Abstract
Purpose:
Few studies have described ocular surface squamous neoplasia (OSSN) and its association with atopic diseases and there is no consensus on the course of OSSN in atopic patients. We thereby report 3 patients with atopy and OSSN.
Methods:
Retrospective case series.
Results:
Three male patients with mean age of 73 presented with OSSN and history of atopy treated with immunosuppressant therapy. Their histories included atopic dermatitis and keratoconjunctivitis. All patients had treatment complicated by multiple surgeries, recurrences, or advanced disease. The patients initially received medical treatment with topical interferon-alpha-2b (IFNα2b). However, all patients had recurrences and required modification of treatment including topical 5-fluorouracil (5-FU).
Conclusion:
We report on three patients with a history of atopy whose OSSN presentation and course was challenging. Our cases responded better to topical 5-fluorouracil compared to topical interferon-alpha-2b, but recurrences were common. These patients may benefit from more aggressive and long-term treatment.
Keywords: ocular surface squamous neoplasia, OSSN, atopic disease, 5- fluorouracil, interferon-alpha-2b, atopy
Introduction
Ocular surface squamous neoplasia (OSSN) represents a spectrum of epithelial squamous malignancies ranging from dysplasia to invasive carcinoma.1 OSSN can be treated surgically2–5 or medically with topical interferon-alpha-2b (IFNα2b), 5-fluorouracil (5-FU), or mitomycin-C (MMC)3,6–14 and in general, treatment outcomes are excellent. With respect to primary medical therapy, approximately 80–90% of tumors completely resolve with IFN, 5FU, and/or MMC.6–14 As such, OSSN is generally considered a slow growing and treatable tumor. However certain individuals have been reported to have a more aggressive disease course, such as those with human immunodeficiency virus (HIV) in Africa14 and those with systemic immunosuppression.15 It is our experience that patients with OSSN and atopic dermatitis have a more aggressive form of OSSN that is more resistant to treatment.
Atopy is characterized by a tendency to produce immunoglobulin E (IgE) antibodies, usually with an increased risk of developing one or more of the following: atopic dermatitis (eczema), allergic rhinitis (hay fever), or allergic asthma. Atopic diseases have been associated with an overactive TH2 immune response leading to hypersensitivity to a range of common environmental antigens. The increased TH2 differentiation of naïve T cells leads to increased production of cytokines such as IL-4, IL-5, IL-9 and IL-13 and support humoral, anti-helminthic, and allergic responses.16 The overall result is excessive IgE production and inhibited TH1 response.
Interestingly, the relationship between atopy and cancer development is unclear.17,18 There are several hypotheses and mechanisms for how atopy may protect against or enhance carcinogenesis.17 The atopic state can cause chronic inflammation. This may enhance cancer risk in tissues where atopy principally manifests such as lung and skin.17,18 The atopic immune state has also been suggested to be protective against cancer as the heightened surveillance decreases the possibility for proliferation of tumor cells.18 This could explain the decreased relative risk of some cancers including brain, pancreatic, lymphatic, and hematopoietic in patients with atopy.17 Thus, the connection between atopy and cancer, including OSSN, remains complex.19
Current research has focused on the correlation between atopy and cancer, with little focus on how cancer treatment options and responses differ in patients with atopic diseases. Some studies have described the course of OSSN and its association with these patients but there is no consensus on how these patients present and respond.20,21 To bridge this knowledge gap, we herein report on individuals with atopic dermatitis and atopic keratoconjunctivitis who presented with OSSN, focusing on unique response to therapy.
Materials and Methods
Retrospective chart review of patients with a clinical history of atopic disease (atopic dermatitis, atopic keratoconjunctivitis, and asthma) who presented with OSSN to the Bascom Palmer Eye Institute. Chart review from 2014 to 2016 captured history, ophthalmic findings, and clinical course. The University of Miami institutional review board approved the retrospective review of patient charts and the study was conducted in accordance to the principles of the Declaration of Helsinki.
Results
Three male patients with mean age of 73 with OSSN and history of atopy with ocular manifestations were identified. Their tumors were more aggressive and less amenable to therapy.
Case 1:
A 61-year-old man with a history of atopic dermatitis and asthma since birth had a chronic history of severe keratoconjunctivitis with associated lid thickening in both eyes and symblephara. For the 5 years prior to presentation, he was treated with systemic prednisone (5mg) daily and tacrolimus. For his eyelids, he also used topical tacrolimus and methylprednisolone.
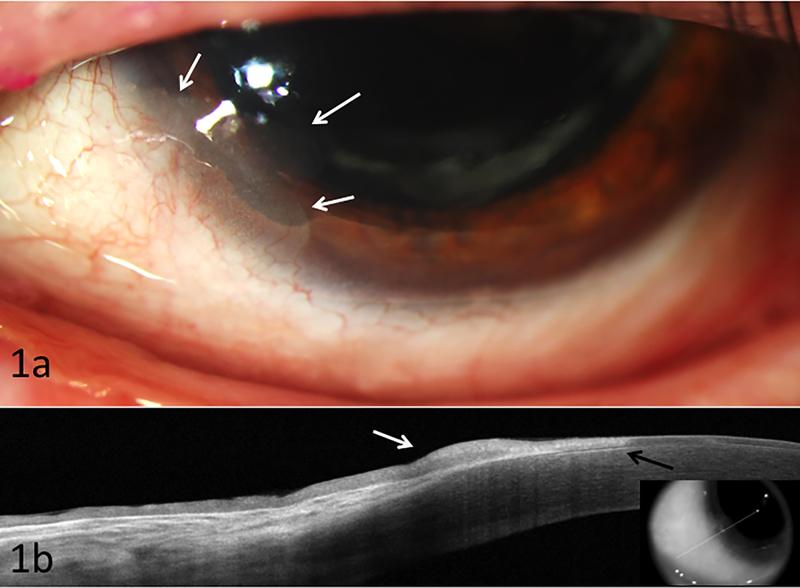
The patient initially presented with right large conjunctival mass associated with loss of vision, and proptosis. Concurrently in his left eye, an inferonasal limbal conjunctival lesion was detected at 7 o’clock which stained with rose bengal and extended 3 mm into the cornea. Computed tomography (CT) scan was consistent with conjunctival and orbital mass on the right. This was confirmed by biopsy to be invasive squamous cell carcinoma. The right orbit was subsequently exenterated with node dissection and radiotherapy was initiated for positive periosteum and pre-auricular node involvement. For the left eye, the conjunctival lesion was biopsied and found to be conjunctival intraepithelial neoplasia. The patient was started on interferon-alpha-2b (IFN, 1 million units/mL) four times daily to the left eye. After 9 weeks, the lesion was noted to be resolving clinically and by ultra high resolution optical coherence tomography (UHR OCT). He continued the IFN for 5 months with clinical tumor resolution in the left eye. Three months later, however, recurrent OSSN was noted at in the same 7 o’clock location (Figure 1a). UHR OCT (Figure 1b) images were consistent with recurrent OSSN. The patient was restarted on IFN therapy for which the tumor was unresponsive and grew in size. The patient was then switched to 5-FU, 1% four times daily weekly cycles with a 3 week holiday. He received a total of 4 cycles with resolution.
Figure 1:
Biomicroscopy of case 1 illustrating (a) recurrent inferonasal OSSN at 7 o’clock (white arrows) with subepithelial scarring; (b) High resolution ocular coherence tomography demonstrating thickened hyper-reflective epithelium (white arrow) with an abrupt transition from normal to abnormal epithelium (black arrow), consistent with ocular surface squamous neoplasia (OSSN). Note subepithelial scaring under the abnormal epithelium. Biopsy confirmed findings.
Case 2:
An 86-year-old man had a long history of atopic keratoconjunctivits and atopic dermatitis for which he used topical tacrolimus and flucinonide (0.05%). He also had a history of multiple squamous and basal cell carcinomas (hands and face). In 2011, he presented with a left limbal lesion from 3 to 9 o’clock which was excised and confirmed to be intraepithelial neoplasia. Recurrent disease was noted two years later and was initially treated with 5 monthly IFN injections (10 million international units/0.5 cc) and subsequent topical IFN 1 MIU/ml four times daily and retinoic acid every other day for 2 years. There was some response, but not resolution. He was then referred to our institution for evaluation.
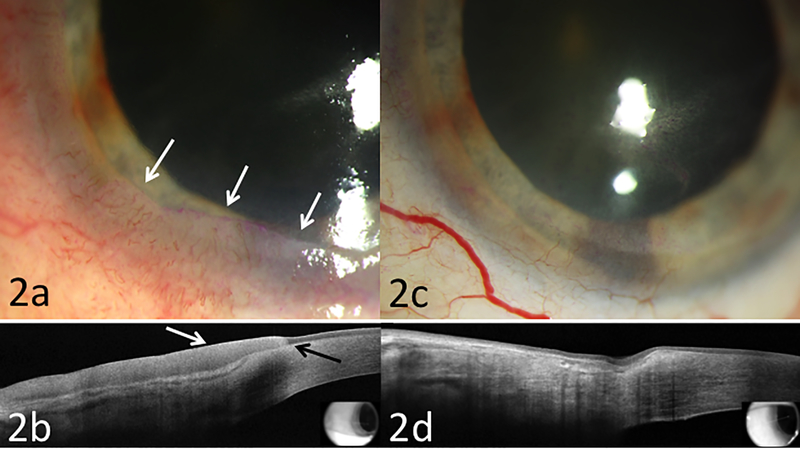
Slit lamp examination revealed a diffuse conjunctival and corneal tumor from 3 to 10 o’clock with minimal vascularity and with associated rose bengal staining (Figure 2a). UHR OCT supported the diagnosis of persistent OSSN (Figure 2b). Due to the incomplete response to the interferon, this was discontinued and the patient was switched to 5-FU 1%. This was given four times daily for a week with a 3 week drug holiday. After 2 cycles of 5-FU, there was dramatic clinical improvement. UHR OCT images confirmed improvement with one remaining area of corneal epithelial thickening at 6 o’clock (Figure 2c). 5-FU was therefore continued for a total of 4 cycles. Clinical examination and UHR OCT imaging were normal at 9 months following completion of 5-FU course (Figure 2d).
Figure 2:
Biomicroscopy of case 2 illustrating (a) recurrent, biopsy proven OSSN with an opalescent and gelatinous tumor from 3 to 10 o’clock with vascularity and rose bengal staining (white arrows); (b) High resolution ocular coherence tomography with typical features of OSSN of thickened hyper-reflective epithelium (white arrows), and an abrupt transition from normal to abnormal epithelium (black arrow); (c) following 2 months of 5-FU, there was dramatic improvement with mild subepithelial haze (d) HR-OCT demonstrated normalization of epithelium thickness with mild residual hyper-reflectivity and no abrupt transition zone.
Case 3:
A 73-year-old man had a 50-year history of atopic dermatitis and keratoconjunctivitis, with resulting forniceal foreshortening, symblepharon, and corneal scarring in both eyes. His past ocular history was significant for left eye cataract surgery, and right eye penetrating keratoplasty complicated by endophthalmitis and eventual enucleation in 1995. In 2002, his left eye was diagnosed with limbal stem cell deficiency. In 2010, he underwent living related conjunctival limbal allograft followed by keratolimbal allograft transplantation (KLAL) 3 months later in his left eye. He was initially treated with oral mycophenolate and tacrolimus. Topically, he was using corticosteroids and cyclosporine.
In 2013, three years after his transplant, a limbal lesion was noted from 3 to 9 o’clock at the edge of the KLAL, consistent with OSSN. Biopsy was performed, and conjunctival intraepithelial neoplasia was confirmed. Due to monocular status, he was initiated on topical IFN (1 million units/mL) four times daily. However, the tumor did not resolve and he underwent excisional surgery. The tumor recurred again and was treated with topical interferon and 2 rounds MMC. There was again poor response and another excisional biopsy with cryotherapy was performed. Pathology again confirmed conjunctival intraepithelial neoplasia. The tumor then recurred for the fourth time and he was referred for management.
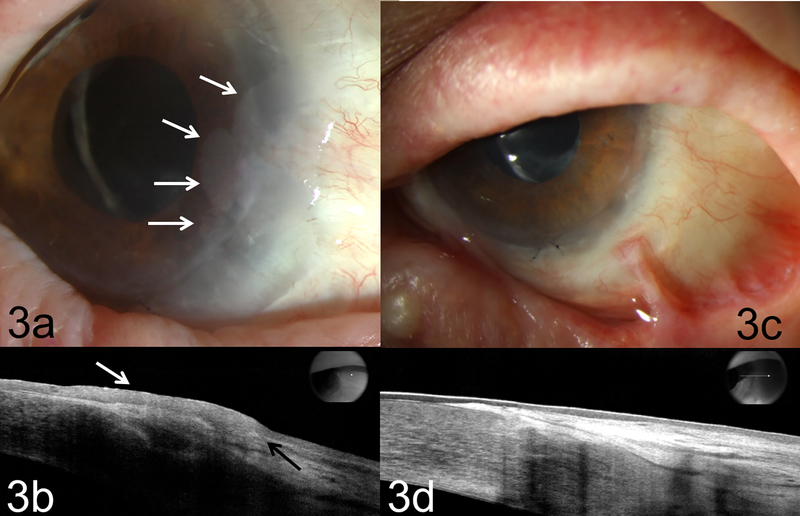
Slit lamp examination of the left eye revealed a gelatinous lesion at the limbus from 2 to 4:30 o’clock with 4 mm extension into the corneal surface (Figure 3a). Another conjunctival gelatinous lesion was identified inferiorly at 5 to 6 o’clock with no corneal extension. UHR-OCT supported the diagnosis of recurrent OSSN. Due to the ineffective IFN and MMC therapy, 5-FU and retinoic acid were initiated. The patient was treated with one week cycles of 5-FU four times daily followed by a 3 week holiday. Retinoic acid (.01%) was also given every other day as it has shown activity against epithelial lesions. Following 4 cycles of 5-FU and the retinoic acid treatment, the OSSN resolved (Figure 3c). The patient was tumor free for 2 years but presented with recurrence and was restarted on 5-FU.
Figure 3:
Biomicroscopy of case 3 illustrating (a) a biopsy proven recurrent OSSN with an opalescent and gelatinous lesion at 2 to 4:30 o’clock limbus. There was extension onto corneal surface about 4 mm (white arrows) at edge of the limbal autograft; (b) high resolution OCT (HR-OCT) confirmed recurrent OSSN with thickened, hyper-reflective epithelium (white arrow) and an abrupt transition from normal to abnormal epithelium (black arrow). (c) Tumor resolved OSSN after 4 rounds of 5-FU and retinoic acid; note eyelid scarring from atopic dermatitis. (d) HR-OCT confirms thin, normalized epithelium at resolution of treatment.
Discussion
We report on three patients with a history of atopic dermatitis and atopic keratoconjunctivitis whose OSSN presentation and course was challenging. All were associated with aggressive disease and frequent recurrences and required multiple treatment modalities. Treatment with interferon was marked by partial response and recurrences. These cases suggest that the immune hyperactivity of atopic diseases affects how medical therapies combat OSSN and the aggressivity of the disease.
Despite numerous epidemiological studies on the association between atopy and cancer, the data is largely inconclusive. Some cancers are found to be at increased risk (lung, skin, and prostate)22 while others seem to be at a lower risk (childhood leukemia, pancreatic, and brain tumors) in individuals with atopic diseases.17,18 Organs where atopy tends to manifest have been associated with an increased relative risk of respective cancer including the respiratory tract, gastrointestinal tract, skin.17 Pertinent to the eye, a history of allergic conjunctivitis is a reported risk factor for OSSN23, further suggesting inflammation in atopic target organs may promote cancer. However, in mice, mutations in epidermal barrier proteins that predispose individuals to atopic dermatitis and increased asthma severity were found to be protective against skin cancers.24,25 Our patients had ocular manifestations of their atopy and the chronic inflammation associated may have contributed to the development of their OSSN.
In this small series, it seems that IFN treatment was less effective in treating OSSN. This is contrast to previous publications which found topical IFN to be an effective treatment in most OSSN lesions, including those with systemic immunosuppression.15,26 The OSSN recurrence rate with topical IFN-a2b has been reported to be 6% in follow-up ranging between 7 to 55 months.27 All of our atopic patients had recurrences of their OSSN and some with multiple recurrences.
Individuals with atopic diseases have a predominantly TH2 response, which suppresses the tumor-eradicating TH1 response. Thus, tumor regression with topical IFN may be dependent on having a TH1 immunity profile not found in patients with atopy. While no studies have specifically focused on mechanisms by which IFN resolves OSSN, a few studies have examined this issue with regards to cervical intraepithelial neoplasia. In a cervical model, a local TH1 profile (versus a TH2 profile) was associated with better response to IFN.28 Other factors found to effect IFN response included non-smoking status28,29, reduction in human papilloma viral load28–30, lower levels of local inflammatory cytokines (e.g. IL-6)29 and increased peripheral natural killer cell (NK) activity.31 Thus, the suppressed TH1 profile and increased levels of local inflammatory mediators in atopy may explain the poor response of our patients to traditionally successful IFN therapy.
Our three cases of OSSN in atopic patients responded better to 5-FU over IFN. 5-FU is a structural analogue of uracil, which inhibits DNA replication by blocking the enzyme thymidylate synthase. Further, metabolic derivatives of 5-FU also disrupt RNA synthesis in the cell. Rapidly multiplying cells, such as tumor cells, require the replication of DNA and synthesis of large amount of RNA in each cell cycle. Thus, 5-FU can cause much more damage in tumor cells than normal cells, allowing selective targeting of cancerous lesions. 5-FU’s mechanism as an antimetabolite rather than relying upon a specific immune profile may explain its success in eliciting OSSN regression in our patients with atopy and immune dysregulation.
The complex, varied and broad nature of atopic diseases and tumorigenesis likely explains the conflicting conclusions in literature and the challenging treatment responses of our OSSN cases. For example, the eosinophilia usually accompanying atopic disease can be pro-inflammatory, anti-inflammatory, or immunoregulatory through their activation and regulation of cytokines and growth factors.32 Eosinophils promote inflammation and tissue destruction in atopic and allergic diseases, but they also regulate wound healing through fibroblast proliferation and matrix metalloproteinases. They have also been found to be immune effector cells against tumors through reactions such as eosinophil-derived EPO synergizing with macrophage reactive oxygen species to kill tumor cells.32 Thus, the multifaceted nature of atopic immunity and intricacies of carcinogenesis complicate finding a direct relationship between the two.
Our study findings must be considered bearing in mind its limitations which were a small population and potential confounders (immunosuppressive medication including tacrolimus, genetics). Despite these limitations, this case series highlights the complexity of OSSN treatment in patients with underlying atopic disease. In our 3 cases, individuals developed multiple recurrences which were less responsive to traditional medical therapy. In our experience, the clinical course of our 3 patients was much more aggressive than typical for OSSN in our institution. This may be because patients with atopy have several issues: immune dysregulation, induced inflammation and its associated risks of malignant transformation, and immunosuppression from drugs used to treat atopic disease. Further research is needed to better determine how atopy and inflammation affects cancer presentation and treatment, especially in relation to OSSN. Nevertheless, it may be beneficial to consider a varied and more aggressive treatment and increased vigilance for these patients with atopy and OSSN.
Acknowledgments
Funding: Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006–15S (Dr. Galor), NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, The Ronald and Alicia Lepke Grant, The Lee and Claire Hager Grant, The Jimmy and Gaye Bryan Grant, The H. Scott Huizenga Grant, The Robert Baer Family Grant, The Gordon Charitable Foundation, Emilyn Page and Mark Feldberg Grant, and the Richard Azar Family Grant(institutional grants).
Footnotes
Conflict of interest: None
References
- 1.Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39(6):429–450. [DOI] [PubMed] [Google Scholar]
- 2.Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumors. The 1994 Lynn B. McMahan Lecture. Archives of ophthalmology. 1997;115(6):808–815. [DOI] [PubMed] [Google Scholar]
- 3.Midena E, Angeli CD, Valenti M, de Belvis V, Boccato P. Treatment of conjunctival squamous cell carcinoma with topical 5-fluorouracil. The British journal of ophthalmology. 2000;84(3):268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peksayar G, Altan-Yaycioglu R, Onal S. Excision and cryosurgery in the treatment of conjunctival malignant epithelial tumours. Eye (London, England). 2003;17(2):228–232. [DOI] [PubMed] [Google Scholar]
- 5.Siganos CS, Kozobolis VP, Christodoulakis EV. The intraoperative use of mitomycin-C in excision of ocular surface neoplasia with or without limbal autograft transplantation. Cornea. 2002;21(1):12–16. [DOI] [PubMed] [Google Scholar]
- 6.Ballalai PL, Erwenne CM, Martins MC, Lowen MS, Barros JN. Long-term results of topical mitomycin C 0.02% for primary and recurrent conjunctival-corneal intraepithelial neoplasia. Ophthalmic plastic and reconstructive surgery. 2009;25(4):296–299. [DOI] [PubMed] [Google Scholar]
- 7.Frucht-Pery J, Sugar J, Baum J, et al. Mitomycin C treatment for conjunctival-corneal intraepithelial neoplasia: a multicenter experience. Ophthalmology. 1997;104(12):2085–2093. [DOI] [PubMed] [Google Scholar]
- 8.Galor A, Karp CL, Chhabra S, Barnes S, Alfonso EC. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: a dose comparison study. Br J Ophthalmol. 2010;94(5):551–554. [DOI] [PubMed] [Google Scholar]
- 9.Karp CL, Galor A, Chhabra S, Barnes SD, Alfonso EC. Subconjunctival/perilesional recombinant interferon alpha2b for ocular surface squamous neoplasia: a 10-year review. Ophthalmology. 2010;117(12):2241–2246. [DOI] [PubMed] [Google Scholar]
- 10.Karp CL, Galor A, Lee Y, Yoo SH. Pegylated interferon alpha 2b for treatment of ocular surface squamous neoplasia: a pilot study. Ocul Immunol Inflamm. 2010;18(4):254–260. [DOI] [PubMed] [Google Scholar]
- 11.Adler E, Turner JR, Stone DU. Ocular surface squamous neoplasia: a survey of changes in the standard of care from 2003 to 2012. Cornea. 2013;32(12):1558–1561. [DOI] [PubMed] [Google Scholar]
- 12.Yeatts RP, Engelbrecht NE, Curry CD, Ford JG, Walter KA. 5-Fluorouracil for the treatment of intraepithelial neoplasia of the conjunctiva and cornea. Ophthalmology. 2000;107(12):2190–2195. [DOI] [PubMed] [Google Scholar]
- 13.Yeatts RP, Ford JG, Stanton CA, Reed JW. Topical 5-fluorouracil in treating epithelial neoplasia of the conjunctiva and cornea. Ophthalmology. 1995;102(9):1338–1344. [DOI] [PubMed] [Google Scholar]
- 14.Kaimbo Wa Kaimbo D, Parys-Van Ginderdeuren R, Missotten L. Conjunctival squamous cell carcinoma and intraepithelial neoplasia in AIDS patients in Congo Kinshasa. Bull Soc Belge Ophtalmol. 1998;268:135–141. [PubMed] [Google Scholar]
- 15.Shields CL, Ramasubramanian A, Mellen PL, Shields JA. Conjunctival squamous cell carcinoma arising in immunosuppressed patients (organ transplant, human immunodeficiency virus infection). Ophthalmology. 2011;118(11):2133–2137 e2131. [DOI] [PubMed] [Google Scholar]
- 16.Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josephs DH, Spicer JF, Corrigan CJ, Gould HJ, Karagiannis SN. Epidemiological associations of allergy, IgE and cancer. Clin Exp Allergy. 2013;43(10):1110–1123. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy. 2005;60(9):1098–1111. [DOI] [PubMed] [Google Scholar]
- 19.Gichuhi S, Sagoo MS, Weiss HA, Burton MJ. Epidemiology of ocular surface squamous neoplasia in Africa. Trop Med Int Health. 2013;18(12):1424–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn TH, Manzouri B, Tuft SJ. Ocular surface squamous neoplasia in an immunosuppressed patient with atopic keratoconjunctivitis. Int Ophthalmol. 2012;32(5):471–473. [DOI] [PubMed] [Google Scholar]
- 21.Rundle P, Mudhar HS, Rennie I. Conjunctival intra-epithelial neoplasia occurring in young patients with asthma. Eye (Lond). 2010;24(7):1182–1185. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Rothenbacher D, Low M, Stegmaier C, Brenner H, Diepgen TL. Atopic diseases, immunoglobulin E and risk of cancer of the prostate, breast, lung and colorectum. Int J Cancer. 2006;119(3):695–701. [DOI] [PubMed] [Google Scholar]
- 23.Gichuhi S, Macharia E, Kabiru J, et al. Risk factors for ocular surface squamous neoplasia in Kenya: a case-control study. Trop Med Int Health. 2016;21(12):1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cipolat S, Hoste E, Natsuga K, Quist SR, Watt FM. Epidermal barrier defects link atopic dermatitis with altered skin cancer susceptibility. Elife. 2014;3:e01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–446. [DOI] [PubMed] [Google Scholar]
- 26.Karp CL, Moore JK, Rosa RH Jr. Treatment of conjunctival and corneal intraepithelial neoplasia with topical interferon alpha-2b. Ophthalmology. 2001;108(6):1093–1098. [DOI] [PubMed] [Google Scholar]
- 27.Nanji AA, Sayyad FE, Karp CL. Topical chemotherapy for ocular surface squamous neoplasia. Curr Opin Ophthalmol. 2013;24(4):336–342. [DOI] [PubMed] [Google Scholar]
- 28.Ramos MC, Mardegan MC, Peghini BC, Adad SJ, Michelin MA, Murta EF. Expression of cytokines in cervical stroma in patients with high-grade cervical intraepithelial neoplasia after treatment with intralesional interferon alpha-2b. European journal of gynaecological oncology. 2010;31(5):522–529. [PubMed] [Google Scholar]
- 29.Mardegan MC, Ramos MC, Adad SJ, Michelin MA, Shimba D, Murta EF. Immunological evaluation of vaginal secretion in patients with high-grade cervical intraepithelial neoplasia treated with intralesional interferon alpha-2b. Eur J Gynaecol Oncol. 2011;32(3):297–302. [PubMed] [Google Scholar]
- 30.Chakalova G, Ganchev G. Local administration of interferon-alpha in cases of cervical intraepithelial neoplasia associated with human papillomavirus infection. J Buon. 2004;9(4):399–402. [PubMed] [Google Scholar]
- 31.Garzetti GG, Ciavattini A, Romanini C, et al. Interferon alpha 2b treatment of cervical intraepithelial neoplasia grade 2: modulation of natural killer cell. Gynecol Obstet Invest. 1994;37(3):204–209. [DOI] [PubMed] [Google Scholar]
- 32.Munitz A, Levi-Schaffer F. Eosinophils: ‘new’ roles for ‘old’ cells. Allergy. 2004;59(3):268–275. [DOI] [PubMed] [Google Scholar]