Abstract
The genetic etiologies of many rare disorders, including early infantile epileptic encephalopathies, are largely undiagnosed. A 6-year old girl was admitted to the National Institutes of Health Undiagnosed Diseases Program with profound intellectual disability, infantile onset seizures, chronic respiratory failure, facial dysmorphisms, skeletal abnormalities and atrial septum defect. A large region of homozygosity was discovered on chromosome 16, spanning 16q22.1–16q24.3 caused by uniparental disomy (UPD) that included a maternally inherited homozygous microdeletion covering exon 6 of WWOX (NM_016373.3). mRNA expression analysis revealed that the deletion led to nonsense-mediated decay of the NM_016373.3 transcript; the exon 6 of an alternative transcript (NM_130791.3), lacking the short-chain dehydrogenase, was utilized. The microdeletion in WWOX explains the seizures and intellectual disability, while pathogenic variants in another gene, HSPG2, are likely responsible for the patient’s skeletal abnormalities. This report describes a rare autosomal recessive disorder with multiple genetic etiologies, one of which involves UPD.
Keywords: Uniparental disomy, blended phenotype, epileptic encephalopathy, perlecan, rare diseases
The past decade has seen a surge in the application of next generation sequencing to the diagnosis of many rare disorders, including infantile onset epileptic encephalopathies. Nevertheless, several cases remain unsolved, in part due to unusual inheritance patterns that are not routinely evaluated. For example, chromosomal rearrangements may lead to uniparental disomy (UPD), creating a region of homozygosity that can be searched for disease-causing variants. Such regions of homozygosity can be detected using single nucleotide polymorphism (SNP) arrays.
Here we report a case with a maternally inherited homozygous microdeletion within a region of UPD on chromosome 16q22.1–24.3 detected by SNP analysis. The microdeletion occurred within the WW-domain containing oxidoreductase gene (WWOX; MIM# 605131), associated with early infantile epileptic encephalopathy 28 (EIEE28; MIM# 616211). The proband had phenotypic features associated with WWOX mutations (Supp. Table S1 and Supp. Table S2), such as severe growth retardation, microcephaly, epileptic seizures and retinopathy (Abdel-Salam, et al., 2014; Ben-Salem, et al., 2015; Elsaadany, et al., 2016; Gribaa, et al., 2007; Mallaret, et al., 2014; Mignot, et al., 2015; Tabarki, et al., 2015; Valduga, et al., 2015). However, additional clinical findings in our patient (Supp. Table S2 and Supp. Table S3), including osteoporosis, abnormalities of the hip and elbow, and ventricular and atrial septal defects, produced a blended phenotype, suggesting involvement of an additional pathogenic variant in the genome.
The proband is a 10-year old female of non-consanguineous Caucasian and Middle Eastern descent born at 39-weeks gestation to a 39-year old G2P2 mother by caesarian section. Pregnancy was complicated by a maternal weight gain, gestational diabetes, and less active fetal movements. APGAR scores were six and eight, respectively, at one and five minutes of life. Occipitofrontal circumference was 33.7cm (10%), weight was 2.8kg (15%), and length was 47.0cm (12%). On newborn exam, the proband was jaundiced with a poor respiratory drive and inability to suckle or swallow. She required nasophayngeal suction, oxygenation by positive pressure, and tactile stimulation. Laboratory results demonstrated polycythemia of the newborn, neutropenia, thrombocytopenia, abnormal buffy coat cellular inclusions, hypoglycemia, septicemia that prompted IV antibiotic and fluid administration. Auscultation revealed murmurs of atrial-septal and ventricular-septal defects and echocardiography identified Wolff-Parkinson-White syndrome. She was discharged in stable condition on nectar-consistency feeding formula.
The infant continued to have frequent choking spells at home and was readmitted to the NICU within two weeks for respiratory infection secondary to laryngomalacia and had a seizure; electroencephalography (EEG) and magnetic resonance imaging (MRI) returned normal at that time. Over the next several weeks her muscle tone became increasingly rigid. By five-weeks of age, she began to regularly demonstrate moderate myoclonic jerks; repeat EEG revealed epileptiform discharges. MRI showed moderate bilateral, periventricular T2-hyperintensity, delayed myelination with gliosis, and reduced volume of the splenium and corpus callosum, considered consistent with a periventricular leukomalacia. Microcephaly and right-sided positional plagiocephaly were noted. Pharmacotherapeutics provided little relief to seizure activity but the child was optimized to a regimen of phenobarbital, topiramate, and pyridoxine. During her first two years of life, she had recurrent gastric reflux and feeding difficulties that required gastrostomy, and recurrent respiratory distress that required tracheostomy.
At age six the proband was enrolled in the NIH Undiagnosed Diseases Program (Gahl, et al., 2012; Gahl, et al., 2016; Gahl and Tifft, 2011) and admitted to the National Institutes of Health (NIH) Clinical Center under clinical protocol #76-HG-0238, “Diagnosis and Treatment of Patients with Inborn Errors of Metabolism or Other Genetic Disorders,” approved by the Institutional Review Board of the National Human Genome Research Institute. Written informed consent was obtained from the proband’s parents.
At this time the proband weighed 17kg (13%) and stood 93.2cm tall (<5%) with a BMI 19.6 (97%). She appeared awake and alert, and responded to questions by blinking. She was brachycephalic and microcephalic with a head circumference of 48cm (1%). She had hypotonic facies with frontal bossing and bi-temporal narrowing but normally placed and rotated ears. Her inner canthal (+2SD) and outer canthal (+3SD) distances were increased. She was myopic with left eye exotropia; she wore corrective lenses. She demonstrated a barrel-chest with widely-spaced nipples. She had chronic eczema and blepharitis. Examination of the cranial nerves revealed pupils that were equal, round and reactive to light. She had normal optic nerves and retinae. Her eyes tracked well with only short periodic lapses. There was no facial asymmetry and the tongue was midline.
The child’s motor skills were limited since birth. She was unable to turn her head or hold hear head without support and had a left-sided thoracic scoliosis. She had truncal hypotonia, but with hypertonic extremities, consistent with spastic quadriparesis, which was complicated by progressive arthrogryposis with fixed contractures at the elbows, knees, and feet with crepitus. Her legs remain in a frog-leg abducted posture at the hips. Nonetheless, she can occasionally move all extremities but not significantly against full gravity. She had upper motor neuron signs on bilateral Achilles with increased deep tendon reflexes and sustained clonus.
Electromyography (EMG)-NCV revealed normal sensory and motor nerve conduction studies. Limited needle EMG of proximal arm and leg muscles showed membrane irritability with small, polyphasic motor units, suggesting a primary muscle disorder. Skeletal surveys demonstrated thickening of the skull base and calvarium, and small-for-age radial-ulnar metaphyses. She had bilateral cox valga and club foot deformity and there was generalized decreased mineralization consistent with osteopenia but with no evidence of fractures.
Neuroimaging showed moderate cortical atrophy, thin corpus callosum, minor posterior thinning of the cerebellar vermis, enlargement of ventricles, and prominent cisterns. Basal ganglia and thalami showed normal signal despite small size, while the globus pallidus had iron deposits. There were few imaging changes between infancy and her most recent study. MR spectroscopy for non-essential amino acids was decreased globally and lactate levels were extremely low.
Serial 48-hour EEG identified consistently abnormal patterns consistent with tonic-clonic and myoclonic seizures. Awake EEG showed diffuse delta frequencies at approximately four hertz. Posteriorly, dominant six hertz theta waves were identified, and anteriorly faster frequencies were seen. In drowsy states, background activity was a mixture of delta frequencies. No significant sleep architecture was seen in sleep phases. The spike-and-wave discharges and bursts of spike-and-waves are decreased during sleep. There was an episode of myoclonic jerk showing generalized spike-and-wave discharges, lasting five seconds. There were also brief periods of generalized spike-and-wave discharge without clinically evident seizures.
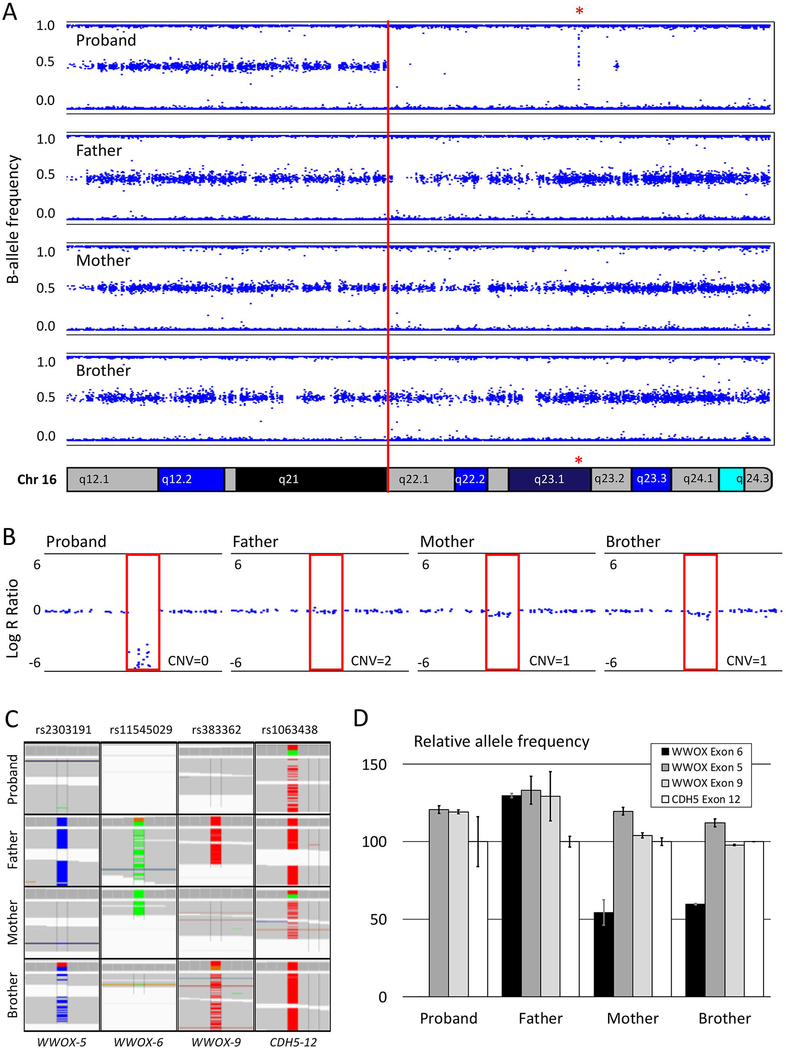
SNP array analysis revealed a large region of homozygosity on chromosome 16 stretching from 16q22.1 through 16q24.3 (Figure 1A) that includes a 22kb deletion (Figure 1B) involving exon 6 of WWOX (NM_016373.3). Further analysis of the exome data (parameters listed in Supp. Table S4) revealed non-Mendelian inheritance in this area of homozygosity; at locations where the father and mother are homozygous for different SNPs, the unaffected brother was heterozygous as expected, but the proband was consistently homozygous for the allele inherited from the mother (Figure 1C). A genotyping assay performed on three SNPs in WWOX (rs2303191, rs11545029, and rs383362) with non-Mendelian inheritance and an additional SNP (rs1063438) in CDH5 (MIM# 601120) just before the region of homozygosity with normal Mendelian inheritance to serve as control, confirmed the inheritance modes (Supp. Figure S1). Digital droplet PCR specifically amplifying the SNP in exon 6 showed a loss of allele (Figure 1D and Supp. Figure S2), consistent with a CNV=0 in the proband, and a CNV=1 in the mother and brother. These results suggest that the stretch of homozygosity is caused by maternal uniparental disomy (UPD), which was also confirmed by GeneDX (Gaithersburg, MD) through a whole genome oligonucleotide array.
Figure 1: Genetic analysis of the homozygous region on chromosome 16.
A. B-allele frequency plot of a single-nucleotide-polymorphism (SNP)-array showing a large stretch of homozygosity from 16q22.1–16q24.3 present in the affected proband but not in the unaffected family members. The red asterisk indicates the location of the exon 6 deletion in WWOX. B. The Log-R ratio on the SNP-array shows the copy number variation (CNV) at WWOX exon 6. The proband has a CNV of 0 and the mother and brother have a CNV of 1, whereas the father has both alleles at this location. C. Three SNPs in and near WWOX exon 5, 6 and 9 reveal non-Mendelian inheritance for the proband, who consistently inherited only the variant from her mother. The SNP in exon 12 of CDH5, located at 16q21 shows normal Mendelian inheritance for the proband. D. Relative mRNA expression reflecting allele frequencies for the three non-Mendelian SNPs in WWOX determined by digital droplet PCR. No loss of alleles for WWOX exons 5 and 9 compared to exon 12 of CDH5 confirmed the bi-allelic deletion of exon 6 observed in our proband and the mono-allelic deletion in the mother and the brother.
WWOX is located on second most fragile area of the genome (FRA16D), which is prone to genomic rearrangements (Bednarek, et al., 2000; Dayan, et al., 2013). WWOX is often deleted in cancer cells (Dayan, et al., 2013), but conditional deletions in mice and rats have not confirmed its role in carcinogenesis (Abu-Remaileh, et al., 2015; Ludes-Meyers, et al., 2009; Suzuki, et al., 2009). These animal models do show a link between the deletion of Wwox and epilepsy, supporting the association between autosomal recessive mutations in WWOX and EIEE28 (Mallaret, et al., 2014; Suzuki, et al., 2009). Thus far, 23 patients suffering from EIEE28 have been described with changes in WWOX (Supp. Table S1), involving deletions or variants that introduce a premature stop (Abdel-Salam, et al., 2014; Ben-Salem, et al., 2015; Elsaadany, et al., 2016; Gribaa, et al., 2007; Mallaret, et al., 2014; Mignot, et al., 2015; Tabarki, et al., 2015; Valduga, et al., 2015) as well as a case with a complete duplication of the gene (Szymanska, et al., 2014).
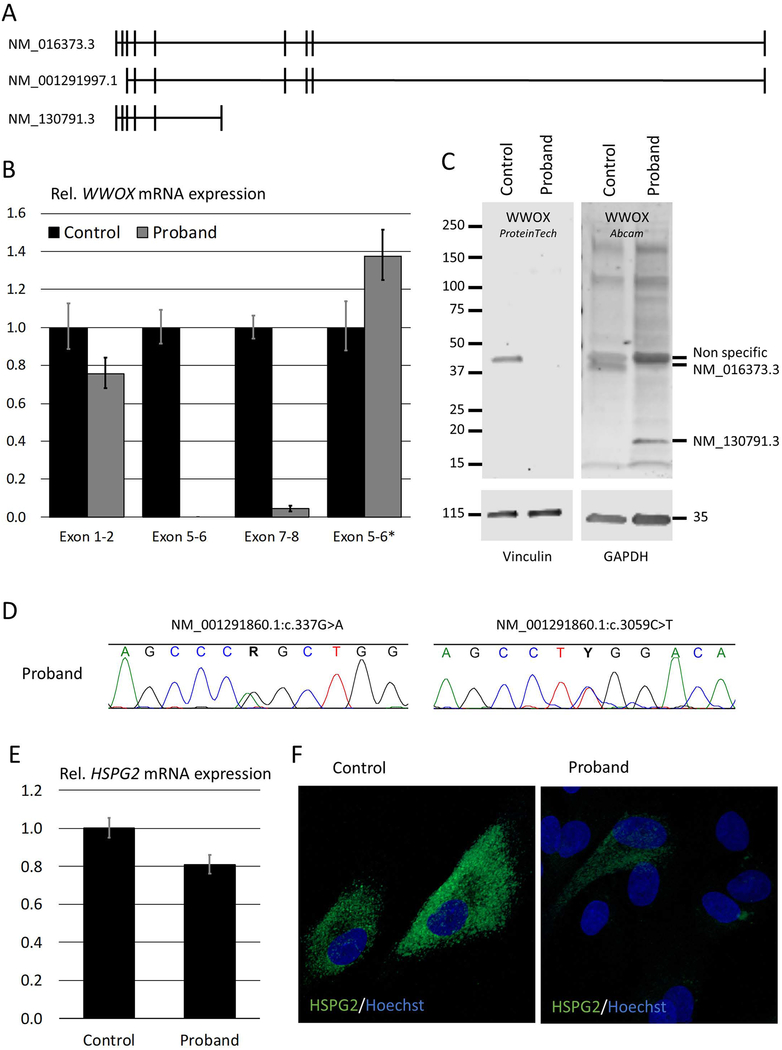
To determine the effect of the exon 6 deletion in our patient on both mRNA and protein expression, qPCR and western blot analyses were performed. Since there are three distinct transcripts for WWOX (Figure 2A), mRNA expression levels were analyzed with four different assays, covering exon 1–2, exon 5–6 and exon 7–8 junctions of transcript NM_016373.3, and exon 5–6 junction of transcript NM_130791.3 (exon 5–6*), respectively (Figure 2B). As expected, the assay spanning the deleted exon 6 was not detected in the proband. The exon 1–2 junction, however, was detected and was only slightly reduced in our patient, whereas the exon 7–8 junction was barely detectable, suggesting that the two longer transcripts were not expressed or were degraded. The residual expression of the exon 1–2 junction may be explained by the amplification of NM_130791.3, which has increased expression of its exon 5–6 junction. Protein expression analysis (Figure 2C and Supp. Figure S3) also showed the loss of the longer isoform (NM_016373.3/NP_057457.1), which contains the short-chain dehydrogenase/reductase domain, and the increased expression of the shorter isoform (NM_130791.3/NP_570607.1), which only contains the WW-domains. The third isoform (NM_001291997.1/NP_001278926.1), which should encode a 33kDa protein, was not detected by either antibody in the control cells and is likely not expressed in fibroblasts.
Figure 2: WWOX and HSPG2 expression analysis.
A. There are three known transcripts for WWOX: NM_016373.3 is the longest; NM_001291997.1 has an alternate start site and lacks the first two exons; and NM_130791.3 utilizes an alternate exon 6 that includes a stop codon and codes for a shorter transcript. B. Quantitative polymerase chain reaction (qPCR) analysis of WWOX mRNA shows a decrease in expression of the exon-1–2 junction in fibroblasts of the proband, no expression of the exon 5–6 junction and negligible expression of the exon 7–8 junction, indicating that the deletion causes nonsense mediated decay of the two longer transcripts. Expression of the junction between exon 5 and the alternate exon 6 (6*) of shorter transcript is increased. C. Western blot analysis shows the lack of expression of the longest transcript at 46kDa in the proband, both by probing with an antibody raised against a peptide translated from exons 1–7 (ProteinTech) and one raised against a peptide translated from exons 1–5 (Abcam). The latter shows the increased expression of the short isoform at 19kDa, whereas neither was able to detect the 33kDa isoform in patient or control. Full-size western blots are available in the online supporting information (Fig. S3). D. Chromatograms from Sanger sequencing reads around the maternally inherited variant NM_001291860.1:c.337G>A and the paternally inherited variant NM_001291860.1:c.3059C>T in HSPG2 that segregate with disease (also see Supp. Figure S4). E. qPCR of HSPG2 shows a mild reduction in mRNA expression levels. F. Immunofluorescence confocal microscopy shows a marked reduction of HSPG2 (green) on the membrane of the proband’s fibroblasts compared to control. Nuclei are stained with Hoechst 33342 (blue).
In our proband, the WWOX exon 6 deletion leads to loss of the full-length, functional protein containing the short-chain dehydrogenase/reductase domain (Abu-Remaileh, et al., 2015; Mignot, et al., 2015). Hence, our patient’s phenotype resembled that of previously reported cases of WWOX loss-of-function. Our patient also exhibited some findings of the metabolic syndrome reported in Wwox-deficient mice (Abu-Remaileh, et al., 2015; Aldaz, et al., 2014; Ludes-Meyers, et al., 2009). As the risk for UPD is mostly dependent on the karyotype and its formation, the identification of additional cases by molecular genotyping will likely clarify the incidence and risk for UPD-associated EIEE28.
Other clinical features in our patient, including skeletal dysplasia, osteoporosis, and flexion contractures, could not be attributed to the variant in WWOX. Therefore, exome sequences were analyzed to identify additional genetic causes of the patient’s phenotype. Two variants in HSPG2 (MIM# 142461; NM_001291860.1; c.337G>A, p.Glu113Lys and c.3059C>T, p.Pro1020Leu) were validated by Sanger sequencing and segregated with disease (Figure 2D and Supp. Figure S4). Pathogenicity scores indicate that these variants are relatively pathogenic (CADD = 24.1 and 19.2) but seem to be relatively frequent (gnomAD All = 0.025 and 0.642). We see conflicting interpretation for the p.Pro1020Leu, because thirteen presumably normal homozygotes have been reported in gnomAD; however, it is important to note that the gnomAD population can include relatively young parents of affected children, and thus subtle changes that occur late in life are easily missed in a rare instances of homozygous cases. Nonetheless, there are reports associating the p.Pro1020Leu variant with two different skeletal dysplasias, i.e., Schwartz-Jampel syndrome, type 1 (MIM# 255800) and Silverman-Handmaker type dyssegmental dysplasia (MIM# 224410) (Iwata, et al., 2015; Stum, et al., 2006). The effect of p.Glu113Lys, which has a higher pathogenicity score, in combination with the p.Pro1020Leu, can only be ascertained by additional functional studies. While the two missense HSPG2 variants did not significantly alter mRNA expression (Figure 2E), there was a marked reduction in membrane bound HSPG2 protein (i.e., perlecan) in the patient’s cultured fibroblasts (Figure 2F), which is consistent with other variants in HSPG2 domain III (Stum, et al., 2006).
Here we described a 10-year old female who presented with intractable epilepsy, microcephaly, severe developmental delays, cerebral palsy with spastic quadriplegia, laryngomalacia, skeletal dysplasia and Wolff-Parkinson-White syndrome. The combination of SNP-array and exome sequencing revealed a maternally inherited homozygous deletion covering WWOX exon 6. Digital droplet PCR confirmed there was no loss of alleles other than the copy number variation at exon 6 for the proband, mother and brother, confirming non-Mendelian inheritance caused by UPD. Additionally, variants in HSPG2 led to the decrease in membrane bound protein in the proband’s fibroblasts and accounted for some of the additional phenotypes.
Our report underlines the importance of careful analysis of different types of genomic data in rare diseases, since a combination of rare variants may contribute to disease. This case also supports the importance of UPD as an underlying mechanism of rare autosomal recessive disorders.
Supplementary Material
Supp. Figure S1: Results genotyping assays for SNPs rs2303191, rs11545029, and rs383362 in WWOX and SNP rs1063438 in CDH5.
Supp. Figure S2: Example of digital droplet PCR output for SNP rs11545029 in WWOX exon 6.
Supp. Figure S3: Full-size western blots for WWOX
Supp. Figure S4: Sanger sequencing and confirmation of segregation for the HSPG2 variants
Supp. Table S1: Phenotypes observed in the proband compared to published WWOX patients
Supp. Table S2: Abnormal laboratory results in plasma, urine and CSF
Supp. Table S3: Additional phenotypes not associated with WWOX
Supp. Table S4: Exome outputs, software versions, cut-offs
Supp. Table S5: Primers and probes for digital droplet PCR assays
Supp. Table S6: Setup genotyping and digital droplet PCR assay
ACKNOWLEDGMENTS
We thank the proband and her family for participating in this study. This research was supported by the Intramural Research Program of the National Human Genome Research Institute and the NIH Office of the Director’s Common Fund. We thank Cyrus Keyvanfar for his help in assembling the clinical data, Yan Huang for establishing the primary fibroblast cultures, and Sarah Thomas, Christopher Adams, and Joshi Stephen for their contributions to the bioinformatics analyses.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
REFERENCES
- Abdel-Salam G, Thoenes M, Afifi HH, Korber F, Swan D, Bolz HJ. 2014. The supposed tumor suppressor gene WWOX is mutated in an early lethal microcephaly syndrome with epilepsy, growth retardation and retinal degeneration. Orphanet J Rare Dis 9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Remaileh M, Joy-Dodson E, Schueler-Furman O, Aqeilan RI. 2015. Pleiotropic Functions of Tumor Suppressor WWOX in Normal and Cancer Cells. J Biol Chem 290(52):30728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz CM, Ferguson BW, Abba MC. 2014. WWOX at the crossroads of cancer, metabolic syndrome related traits and CNS pathologies. Biochim Biophys Acta 1846(1):188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek AK, Laflin KJ, Daniel RL, Liao Q, Hawkins KA, Aldaz CM. 2000. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3–24.1, a region frequently affected in breast cancer. Cancer Res 60(8):2140–5. [PubMed] [Google Scholar]
- Ben-Salem S, Al-Shamsi AM, John A, Ali BR, Al-Gazali L. 2015. A novel whole exon deletion in WWOX gene causes early epilepsy, intellectual disability and optic atrophy. J Mol Neurosci 56(1):17–23. [DOI] [PubMed] [Google Scholar]
- Dayan S, O’Keefe LV, Choo A, Richards RI. 2013. Common chromosomal fragile site FRA16D tumor suppressor WWOX gene expression and metabolic reprograming in cells. Genes Chromosomes Cancer 52(9):823–31. [DOI] [PubMed] [Google Scholar]
- Elsaadany L, El-Said M, Ali R, Kamel H, Ben-Omran T. 2016. W44X mutation in the WWOX gene causes intractable seizures and developmental delay: a case report. BMC Med Genet 17(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl WA, Markello TC, Toro C, Fajardo KF, Sincan M, Gill F, Carlson-Donohoe H, Gropman A, Pierson TM, Golas G and et al. 2012. The National Institutes of Health Undiagnosed Diseases Program: insights into rare diseases. Genet Med 14(1):51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl WA, Mulvihill JJ, Toro C, Markello TC, Wise AL, Ramoni RB, Adams DR, Tifft CJ, Udn. 2016. The NIH Undiagnosed Diseases Program and Network: Applications to modern medicine. Mol Genet Metab 117(4):393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahl WA, Tifft CJ. 2011. The NIH Undiagnosed Diseases Program: lessons learned. Jama 305(18):1904–5. [DOI] [PubMed] [Google Scholar]
- Gribaa M, Salih M, Anheim M, Lagier-Tourenne C, H’Mida D, Drouot N, Mohamed A, Elmalik S, Kabiraj M, Al-Rayess M and et al. 2007. A new form of childhood onset, autosomal recessive spinocerebellar ataxia and epilepsy is localized at 16q21-q23. Brain 130(Pt 7):1921–8. [DOI] [PubMed] [Google Scholar]
- Iwata S, Ito M, Nakata T, Noguchi Y, Okuno T, Ohkawara B, Masuda A, Goto T, Adachi M, Osaka H and et al. 2015. A missense mutation in domain III in HSPG2 in Schwartz-Jampel syndrome compromises secretion of perlecan into the extracellular space. Neuromuscul Disord 25(8):667–71. [DOI] [PubMed] [Google Scholar]
- Ludes-Meyers JH, Kil H, Parker-Thornburg J, Kusewitt DF, Bedford MT, Aldaz CM. 2009. Generation and characterization of mice carrying a conditional allele of the Wwox tumor suppressor gene. PLoS One 4(11):e7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallaret M, Synofzik M, Lee J, Sagum CA, Mahajnah M, Sharkia R, Drouot N, Renaud M, Klein FA, Anheim M and et al. 2014. The tumour suppressor gene WWOX is mutated in autosomal recessive cerebellar ataxia with epilepsy and mental retardation. Brain 137(Pt 2):411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot C, Lambert L, Pasquier L, Bienvenu T, Delahaye-Duriez A, Keren B, Lefranc J, Saunier A, Allou L, Roth V and et al. 2015. WWOX-related encephalopathies: delineation of the phenotypical spectrum and emerging genotype-phenotype correlation. J Med Genet 52(1):61–70. [DOI] [PubMed] [Google Scholar]
- Stum M, Davoine CS, Vicart S, Guillot-Noel L, Topaloglu H, Carod-Artal FJ, Kayserili H, Hentati F, Merlini L, Urtizberea JA and et al. 2006. Spectrum of HSPG2 (Perlecan) mutations in patients with Schwartz-Jampel syndrome. Hum Mutat 27(11):1082–91. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Katayama K, Takenaka M, Amakasu K, Saito K, Suzuki K. 2009. A spontaneous mutation of the Wwox gene and audiogenic seizures in rats with lethal dwarfism and epilepsy. Genes Brain Behav 8(7):650–60. [DOI] [PubMed] [Google Scholar]
- Szymanska K, Szczaluba K, Lugowska A, Obersztyn E, Radkowski M, Nowakowska BA, Kusmierska K, Tryfon J, Demkow U. 2014. The analysis of genetic aberrations in children with inherited neurometabolic and neurodevelopmental disorders. Biomed Res Int 2014:424796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarki B, AlHashem A, AlShahwan S, Alkuraya FS, Gedela S, Zuccoli G. 2015. Severe CNS involvement in WWOX mutations: Description of five new cases. Am J Med Genet A 167a(12):3209–13. [DOI] [PubMed] [Google Scholar]
- Valduga M, Philippe C, Lambert L, Bach-Segura P, Schmitt E, Masutti JP, Francois B, Pinaud P, Vibert M, Jonveaux P. 2015. WWOX and severe autosomal recessive epileptic encephalopathy: first case in the prenatal period. J Hum Genet 60(5):267–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Figure S1: Results genotyping assays for SNPs rs2303191, rs11545029, and rs383362 in WWOX and SNP rs1063438 in CDH5.
Supp. Figure S2: Example of digital droplet PCR output for SNP rs11545029 in WWOX exon 6.
Supp. Figure S3: Full-size western blots for WWOX
Supp. Figure S4: Sanger sequencing and confirmation of segregation for the HSPG2 variants
Supp. Table S1: Phenotypes observed in the proband compared to published WWOX patients
Supp. Table S2: Abnormal laboratory results in plasma, urine and CSF
Supp. Table S3: Additional phenotypes not associated with WWOX
Supp. Table S4: Exome outputs, software versions, cut-offs
Supp. Table S5: Primers and probes for digital droplet PCR assays
Supp. Table S6: Setup genotyping and digital droplet PCR assay