Introduction
Topical corticosteroid addiction results from prolonged use of topical corticosteroids (TCS) resulting in flaring of an acneiform, scaling, and erythematous rash with attempts at discontinuation. The resulting cycle of TCS use, withdrawal, flare of symptoms, and resumed use is driven by both a psychological and physical dependence. We report a novel case of 2 family members simultaneously having an addiction from a shared TCS prescription. The simultaneous withdrawal and shared flares in their repetitive cycle of TCS misuse sheds light on the danger of topical corticosteroid addiction and the folly of prescription sharing.
Case
A 34-year-old white woman and her 6-year-old son presented with a 6-month history of central facial rashing. One year prior, the child had nummular eczema for which an urgent care clinic provider prescribed hydrocortisone 2.5% cream applied twice daily. The patient and mother were cautioned to not use topical steroids for more than a few weeks, but there was no further discussion of potential side effects. After several months without relief, the pair visited a different provider who escalated the child's treatment to triamcinolone 0.1% cream and subsequently betamethasone 0.05% cream at another visit. Each of these prescriptions came with several refills. During this time, the mother had a scaling rash in the nasolabial folds for which her son's topical steroids yielded relief. However, the pair would routinely discontinue use after several weeks per the instruction of the first prescribing provider. After discontinuation, they would experience intense flaring of their rashes, leading them to believe some underlying skin condition was to blame and topical steroids were the treatment. This situation evolved into a cycle of withdrawal, resumed TCS use, improvement, discontinuation, and worsening rebound symptoms.
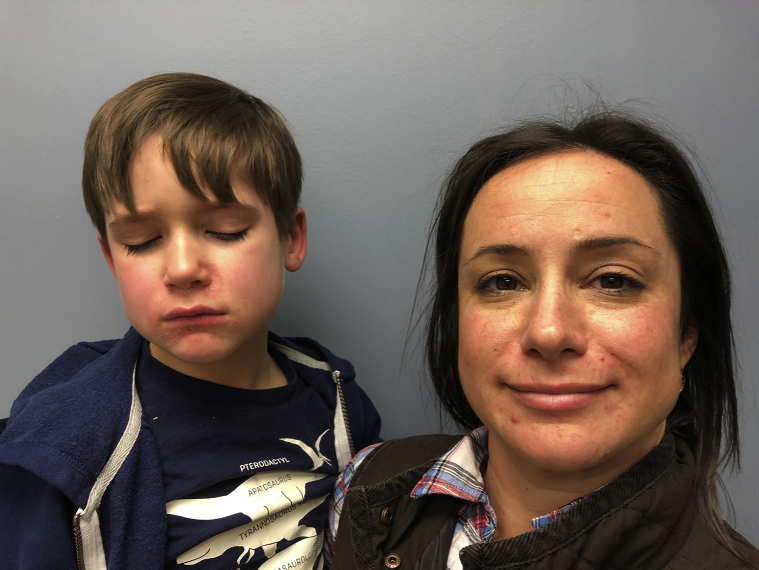
Desperate for relief, the pair visited yet another provider who prescribed a short course of oral steroids, which provided quick and complete resolution. As expected, their rashes emerged several days later. With this, the pair presented to our clinic where physical examination found erythematous acneiform papules with background scaling in the perioral region of both patients (Fig 1). With a diagnosis of perioral dermatitis, all topical steroids were discontinued, and a combination of systemic and topical antimicrobials was initiated (Table I). Despite this treatment, both patients reported worsening of symptoms for about 10 days after topical steroid termination (Fig 2). Gradually, with strict TCS abstinence, both patients reported full resolution of symptoms in the following weeks (Fig 3).
Fig 1.
Initial presentation. Both patients exhibit erythematous acneiform papules with background scaling in the perioral region.
Table I.
Topical corticosteroid withdrawal treatment and rationale
| Prescribed regimen at initial encounter | Rationale | |
|---|---|---|
| Patient 1 (mother) | Discontinuation of all TCS use | A significant majority of TCS addiction improves within 2-3 weeks with a cold turkey approach.1 |
| Doxycycline, 100 mg PO BID × 30 d | Suppression of follicular infection caused by to TCS-mediated microbial overgrowth2 and anti-inflammatory effects of tetracyclines at lower doses (20-40 mg) | |
| Clindamycin 1% lotion applied bid to affected areas of face and perioral region | Moisturizing and antibiotic effects | |
| Ketoconazole 2% cream once weekly × 2 wks | History consistent with seborrheic dermatitis and suppression of microbial overgrowth encouraged by TCS-mediated immune suppression | |
| Pramoxine lotion PRN for itching | Relief of burning and itching with low sensitizing potential1, 2 | |
| Patient 2 (child) | Discontinuation of all TCS use | See above |
| Clindamycin 1% lotion applied bid to affected areas of face and perioral region | See above | |
| Amoxicillin 250 mg PO BID × 1 month | Suppression of follicular infection2 in young children who cannot take tetracyclines |
Fig 2.
Two weeks postwithdrawal. Patient 1 (right) has mild background scaling in the perioral region with few erythematous nodules on the chin. Patient 2 (left) has persistent perioral scaling with involvement of the nasal folds.
Fig 3.
Two months postwithdrawal. Both patients have complete recovery from TCS addiction.
Discussion
The folly of prescription sharing
To our knowledge, a case of parent-child TCS addiction caused by prescription sharing has never been reported. Furthermore, although most TCS addiction cases are in women using facial TCS, only 7% of reported cases involve children.3 The psychological aspect of TCS addiction combined with the parent-child relationship make this case an interesting extrapolation of folie à deux—one that we have colloquially termed “folly” à deux—in which both mother and son experienced the physical and psychological discomfort of withdrawal. Folie à deux is a French term meaning shared madness, or madness for two. According to the Diagnostic and Statistical Manual of Mental Disorders, folie à deux is defined as delusional symptoms in the partner of an individual with a delusional disorder.4 While our patients had no psychiatric comorbidities and certainly do not have a delusional disorder, an extrapolation of this concept can be applied in which the mother began using her son's TCS prescription because of perceived similarity in cutaneous symptoms. Furthermore, both endured the psychological distress of the physical symptoms of withdrawal: erythema, scaling, pustules, and sensations of burning, stinging, intense itching, pain, and facial warmth.3 To add insult to injury, the otherwise desirable anti-inflammatory effects of TCS allow microbial overgrowth, which triggers an intense immune response immediately with TCS discontinuation.1, 2 Together, these reactions induce psychological distress and make it difficult to maintain normal social functioning.3 One can imagine that the psychological distress experienced by a mother is only compounded by the same experience in a child and that the choice to resume TCS use seemed logical to the pair. The double entendre “folly” à deux reflects both the foolishness of prescription sharing and the challenge introduced by the duality of the parent-child relationship. Together, they entered a futile cycle of classic TCS addiction.
Not surprisingly, TCS are one of the most commonly shared dermatologic prescription medications.5 Patients are more willing to borrow medication if it is prescribed to a family member,6 and one of the most common reasons cited for prescription sharing is having the same medical problem.7 Of course, to a patient browsing the internet, all skin conditions may look the same. The consequences of prescription sharing and parental self-treatment of their children include unanticipated adverse events, complications of incorrect use, delay in seeking professional help, and misuse leading to addiction,8 all of which characterize the pair's experience.
Fragmentation of care is a hallmark of this case that highlights a contributing factor to TCS addiction. When prescribing, it is imperative to inquire about past and current use of other TCS and any symptoms involved with either use or discontinuation. Furthermore, education regarding the hazards of TCS use are important because many patients view them as innocuous. They should be informed that misuse can cause atrophy, striae, fragile skin, and addiction. Our patients were told to discontinue use after several weeks but received no instruction regarding side effects or signs of withdrawal; although well intentioned, this information led the patients to routinely discontinue use every few weeks, inducing their own withdrawal and priming the cycle of addiction. Therefore, health care providers must monitor TCS use and identify patients who may be at a higher risk of misuse and addiction based on their medical history.3 Of course, patient education must be balanced with information about the safety of appropriate TCS use to minimize TCS phobia, a recent social media–driven phenomenon that has likely arisen from ubiquitous TCS abuse.9
Management of topical corticosteroid addiction
We favor immediate elimination of all topical and systemic steroids in our patients with TCS addiction. Patients must be ardently informed that they will suffer the physical signs, symptoms, and psychological effects of withdrawal and rebound flaring for 10 to 14 days despite maximal medical therapy.2, 9 Depression and doubt are hallmarks of this phase as patients may feel their doctor is failing them and subsequently desire to resume TCS use. Even as they begin to improve, patients may be quite sensitive to seemingly insignificant stimuli causing heightened anxiety.10 Although the time required for complete resolution depends on the potency and duration of TCS misuse, and perhaps factors unique to specific patients, a significant majority see the improvement they desire within 2 to 3 weeks.1 Reassurance at biweekly visits with a phone call from the physician or nurse 1 week after the visit is critical. Given the compounded parent-child relationship of our patients, this was particularly important considering the mother's feelings of guilt and responsibility for her child's suffering. We stress that time may be the greatest medicine in these situations.10 We also recognize that some experts favor slow tapering of TCS or the use of pimecrolimus 1% cream or tacrolimus 0.1% ointment for their anti-inflammatory effects; however, we feel this prolongs the inevitable flaring that uniformly resolves within just a few weeks with our “cold turkey” approach.1, 3, 10
Maximal medical therapy of TCS addiction is detailed in Table I and includes (1) doxycycline or other systemic antibiotics in children too young to receive tetracyclines,7 (2) clindamycin lotion twice daily, and (3) moisturizing lotions containing 1% pramoxine.2, 10
Footnotes
Funding sources: None.
Conflicts of interest: Robert T. Brodell, MD, discloses the following potential conflicts of interest: Advisory Board, Novartis; Clinical trials, Genentech, Novartis, and Janssen Biotech, Inc. Bonnie Hodge and Dr Huynh have no conflicts to disclose.
References
- 1.Wells K., Brodell R.T. Topical corticosteroid “addiction”: a cause of perioral dermatitis. Postgrad Med. 1993;93(5):225–230. doi: 10.1080/00325481.1993.11701671. [DOI] [PubMed] [Google Scholar]
- 2.Brodell R.T., O'Brien M.J. Topical corticosteroid-induced acne: Three treatment strategies to break the ‘addiction’ cycle. Postgrad Med. 1999;106(6):225–229. doi: 10.3810/pgm.1999.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Hajar T., Leshem Y.A., Hanifin J.M. A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J Am Acad Dermatol. 2015;72:541–549. doi: 10.1016/j.jaad.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition 5. Arlington, VA: American Psychiatric Association; 2013.
- 5.Hogan D.J., Moreland A., Lane P., Lal S. Exchange of prescription medications by dermatology outpatients. J Am Acad Dermatol. 1990;23(5 pt 1):953. doi: 10.1016/s0190-9622(08)80715-3. [DOI] [PubMed] [Google Scholar]
- 6.Daniel K.L., Honein M.A., Moore C.A. Sharing prescription medication among teenage girls: potential danger to unplanned/undiagnosed pregnancies. Pediatrics. 2003;111(5 pt 2):1167–1170. [PubMed] [Google Scholar]
- 7.Goldsworthy R.C., Schwartz N.C., Mayhorn C.B. Beyond abuse and exposure: framing the impact of prescription-medication sharing. Am J Public Health. 2008;98(6):1115–1121. doi: 10.2105/AJPH.2007.123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis J., Mullan J. Prescription medication borrowing and sharing--risk factors and management. Aust Fam Physician. 2009;38(10):816–819. [PubMed] [Google Scholar]
- 9.Ghosh A., Sengupta S., Coondoo A., Jana A.K. Topical corticosteroid addiction and phobia. Indian J Dermatol. 2014;59(5):465–468. doi: 10.4103/0019-5154.139876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukaya M., Sato K., Sato M. Topical corticosteroid addiction in atopic dermatitis. Drug Healthc Patient Saf. 2014;6:131–138. doi: 10.2147/DHPS.S6920. [DOI] [PMC free article] [PubMed] [Google Scholar]