. [DOI] [PubMed] [Google Scholar]
- 25.Tufail M & Takeda M (2008) Molecular characteristics of insect vitellogenins. J Insect Physiol 54, 1447–1458, doi: 10.1016/j.jinsphys.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Warren TG & Mahowald AP (1979) Isolation and partial chemical characterization of the three major yolk polypeptides from Drosophila melanogaster. Dev Biol 68, 130–139. [DOI] [PubMed] [Google Scholar]
- 27.Sreelatha A, Kinch LN & Tagliabracci VS (2015) The secretory pathway kinases. Biochim Biophys Acta 1854, 1687–1693, doi: 10.1016/j.bbapap.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H, Zhu Q, Cui J, Wang Y, Chen MJ, Guo X, Tagliabracci VS, Dixon JE & Xiao J (2018) Structure and evolution of the Fam20 kinases. Nat Commun 9, 1218, doi: 10.1038/s41467-018-03615-z10.1038/s41467-018-03615-z [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasa-Benito M, Marin O, Meggio F & Pinna LA (1996) Golgi apparatus mammary gland casein kinase: monitoring by a specific peptide substrate and definition of specificity determinants. FEBS Lett 382, 149–152, doi: 0014-5793(96)00136-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 30.Xiao J, Tagliabracci VS, Wen J, Kim SA & Dixon JE (2013) Crystal structure of the Golgi casein kinase. Proc Natl Acad Sci U S A 110, 10574–10579, doi: 10.1073/pnas.13092111101309211110 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui J, Xiao J, Tagliabracci VS, Wen J, Rahdar M & Dixon JE (2015) A secretory kinase complex regulates extracellular protein phosphorylation. Elife 4, e06120, doi: 10.7554/eLife.06120. [DOI] [PMC free article] [PubMed] [Google Scholar]