Abstract
Tropomodulins (Tmods) are proteins that cap the slow-growing (pointed) ends of actin filaments (F-actin). The basis for our current understanding of Tmod function comes from studies in cells with relatively stable and highly organized F-actin networks, leading to the view that Tmod capping functions principally to preserve F-actin stability. However, not only is Tmod capping dynamic, but it also can play major roles in regulating diverse cellular processes involving F-actin remodeling. Here, we highlight the multifunctional roles of Tmod with a focus on Tmod3. Like other Tmods, Tmod3 binds tropomyosin (Tpm) and actin, capping pure F-actin at submicromolar and Tpm-coated F-actin at nanomolar concentrations. Unlike other Tmods, Tmod3 can also bind actin monomers and its ability to bind actin is inhibited by phosphorylation of Tmod3 by Akt2. Tmod3 is ubiquitously expressed and is present in a diverse array of cytoskeletal structures, including contractile structures such as sarcomere-like units of actomyosin stress fibers and in the F-actin network encompassing adherens junctions. Tmod3 participates in F-actin network remodeling in lamellipodia during cell migration and in the assembly of specialized F-actin networks during exocytosis. Furthermore, Tmod3 is required for development, regulating F-actin mesh formation during meiosis I of mouse oocytes, erythroblast enucleation in definitive erythropoiesis, and megakaryocyte morphogenesis in the mouse fetal liver. Thus, Tmod3 plays vital roles in dynamic and stable F-actin networks in cell physiology and development, with further research required to delineate the mechanistic details of Tmod3 regulation in the aforementioned processes, or in other yet to be discovered processes.
Keywords: Actin, Tropomodulin, Tropomyosin, Actin filament, Pointed-end capping, Actin sequestering
Introduction
Diverse cytoskeletal structures required for cellular processes are formed through the dynamic regulation of actin filament (F-actin) assembly and associations. Fine tuning of F-actin assembly/disassembly is achieved through its interaction with specialized actin-binding proteins. There are a host of proteins that nucleate new filaments in linear or branched arrays, stabilize actin by binding along filaments, cap the fast-growing (barbed) end to prevent filament growth, or bind monomeric actin (G-actin) to control available monomer pools (Pollard et al. 2000; Pollard and Cooper 2009). At the slow-growing (pointed) end of F-actin, the tropomodulin (Tmod) family of proteins (~ 40 kDa) cap F-actin to prevent actin monomer association or disassociation (Fowler and Dominguez 2017; Yamashiro et al. 2012). The leiomodins (Lmods) are a group of larger (~ 65–70 kDa) Tmod-related proteins present mainly in striated and smooth muscles. Lmods also bind to the pointed end, but are potent nucleators of new filaments which grow rapidly from their barbed ends, unlike Tmods which inhibit filament growth by capping pointed ends (Fowler and Dominguez 2017). While the actin-related protein 2/3 complex (Arp2/3) can also cap F-actin at the pointed end, Arp2/3 simultaneously binds to the side of another filament, nucleating a new filament to create a branched F-actin network (Mullins et al. 1998). Tmods do not bind along the sides of F-actins and function predominantly as F-actin pointed-end capping proteins.
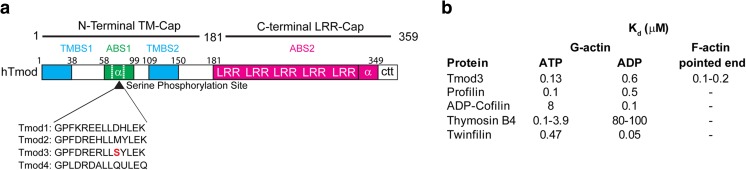
The vertebrate tropomodulin (Tmod) family consists of four isoforms (Tmod1, Tmod2, Tmod3, Tmod4) which share high (~ 80%) amino acid sequence similarity in mammals, with somewhat lower similarity in chickens, frogs, and zebrafish. Tmods are also present in Drosophila melanogaster (tmod), Caenorhabditis elegans (unc-94), and other invertebrates (Yamashiro et al. 2012). Tmods contain two major structural and functional domains that work together to cap F-actin pointed ends (Fig. 1a) (Colpan et al. 2013; Fowler and Dominguez 2017; Yamashiro et al. 2012). The N-terminal domain is mostly unstructured and contains an actin-binding site (ABS1) flanked by two tropomyosin (Tpm)-binding sites (TMBS1 and TMBS2) required for high-affinity Tmod capping of Tpm-coated F-actin pointed ends. The ABS1 spans residues 58–99 and contains a short α-helix (residues 65–75), whereas TMBS1 (residues 1–38) and TMBS2 (residues 109–150) are intrinsically disordered, with short portions adopting an α-helical conformation upon binding to Tpm (residue numbering from Tmod1) (Colpan et al. 2013; Fowler and Dominguez 2017). A second actin-binding site (ABS2) is contributed by the globular C-terminal domain, consisting of five leucine-rich repeats followed by a nonhomologous α-helix and a C-terminal tail, which allows for F-actin pointed-end capping. Tmod caps pure F-actin pointed ends at submicromolar concentrations (Kd ~ 0.1–0.2 μm), but caps Tpm-coated F-actin pointed ends at concentrations at least an order of magnitude lower (Kd < 20 nm) (Fowler and Dominguez 2017; Weber et al. 1994; Yamashiro et al. 2012). All Tmods have the capacity to bind Tpm and are high-affinity caps for Tpm-coated F-actin pointed ends, but Tmod isoforms have preferential Tpm binding partners that influence their capping activity (Colpan et al. 2016; Gokhin and Fowler 2011a; Lewis et al. 2014; Lim et al. 2015; Uversky et al. 2011; Yamashiro et al. 2012; Yamashiro et al. 2014).
Fig. 1.
Tmod structural domains and actin dissociation constants. a Structure of Tmod highlighting important regions for actin-binding and capping as well as Tpm binding (residue numbering from human Tmod1). Tmod3 is unique as it contains a phosphorylation site at Ser71, which is a substrate for Akt2 phosphorylation. The structure schematic is redrawn based on both Yamashiro et al. 2012 (N-terminal CAP and C-terminal CAP terminology) and from Fowler and Dominguez 2007 (updated domain assignment and residue numbering). b Table showing the dissociation constants (Kd) of Tmod3 and select actin-binding proteins for G-actin (Carlier et al. 1997; Fischer et al. 2006; Hertzog et al. 2004; Ojala et al. 2002; Pollard et al. 2000; Yu et al. 1993) and of Tmod3 for pure F-actin pointed ends (Fischer et al. 2003; Fischer et al. 2006)
Whereas Tmod3 is ubiquitously expressed in mammalian cells and tissues, the expression patterns of Tmod1, Tmod2, and Tmod4 are tissue-specific (Cox and Zoghbi 2000; Yamashiro et al. 2012). Tmod1 is expressed in terminally differentiated, post-mitotic cells including striated muscle, red blood cells, lens fiber cells, and neurons. Tmod2 and Tmod4 are predominantly expressed in neurons and skeletal muscle, respectively. A great deal of our understanding of Tmods is based on seminal findings for Tmod1 in the red blood cell membrane skeleton (Fowler 1987) and striated muscle sarcomeres (Fowler et al. 1993). In red blood cells, Tmod1 binds to the pointed ends of the short F-actins in the membrane skeleton, and in striated muscle, Tmod1 binds to the pointed ends of the long F-actins (thin filaments) in sarcomeres. In both F-actin networks, Tmod1 capping blocks actin monomer association and dissociation at pointed ends to regulate filament length and preserve F-actin stability (Fowler 1996; Fowler and Dominguez 2017; Gokhin and Fowler 2011c; Gregorio et al. 1995; Moyer et al. 2010). As F-actin is relatively stable in the red blood cell membrane skeleton and in striated muscle sarcomeres, this has led to the general view that Tmods function principally to regulate F-actin length and stability in highly organized and stable actin cytoskeletal structures. Nevertheless, in both red blood cells and in striated muscle thin filaments, Tmod1 capping is dynamic and actin subunits exchange at filament ends, hinting at functions for Tmods in dynamic actin cytoskeleton remodeling (Fowler and Dominguez 2017; Gokhin and Fowler 2016; Littlefield et al. 2001).
In this review, we highlight the function of Tmods in the diverse F-actin networks of non-muscle cells. The developmental expression and cellular functions of Tmod1, Tmod2, and Tmod4 isoforms have been reviewed elsewhere (Fowler and Dominguez 2017; Gokhin and Fowler 2011c; Gokhin and Fowler 2013; Gray et al. 2017; Yamashiro et al. 2012) and will not be discussed in great detail. Rather, here we focus on the role(s) of Tmod3. Apart from being ubiquitously expressed, Tmod3 has unique molecular properties that position it as a multifunctional Tmod with the ability to regulate actin cytoskeletal structures across a broad swath of biological contexts.
Tmod3 is a unique Tmod
Despite the similarities in sequence and structure between the Tmod isoforms, Tmod3 has several key differences. In addition to capping F-actin pointed ends, Tmod3 can also bind globular (G-) actin in vitro and in cells (Fischer et al. 2006; Yamashiro et al. 2010). In vitro, kinetic modeling and steady-state polymerization assays indicate that Tmod3 sequesters monomeric G-actin at submicromolar concentrations, similar to Tmod3 capping of F-actin pointed ends. The ability to sequester G-actin is exclusive for Tmod3 as Tmod2 has only a small effect on steady-state monomer levels, and Tmod1 and Tmod4 have no effect. Binding assays with truncated and mutant Tmod3 fragments and cross-linked peptides identified by EDC/sulfo-NHS and mass spectrometry indicate that the G-actin-binding site of Tmod3 includes ABS1, which interacts with actin subdomains 2 and 4 (Fig. 1a) (Yamashiro et al. 2010). Interestingly, the crystal structure of a Tmod1-gelsolin segment 1-actin complex shows that a Tmod1 ABS1 peptide extends across actin subdomains 4, 2, and 1 (from amino- to carboxy terminal) (Rao et al. 2014), but the structural basis for G-actin binding (Tmod3) versus F-actin capping (Tmod1 and Tmod3) is unclear. Tmod3 has a preference for binding ATP-G-actin (Kd ~ 0.1 μM) over ADP-G-actin (Kd ~ 0.6 μM) (Fischer et al. 2006), and the affinity of Tmod3 for ATP-G-actin is comparable to other G-actin sequestering molecules, such as profilin or thymosin β4 (Tβ4) (Fig. 1b). Some experiments suggest that Tmod3 may interact better with non-muscle β- or γ-G-actin than with α-skeletal muscle G-actin, but this has not been explored fully (Gokhin and Fowler 2011a; Yamashiro et al. 2014).
Tmod3 is also unique among Tmods in that it is a substrate for Akt2 phosphorylation (Lim et al. 2015) placing it downstream of PI3K mediated activation of PIP2 to PIP3 by insulin receptor signaling (Gonzalez and McGraw 2009). Sequence analysis demonstrates a unique Akt2 consensus motif at Ser71 within the ABS1 α-helix of Tmod3 not present in other Tmods (Fig. 1a). Chemical cross-linking of recombinant phospho-mimetic (S71D) or phospho-defective (S71A) Tmod3 to actin followed by SDS-PAGE reveals that formation of Tmod3-actin complexes is reduced for the S71D mutant, suggesting that Akt2 phosphorylation at Ser71 inhibits Tmod3-actin interactions. However, since this assay detects Tmod3 cross-linking to actin and oligomeric complexes, it remains unclear whether Akt2 phosphorylation modulates Tmod3 G-actin-binding and/or Tmod3 F-actin-capping activity.
In tissues where several Tmods are present, Tmod3 preferentially regulates some F-actin networks via specific binding partners. In skeletal muscle, Tmod3 stabilizes the sarcoplasmic reticulum (SR) and maintains myofibril alignment by capping γ-actin filaments and binding to small ankyrin 1.5 (sAnk1.5), a SR-associated membrane protein at the M line in skeletal muscle (Gokhin and Fowler 2011a). This is unique to Tmod3, as Tmod1, which is also present in skeletal muscle, does not coimmunoprecipitate with sAnk1.5 or γ-actin filaments, or colocalize with sAnk1.5 at the M line. In addition, unlike Tmod1, Tmod3 is present in a complex with Tpm3.1 and Tpm4 that are associated with the γ-actin filaments. Thus, Tmod3 participates in capping γ-actin filament networks in the SR, while Tmod1 caps the α-actin filaments in muscle sarcomeres (Gokhin and Fowler 2011b).
Tmod3 is ubiquitously expressed and has the ability to cap Tpm-F-actin and pure F-actin pointed ends and can selectively regulate specialized Tpm-F-actin networks. It can also bind and sequester actin monomers. Furthermore, binding of Tmod3 to actin is modulated by Akt2 phosphorylation. This suggests that Tmod3 has the potential to play multifunctional roles in regulating dynamic actin-based processes. Indeed, accumulating evidence demonstrates Tmod3 is associated with diverse actin cytoskeletal and contractile structures, implicating a broad role for Tmod3 in regulating many cellular processes.
Actin cytoskeletal structures and cellular processes that depend upon Tmod3
Tmod3 is associated with contractile stress fibers
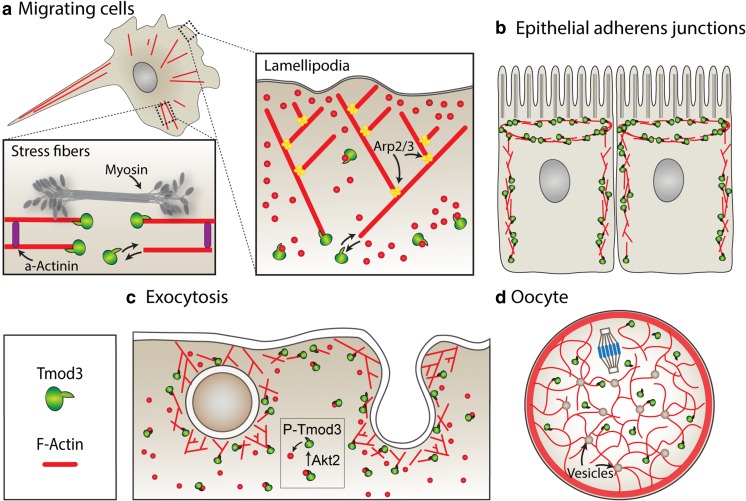
In nonmuscle cells adherent on stiff substrates, F-actin and nonmuscle myosin II (NMII) assemble into large contractile bundles termed stress fibers, in which NMII bipolar filaments alternate with α-actinin-cross-linked F-actin in periodic arrays of sarcomere-like contractile units (Naumanen et al. 2008; Pellegrin and Mellor 2007). In fibroblasts, super-resolution imaging of GFP-Tmod3 using structured illumination microscopy (SIM) reveals narrow stripes of GFP-Tmod3 flanked by closely spaced doublets of NMII motor domains at ends of bipolar filaments (Hu et al. 2017) (Fig. 2a). The GFP-Tmod3 stripes alternate with broader α-actinin bands that are coincident with F-actin-free barbed ends labeled by incorporation of fluorescent-tagged G-actin. This suggests that Tmod3 caps the F-actin pointed ends located in the middle of the sarcomere-like units, similar to Tmod1 in striated muscle sarcomeres (Fowler and Dominguez 2017; Gokhin and Fowler 2011c; Gokhin and Fowler 2013). A function for Tmod3 in contractile NMII-F-actin bundles is suggested by the phenotype of Tmod3−/− mouse fetal liver megakaryocytes spreading on collagen I, where the robust F-actin bundles associated with periodic NMIIA stripes fail to assemble normally (Sui et al. 2015).
Fig. 2.
Tmod3 regulates diverse actin cytoskeletal structures. The regulation of these cytoskeletal structures requires multiple actin-binding proteins; however, only the minimal number of binding proteins are drawn for simplicity and to highlight the role of Tmod3. a Tmod3 is present in stress fibers where it caps F-actin pointed ends within the NMII-F-actin sarcomere-like units (pictured here within a migrating cell). Tmod3 is also present in lamellipodia where it may cap F-actin pointed ends to stabilize filaments, and/or sequester G-actin. By stabilizing the pointed end and/or sequestering actin monomers, Tmod3 acts as a negative regulator of lamellipodia actin assembly. b In polarized epithelial cells, Tmod3 is associated with F-actin at lateral membranes and in the apical domain where it may cap F-actin pointed ends in the circumferential NMII-F-actin belt. The stabilization of F-actin by Tmod3 is critical to maintaining the cellular tension required for cell shape as well as for withstanding mechanical stresses associated with morphogenesis of epithelial sheets. c Tmod3 is associated with the F-actin cortex in adipocytes where it facilitates vesicle docking and fusion during insulin-mediated GLUT4 exocytosis. Insulin-mediated signaling to Akt2 leads to phosphorylation of Tmod3 at S71, which inhibits Tmod3 binding to actin and releases G-actin for cortical F-actin assembly. d Tmod3 is associated with the cytoplasmic F-actin mesh in mouse oocytes. During oocyte maturation, Tmod3 is required for assembly of the F-actin mesh and is critical for proper spindle migration and asymmetric division. These schematics are not drawn to scale
A role for Tmod3 in contractile force generation is also suggested by observations from striated muscle. In Tmod1−/− mouse skeletal muscles, Tmod3 relocalizes from the sarcoplasmic reticulum-associated γ-actin cytoskeleton to cap the thin filament pointed ends in sarcomeres, where it substitutes for Tmod1 in regulating thin filament lengths (Gokhin and Fowler 2011a; Gokhin et al. 2010). However, Tmod1−/− sarcomeres with Tmod3-capped thin filaments (with normal thin filament lengths) produce less force in skinned fiber assays, due to impaired Tpm strand movement and reduced skeletal muscle myosin cross-bridge binding to the thin filaments (Ochala et al. 2014). This suggests the unexpected idea that Tmod capping of Tpm-F-actin pointed ends may either permit (Tmod1) or restrict (Tmod3) the azimuthal movement of striated muscle Tpm strands necessary for thin filament activation and actomyosin contraction. One may speculate that Tmod3 capping of Tpm-F-actin may contribute to activation or inhibition of NMIIs by recruiting different Tpm isoforms (Barua et al. 2014; Clayton et al. 2015; Gateva et al. 2017; Pathan-Chhatbar et al. 2018).
Tmod3 associates with F-actin at adherens junctions and contributes to epithelial cell morphogenesis
Adherens junctions form the interface between epithelial cells and function to coordinate cell-cell interactions and integrate the NMII-F-actin networks across neighboring cells (Charras and Yap 2018; Lecuit and Yap 2015; Pilot and Lecuit 2005). These E-cadherin-mediated cell-cell junctions are critical to coordinate cellular contraction as well as generate tissue-level tension and patterning to drive developmental morphogenic processes. In mature junctions, F-actin is stabilized by Tpm (Caldwell et al. 2014; McKeown et al. 2014; Weber et al. 2007) and organized with NMII bipolar filaments into a circumferential belt that runs parallel to the plasma membrane, located near the apical portion of polarized epithelial cells (Mege and Ishiyama 2017; Zhang et al. 2005). In primary epithelial cells in tissues, the circumferential belt of NMII and F-actin is organized into striking periodic arrays of sarcomere-like contractile units, similar to the NMII-F-actin stress fibers in fibroblasts (Ebrahim et al. 2013). While these structures have not been studied in a large number of epithelial cell types or in cultured cell lines, they were characterized in organ of Corti epithelial cells and have also been observed in intestinal enterocytes and stomach epithelial cells in tissues.
Tmod3 is present in polarized epithelial cells, where it associates with F-actin at the lateral membranes encompassing the adherens junctions, and with the F-actin-rich terminal web underlying the apical membrane (Cox-Paulson et al. 2014; Cox-Paulson et al. 2012; Guo et al. 2014; Weber et al. 2007) (Fig. 2b). In cultured human intestinal epithelial cells (Caco2), knockdown of Tmod3 results in dissociation of Tpm and disassembly of F-actin from lateral membranes, along with disruption of αII-spectrin organization. The disassembly of Tpm, F-actin, and αII-spectrin is accompanied by a decrease in epithelial cell height and concomitant expansion of cell apical and basal surface area, but no effects on apical-basal cell polarity. Tmod3 may stabilize the mechanically resilient αII-spectrin-F-actin network (membrane skeleton) that is linked to E-cadherin via ankyrinG and controls lateral membrane biogenesis and cell height (Bennett and Healy 2009). This would be analogous to the function of Tmod1 in stabilizing the spectrin-F-actin membrane skeleton in red blood cells (Moyer et al. 2010).
In C. elegans intestinal epithelial cells, loss of UNC-94/Tmod function results in reduced F-actin in the terminal web and a flattened intestinal lumen (Cox-Paulson et al. 2014). Strikingly, the defects due to the loss of UNC-94 function are rescued when NMII-F-actin contractility is increased by RNAi depletion of the myosin phosphatase regulatory subunit, mel-11. While the nanoscale level organization of Tmod3 along F-actin at adherens junctions or in the terminal web is unknown, it is attractive to consider that Tmod3 caps F-actin pointed ends in sarcomere-like contractile structures of the circumferential belts at adherens junction, similar to Tmod3 in stress fibers of fibroblasts (Fig. 2a). We would hypothesize that the absence of Tmod3 would lead to a disorganization or disassembly of these sarcomere-like structures, leading to cellular relaxation. This could explain the reduction in cell height and increase in cell area with Tmod3 knockdown in epithelial cells (Weber et al. 2007) and the flattening of the intestinal lumen in C. elegans (Cox-Paulson et al. 2014). A role for Tmod3 in circumferential NMII-F-actin belts also does not exclude a role for Tmod3 in the spectrin-F-actin membrane skeleton, with both of these F-actin structures contributing to epithelial cell morphology.
Further supporting a role for Tmod in maintaining tissue-level epithelial cell tension at adherens junctions, C. elegans UNC-94/Tmod was identified as a synthetic lethal enhancer of a weak loss-of-function allele of hmp-1, an α-catenin homolog (Cox-Paulson et al. 2012). HMP-1/α-catenin is a component of the cadherin-catenin complex that protects epidermal adherens junctions from contractile stresses imposed by circumferential NMII-F-actin bundles during embryonic morphogenesis (Vuong-Brender et al. 2018). UNC-94 is localized to the adherens junction in proximity to, but not directly interacting with, HMP-1 (Cox-Paulson et al. 2012; Stevenson et al. 2007). High-speed imaging of GFP-JAC1/p120 catenin-labeled adherens junctions during embryonic elongation in epidermal morphogenesis demonstrated that, as compared to loss of function for HMP-1 alone, the loss of function for both HMP-1 and UNC-94 resulted in a much greater extent of junctional disruption. Therefore, UNC-94/Tmod is likely recruited to F-actin at adherens junctions to withstand contractile mechanical stresses associated with morphogenesis. A contractile NMII-F-actin network, which requires Tmod3, may provide the necessary intrinsic cellular tension that would be resistant to pulling forces.
Tmod3 associates with lamellipodia F-actin to regulate cell migration
Actin assembly/disassembly dynamics are critical for the extension of the plasma membrane in the lamellipodia at the leading edge of migrating cells (Abercrombie et al. 1970; Carlier and Shekhar 2017; Pollard and Borisy 2003; Pollard and Cooper 2009; Ridley 2011). In brief, Arp2/3 nucleates new actin filaments from the sides of pre-existing filaments to form a branched dendritic network in lamellipodia. New ATP- and ADP-Pi-rich filaments grow rapidly at their free barbed ends towards the membrane, until they are capped by heterodimeric capping protein, while older ADP filaments are severed by actin depolymerizing factor (ADF)/cofilin to promote actin disassembly and recycle monomers for new network growth driving lamellipodia protrusion.
While barbed end capping is a positive regulator of actin assembly dynamics in lamellipodia protrusion and cell migration (Carlier and Shekhar 2017; Pollard and Borisy 2003), Tmod3 is a negative regulator of lamellipodia actin assembly and cell migration (Fischer et al. 2003). In cultured human microvascular endothelial cells (HMEC-1), Tmod3 is localized to the F-actin-rich lamellipodia of migrating cells (Fig. 2a). siRNA knockdown of Tmod3 increases rates of cell migration, while overexpression of GFP-Tmod3 reduces cell migration rates and leads to loss of cell polarity in random migration assays (Fischer et al. 2003). Tmod3 levels are inversely correlated with free pointed ends and with levels of F-actin, free barbed ends and Arp2/3 levels in lamellipodia. This suggests that increased pointed end capping by Tmod3 and inhibition of F-actin depolymerization could prevent network turnover and reduce the pool of G-actin available for assembly onto barbed ends, thereby decreasing the lamellipodia F-actin network (Fischer and Fowler 2003). Conversely, Tmod3 depletion could reduce capping and enhance F-actin pointed end disassembly, providing more G-actin for network assembly and increasing the lamellipodia F-actin network.
Tmod3 capping of Tpm-F-actin pointed ends may also prevent migration by enhancing the binding of Tpm along F-actin, independently stabilizing F-actin and reducing network turnover (Fischer and Fowler 2003; Ono 2010; Yamashiro et al. 2012). In general, Tpms are also thought to be negative regulators of lamellipodia extension (Blanchoin et al. 2001; DesMarais et al. 2002; Gupton et al. 2005). Microinjection of rabbit skeletal muscle α-Tpm (Tpm1.1) in motile cells prevents lamellipodia formation by preventing ADF/cofilin severing as well as Arp2/3 branching (Gupton et al. 2005). One particular Tpm isoform, Tpm2.1, is absent from the leading edge of lamellipodia in mouse embryonic fibroblasts (Brayford et al. 2016) and is restricted to more stable populations of F-actin in the lamella, behind the lamellipodia. Due to the roles Tmod3 and Tpm2.1 play in reducing the lamellipodia F-actin network, it may be presumed that Tmod3 reinforces the interaction of Tpm2.1 with F-actin; however, this remains to be tested in cells. Of note, not all Tpm isoforms are negatively correlated with motility and lamellipodia generation. Tpm 1.8/1.9 is present at the leading edge of lamellipodia and enables stabilization and persistence of lamellipodia protrusions (Brayford et al. 2016; Hillberg et al. 2006). Thus, the network arrangement of F-actin may also depend on the spatial distribution of specific Tpm isoforms (Gateva et al. 2017).
Another way that Tmod3 could interfere with F-actin assembly in lamellipodia would be by Tmod3 sequestration of G-actin (Fig. 2a), reducing the available pool of monomers (Fischer and Fowler 2003). Tmod3 could also reduce available monomers by competing for binding with thymosin β4, as shown in vitro (Fischer et al. 2006), or with profilin, two other G-actin sequestering proteins essential for dendritic actin network assembly and turnover at the leading edge of lamellipodia (Lee et al. 2013; Vitriol et al. 2015). Indeed, the endogenous concentration of Tmod3 in endothelial cells (~ 0.5 μM) (Fischer et al. 2003) is sufficient for Tmod3 to bind G-actin in cells (Kd ~ 0.1 μM for ATP-actin) (Fischer et al. 2006). Diffuse Tmod3 staining throughout the cytoplasm also suggests that Tmod3 exists in a soluble pool within motile cells, and fractionation studies indicate ~ 30–40% of endogenous Tmod3 is associated with the TX-100 insoluble cytoskeleton (Fischer et al. 2003). However, Tmod3 has a nanomolar affinity for Tpm-F-actin pointed ends but only a submicromolar affinity for G-actin. This implies that Tmod3 will preferentially cap the Tpm-F-actin pointed ends in cells, reducing the cytosolic levels of Tmod3 available to bind monomers and influence the monomer pool. The number of Tpm-F-actin pointed ends in cells has not been determined. Future studies of actin dynamics and F-actin network structure in lamellipodia of cells expressing Tmod mutants with disabled monomer binding, F-actin capping, or Tpm binding sites (Colpan et al. 2013; Gray et al. 2017; Yamashiro et al. 2012) are required to reveal the mechanistic details of Tmod3 regulation of F-actin network assembly in lamellipodia protrusion and cell migration.
Tmod3 regulates cortical actin remodeling during exocytosis
The actin cytoskeleton plays crucial roles in the distal stages of exocytosis at the plasma membrane by tethering vesicles as well as facilitating membrane docking and fusion (Lopez et al. 2009). During vesicle docking and fusion, various actin-associated molecules promote assembly of cortical F-actin as well as form a contractile NMII-F-actin network surrounding vesicles to enable their docking and subsequent fusion to the plasma membrane. In this process, ADF/cofilin is required to disassemble F-actin to provide G-actin for actin assembly (Miklavc et al. 2015). F-actin is then polymerized by Wasp activation of Arp2/3 nucleation, forming a branched dendritic network, similar to lamellipodia (Tran et al. 2015; Yang et al. 2014). Furthermore, NMII is incorporated into the cortical F-actin surrounding exocytic vesicles, where it cross-links F-actin and generates contractile force (Chiu et al. 2010; Chung le et al. 2010; Miklavc et al. 2012).
A role for Tmod3 in exocytosis has been elucidated in insulin-stimulated GLUT4 insertion at the plasma membrane in 3T3-L1 adipocytes (Lim et al. 2015) (Fig. 2c). In this system, insulin stimulates Akt2-dependent phosphorylation of Tmod3 at Ser71. shRNA-mediated knockdown of Tmod3 or expression of a Ser71 phospho-defective Tmod3 (Tmod3-S71D) interferes with cortical F-actin enrichment and prevents GLUT4 insertion at the plasma membrane, reducing glucose uptake. This provides the first evidence that signaling pathways modulate Tmod3 function to alter actin assembly and regulate a cellular function. However, the exact molecular role(s) of Tmod3 during actin remodeling in exocytosis remain unclear. It was suggested that non-phosphorylated Tmod3 sequesters G-actin, based on diffuse cytoplasmic staining for Tmod3 in untreated cells, and the ability of Ser71 phosphorylation to inhibit association of Tmod3 with actin in vitro. In this case, Akt2-dependent Ser71 phosphorylation would function to release G-actin from Tmod3 to increase the monomer pool and promote F-actin assembly (Fig. 2d). While insulin stimulation does lead to an increase in Tmod3 association with cortical F-actin, it is unclear if phosphorylation promotes this association (Lim et al. 2015). In addition to Tmod3, Tpm3.1 is assembled along cortical F-actin following insulin stimulation (Kee et al. 2015). Binding of Tmod3 to Tpm3.1 is important for GLUT4 exocytosis, as cells overexpressing a Tmod3 mutant unable to bind Tpm3.1, with disabled TMBS1 and TMBS2, have reduced GLUT4 insertion to the plasma membrane (Lim et al. 2015). This mechanism may operate in vivo, based on studies in white adipose tissue and skeletal muscle of Tpm3.1-overexpressing or Tpm3.1-null mice, which demonstrate alterations in F-actin corresponding to increased or decreased insulin-stimulated glucose uptake, respectively (Kee et al. 2015). Tmod3 capping of cortical F-actin pointed ends could promote Tpm3.1 binding, or binding of Tpm3.1 along F-actin could facilitate Tmod3 capping (Yamashiro et al. 2012). Together, Tmod3 and Tpm3.1 likely cooperate to preserve newly assembled cortical F-actin to facilitate vesicle docking and fusion. Tpm3.1 also recruits NMIIA which cross-links F-actin and provides contractile forces that may aid in vesicle fusion (Chung le et al. 2010; Kee et al. 2015). Thus, Tmod3 may assist in promoting contractile NMII-F-actin networks to facilitate exocytic vesicle docking and fusion, similar to proposed roles of Tmod3 in stabilizing the contractile NMII-F-actin networks in stress fibers and adherens junctions as discussed above.
Tmod3 associates with the cytoplasmic F-actin mesh required for asymmetric division of mouse oocytes
Asymmetric division is critical in mammalian development. To maintain the maternal components in oocyte maturation, asymmetric divisions occur during meioses I and II to form totipotent large haploid oocytes and smaller polar bodies. In mouse oocyte maturation, formation of a cytoplasmic F-actin mesh coordinates with cortical F-actin rearrangements to control central positioning of the nucleus in the prophase oocyte and promote spindle migration to the cortex that occurs in meiosis I (Almonacid et al. 2014; Almonacid et al. 2018; Namgoong and Kim 2016). Failure to form the F-actin mesh impairs nucleus centering and spindle migration, leading to defects in asymmetric division and polar body formation. Several crucial actin regulators are required for formation of the F-actin mesh and spindle migration in mouse oocytes, including spire and formin actin nucleators, capping protein, Tpm3, cofilin, and filamin. Arp2/3 nucleation of actin assembly also drives cytoplasmic streaming of a dynamic actin network that assists spindle migration and maintain the asymmetric position of the spindle at the cortex after the first meiosis (Yi et al. 2011).
Tmod3 capping of F-actin pointed ends is also critical for assembly of the cytoplasmic F-actin mesh in mouse oocytes (Jo et al. 2016) (Fig. 2d). Tmod3 protein is present throughout oocyte maturation and is in a punctate pattern in the cytoplasm. siRNA knockdown of Tmod3 results in a reduced cytoplasmic F-actin mesh, accompanied by impaired chromatin migration and aberrant asymmetric division with formation of an abnormally large polar body. Since Tmod3 depletion in oocytes decreases the F-actin mesh, this suggests that Tmod3 does not function solely by sequestering G-actin, as then Tmod3 depletion would have been expected to lead to an increase in the F-actin mesh, which is not observed. Instead, Tmod3-capping of Tpm-coated F-actin pointed ends is likely to be important, since the expression of a truncated Tmod3 lacking the C-terminal F-actin capping domain (which binds to Tpm but not to pointed ends) has a dominant negative effect, reducing the F-actin mesh. In addition, knockdown of both Tmod3 and Tpm3 leads to a greater reduction in F-actin mesh formation than does either alone. By contrast, overexpression of full-length GFP-Tmod3 enhances F-actin mesh formation and interferes with asymmetric cell division, indicating that excessive F-actin mesh formation may impede spindle migration. Tmod3 capping of Tpm3-F-actin pointed ends likely stabilizes the F-actin mesh by reducing pointed-end depolymerization and protecting filaments from cofilin severing, which would reduce the available pool of monomers for dynamic mesh turnover, similar to Tmod3 in lamellipodia, as discussed above. Future studies will be required to establish how Tmod3 activity is integrated with the other actin regulators to coordinate the complex events of spindle migration and asymmetric division, including actin-dependent cytoplasmic streaming, vesicular movements, and cortical F-actin rearrangements (Almonacid et al. 2014; Almonacid et al. 2018; Namgoong and Kim 2016).
Tmod3 regulation of F-actin in embryonic development
Tmod3 is ubiquitously expressed and thus has essential functions in many developmental processes, not limited to those mentioned above. For instance, Tmod3 null embryos have severe defects in fetal liver erythropoiesis resulting in anemia and embryonic lethality at E14.5–16.5 (Sui et al. 2014). The absence of Tmod3 profoundly affects definitive erythropoiesis at multiple levels including reduced progenitors, inability to form erythroblast-macrophage islands, and impaired erythroblast cell survival, cell cycle progression, and enucleation (Sui et al. 2014). Tmod3-null erythroblasts demonstrate aberrant F-actin organization that may explain their defects in enucleation, which is an F-actin-dependent cellular process likened to asymmetric division (Ji et al. 2011; Li 2013; Nowak et al. 2017). Additionally, Tmod3 null embryos have macrothrombocytopenia (fewer and abnormally large platelets), due to defects in megakaryocyte morphogenesis and proplatelet formation. Tmod3 null megakaryocytes differentiate normally but have altered demarcation membrane systems with uneven cytoplasmic organelle distribution, producing abnormally large proplatelet buds with variable organelle content and aberrant Tpm4 and F-actin distributions (Sui et al. 2015). It was proposed that Tmod3 capping of Tpm4-F-actin may stabilize the α2-spectrin membrane skeleton that is known to control the formation of the demarcation membrane system, required for normal proplatelet biogenesis (Patel-Hett et al. 2011).
Observation of Tmod3 null mouse embryos at midgestation also shows anomalies in other tissue types, from bone to brain (R.B. Nowak and V.M. Fowler, unpublished), suggesting that Tmod3 is involved in morphogenesis of other specialized cells and tissues during embryonic development. However, whether defects in biological processes within these other tissues are directly due to deletion of Tmod3, or instead to secondary systemic effects, has not been investigated (i.e., with tissue-specific knock-outs). It is also possible that some of these developmental defects may originate from earlier defects in asymmetric division during oocyte maturation, discussed above. Nevertheless, the growing evidence from exploratory expression/biomarker studies demonstrates likely Tmod3 involvement in other specialized cellular phenomena (Lopez-Ubeda et al. 2015; Lu et al. 2017; Paez et al. 2016; Qiu et al. 2010) and pathologies (Gajbhiye et al. 2017; Gajbhiye et al. 2012; Paez et al. 2016), providing evidence of Tmod3’s broad and relatively unexplored roles.
Conclusions
While it is evident that Tmod3 is associated with many different types of actin cytoskeletal networks in cells and plays vital roles in numerous dynamic biological processes, many questions remain open. For example, it is unclear to what extent Tmod3’s functions in complex biological processes of erythropoiesis or megakaryocyte proplatelet formation might be due to Tmod3 regulation of specific types of F-actin networks, such as lamellipodia dendritic networks, cortical F-actin, contractile NMII-F-actin bundles, or membrane-associated spectrin-F-actin networks. Indeed, Tmod3 localization and F-actin network composition and organization in these and other specialized cells during embryonic development are not well-defined. It is possible that specific Tmod3-binding partners may selectively target Tmod3 to different F-actin network structures in these specialized cell types, analogous to sAnk1.5 in the SR of skeletal muscle. The molecular mechanisms of Tmod3 function in these F-actin networks and cellular processes also require further examination. In comparison to the other, tissue-specific Tmod isoforms, Tmod3 is unique in that it can bind and sequester G-actin. However, the importance of Tmod3 G-actin binding in vivo within cells and/or tissues has not been definitively demonstrated. Additionally, we suggested that a competition between Tmod3 and other actin sequestering proteins could be important; reconstituted F-actin assembly systems could help investigate this possibility. Tmod3 function is linked to Akt2 phosphorylation in adipocytes, but how Akt2 phosphorylation or other signaling pathways may regulate Tmod3 function in other cell types, and whether they affect G-actin sequestering or F-actin capping, is unclear. New specialized tools (i.e., conditional knock-outs/knock-ins/mutant mice) and improved methodology (i.e., Tmod3 visualization using Tmod3-GFP or Tmod3-specific antibodies in cell/tissues with super-resolution imaging) will provide opportunities to achieve a greater understanding of Tmod3 regulation and dynamics of F-actin networks in diverse biological processes.
Acknowledgements
We are grateful to Roberta B. Nowak for the preparation of Fig. 2.
Funding information
This work was funded by the National Institutes of Health (NIH) Grants R01 HL083464 and R01 EY017724 to V.M.F. J.P. was supported by a fellowship from the Natural Science and Engineering Research Council of Canada.
Conflict of interest
Justin Parreno declares that he has no conflict of interest. Velia M. Fowler declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. II. “RRuffling”. Exp Cell Res. 1970;60:437–444. doi: 10.1016/0014-4827(70)90537-9. [DOI] [PubMed] [Google Scholar]
- Almonacid M, Terret ME, Verlhac MH. Actin-based spindle positioning: new insights from female gametes. J Cell Sci. 2014;127:477–483. doi: 10.1242/jcs.142711. [DOI] [PubMed] [Google Scholar]
- Almonacid M, Terret ME, Verlhac MH (2018) Control of nucleus positioning in mouse oocytes. Semin Cell Dev Biol 82:34–40 [DOI] [PubMed]
- Barua B, Nagy A, Sellers JR, Hitchcock-DeGregori SE. Regulation of nonmuscle myosin II by tropomyosin. Biochemistry. 2014;53:4015–4024. doi: 10.1021/bi500162z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V, Healy J. Membrane domains based on ankyrin and spectrin associated with cell-cell interactions. Cold Spring Harb Perspect Biol. 2009;1:a003012. doi: 10.1101/cshperspect.a003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchoin L, Pollard TD, Hitchcock-DeGregori SE. Inhibition of the Arp2/3 complex-nucleated actin polymerization and branch formation by tropomyosin. Curr Biol. 2001;11:1300–1304. doi: 10.1016/S0960-9822(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Brayford S, Bryce NS, Schevzov G, Haynes EM, Bear JE, Hardeman EC, Gunning PW. Tropomyosin promotes lamellipodial persistence by collaborating with Arp2/3 at the leading edge. Curr Biol. 2016;26:1312–1318. doi: 10.1016/j.cub.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BJ, et al. Tropomyosin isoforms support actomyosin biogenesis to generate contractile tension at the epithelial zonula adherens. Cytoskeleton (Hoboken) 2014;71:663–676. doi: 10.1002/cm.21202. [DOI] [PubMed] [Google Scholar]
- Carlier MF, et al. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Shekhar S. Global treadmilling coordinates actin turnover and controls the size of actin networks. Nat Rev Mol Cell Biol. 2017;18:389–401. doi: 10.1038/nrm.2016.172. [DOI] [PubMed] [Google Scholar]
- Charras G, Yap AS. Tensile forces and mechanotransduction at cell-cell junctions. Curr Biol. 2018;28:R445–R457. doi: 10.1016/j.cub.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Chiu TT, Patel N, Shaw AE, Bamburg JR, Klip A. Arp2/3- and cofilin-coordinated actin dynamics is required for insulin-mediated GLUT4 translocation to the surface of muscle cells. Mol Biol Cell. 2010;21:3529–3539. doi: 10.1091/mbc.E10-04-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung le TK, et al. Myosin IIA participates in docking of Glut4 storage vesicles with the plasma membrane in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2010;391:995–999. doi: 10.1016/j.bbrc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Clayton JE, Pollard LW, Murray GG, Lord M. Myosin motor isoforms direct specification of actomyosin function by tropomyosins. Cytoskeleton (Hoboken) 2015;72:131–145. doi: 10.1002/cm.21213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpan M, Moroz NA, Gray KT, Cooper DA, Diaz CA, Kostyukova AS. Tropomyosin-binding properties modulate competition between tropomodulin isoforms. Arch Biochem Biophys. 2016;600:23–32. doi: 10.1016/j.abb.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpan M, Moroz NA, Kostyukova AS. Tropomodulins and tropomyosins: working as a team. J Muscle Res Cell Motil. 2013;34:247–260. doi: 10.1007/s10974-013-9349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Paulson E, et al. The minus-end actin capping protein, UNC-94/tropomodulin, regulates development of the Caenorhabditis elegans intestine. Dev Dyn. 2014;243:753–764. doi: 10.1002/dvdy.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox-Paulson EA, et al. Tropomodulin protects alpha-catenin-dependent junctional-actin networks under stress during epithelial morphogenesis. Curr Biol. 2012;22:1500–1505. doi: 10.1016/j.cub.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PR, Zoghbi HY. Sequencing, expression analysis, and mapping of three unique human tropomodulin genes and their mouse orthologs. Genomics. 2000;63:97–107. doi: 10.1006/geno.1999.6061. [DOI] [PubMed] [Google Scholar]
- DesMarais V, Ichetovkin I, Condeelis J, Hitchcock-DeGregori SE. Spatial regulation of actin dynamics: a tropomyosin-free, actin-rich compartment at the leading edge. J Cell Sci. 2002;115:4649–4660. doi: 10.1242/jcs.00147. [DOI] [PubMed] [Google Scholar]
- Ebrahim S, et al. NMII forms a contractile transcellular sarcomeric network to regulate apical cell junctions and tissue geometry. Curr Biol. 2013;23:731–736. doi: 10.1016/j.cub.2013.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RS, Fowler VM. Tropomodulins: life at the slow end. Trends Cell Biol. 2003;13:593–601. doi: 10.1016/j.tcb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Fischer RS, Fritz-Six KL, Fowler VM. Pointed-end capping by tropomodulin3 negatively regulates endothelial cell motility. J Cell Biol. 2003;161:371–380. doi: 10.1083/jcb.200209057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RS, Yarmola EG, Weber KL, Speicher KD, Speicher DW, Bubb MR, Fowler VM. Tropomodulin 3 binds to actin monomers. J Biol Chem. 2006;281:36454–36465. doi: 10.1074/jbc.M606315200. [DOI] [PubMed] [Google Scholar]
- Fowler VM. Identification and purification of a novel Mr 43,000 tropomyosin-binding protein from human erythrocyte membranes. J Biol Chem. 1987;262:12792–12800. [PubMed] [Google Scholar]
- Fowler VM. Regulation of actin filament length in erythrocytes and striated muscle. Curr Opin Cell Biol. 1996;8:86–96. doi: 10.1016/S0955-0674(96)80052-4. [DOI] [PubMed] [Google Scholar]
- Fowler VM, Dominguez R. Tropomodulins and leiomodins: actin pointed end caps and Nucleators in muscles. Biophys J. 2017;112:1742–1760. doi: 10.1016/j.bpj.2017.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler VM, Sussmann MA, Miller PG, Flucher BE, Daniels MP. Tropomodulin is associated with the free (pointed) ends of the thin filaments in rat skeletal muscle. J Cell Biol. 1993;120:411–420. doi: 10.1083/jcb.120.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajbhiye R, et al. Panel of autoimmune markers for noninvasive diagnosis of minimal-mild endometriosis. Reprod Sci. 2017;24:413–420. doi: 10.1177/1933719116657190. [DOI] [PubMed] [Google Scholar]
- Gajbhiye R, et al. Identification and validation of novel serum markers for early diagnosis of endometriosis. Hum Reprod. 2012;27:408–417. doi: 10.1093/humrep/der410. [DOI] [PubMed] [Google Scholar]
- Gateva G, et al. Tropomyosin isoforms specify functionally distinct actin filament populations in vitro. Curr Biol. 2017;27:705–713. doi: 10.1016/j.cub.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, Fowler VM. Cytoplasmic gamma-actin and tropomodulin isoforms link to the sarcoplasmic reticulum in skeletal muscle fibers. J Cell Biol. 2011;194:105–120. doi: 10.1083/jcb.201011128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, Fowler VM. The sarcoplasmic reticulum: actin and tropomodulin hit the links. Bioarchitecture. 2011;1:175–179. doi: 10.4161/bioa.1.4.17533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, Fowler VM. Tropomodulin capping of actin filaments in striated muscle development and physiology. J Biomed Biotechnol. 2011;2011:103069. doi: 10.1155/2011/103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, Fowler VM. A two-segment model for thin filament architecture in skeletal muscle. Nat Rev Mol Cell Biol. 2013;14:113–119. doi: 10.1038/nrm3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, Fowler VM. Feisty filaments: actin dynamics in the red blood cell membrane skeleton. Curr Opin Hematol. 2016;23:206–214. doi: 10.1097/MOH.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhin DS, et al. Tropomodulin isoforms regulate thin filament pointed-end capping and skeletal muscle physiology. J Cell Biol. 2010;189:95–109. doi: 10.1083/jcb.201001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KT, Kostyukova AS, Fath T. Actin regulation by tropomodulin and tropomyosin in neuronal morphogenesis and function. Mol Cell Neurosci. 2017;84:48–57. doi: 10.1016/j.mcn.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio CC, Weber A, Bondad M, Pennise CR, Fowler VM. Requirement of pointed-end capping by tropomodulin to maintain actin filament length in embryonic chick cardiac myocytes. Nature. 1995;377:83–86. doi: 10.1038/377083a0. [DOI] [PubMed] [Google Scholar]
- Guo Z, Neilson LJ, Zhong H, Murray PS, Zanivan S, Zaidel-Bar R. E-cadherin interactome complexity and robustness resolved by quantitative proteomics. Sci Signal. 2014;7:rs7. doi: 10.1126/scisignal.2005473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, et al. Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J Cell Biol. 2005;168:619–631. doi: 10.1083/jcb.200406063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzog M, et al. The beta-thymosin/WH2 domain; structural basis for the switch from inhibition to promotion of actin assembly. Cell. 2004;117:611–623. doi: 10.1016/S0092-8674(04)00403-9. [DOI] [PubMed] [Google Scholar]
- Hillberg L, Zhao Rathje LS, Nyakern-Meazza M, Helfand B, Goldman RD, Schutt CE, Lindberg U. Tropomyosins are present in lamellipodia of motile cells. Eur J Cell Biol. 2006;85:399–409. doi: 10.1016/j.ejcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Hu S, et al. Erratum: long-range self-organization of cytoskeletal myosin II filament stacks. Nat Cell Biol. 2017;19:258. doi: 10.1038/ncb3479. [DOI] [PubMed] [Google Scholar]
- Ji P, Murata-Hori M, Lodish HF. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol. 2011;21:409–415. doi: 10.1016/j.tcb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YJ, Jang WI, Kim NH, Namgoong S. Tropomodulin-3 is essential in asymmetric division during mouse oocyte maturation. Sci Rep. 2016;6:29204. doi: 10.1038/srep29204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee AJ, et al. An actin filament population defined by the tropomyosin Tpm3.1 regulates glucose uptake. Traffic. 2015;16:691–711. doi: 10.1111/tra.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T, Yap AS. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat Cell Biol. 2015;17:533–539. doi: 10.1038/ncb3136. [DOI] [PubMed] [Google Scholar]
- Lee CW, Vitriol EA, Shim S, Wise AL, Velayutham RP, Zheng JQ. Dynamic localization of G-actin during membrane protrusion in neuronal motility. Curr Biol. 2013;23:1046–1056. doi: 10.1016/j.cub.2013.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RA, Yamashiro S, Gokhin DS, Fowler VM. Functional effects of mutations in the tropomyosin-binding sites of tropomodulin1 and tropomodulin3. Cytoskeleton (Hoboken) 2014;71:395–411. doi: 10.1002/cm.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R. The art of choreographing asymmetric cell division. Dev Cell. 2013;25:439–450. doi: 10.1016/j.devcel.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Lim CY, Bi X, Wu D, Kim JB, Gunning PW, Hong W, Han W. Tropomodulin3 is a novel Akt2 effector regulating insulin-stimulated GLUT4 exocytosis through cortical actin remodeling. Nat Commun. 2015;6:5951. doi: 10.1038/ncomms6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield R, Almenar-Queralt A, Fowler VM. Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol. 2001;3:544–551. doi: 10.1038/35078517. [DOI] [PubMed] [Google Scholar]
- Lopez-Ubeda R, Garcia-Vazquez FA, Romar R, Gadea J, Munoz M, Hunter RH, Coy P. Oviductal transcriptome is modified after insemination during spontaneous ovulation in the sow. PLoS One. 2015;10:e0130128. doi: 10.1371/journal.pone.0130128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JA, et al. Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol Biol Cell. 2009;20:3918–3929. doi: 10.1091/mbc.E09-03-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Ye Y, Bao W, Yang Q, Wang J, Liu Z, Shi S. Genome-wide identification of genes essential for podocyte cytoskeletons based on single-cell RNA sequencing. Kidney Int. 2017;92:1119–1129. doi: 10.1016/j.kint.2017.04.022. [DOI] [PubMed] [Google Scholar]
- McKeown CR, Nowak RB, Gokhin DS, Fowler VM. Tropomyosin is required for cardiac morphogenesis, myofibril assembly, and formation of adherens junctions in the developing mouse embryo. Dev Dyn. 2014;243:800–817. doi: 10.1002/dvdy.24115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege RM, Ishiyama N (2017) Integration of cadherin adhesion and cytoskeleton at Adherens junctions Cold Spring Harb Perspect Biol 9 10.1101/cshperspect.a028738 [DOI] [PMC free article] [PubMed]
- Miklavc P, Ehinger K, Sultan A, Felder T, Paul P, Gottschalk KE, Frick M. Actin depolymerisation and crosslinking join forces with myosin II to contract actin coats on fused secretory vesicles. J Cell Sci. 2015;128:1193–1203. doi: 10.1242/jcs.165571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklavc P, Hecht E, Hobi N, Wittekindt OH, Dietl P, Kranz C, Frick M. Actin coating and compression of fused secretory vesicles are essential for surfactant secretion--a role for Rho, formins and myosin II. J Cell Sci. 2012;125:2765–2774. doi: 10.1242/jcs.105262. [DOI] [PubMed] [Google Scholar]
- Moyer JD, et al. Tropomodulin 1-null mice have a mild spherocytic elliptocytosis with appearance of tropomodulin 3 in red blood cells and disruption of the membrane skeleton. Blood. 2010;116:2590–2599. doi: 10.1182/blood-2010-02-268458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RD, Heuser JA, Pollard TD. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci U S A. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namgoong S, Kim NH. Roles of actin binding proteins in mammalian oocyte maturation and beyond. Cell Cycle. 2016;15:1830–1843. doi: 10.1080/15384101.2016.1181239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J Microsc. 2008;231:446–454. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- Nowak RB, et al. Tropomodulin 1 controls erythroblast enucleation via regulation of F-actin in the enucleosome. Blood. 2017;130:1144–1155. doi: 10.1182/blood-2017-05-787051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochala J, Gokhin DS, Iwamoto H, Fowler VM. Pointed-end capping by tropomodulin modulates actomyosin crossbridge formation in skeletal muscle fibers. FASEB J. 2014;28:408–415. doi: 10.1096/fj.13-239640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala PJ, Paavilainen VO, Vartiainen MK, Tuma R, Weeds AG, Lappalainen P. The two ADF-H domains of twinfilin play functionally distinct roles in interactions with actin monomers. Mol Biol Cell. 2002;13:3811–3821. doi: 10.1091/mbc.e02-03-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. Dynamic regulation of sarcomeric actin filaments in striated muscle. Cytoskeleton (Hoboken) 2010;67:677–692. doi: 10.1002/cm.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez AV, et al. Heme oxygenase-1 in the forefront of a multi-molecular network that governs cell-cell contacts and filopodia-induced zippering in prostate cancer. Cell Death Dis. 2016;7:e2570. doi: 10.1038/cddis.2016.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-Hett S, et al. The spectrin-based membrane skeleton stabilizes mouse megakaryocyte membrane systems and is essential for proplatelet and platelet formation. Blood. 2011;118:1641–1652. doi: 10.1182/blood-2011-01-330688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan-Chhatbar S, Taft MH, Reindl T, Hundt N, Latham SL, Manstein DJ. Three mammalian tropomyosin isoforms have different regulatory effects on nonmuscle myosin-2B and filamentous beta-actin in vitro. J Biol Chem. 2018;293:863–875. doi: 10.1074/jbc.M117.806521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232:685–694. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu Rev Biophys Biomol Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, et al. Gene expression profiles of adipose tissue of high-fat diet-induced obese rats by cDNA microarrays. Mol Biol Rep. 2010;37:3691–3695. doi: 10.1007/s11033-010-0021-6. [DOI] [PubMed] [Google Scholar]
- Rao JN, Madasu Y, Dominguez R. Mechanism of actin filament pointed-end capping by tropomodulin. Science. 2014;345:463–467. doi: 10.1126/science.1256159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Stevenson TO, Mercer KB, Cox EA, Szewczyk NJ, Conley CA, Hardin JD, Benian GM. unc-94 encodes a tropomodulin in Caenorhabditis elegans. J Mol Biol. 2007;374:936–950. doi: 10.1016/j.jmb.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, et al. Tropomodulin3-null mice are embryonic lethal with anemia due to impaired erythroid terminal differentiation in the fetal liver. Blood. 2014;123:758–767. doi: 10.1182/blood-2013-03-492710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, Nowak RB, Sanada C, Halene S, Krause DS, Fowler VM. Regulation of actin polymerization by tropomodulin-3 controls megakaryocyte actin organization and platelet biogenesis. Blood. 2015;126:520–530. doi: 10.1182/blood-2014-09-601484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DT, Masedunskas A, Weigert R, Ten Hagen KG. Arp2/3-mediated F-actin formation controls regulated exocytosis in vivo. Nat Commun. 2015;6:10098. doi: 10.1038/ncomms10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Shah SP, Gritsyna Y, Hitchcock-DeGregori SE, Kostyukova AS. Systematic analysis of tropomodulin/tropomyosin interactions uncovers fine-tuned binding specificity of intrinsically disordered proteins. J Mol Recognit. 2011;24:647–655. doi: 10.1002/jmr.1093. [DOI] [PubMed] [Google Scholar]
- Vitriol EA, McMillen LM, Kapustina M, Gomez SM, Vavylonis D, Zheng JQ. Two functionally distinct sources of actin monomers supply the leading edge of lamellipodia. Cell Rep. 2015;11:433–445. doi: 10.1016/j.celrep.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong-Brender TTK, Boutillon A, Rodriguez D, Lavilley V, Labouesse M. HMP-1/alpha-catenin promotes junctional mechanical integrity during morphogenesis. PLoS One. 2018;13:e0193279. doi: 10.1371/journal.pone.0193279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A, Pennise CR, Babcock GG, Fowler VM. Tropomodulin caps the pointed ends of actin filaments. J Cell Biol. 1994;127:1627–1635. doi: 10.1083/jcb.127.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KL, Fischer RS, Fowler VM. Tmod3 regulates polarized epithelial cell morphology. J Cell Sci. 2007;120:3625–3632. doi: 10.1242/jcs.011445. [DOI] [PubMed] [Google Scholar]
- Yamashiro S, Gokhin DS, Kimura S, Nowak RB, Fowler VM. Tropomodulins: pointed-end capping proteins that regulate actin filament architecture in diverse cell types. Cytoskeleton (Hoboken) 2012;69:337–370. doi: 10.1002/cm.21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Gokhin DS, Sui Z, Bergeron SE, Rubenstein PA, Fowler VM. Differential actin-regulatory activities of Tropomodulin1 and Tropomodulin3 with diverse tropomyosin and actin isoforms. J Biol Chem. 2014;289:11616–11629. doi: 10.1074/jbc.M114.555128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Speicher KD, Speicher DW, Fowler VM. Mammalian tropomodulins nucleate actin polymerization via their actin monomer binding and filament pointed end-capping activities. J Biol Chem. 2010;285:33265–33280. doi: 10.1074/jbc.M110.144873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, et al. Arp2/3 complex regulates adipogenesis by controlling cortical actin remodelling. Biochem J. 2014;464:179–192. doi: 10.1042/BJ20140805. [DOI] [PubMed] [Google Scholar]
- Yi K, Unruh JR, Deng M, Slaughter BD, Rubinstein B, Li R. Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat Cell Biol. 2011;13:1252–1258. doi: 10.1038/ncb2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Lin SC, Morrison-Bogorad M, Atkinson MA, Yin HL. Thymosin beta 10 and thymosin beta 4 are both actin monomer sequestering proteins. J Biol Chem. 1993;268:502–509. [PubMed] [Google Scholar]
- Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP, Braga VM. Actin at cell-cell junctions is composed of two dynamic and functional populations. J Cell Sci. 2005;118:5549–5562. doi: 10.1242/jcs.02639. [DOI] [PubMed] [Google Scholar]