Abstract
Breast cancer (BCa) bone metastases (BMETs) drive osteolysis via a feed-forward loop involving tumoral secretion of osteolytic factors (e.g., PTHrP) induced by bone matrix-derived growth factors (e.g., TGFβ). In prior experiments, turmeric-derived curcumin inhibited in vivo BMET progression and in vitro TGFβ/Smad-signaling in a TGFβ-stimulated PTHrP-dependent human xenograft BCa BMET model (MDA-SA cells). However, it is unclear whether curcumin or curcumin-glucuronide mediates in vivo protection since curcumin-glucuronide is the primary circulating metabolite in rodents and in humans. Thus, effects of curcumin vs. curcumin-glucuronide on Smad-dependent TGFβ signaling were compared in a series of BCa cell lines forming TGFβ-dependent BMET in murine models, and tissue-specific metabolism of curcumin in mice was examined by LC-MS. While curcumin inhibited TGFβ-receptor-mediated Smad2/3 phosphorylation in all BCa cells studied (human MDA-SA, MDA-1833, MDA-2287, and murine 4T1 cells), curcumin-glucuronide did not. Similarly, curcumin, but not curcumin-glucuronide, blocked TGFβ-stimulated secretion of PTHrP from MDA-SA and 4T1 cells. Because the predominant serum metabolite, curcumin-glucuronide, lacked bioactivity, we examined tissuespecific metabolism of curcumin in mice. Compared to serum and other organs, free curcumin (both absolute and percentage of total) was significantly increased in bone, which was also a rich source of enzymatic deglucuronidation activity. Thus, curcumin, and not curcumin-glucuronide, appears to inhibit bone-tropic BCa cell TGFβ-signaling and undergo site specific activation (deconjugation) within the bone microenvironment. These findings suggest that circulating curcumin-glucuronide may act as a pro-drug that preferentially targets bone, a process that may contribute to the bone-protective effects of curcumin and other highly glucuronidated dietary polyphenols.
Keywords: curcumin, glucuronide, breast cancer, bone metastasis, TGFβ
1. INTRODUCTION
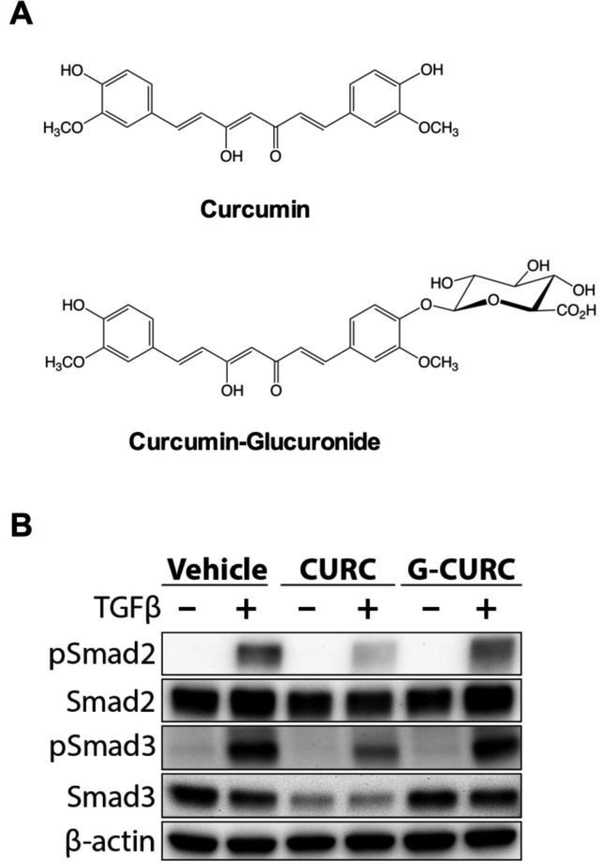
One in eight women will develop breast cancer during their lifetime [1]. A third will be diagnosed with stage IV breast cancer and the majority of these women will develop osteolytic bone metastases (BMETs), which, despite the use of chemotherapeutic agents and osteoclast-targeting drugs, are essentially incurable. The propensity of breast cancer to form osteolytic bone lesions appears to be driven by interactions between bone-metastatic tumor cells and the bone microenvironment [2–5]. This crosstalk within the tumor-bone niche is mediated via multiple signaling pathways, including tumoral effects of growth factors, such as transforming growth factor β (TGFβ), released from resorbed bone matrix [2,6–8]. TGFβ signaling in bone metastatic breast cancer cells, primarily mediated by Smad-dependent pathways, is thought to promote bone metastasis progression via induction of factors, such as parathyroid hormone-related protein (PTHrP), which promote osteoclast-mediated bone resorption [2,6,8,9]. Thus, TGFβ, Smads, and PTHrP enter into a feed-forward cycle that promotes metastasis of breast cancer cells within the bone microenvironment. Current bone-specific therapies (e.g., bisphosphonates or denosumab) attempt to break this cycle by targeting the osteoclast. In contrast, abrogation of tumoral TGFβ signaling by curcumin, a bioactive polyphenol derived from the turmeric rhizome (Curcuma longa L.) (Fig. 1A, top), has also shown promising results in pre-clinical studies using an experimental human-xenograft model of breast cancer bone metastasis (MDA-MB-231 [MDA-SA]). This model is dependent on TGFβ-stimulated PTHrP secretion [9–13], where curcumin inhibited bone metastasis progression in vivo and blocked TGFβ-stimulated PTHrP secretion in vitro by inhibition of receptor-regulated Smad2/3 activation [14,15]. However, the curcumin metabolite responsible for bone-protective effects in vivo is unclear, as curcumin-glucuronide (Fig. 1A, bottom), a phase 2 metabolite that is assumed to lack biological activity [16,17], is the predominant circulating form in vivo, whereas free curcumin is barely detectable in serum of both rodents and humans [16–20].
Figure 1.
A) Structures of curcumin and curcumin-glucuronide. B) Comparative effects of curcumin and curcumin-glucuronide on TGFβ-stimulated phosphorylation of receptor-regulated Smad2 and Smad3 in bone-tropic MDA-SA breast cancer cells. MDA-SA cells were stimulated for 1 hour with TGFβ−1 (5 ng/ml) after 16 h pretreatment with 30 μM curcumin, curcuminglucuronide, or vehicle. Phosphorylated (p)-Smad2, pSmad3, Smad2, and Smad3 protein levels were determined by Western blot analysis of protein from whole cell lysates, with verification of protein loading by tryptophan labeling (data not shown) and quantification of β-actin.
Therefore, studies were undertaken to determine whether curcumin or its major serum metabolite, curcumin-glucuronide, are responsible for inhibition of Smad-dependent TGFβ signaling in bone metastatic breast cancer cells. Furthermore, to explore and extend the possible clinical significance of previous findings, which focused on a single cell model [14,15], inhibitory effects of curcumin vs curcumin-glucuronide on TGFβ receptor-mediated activation of Smad signaling were compared using four complementary bone-tropic breast cancer cell lines (human MDA-SA, MDA-1833, MDA-2287; murine 4T1), which share the common feature of forming bone metastases in vivo with evidence of tumoral Smad-mediated TGFβ dependence [6,7,9–11,21,22]. In addition, possible site-specific curcumin metabolism in vivo was queried by comparing levels of curcumin and curcumin-glucuronide in the circulation vs bone and other tissues.
2. MATERIALS AND METHODS
2.1. Cell lines and chemicals
Three distinct bone-tropic MDA-MB-231-derived human breast cancer cells were kindly provided by Dr. Theresa Guise, Indiana University (MDA-SA) [9,10] and Dr. Joan Massagué, Sloan-Kettering (MDA-1833 and MDA-2287) [6]. All human cell lines were authenticated using short tandem repeat profiling by the University of Arizona Genetics Core [23]. Bone-tropic murine 4T1 cells, frequently used to model breast cancer bone metastases in immunocompetent mice [13,22,24–26], were obtained directly from American Type Culture Collection (#CRL-2539, ATCC) and used within 10 passages. The chemical content of commercially obtained curcumin (#218580100, Fisher; 80.6% curcumin, 13.5% demethoxycurcumin, and 2.4% bisdemethoxycurcumin by weight) and curcumin-glucuronide (#C838510, Toronto Research Chemicals; curcumin-glucuronide devoid of free curcumin) was verified using standard methods (see below) [27,28] with stock solutions prepared in DMSO. Cells were stimulated with recombinant human TGFβ1 (#240-B, R&D Systems).
2.2. Cell culture
Cells were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin. For analysis of TGFβ-induced Smad signaling, cells were pretreated for 16 hours with vehicle (DMSO), curcumin (30 μM), or curcumin-glucuronide (30 μM) followed by 1 hour of TGFβ1 (5 ng/ml) stimulation prior to isolation of whole cell lysates. For analysis of TGFβ-induced PTHrP secretion, cells, plated in 24-well plates at 1×105 cells/well, were pre-incubated with varying doses of curcumin, curcumin-glucuronide, or vehicle for 4 hours prior to simulation with TGFβ1 (5 ng/ml) for 24 hours. Conditioned media, stored at −80°C after addition of protease inhibitors (#P8340, Sigma), was assayed for secreted PTHrP using a commercial immunoradiometric assay (#DSL-8100, Beckman Coulter). Effects of curcumin or curcumin-glucuronide on cell viability were assessed using a commercial MTT assay (#30–1010K, ATCC).
2.3. Western blot analysis
Proteins, isolated from whole cell lysates and quantitated by Bradford assay for normalized loading (#5000002, Bio-Rad), were separated on Mini-PROTEAN TGX-PAGE gels (BioRad), and transferred to Immobilon-FL PVDF membranes (Millipore), with even protein loading confirmed by stain-free imaging of UV-activated binding of gel trihalo compound binding to protein tryptophan residues (BioRad). Blots were probed with primary antibodies directed against Smad2 (#5339), pSmad2 (S465/467, #3108), Smad3 (#9523), pSmad3 (S423/425, #9520), or β-actin (#4967, Cell Signaling Technology [CST]), followed by HRP-conjugated secondary antibody (#7074, CST) and chemiluminescent visualization of SuperSignal West Femto ECL substrate (#34095, ThermoFisher). Densitometry was performed in ImageJ (v2.0.0, NIH) and normalized to β-actin expression, which was statistically unchanged by curcumin or curcumin-glucuronide treatment (data not shown).
2.4. Glucuronide deconjugation activity assay
Deglucuronidation activity in bone and BCa cell lines was determined by fluorometric assay using standard methods [29]. Briefly, lysates of bone marrow or breast cancer cells, normalized to protein, were incubated with 4-methylumbelliferyl-glucuronide (4-MUG) in pH 4.8 assay buffer at 37°C for 1 hour. The fluorescent cleavage product, 4-methylumelliferone (4-MU), was quantified using a spectrophotometer (Ex/Em 360 nm/470 nm) and concentration determined by comparison to a standard curve (limit of detection, 2.32 nmol 4-MU/g protein/min).
2.5. In vivo studies
Female, 4-week-old, C57BL/6J mice (#000664, Jackson Labs) were maintained at constant temperature and humidity in a central animal facility, in sterile individual ventilated cages, at 4–5 mice/cage, on a 14/10 hour light/dark cycle. Mice were provided water (reverse osmosis automatic system) and diet (NIH-31 #7913, Teklad) ad libitum. In experiments approved by the University of Arizona Institutional Animal Care and Use Committee (IACUC), mice were treated orally by gavage with 500 mg/kg curcumin, a dose, corrected for body surface area (human equivalent dose [HED], 2.5 g) [30], that has been shown to have bone effects in a rat model of arthritis [27]. Serum and bone marrow were collected at 30 minutes, the reported time of Cmax [31–33]. Serum was prepared from blood gathered by cardiac puncture of isoflurane-anesthetized mice and centrifuged at 8,000 g for 10 minutes after clotting. Following sacrifice by cervical dislocation, bone marrow was isolated from excised hind leg bones (two femurs and tibia per animal) stripped of soft tissue, which were placed in microfuge tubes and centrifuged at 10,000 g for 15 seconds as previously described [34]. Bone marrow pellets (combined marrow from 4 hind leg bones per animal) were weighed, suspended in 300 μL of sodium acetate buffer (50 mM, pH 5.0), centrifuged, and supernatant collected. Skeletal muscle, collected from proximal hind limbs (adjacent to femur), cardiac tissue, or kidney were collected in ice-cold PBS and rinsed to remove blood. Serum, tissues, and marrow supernatants were stored at −80°C until assayed.
2.6. LC-MS analysis of curcumin and curcumin-glucuronide
Serum, was acidified to pH 5 using HCl (1 M), and bone marrow samples were diluted in 20 mM sodium acetate pH 5, extracted using 30-mg Waters HLB (hydrophilic-lipophilic balance) cartridges, and eluted with methanol. Eluates were evaporated under a stream of nitrogen and dissolved in water/acetonitrile (50:50). Tissues were lyophilized and extracted using water/acetonitrile (30:70). Samples were sonicated and filtered before analysis. LC-MS analyses were performed using a Thermo Finnigan TSQ Vantage triple stage quadrupole mass spectrometer operated in the positive ion mode. Curcumin and curcumin-glucuronide were separated using a Waters Symmetry Shield C18 column (2.1 × 50 mm, 1.8 μm) at room temperature, eluted with a gradient of acetonitrile in water/0.1% formic acid changed from 15% acetonitrile to 85% in 3 min and further increased to 95% in 1 min at a flow rate of 0.4 ml/min. The SRM transitions were for curcumin m/z 369 → 177, d6-curcumin m/z 375 → 180, curcumin-glucuronide m/z 545 → 369 and d6-curcumin-glucuronide m/z 551 → 375. The limits of detection for curcumin and curcumin-glucuronide were 14.9 nM and 2.9 nM, respectively. The percentage of curcumin in serum and bone that was unconjugated (aglycone, or “free”, curcumin) was calculated as the quotient of free curcumin divided by (free curcumin + curcumin-glucuronide). Curcumin and curcumin-glucuronide concentrations in bone marrow were corrected for sample dilution in buffer, using pellet weights and assuming a density of 1.06 g/mL [35].
2.7. Statistical Analysis
Statistical analyses were performed using Prism v6.0h software (GraphPad, San Diego, CA) with data expressed as mean ± SEM. Half-maximal inhibitory concentrations (IC50) were determined by analyzing concentration-response data using a four-parameter logistic equation. Statistical significance was determined using one-way ANOVA with post hoc testing (Western blot densitometry and PTHrP secretion), or paired Student’s t-test (curcumin metabolite quantification), as appropriate. Data are representative of at least 3 independent experiments, with 4–6 animals/group for in vivo studies.
RESULTS
3.1. Effects of curcumin and curcumin-glucuronide on TGFβ-stimulated Smad signaling in bone tropic breast cancer cells
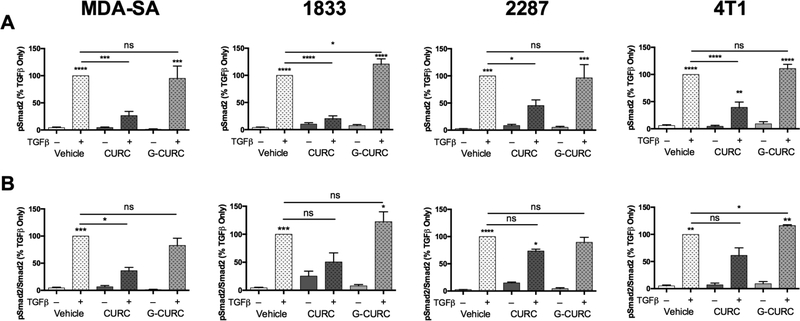
Bone-tropic MDA-SA cells neither glucuronidated curcumin nor de-conjugated curcumin-glucuronide as determined by LC-MS analysis of media of cells treated with the respective compounds (data not shown). Lack of metabolic transformation by the cells enabled direct testing of the activity of curcumin and curcumin-glucuronide. Curcumin inhibited TGFβstimulated increase in phosphorylated receptor-regulated Smad levels (pSmad2 and pSmad3) while curcumin-glucuronide did not (Fig. 1B). Similar differential effects of free vs glucuronidated curcumin were demonstrated in human MDA-1833 and MDA-2287 and murine 4T1 breast cancer cells; TGFβ-stimulated pSmad2 levels were decreased in response to curcumin treatment, while curcumin-glucuronide appeared to have no effect (Fig. 2). Because variable reductions in constitutive Smad2 levels were also evident in all four cell lines in response to curcumin treatment (34.2%−44.3% reduction [n=3–5/group], p < 0.05, data not shown), effects of curcumin and curcumin-glucuronide on both absolute and relative (pSmad2/Smad2 ratio) levels of Smad2 phosphorylation in response to TGFβ stimulation were determined. Statistically significant decreases (54.5–79.4%) in pSmad2 were documented in all TGFβ-stimulated bone-tropic breast cancer cell lines in response to curcumin treatment (Fig. 3A), while curcumin-glucuronide was without effect, except for a statistically significant increase in pSmad2 in a single cell line (MDA-1833) (Fig. 3A). Ratios of pSmad2/Smad2 were also decreased by 26.463.8% in all curcumin-treated TGFβ-stimulated breast cancer cell lines (Fig. 3B), although these changes only reached statistical significance in the human breast cancer cell line (MDA-SA) exhibiting the greatest inhibitory effect (63.8% decrease, p < 0.05). In contrast, treatment with curcumin-glucuronide either did not alter pSmad2/Smad2 ratios, or resulted in a small but statistically significant increase in pSmad2/Smad2 in murine 4T1 cells (Fig. 3B).
Figure 2.
Comparative effects of curcumin and curcumin-glucuronide on TGFβ-stimulated phosphorylation of receptor-regulated Smad2 in bone-tropic human MDA-1833, human MDA2287 and murine 4T1 breast cancer cells. Cells were pretreated with 30 μM curcumin, curcumin-glucuronide, or vehicle (16h) prior to stimulation for 1 hour with TGFβ−1 (5 ng/ml). Phosphorylated (p)-Smad2 and Smad2 protein levels were determined by western analysis with verification of protein loading by tryptophan labeling (data not shown) and normalization to βactin. Data are representative of 3–5 independent experiments.
Figure 3.
Effect of curcumin vs. curcumin-glucuronide on p-Smad2 and ratio of p-Smad2/Smad2 in TGFβ-stimulated bone tropic MDA-SA, MDA-1833, MDA-2287 or 4T1 breast cancer cells. A) pSmad2, normalized to β-actin. B) pSmad2/Smad2 ratios, normalized to β-actin. Data are reported as mean ± SEM (n = 3–5/group). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 vs control or other condition indicated by bars.
3.2. Effects of curcumin and curcumin-glucuronide on TGFβ-stimulated secretion of osteolytic PTHrP from bone tropic breast cancer cells
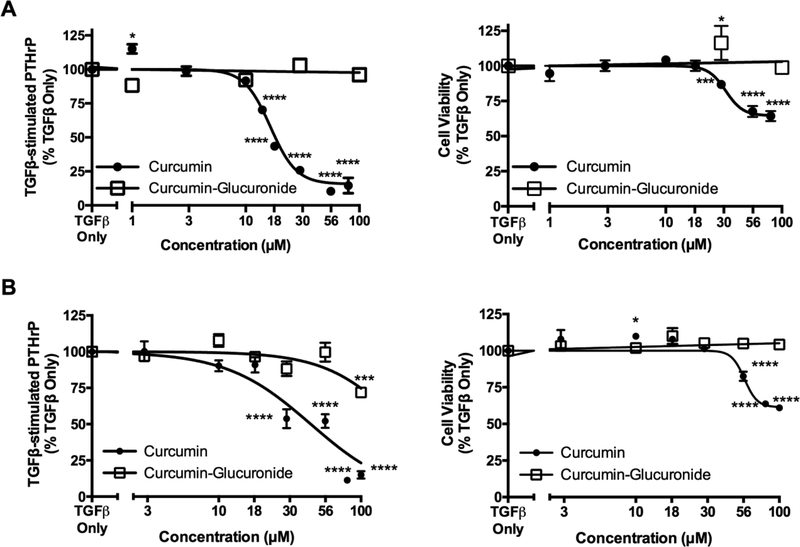
To determine whether observed changes in TGFβ signaling were associated with altered expression of TGFβ-regulated genes, dose-dependent effects of curcumin vs curcumin-glucuronide on TGFβ-stimulated secretion of PTHrP were determined. In MDA-SA cells, which exhibited statistically significant decreases in TGFβ-stimulated pSmad2 and pSmad2/Smad2 in response to curcumin but were unresponsive to curcumin-glucuronide, TGFβ-induced secretion of PTHrP was inhibited by curcumin (Fig. 4A, left panel; IC50 = 16.5 μM) at concentrations that had minimal effect on cell viability (Fig. 4A, right panel). In contrast, curcumin-glucuronide was without effect (Fig. 4A, open boxes). In murine 4T1 cells, which exhibited statistically significant decreases in TGFβ-induced pSmad2 in response to curcumin but no inhibition in response to curcumin-glucuronide, curcumin also inhibited TGFβ-stimulated PTHrP secretion (Fig. 4B, left panel; IC50 = 44.4 μM) at concentrations having minimal effect on cell viability (Fig. 4B, right panel). In contrast, curcumin-glucuronide only decreased (28.1%) PTHrP secretion at the highest concentration tested (100 μM) without altering cell viability (Fig. 4B, open boxes).
Figure 4.
Concentration-dependent effects of curcumin or curcumin-glucuronide on TGFβ-stimulated PTHrP secretion from bone-tropic breast cancer cells. A) Human MDA-MB cell TGFβ-stimulated PTHrP secretion (4.1-fold increase vs. control [p < 0.0001], left panel) and cell viability (right panel). B) Murine 4T1 cell TGFβ-stimulated PTHrP secretion (1.8-fold increase [p < 0.0001 vs control], left panel) and cell viability (right panel). Data are expressed as mean ± SEM (n = 4–36/group) relative to TGFβ-only cells. * p < 0.05, *** p < 0.001, **** p < 0.0001 vs TGFβ only.
3.3. Deglucuronidation activity in breast cancer cells and in bone
Glucuronide deconjugation activity was high in mouse bone marrow cells (238.3 ⩲ 13.7 nmol/g protein/minute, n = 3/group). In contrast, deglucuronidation activity in bone tropic breast cancer cells was either low (6.39 ⩲ 0.06 nmol/g protein/minute, n = 3 for 4T1 cells) or undetectable (< 2.32 nmol/g protein/minute for MDA-SA, 1833, and 2287 cells [n = 3/group]).
3.4. Curcumin metabolite levels in serum vs tissues following oral curcumin treatment
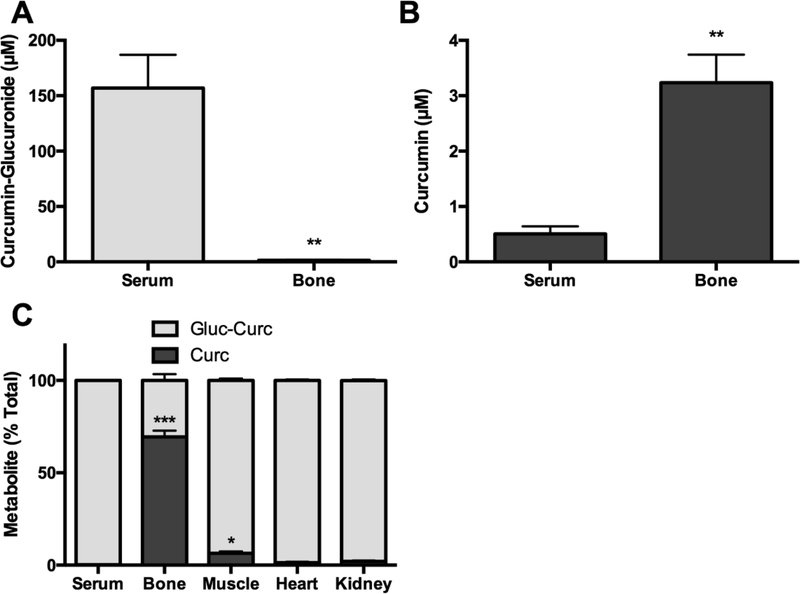
Following oral administration of curcumin to mice curcumin-glucuronide was the primary metabolite detected in serum (Fig. 5A, 156.8 μM), as compared to aglycone curcumin (Fig. 5B, 0.5 μM), accounting for 99.7% of total curcumin in the circulation (Fig. 5C). In contrast, aglycone curcumin (3.2 μM curcumin [Fig 5B]) was the major metabolite in bone (vs 1.5 μM curcuminglucuronide [Fig 5A]), accounting for 69.4% of total curcumin (Fig. 5C). Aglycone curcumin concentrations in bone were 6.4-fold higher than in the circulation (Fig 5B, p < 0.01). In contrast, aglycone curcumin comprised only 6.4%, 1.3%, or 2.0% of total curcumin in adjacent skeletal muscle, heart, or kidney respectively (Fig 5C).
Figure 5.
Curcumin and curcumin-glucuronide levels in serum, bone, thigh muscle, heart, and kidney following oral curcumin administration. Tissues (n = 5/group) were harvested from female C57BL/6 mice 30 minutes after oral gavage with curcumin (500 mg/kg [human equivalent dose, 2.5 g]). A) Concentration of curcumin-glucuronide or B) curcumin in bone and serum. C) Relative contribution of free vs glucuronidated curcumin, expressed as % of total to total curcumin in serum, bone, skeletal muscle, heart, and kidney. * p < 0.05,** p < 0.01, *** p
< 0.001 vs serum.
3. DISCUSSION
In clinical breast cancer bone metastases, Smad2 activation (pSmad2) has been reported in 75% of samples, while 68–100% express the Smad-inducible osteolytic factor, PTHrP [7,36–39]. When coupled with evidence that these pathways can mediate metastatic progression in MDA-SA, MDA-1833, MDA-2287, and 4T1 preclinical bone metastases models [6,7,9–11,21,22,24], this suggests that targeting tumoral Smad signaling within the bone microenvironment could be a clinically relevant approach. It is therefore notable that treatment with curcumin, a natural product that has been used medicinally for centuries, significantly reduced receptor-mediated phosphorylation of Smad2 levels in each of these bone-tropic breast cancer cell lines subsequent to TGFβ stimulation. While the exact mechanism of curcumin’s inhibitory effect on Smad-dependent TGFβ signaling remains an active area of research in our laboratory, a key finding in the studies reported here is the comparative ineffectiveness of curcumin-glucuronide in mediating this protective effect across all TGFβ-dependent bone-metastatic BCa cell lines.
The physiologic relevance of curcumin inhibition of TGFβ-inducible Smad signaling in the context of osteolytic bone metastases and requirement for the aglycone metabolite, was further demonstrated by experiments documenting curcumin inhibition of TGFβ-stimulated PTHrP secretion in BCa cell lines forming pSmad2- and PTHrP-positive bone metastases in vivo (MDASA and 4T1) [9–11,21,22,24], while curcumin-glucuronide was either without effect (MDA-SA) or was much less potent (4T1, projected IC50 = 191.4 μM, with inhibition only at the highest tested dose [100 μM]). In toto, these findings suggest that aglycone curcumin, rather than the prevailing circulating metabolite, curcumin-glucuronide (>99% of total), would be required for effective blockade of BCa cell Smad signaling within the bone microenvironment. Our finding of site-specific increases in curcumin within bone, accounting for nearly 70% of local metabolites (vs. <1% in circulation or ≤ 7% in other tissues), with a concentration >6-fold higher than circulating levels perfusing the tissue, is thus quite remarkable and consistent with bone-specific bioactivation of curcumin-glucuronide. The fact that deglucuronidation activity was high in bone marrow cells, consistent with previous reports that cells of hematopoietic origin express enzymes capable of deconjugating glucuronides [40], while activity in the bone metastatic BCa tumor cells, was either low (4T1 cells) or undetectable, suggests a likely role for bone hematopoietic cells in mediating the local activation of curcumin within the tumor/bone microenvironment. Of note, however, while MDA-SA cells did not appear capable of deconjugating curcumin-glucuronide (no detectable deconjugation activity or free curcumin in conditioned media from curcumin-glucuronide treated cells), the low level of deconjugation activity detected in murine 4T1 cells could contribute to the inhibitory effect on PTHrP secretion that only occurred with highest curcumin-glucuronide concentration tested (100 μM), suggesting possible tumor-specific effects on curcumin bioactivation in the tumor/bone microenvironment, as well.
Inhibition of tumoral TGFβ signaling has previously been proposed as a means of blocking breast cancer bone metastasis progression, which could be complementary to the osteoclast-directed effects of currently available bone-specific treatments [41]. However, the opposing roles of TGFβ in carcinogenesis, which can be anti-proliferative during primary tumorigenesis, while promoting tumor progression in advanced disease, provides a theoretical barrier to the development of systemic chemotherapeutic treatments targeting the TGFβ pathway [42–44]. Enrichment of bioactive curcumin within the bone microenvironment, as demonstrated in these studies, suggests that circulating curcumin-glucuronide may be functioning as a prodrug and that site-specific deconjugation of curcumin within bone may allow for tissue-specific inhibition of Smad signaling in adjacent tumor cells, avoiding issues related to systemic inhibition of TGFβ signaling [45,46].
Importantly, curcumin concentrations in vivo, when averaged over the entire bone compartment, were approximately 5-fold lower than the IC50 documented for inhibition of BCa TGFβ signaling in vitro. However, because of the well documented heterogeneity of bone blood flow [47,48], regional differences in curcumin-glucuronide delivery and distribution within bone seem likely and could facilitate paracrine effects of curcumin formed locally in situ within particular regions, such as the highly vascularized, well-oxygenated metaphyseal niche where bone metastases preferentially form in pre-clinical models [49], a postulate currently being tested in ongoing studies in our laboratory.
What is abundantly clear from the studies presented here, is the remarkable enrichment of bioactive aglycone curcumin that occurs in vivo within a tumor-free bone microenvironment and the need for this metabolite in order to block TGFβ-driven BCa bone metastasis progression. While it has been previously postulated that the biological effects of curcumin may require its deconjugation at sites of action [50], to our knowledge, this is the first demonstration of tissue-specific metabolism of curcumin, a compound of great medicinal interest [51]. Thus, just as BCa cells are thought to induce the site-specific release of TGFβ within bone, which drives metastases progression, bone marrow cells may similar facilitate a microenvironment-specific treatment, mediating the local production of bioactive curcumin. As bone-protective effects of curcumin have also been reported for other disease states, such as arthritis, deconjugation of this polyphenol within bone may be a pre-requisite for its bioactivity in other bone diseases as well [52–55]. Furthermore, these findings suggest that a physiologic role for tissue-specific deconjugation may also extend to other highly glucuronidated naturally-occurring polyphenols with bone-protective effects.
Curcumin inhibits TGFβ signaling in TGFβ-dependent bone-tropic breast cancer cells.
Curcumin-glucuronide, the primary circulating curcumin metabolite, does not.
Aglycone curcumin is enriched in mouse bone marrow (vs serum or other tissues) after oral dosing.
Curcumin-glucuronide may be a pro-drug that is activated in bone by deconjugation.
ACKNOWLEDGEMENTS
We kindly thank Dr. Theresa Guise (Indiana University) for kindly providing the MDA-SA cell lines and Dr. Joan Massagué (Sloan-Kettering) for providing the MDA-1833 and MDA-2287 cell lines.
Footnotes
Conflict of Interest/Financial Support: The authors have no conflict of interest to report. This work was supported by the National Cancer Institute (NCI), the National Center for Complementary and Integrative Health (NCCIH), and the Office of Dietary Supplements (ODS) at the National Institutes of Health (NIH) (R01CA174926 and R34 AT007837 to JLF, R01AT006896 to CS and F31AT009938 to AK); the United States Department of Agriculture (2014–38420-21799 national Needs Fellowship to AK); and the American Heart Association (16POST27250138 post-doctoral fellowship to PBL). Mass spectrometric analyses were performed in part through Vanderbilt University Medical Center’s Digestive Disease Research Center supported by NIH grant P30DK058404 Core Scholarship.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Altekruse S, et al. SEER Cancer Statistics Review, 1975–2013 n.d. https://seer.cancer.gov/csr/1975_2013/ (accessed January 12, 2016).
- [2].Käkönen S-M, Mundy GR. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003;97:834–9. doi: 10.1002/cncr.11132. [DOI] [PubMed] [Google Scholar]
- [3].Sethi N, Kang Y. Dysregulation of developmental pathways in bone metastasis. Bone 2011;48:16–22. doi: 10.1016/j.bone.2010.07.005. [DOI] [PubMed] [Google Scholar]
- [4].Ell B, Kang Y. SnapShot: Bone metastasis. Cell 2012;151:690–690.e1. doi: 10.1016/j.cell.2012.10.005. [DOI] [PubMed] [Google Scholar]
- [5].Ren G, Esposito M, Kang Y. Bone metastasis and the metastatic niche. J Mol Med 2015;93:1203–12. doi: 10.1007/s00109-015-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kang Y, Siegel PM, Shu A, Drobnjak M, Kakonen SM, Cordón C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 2003;3:537–49. [DOI] [PubMed] [Google Scholar]
- [7].Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen C-R, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A 2005;102:13909–14. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buijs JT, Stayrook KR, Guise TA. TGFβ in the Bone Microenvironment: Role in Breast Cancer Metastases. Cancer Microenviron 2011;4:261–81. doi: 10.1007/s12307-011-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Guise TA, Yin JJ, Taylor SD, Kumagai Y, Dallas M, Boyce BF, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 1996;98:1544–9. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Käkönen S-M, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, et al. Transforming Growth Factor-β Stimulates Parathyroid Hormone-related Protein and Osteolytic Metastases via Smad and Mitogen-activated Protein Kinase Signaling Pathways. J Biol Chem 2002;277:24571–8. doi: 10.1074/jbc.M202561200. [DOI] [PubMed] [Google Scholar]
- [12].Korpal M, Yan J, Lu X, Xu S, Lerit DA, Kang Y. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat Med 2009;15:960–7. doi: 10.1038/nm.1943. [DOI] [PubMed] [Google Scholar]
- [13].Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci 2012;109:16618–23. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wright LE, Frye JB, Gorti B, Timmermann BN, Funk JL. Bioactivity of Turmeric-Derived Curcuminoids and Related Metabolites in Breast Cancer. Curr Pharm Des 2013;19:6218–25. doi: 10.2174/1381612811319340013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wright LE, Frye JB, Lukefahr AL, Timmermann BN, Mohammad KS, Guise TA, et al. Curcuminoids Block TGF-β Signaling In Human Breast Cancer Cells And Limit Osteolysis In A Murine Model Of Breast Cancer Bone Metastasis. J Nat Prod 2013;76:316–21. doi: 10.1021/np300663v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pal A, Sung B, Prasad BAB, Schruber PTJ, Prasad S, Aggarwal BB, et al. Curcumin Glucuronides: Assessing the Proliferative Activity against Human Cell Lines. Bioorg Med Chem 2014;22:435–9. doi: 10.1016/j.bmc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shoji M, Nakagawa K, Watanabe A, Tsuduki T, Yamada T, Kuwahara S, et al. Comparison of the effects of curcumin and curcumin glucuronide in human hepatocellular carcinoma HepG2 cells. Food Chem 2014;151:126–32. doi: 10.1016/j.foodchem.2013.11.021. [DOI] [PubMed] [Google Scholar]
- [18].Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas P. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med 1998;64:353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- [19].Ireson C, Orr S, Jones DJL, Verschoyle R, Lim C-K, Luo J, et al. Characterization of Metabolites of the Chemopreventive Agent Curcumin in Human and Rat Hepatocytes and in the Rat in Vivo, and Evaluation of Their Ability to Inhibit Phorbol Ester-induced Prostaglandin E2 Production. Cancer Res 2001;61:1058–64. [PubMed] [Google Scholar]
- [20].Garcea G, Jones DJL, Singh R, Dennison AR, Farmer PB, Sharma RA, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer 2004;90:1011–5. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Z, Hu Z, Gupta J, Krimmel JD, Gerseny HM, Berg AF, et al. Intravenous administration of adenoviruses targeting transforming growth factor beta signaling inhibits established bone metastases in 4T1 mouse mammary tumor model in an immunocompetent syngeneic host. Cancer Gene Ther 2012;19:630–6. doi: 10.1038/cgt.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Futakuchi M, Nannuru KC, Varney ML, Sadanandam A, Nakao K, Asai K, et al. Transforming growth factor-β signaling at the tumor-bone interface promotes mammary tumor growth and osteoclast activation. Cancer Sci 2009;100:71–81. doi: 10.1111/j.1349-7006.2008.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, et al. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci U S A 2001;98:8012–7. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].McEarchern JA, Kobie JJ, Mack V, Wu RS, Meade-Tollin L, Arteaga CL, et al. Invasion and metastasis of a mammary tumor involves TGF-β signaling. Int J Cancer 2001;91:76–82. doi: [DOI] [PubMed] [Google Scholar]
- [25].Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T. Zoledronic Acid Inhibits Visceral Metastases in the 4T1/luc Mouse Breast Cancer Model. Clin Cancer Res 2004;10:4559–67. doi: 10.1158/1078-0432.CCR-03-0325. [DOI] [PubMed] [Google Scholar]
- [26].Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Lowen D, et al. A novel orthotopic model of breast cancer metastasis to bone 1999:163–70. [DOI] [PubMed] [Google Scholar]
- [27].Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, et al. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod 2006;69:351–5. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Luis PB, Gordon ON, Nakashima F, Joseph AI, Shibata T, Uchida K, et al. Oxidative metabolism of curcumin-glucuronide by peroxidases and isolated human leukocytes. Biochem Pharmacol 2017;132:143–9. doi: 10.1016/j.bcp.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Glaser J, Sly WS. β-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med 1973;82:969–77. [PubMed] [Google Scholar]
- [30].Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pan M, Huang T, Lin J. Biotransformation of Curcumin Through Reduction and Glucuronidation in Mice. Drug Metab Dispos 1999;27:486–94. [PubMed] [Google Scholar]
- [32].Zhongfa L, Chiu M, Wang J, Chen W, Yen W, Fan-Havard P, et al. Enhancement of curcumin oral absorption and pharmacokinetics of curcuminoids and curcumin metabolites in mice. Cancer Chemother Pharmacol 2012;69:679–89. doi: 10.1007/s00280-011-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ramalingam P, Ko YT. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm Res 2015;32:389–402. doi: 10.1007/s11095-014-1469-1. [DOI] [PubMed] [Google Scholar]
- [34].Amend SR, Valkenburg KC, Pienta KJ. Murine Hind Limb Long Bone Dissection and Bone Marrow Isolation. J Vis Exp 2016;110. doi: 10.3791/53936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gurkan UA, Akkus O. The mechanical environment of bone marrow: A review. Ann Biomed Eng 2008;36:1978–91. doi: 10.1007/s10439-008-9577-x. [DOI] [PubMed] [Google Scholar]
- [36].Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman J a, Henderson MA, et al. Localization of Parathyroid Hormone-related Protein in Breast Cancer Metastases: Increased Incidence in Bone Compared with Other Sites. Cancer Res 1991;51:3059–61. [PubMed] [Google Scholar]
- [37].Kohno N, Kitazawa S, Sakoda Y, Kanbara Y, Furuya Y, Ohashi O, et al. Parathyroid Hormone-related Protein in Breast Cancer Tissues: Relationship between Primary and Metastatic Sites. Breast Cancer 1994;1:43–9. [DOI] [PubMed] [Google Scholar]
- [38].Linforth R, Anderson N, Hoey R, Nolan T, Downey S, Brady G, et al. Coexpression of parathyroid hormone related protein and its receptor in early breast cancer predicts poor patient survival. Clin Cancer Res 2002;8:3172–7. [PubMed] [Google Scholar]
- [39].Xu C, Wang Z, Cui R, He H, Lin X, Sheng Y, et al. Co-expression of parathyroid hormone related protein and TGF-beta in breast cancer predicts poor survival outcome. BMC Cancer 2015;15:1–10. doi: 10.1186/s12885-015-1873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lorbacher P, Yam LT, Mitus WJ. Cytochemical demonstration of β-glucuronidase activity in blood and bone marrow cells. J Histochem Cytochem 1967;5:680–7. [DOI] [PubMed] [Google Scholar]
- [41].Juárez P, Guise TA. TGF-β in cancer and bone: Implications for treatment of bone metastases. Bone 2011;48:23–9. doi: 10.1016/j.bone.2010.08.004. [DOI] [PubMed] [Google Scholar]
- [42].Bierie B, Moses HL. TGF-β and Cancer. Cytokine Growth Factor Rev 2006;17:29–40. doi: 10.1016/j.cytogfr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- [43].Pardali K, Moustakas A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim Biophys Acta 2007;1775:21–62. doi: 10.1016/j.bbcan.2006.06.004. [DOI] [PubMed] [Google Scholar]
- [44].Massagué J TGFβ in cancer. Cell 2008;134:215–30. doi: 10.1016/j.cell.2008.07.001.TGF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-β targeted cancer therapy. Int Biol Sci 2012;8:964–78. doi: 10.7150/ijbs.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014;507:323–8. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lafage-Proust M-H, Roche B, Langer M, Cleret D, Vanden Bossche A, Olivier T, et al. Assessment of bone vascularization and its role in bone remodeling. Bonekey Rep 2015;4:1–8. doi: 10.1038/bonekey.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rosol TJ, Tannehill-Gregg SH, LeRoy BE, Mandl S, Contag CH. Animal model of bone metastasis. Cancer Treat Res 2004;118:47–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev 2008;17:1411–7. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Curcumin Stanić Z., a Compound from Natural Sources, a True Scientific Challenge – A Review. Plant Foods Hum Nutr 2017;72:1–12. doi: 10.1007/s11130-016-0590-1. [DOI] [PubMed] [Google Scholar]
- [52].Funk JL, Frye JB, Oyarzo JN, Kuscuoglu N, Wilson J, McCaffrey G, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum 2006;54:3452–64. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- [53].Wright LE, Frye JB, Timmermann BN, Funk JL. Protection of trabecular bone in ovariectomized rats by turmeric (Curcuma longa L.) Is dependent on extract composition. Agric Food Chem 2010;58:9498–504. doi: 10.1021/jf101873f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rohanizadeh R, Deng Y, Verron E. Therapeutic actions of curcumin in bone disorders. Bonekey Rep 2016;5:1–7. doi: 10.1038/bonekey.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].French DL, Muir JM, Webber CE. The ovariectomized, mature rat model of postmenopausal osteoporosis: An assessment of the bone sparing effects of curcumin. Phytomedicine 2008;15:1069–78. doi: 10.1016/j.phymed.2008.06.007. [DOI] [PubMed] [Google Scholar]