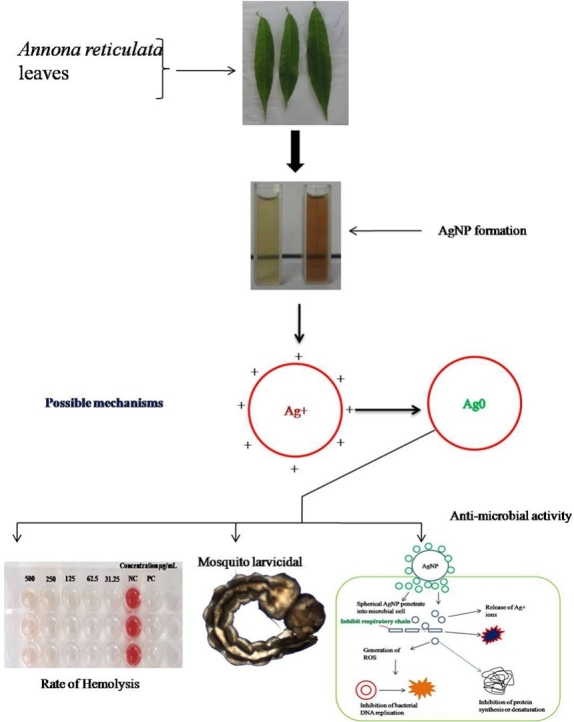
Graphical abstract
Keywords: Aedes aegypti, Anti-microbial, Silver nanoparticle, HR-TEM, Erythrocyte hemolysis
Highlights
-
•
The silver nanoparticles were smaller in size with potent larvicidal activity against Aedes aegypti.
-
•
It also had an efficient inhibition activity against all tested human pathogens.
-
•
To greater extent, it does not harm the human erythrocytes.
Abstract
Silver nanoparticles play a important role in controlling mosquito population as well as multi drug resistant pathogens without causing much harm to humans. In the present study was focused on green synthesis of silver nanoparticles against dengue causing vector (Aedes aegypti) and pathogens affecting humans. The synthesized silver nanoparticle was confirmed using UV- absorption spectrum range obtained at 416 nm, XRD, FTIR and HR-TEM analysis were used to determine the silver nanoparticle morphology and size with ∼6.48 ± 1.2–8.13 ± 0.18 nm and face centered cubic structure. The synthesized silver nanoparticles were exposed to fourth instar larvae of A. aegypti with different concentration (3–20 μg/mL) for 24 h and its elicit maximum mortality (100%) at their final concentration of 20 μg/mL and it’s LC50 value was 4.43 μg/mL and LC90 value was 13.96 μg/mL, respectively. The minimum inhibitory activities of the tested pathogens were 125, 31.25, 62.5, 62.6 and 62.5 μg/mL for the Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Candida albicans respectively. Further, the synthesized silver nanoparticle shows a potent antimicrobial activity against all tested pathogens. Moreover the effect of silver nanoparticle against Red Blood Cells belonging to ‘O’ positive blood group were tested and does not cause higher hemolysis to the cells even at the highest concentration. Based on these finding, we strongly suggested that face centered cubic structured A. reticulata AgNPs is an eco-friendly and potent bio-medical agent and can be apply in wide range of application an alternative chemically synthesized metal nanoparticle.
1. Introduction
Mosquito belongs to the phylum arthropod and is important vector for many vector-borne diseases. It causes millions of death among the population worldwide. It holds life-threatening parasitic pathogens on their thorax region, causes infection by transferring pathogens to humans through the mode of biting [1]. The important genera Aedes, Anopheles and Culex cause several deadliest diseases such as Zika, chikungunya, Dengue fever, yellow fever, Malaria, West Nile, Rift valley fever, Murray valley encephalitis, Japanese encephalitis, Western equine encephalitis, and Dog heartworm [2]. From the above said vectors, Aedes aegypti is a most menace vector for transmitting the viral diseases Zika, Dengue (I to IV types) and chikungunya among the populations. The current most flare-ups of zika virus initially began from Rhesus monkey and later found in human at 1952 in uganda, consequently it was spread to folks by infected female mosquitoes and it has been causing severe infections in all over the countries, especially in Brazil over 4000 instances of microcephaly were recorded to new born infants when this infected female mosquitoes bite the maternal mother [3]. Also, the other viral disease called dengue has been spread persistently owing to frequent climatic changes, increases in impromptu urbanization and international migrations causes deaths throughout the countries. In recent outbreaks of dengue virus over 2.5 billion people are at high risk of infection and an estimated about 3.97 billion dengue infections occurred every year in around 128 nations, mostly in tropical and sub-tropical regions. In India, the instances for dengue fever have been increased and the death rates were also recorded every year. Of all the states and union territories, more than 31,117 people were infected and 48 dengue deaths were reported within the short period of time in 2017. Among the several states of India, the maximum number of dengue cases has been accounted so for, in Kerala with 14,806, followed by Tamil Nadu with 5968 [4]. The emergence of a vast number of mosquitoes vector-borne diseases have been increased with insecticide-resistance developed mosquitoes. In order to control these vectors, the researchers turned into making new and effective products from plant metabolites with insecticidal properties against various vector-borne disease transmitting insects including various genera of mosquito due to resistance and reverse effect on an ecosystem when synthetic insecticides pyrethroids and other synthetic insecticides. In this scenario, many investigations were carried out with the help of plant derived metabolites against various mosquito species [5].
After the discovering of such a antibiotics tetracyclines, cephalosporins, aminoglycosides and macrolides, it has turned resistance towards diverse clinical pathogens, then it leads to shows main problems in chemotherapy in the 1960. When the repeated use of antibiotics to the pathogen, it undergoes losing their efficacy due to their increase in microbial resistance. In this situation, there is an alternative impact were created on multidrug-resistant bacteria for the prevention of these microbes cause problem to the human. For that, the discovery of new antibiotics is an emergence to this microbial world to protect the people from these issues. Hence, the natural products are a one of the major resources for diverse drug molecules discover in today setting [6]. In these circumstances, in recent years the green synthesis of eco-friendly metal nanoparticle from various plant derived metabolites increased interests and demands on nanotechnology and act as good material for the pharmaceutical purpose. The nanoparticle have been valuable properties such as catalytic, optical, electronic, antimicrobial, antiviral, antiplasmodial, insecticidal, magnetic and mosquito larvicidal properties [7,8]. This green synthesis of nanoparticles exhibiting advantages than in chemicals methods without any hazardous to the environment, ensures the safety of it against mosquito vectors [9]. Over the years, several plant extracts are being treated for mosquitocidal activity [10], a few of them were reports the larvicidal activity of green synthesis of silver nanoparticles from the seed extract of Pedalium murex [11], followed which, larvicidal, pesticidal and ovicidal activities of synthesized nickel palladium nanoparticles from C. nucifera [12]. In order to continue this investigation, we are aimed to synthesize silver nanoparticles by utilizing Annona reticulata leaves extract commonly called as ramphal plant or bullock's heart widely dispersed all over India. The A. reticulata leaves being used in medication of Helminthic, insecticides, styptic epilepsy, tumor, toothache, dysentery, fever and it has also been taken superficially as suppurant. The bark of this plant is used to treat vermifuge and antidysenteric. Interestingly, the other parts of the plant such as leaves, stem and root possess isoquinoline alkaloid, which was used in acetylcholinesterase inhibitory effects, antiviral activities and insecticidal activities [13,14]. Moreover the previous studies on A. reticulata mediated synthesized silver nanoparticles also developed antimicrobial activity with some lack of information. Till date as well as to our knowledge there is any specific insecticidal works have been not carried out using silver nanoparticles from A. reticulata mediated synthesized silver nanoparticels. Hence, here, we are aimed to synthesize the AgNPs using aqueous leaves extract of A. reticulata against human pathogen, mosquito larvae and also the effect of toxicity studies were carried out on human Red blood cell towards human welfare.
2. Materials and methods
From the coordinates of 12° 41′N, 78° 39′ E at Vaniyambadi, Vellore district, Tamil Nadu, India, Samples of Annona reticulata leaves were collected and further processed for authentication at Centre for Advanced Studies in Botany, University of Madras, Guindy Campus, Chennai Tamil Nadu, India. Silver nitrate (AgNO3) was purchased from Sigma-Aldrich, India.
2.1. Preparation of A. reticulata aqueous extract
Adopting the method of Yong and soo [15], the aqueous extract was prepared. The collected leaves samples were washed with distilled water and shade dried (28 ± 2 °C) under laboratory conditions for one or two weeks. Once dried, the sample was ground and powdered into fine particles using mechanical grinder. Then fine powder was subjected for extraction using double distilled water in ratio of 10 g in 100 mL under magnetic stirrer at room temperature. The mixture was centrifuged at 15,000 rpm for 15 min at 4 °C. The supernatant was collected and filtered using nylon cloth. Then filtrate was stored in refrigerator for further use.
2.2. Silver nanoparticles synthesis
Biological synthesis of AgNPs was carried out by adopting (Sukirtha et al. [[16]] method with certain modifications. The 1 mM aqueous solution of silver nitrate (AgNO3) was prepared using doubled distilled water and used for the synthesis of silver nanoparticles with help of A. reticulata leaves aqueous extract as reducing agent. The 1 mL of aqueous extract was added with 9 ml of 1 mM silver nitrate aqueous solution for reduction of Ag + ions into Ag°. The mixture was incubated over the two hrs at dark room temperature. The resultant yellowish solution was turned into formation of brownish silver nanoparticles. The synthesized silver nanoparticles was centrifuged at 15,000 rpm for 15 min at 4 °C and three repeated wash with double distilled water was performed to discard a clear supernatant solution. The obtained pellet was dried using lyophilizer, and then lyophilized powder was stored at freezer for further characterization and biological assays.
2.3. Bio-physical characterization of silver nanoparticles
In an initial characterization, the bio reduction of silver nitrate to AgNPs was analyzed by recording the spectra from 200 to 800 nm using UV–vis spectroscopy (LIUV-2500). The existence of functional groups from synthesized nanoparticles was examined using FTIR (Bruker Tensor 27) spectroscopy in the range of 400–5000 cm − 1. The High Resolution- transmission Electron Microscopy (HR-TEM) analysis was used to magnify the lattice arrangements of atoms; shape and size of the Annona reticulata AgNPs. X-ray diffraction analysis was carried out to examine nature of obtained AgNPs formation.
2.4. Mosquito larvae rearing
Larvae were collected from places Pulianthope and Otteri, at Chennai, Tamil Nadu, India. Collected larvae were brought to the laboratory and reared under the ambient temperature (28 ± 2 °C), relative humidity of 55–60%, 12:12 h (light: dark) photoperiod and larvae were fed with 1: 3 ratio, yeast and dog biscuit solution. Pupae were kept in plastic tray and transferred into rearing cage (30 × 30 × 30 cm). After 2 or 3 days pupa got emerged into adult. An emerged adult (male) were fed by cotton soaked in 10% sucrose solution and placed in Petri plate and kept it in cage, whereas female mosquitoes were blood-fed for 2 or 3 days for oviposit development with help of rat kept inside the mosquito rearing cage. For egg lay, water was filled in tray and placed into the cage. The laid eggs were air-dried and used for further bioassays.
2.5. Larvicidal bioassay
The early fourth instar larvae of A. aegypti were used for this bioassay. Larvicidal assay performed in accordance with WHO protocols with certain modifications [17]. For each test, 10 larvae were introduced into plastic cup containing different concentrations (150, 250, 500, 700 and 1000 μg of synthesized AgNPs for the 50 mL (Final concentration) of water and control were set up with tap water. Mortality was assessed after 24 h of exposure; each test was conducted in five replicates.
2.6. Non- toxicity bioassay
The toxicity study was carried out in accordance to our previous studies with slight modification [18]. The Chironomus costatus fourth instar larvae were collected from same mosquitoes breeding site. The larvae were treated with synthesized silver nanoparticles with same concentration followed in larvicidal bioassay (150 to 1000 μg for 50 mL). The control was set up with distilled water and experiment was continued for 24 h exposure.
2.7. Minimum inhibitory concentration (MIC)
The Minimum inhibitory concentration was determined according to method followed by Zhu et al. [19] with minor modification. For this, the 100 μL of Mueller hinton broth (MHB) containing different concentration of (512, 256, 128, 64, 32, 16, 8, 4, 2 and 1 μg /mL) AgNPs was serially diluted in the flat bottom 96 well micro titer plate (Tarson) for the first ten wells and 5 μl of 12 h old test pathogens were added separately to all the wells except control. The plates were incubated at 37 °C for 12 h. After incubation, 10 μL of 0.5% freshly prepared MTT (3-(4, 5-Dimethylthiazol-2-yl)-2,5- Diphenlyltetrazolium Bromide) was added to all the wells and incubated for 2 h at dark condition after that, the 100 μL of 2% DMSO (Dimethyl Sulfoxide) as the solubilization solution was added and it was incubated for 30 min at room temperature (RT). After incubation, the optical density (OD) was measured at 595 nm in ELISA reader to calculate the percentage of cell death. All the pathogens were compared with the commercially available antibiotics, Ampilox for gram positive pathogens, Levofloxacin for gram negative pathogens Fluconazole for Candida albicans were used as the positive controls.
2.8. Antibacterial activity
According to Magaldi et al. [20], the agar well diffusion method was performed for antibacterial activity. The different bacterial strains Staphylococcus aureus (Gram + ve), Pseudomonas aeruginosa (Gram -ve), Bacillus cereus (Gram + ve), and Escherichia coli (Gram -ve) were used as test pathogen and these pathogens were obtained from Unit of Bio control and Metabolites laboratory, Centre for Advance studies in botany University of Madras. The obtained pure cultures of this organism were sub cultured in Mueller–Hinton broth and incubated at 35 °C on a rotary shaker at 200 rpm for 6 hs. After the sub cultured strains was washed with 0.9% saline solution until the intensity of strain was obtained at 0.5 optical density at 570 nm. Then each strain was swabbed uniformly on the individual Mueller-Hinton agar plates using sterile cotton swabs and well was made on agar plates using gel puncture. Then the 100 μl of different concentrations of (50, 75 and 100 μg/mL) silver nanoparticles solution samples was added into the respective well. The zone of inhibition was measured using zone scale after 24 h incubation at 37 °C.
2.9. Antifungal activity
Anti- fungal activity was performed as described in anti-bacterial activity [21]. The pure culture of yeast pathogen Candida albicans was obtained from laboratory of Unit of Bio control and Metabolites, Centre for Advance studies in Botany, University of Madras. The obtained pathogen was subculture and performs the anti fungal activity by 100 μl of different concentrations of (50, 75 and 100 μg/mL) silver nanoparticles was added into the respective well. The zone of inhibition was measured using zone scale after 24 h incubation at 37 °C.
2.9.1. Hemolytic assay
An in vitro hemolytic test was performed in accordance with protocol followed by Mishraa et al. [22], with slight modification. The mammalian blood of O positive group was collected from volunteer and transferred in anticoagulant (Alsever’s) solution. The collected blood was left to 6 h for the complete settlement. The blood was centrifuged at 1500 rpm for 12 min with three repeated washing using 0.9% saline solution. The pellet RBC (2%) was suspended using 299 milliosmole (mOsm) phosphate buffer saline pH 7.4. The suspended RBC treated with various concentrations of (500 to 32.5 μg/mL) AgNPs was dissolved in PBS. As a negative control the Triton X-100 (1%) was used which is capable of rupturing the red blood cells and PBS is used as positive control. For the assay, 200 μl of the dissolved samples and 800 μl of RBC were added and incubated at 37 °C for 2 h. After incubation, the samples were placed in an ice bath for 100 s and centrifuged at 2000 rpm for 10 min. After centrifugation, the supernatants was carefully transferred into 96 well plate used for determining the percentage of hemolysis occurred by taking absorbance at 540 nm in Eliza reader. The percentage of hemolysis was calculated as follows:
Where, AS = Abs of sample treated, AP = Abs of positive and AN = Abs of Negative control respectively.
2.9.2. Statistical analysis
The percentage of larval mortality data were subjected to probit analysis for calculating LC50, LC90 statistics at 95% confidence limits of upper confidence limit (UCL), lower confidence limit (LCL) values and chi-square test was calculated using the Statplus (V.5.00). Results with p ≥ 0.05 were considered to be significant. The percentage of hemolysis calculated using MS-Excel.
3. Result and discussion
3.1. UV–vis spectroscopy analysis
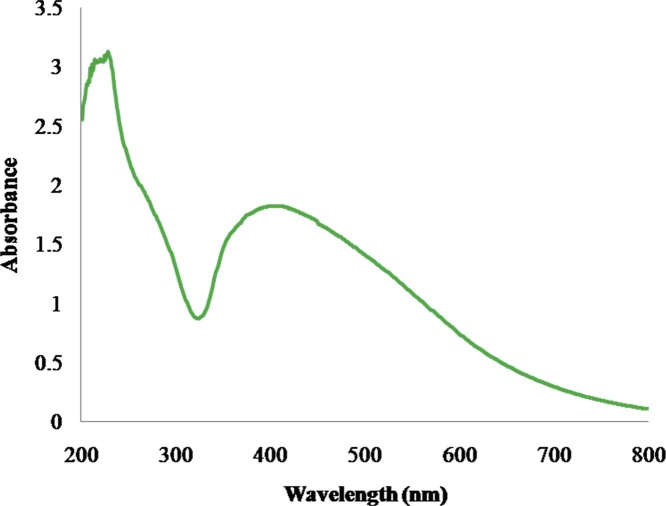
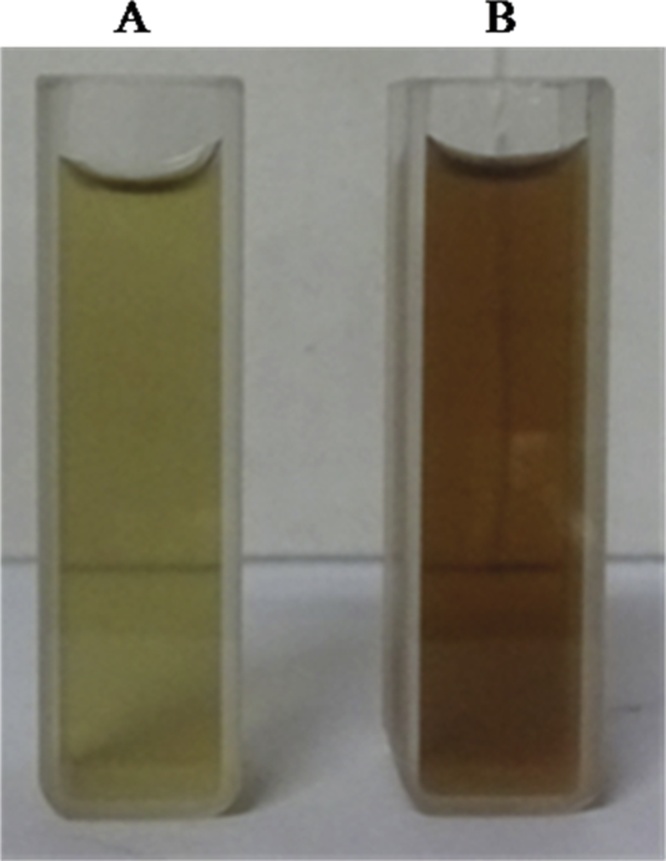
The synthesized silver nanoparticles was confirmed by color formation from yellow to brown due to the reduction of silver salt by presence of reducing agents in aqueous extract of A. reticulata, further the AgNPs formation was reveal UV- absorption spectra was taken from 200 to 800 nm and The obtained absorption spectrum of the synthesized nanoparticles range 416 nm Fig. 2. Further, the incubation time was extended for 24 h to enhance the color intensity of the colloidal solution from transparent to dark brown as shown in (Fig. 1A–B). During the process of nanoparticle synthesis, an inability for silver salt to get reduce to silver ions by adding reducing agent ie., A. reticulata aqueous extract was observed. But interestingly, the silver salt was reduced by changing of aqueous extract pH range from 9.8 to 11.0, and unless this change is done, there is no nanoparticle formation, when at their initial pH range ie., from 6.5 to 7.3. Hence, pH plays an important role in reduce silver salt to silver ions by alterating pH of the bio-molecules present in the aqueous extract of A. reticulata [23]. Accordingly, the result obtained is in accordance with the previous work, done in Annona squamosa leaf extract. This plant belongs to same genus. When synthesis of silver nanoparticle was done under various pH, the pH ≥ 9.8 provided a good environment for the formation of silver nanoparticles [24]. Moreover the formation silver nanoparticles is due to the presence of some trace level of primary and abundant level of secondary metabolites in the aqueous extract (data not shown). Similarly, the Bulut and Rapid [25], reported that reasons for one of the phytochemicals called tannin to form silver nanoparticles is due to its presence in aqueous solution. Hence, this lead to an assumption that silver nanoparticles formation could be due to one of this phytochemical presence in aqueous extract and accompanied by pH 9.8 which is providing the best environment for the formation of silver nanoparticles. In contrast, a study was conducted on this same plant extract, were a synthesis of silver nanoparticles was done without using any pH requirements [26]. This difference of silver nanoparticles formation using aqueous extract of the same species, would be due to influence of place of cultivation and other environmental factors. Indeed, in the present studies, the silver nanoparticles were formed only after the pH altered to 9.8 by using 2 M NaOH. From this result observation, it was an evident, that the formation of AgNPs is mainly relying on the pH in the reaction medium. So, we hypothesized that, the pH may be involved in ionization by electron transfer under this alkaline conditions with different compound present in aqueous solution along with silver salt which helped in formation of silver nanoparticles. An earlier report; showed, that increase in pH of the neem extract immediately reduces silver nitrate into silver nanoparticles as faster than normal phenomenon reaction. This happens by exchange of ionic charge of the molecules present in extract and moreover it act as stabilizing agent for synthesized particles [27]. Based on these mechanisms, we state that, the Annona reticulata extract might contain higher protonated molecules, so which is unable to reduce the silver nitrate into silver nanoparticles at their initial pH around 6.5 to 7.3, but when incorporation of NaOH with molecules in extract, that ionizes the molecules present in extract by alteration particle charges between the molecule and silver nitrate (AgNO3) and it leads to forms the metal nanoparticles. Hence, the A. reticulata silver nanoparticles proved that it needs a higher pH value for their successful reduction process.
Fig. 2.
UV–vis absorption spectra of synthesized silver nanoparticles using A. reticulata. leaves extract.
Fig. 1.
Synthesized silver nanoparticles formed after addition 1 mM Silver nitrate solution into the Aqueous extract (A) and (B) AgNPs synthesized using A. reticulata.
3.2. XRD analysis of silver NPs
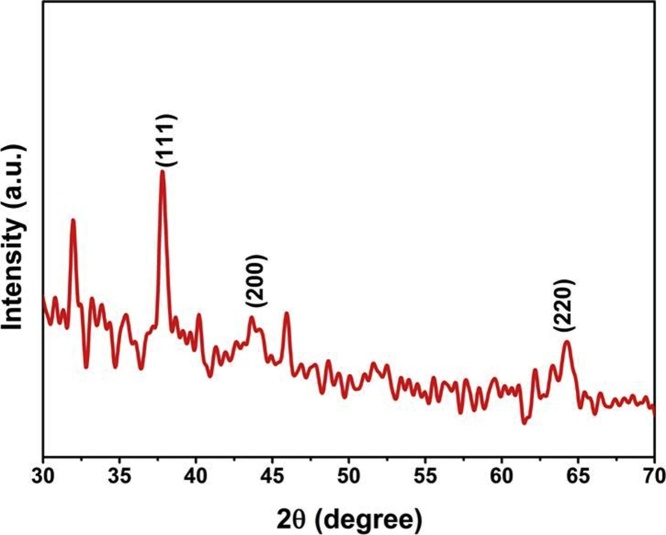
The crystal lattice and structure of the biosynthesized AgNPs was identified by using X-ray powder diffraction (XRD) analysis. The X-ray diffraction studies showed well resolved three diffraction peaks at 2θ angles of approximately, 37.56°, 43.25° and 64.10°, which correspond to the crystallographic planes of faced-centered cubic (FCC) structures of silver nanoparticles 111, 200, and 220, respectively (Fig. 3). The similar reports were also obtained by Santhosh et al. [28], where intense strong and narrow diffraction peaks of nanoparticles at 38.33, 44.28, 64.42, and 77.60 were docked to the 111, 200, 220, and 311 (Bragg's reflections of cubic structure of silver). In this experiment, we got also almost same XRD pattern intensities and confirmed that, the synthesized silver nanoparticles were crystalline in nature and had a face-centered cubic structure (JCPDS Card No. 89-3722).
Fig. 3.
XRD pattern of biosynthesized AgNPs exhibiting the facets of crystalline.
silver.
3.3. FTIR analysis
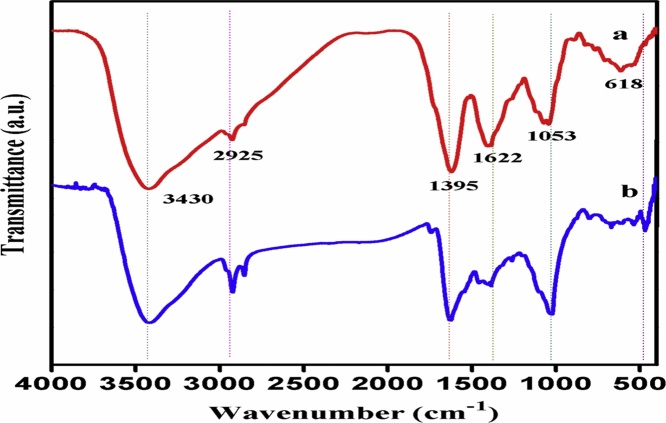
FT-IR spectrum analysis is used to identify interaction between the bio molecules present in leaves extract and silver nitrate. In this regard, as shown in Fig. 4, we were obtained several characteristic peaks 618, 1053, 1395, 1622, 2845, 2925 and 3430, it was assigned to both aqueous extract and silver nanoparticles (Fig. 4a–b), those all characteristic peaks were also obtained in bio-synthesized silver nanoparticles, therefore, all these peaks corresponds molecules might be involved in synthesis and stabilization of silver nanoparticles. The various prominent peaks of 618, 1053, 1395, 1622, 2925 and 3430 cm−1 which corresponds to various functional groups such as C—X – stretching vibration of chloride group, C—O bending vibration of anhydrides group, CH2−CH3 stretching vibration of methyl group, C O bonding vibration of carbonyl group, CH stretching of alkane group and N—H stretching vibrations of amide groups respectively. The peaks at 3430 cm-1, belongs to amide group and aromatic rings were considered as functional groups from flavonoids, triterpenoids, and polyphenols so, the interaction between the metal ion and amide group in the extract of A. reticulata involved in synthesis and stabilization (capping) of silver nanoparticles formation as previously described by Asmathunisha et al., 2010 [29]. On the other hand, the strong band shift at 1622 cm − 1 was attributed to the carbonyl stretch of amides and it would be a related to the proteins molecules present in the aqueous extract so, which is potentially involved in synthesis and capping of silver nanoparticle fromation (Suriyakalaa et al. [30]), respectively. FTIR studies confirms that the carbonyl groups of amino acids and amide groups from flavonoids have a strong affinity towards metal ions and they may be encapsulate the nanoparticles forming by a protective coat-like shell and that prevents their further aggregation as well as stabilization of silver nanoparticles.
Fig. 4.
The FTIR spectra of (a) Aqueous extract and (b) AgNPs synthesized using A. reticulata.
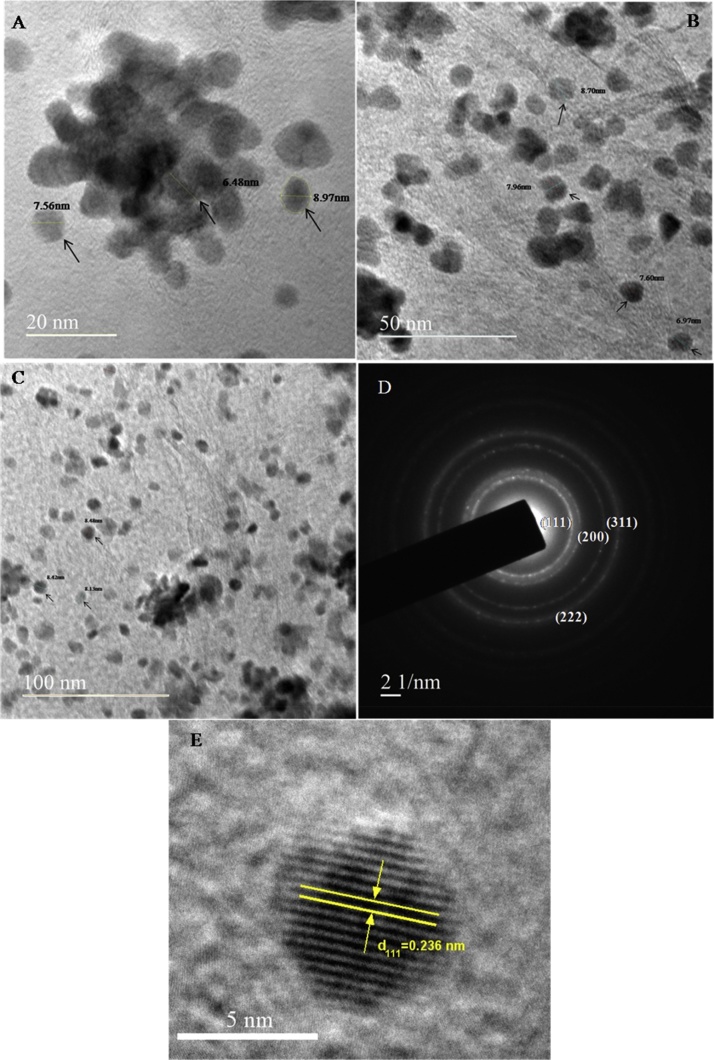
3.4. HR-Transmission electron microscopy
The High Resolution-Transmission Electron Microscope was used for further confirmation of silver nanoparticles (AgNPs) sizes and shape, the obtained silver nanopartices found spherical in shape and these nanoparticles were formed as clear dispersals with few agglomerated. The average size of green synthesized AgNPs was found to be very small in sizes A: 7.67 ± 1.2 nm, B: 8.02 ± 0.59 nm and C: 8.34 ± 0.18 nm as shown in Fig. 5(A–C). The selected area electron diffraction (SAED) demonstrated that the clear specific rings were spotted and it was assigned to the presence of Ag 111, 200, 220, 311, and 222 planes of the face-centered cubic structured (FCC) silver nanoparticles obtained as shown in Fig. 5D and it was confirmed that, these are green route mediated synthesis of AgNPs [31]. Further the crystalline nature of single particles was tested by means of high resolution (HR-TEM) micrographs, which showed the presence of the interplaner spacing lattice fringes value of 0.236 nm (Fig. 5E) correspondent to the 111 plane of Ag, which is good agreeable with other study Khan et al. [32], who also obtained lattice fringes as same manner.
Fig. 5.
HR- TEM micrograph of silver nanoparticles (AgNPs) taken at different magnifications ranging from (A) 20 nm, (B) 50 nm and (C) 100 nm and the spherical size of AgNPs range is 7.67–8.34 nm, (D) Electron diffraction pattern of silver nanoparticles showing respective crystal planes representing of FCC structure and (E) HR-TEM images of Ag- nanoparticles showing the lattice spacing of 0.23 nm.
3.5. Larvicidal bioassay
Larvicidal activity of synthesized silver nanoparticles was tested against early fourth instar larvae of A. aegypti with concentrations of 3–20 μg/mL for 24 h exposure. The data was observed for each concentration in every replicates, the percentages of mortality were as 29 ± 0.54, 60 ± 0.70, 86 ± 0.54, 98 ± 0.44 and 100 ± 0.00% (Table 1). The lethal concentration LC50 value of 4.43 μg/mL and LC90: 13.96 μg/mL, were required to kill 50 and 90% larvae respectively. In our study, the minimum concentration of 15 μg/mL is required to obtain maximum mortality rate. Indeed, our result revealed that A. reticulata silver nanoparticles has more efficient, while compared to other study carried out by Suganya et al. [33], who reported, the larvicidal activity with highest concentration (0.01 to 5 mg /L) requirement to bring larval mortality using Leucas aspera leaf extract route synthesized metal particle for 24 h exposure against A. aegypti and the obtained lethal concentration is LC50: 8.5632 mg/L; LC90: 21.5685 mg/L. The other comparable study of Anil Kumar et al. [34], were also noted, that the usage of green synthesized silver nanoparticles from Excoecaria agallocha leaf extract was tested with higher concentration (2.0 to 14 mg/l) against 3rd and 4th instar larvae of Aedes aegypti and they found highest mortality against third instar larvae of A. aegypti with LC50 = 4.65 and 6.10 mg/L for 24 h of exposure respectively. Ultimately, the present study was showed, that the larvicidal activity is obtained in a very minimal concentration of AgNPs on mosquito larvae and it could be due to the small of size of particles that might be facilitate the penetration through the larval cellular membrane or otherwise binding to S-containing proteins and DNA, that eventually leading to the denaturation of proteins or nucleic acids. Therefore, the strong depletion of membrane and the rupture in proton motive force can cause destruction of cellular functions and finally the cell death was occurred [35].
Table 1.
Larvicidal activity of A. reticulata leaves extract mediated-synthesized AgNPs against fourth instar larvae of Aedes aegypti.
| Treatment | Concentration μg/mL |
Percent mortality ± S.D (24 hrs) | LC 50 (95% LCL-UCL) | LC 90 (95% LCL-UCL) | Chi-square (d.f. = 2) | P- level |
|---|---|---|---|---|---|---|
| 3 | 26 ± 0.54 | |||||
| 5 | 60 ± 0.70 | |||||
| AgNP | 10 | 86 ± 0.54 | 4.43 (3.73- 5.25) | 13.96 (12.73- 15.93) | 0.134 | 0.824 |
| 15 | 98 ± 0.44 | |||||
| 20 | 100 ± 0.00 |
Percent mortality ± S.D (five replicates); Control- nil mortality, LC50- lethal concentration that kills 50% exposed larvae; LC90- lethal concentration that kills 90% of exposed larvae to the plant extract; LCL= lower confidence limit; UCL= upper confidence limit; df = degree of freedom; x2 = chi square; p < 0.05 level of significant.
3.6. Non- toxicity bioassay
The toxicity study of green synthesized nanoparticles was tested against fourth instar larvae chironomus costatus, which was collected from same habitat of mosquito larval collection site. The choice of these organisms is due to its importance in ecological dynamics of aquatic ecosystems and these larvae are bio indicators in studies of monitoring water quality [36]. The importance in analysis of toxicity studies using these synthesized nanoparticles is due to what kind of impacts, which have been created in water bodies like efficiency of biodegradability and other such a factors. Hence, we tested these organisms with followed concentrations of 3 to 20 μg/mL and the mortality of these organisms was confirmed by shaking thus plastic cup containing organisms after 24 h exposured. Interestingly after 24 h exposured, the AgNPs does not cause any mortality against tested aquatic organisms and which was showed us that A. reticulata silver nanoparticle was safe to non target organisms. In a similar way, Patil et al. [37], who reported that, Poecilia reticulata fishes tested for 48 h of exposure to Spergularia rubra synthesized AgNPs and observed that there was any adverse effects were not noticed to the non target organisms. Followed which, Subarani et al. [38], they also were reported that there was no toxicity impact on tested non target organisms when Vinca rosea-synthesized AgNP exposured even after long duration 72 h. In contrary, the Govindarajan and Benelli [39], reported that, the facile synthesized silver nanoparticles from Barleria cristata cause the mortality against D. indicus and A. bouvieri non target aquatic insect tested at their lethal concentration value of 633.26 to 8595.89 μg/mL. In this connection, we suggest that the A. reticualta silver nanoparticle was safe to the environment and eco-friendly for non target aquatic insect.
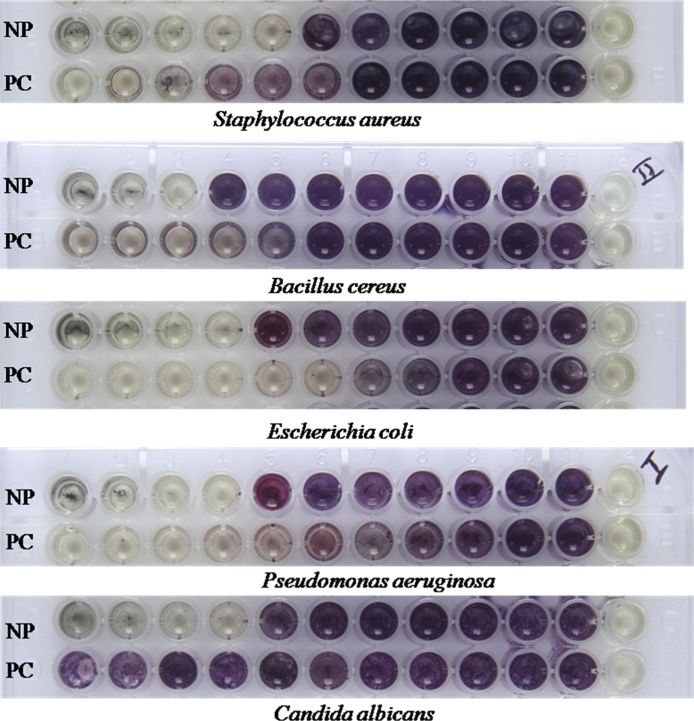
3.7. Minimum inhibitory concentration
The minimal inhibitory concentration (MIC) commonly used to know which is the minimal concentration needed for the microbial growth inhibition. In this context, six human pathogenic strains were tested using green synthesized silver nanoparticles at the range of 1 mg/mL by serial dilution method. As shown in Fig. 6, the microbial growth inhibition was visibly observed in 96 well plates after 24 h treatments and the complete inhibition was found at 125 μg/ml concentration for the Bacillus cereus, 31.2 μg/ml for Staphylococcus aureus, 62.5 μg/ml for Pseudomonas aeruginosa, 62.5 μg/ml for Escherichia coli and 62.5 μg/ml for Candida albicans as its described in Table 2. In this test, the aqueous extract was also tested separately for MIC at the same concentration of 1 mg/mL, but thus not inhibits the any microbial growth (data not shown). The results were in accordance with the some previous investigation by Abalkhil et al. [40], they was reporting, that the minimum inhibitory assay was evaluated for its antibacterial activity using three plant mediated with small size silver nanoparticles from Aloe vera, Portulaca oleracea and Cynodon dactylon against various pathogenic strains among various pathogens, the B. subtilis, S. aureus, and P. aeruginosa growth were inhibited by the all the silver nanoparticles with requiring of higher concentration. The difference in susceptibility of microbial growth inhibition among the various silver nanoparticles is due to the surface and size of the particles variances [41]. Commonly, the AgNPs with smaller sizes have a large surface area, which facilitate the interaction with the bacterial cell membrane and could be alter primary functions, such as permeability and cell respiration eventually, which leads to cell death [42]. Hence, our result suggested, that the A. reticulata mediated synthesized AgNPs has potent growth inhibition against tested all pathogens.
Fig. 6.
Minimum inhibitory concentration activity of AgNP against Staphylococcus aureus, Bacillus cereus, Pseudomonas aeruginosa, Escherichia coli and Candida albicans.
NP - Nanoparticles PC- Positive control.
1–10 wells used 10 fold serial dilution of crude and Positive control, 11 well as 100% of growth 12 well as No growth.
Table 2.
Minimum inhibitory concentration values for silver nanoparticles against different microbial strains; NP: Nanoparticles; PC: Positive control (Antibiotics- Levofloxacin for Gram + ve bacteria; Ampilox for Gram –ve bacteria and fluconazole for Candida albicans).
| Parameter | Minimum inhibitory concentration (MIC) (μg/mL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gram-positive bacterium |
Gram-negative bacterium |
Fungal pathogen |
||||||||
|
S. a |
B. c |
E. coli |
P. aeruginosa |
Candida albicans |
||||||
| NP | PC | NP | PC | NP | PC | NP | PC | NP | PC | |
| MIC value | 31.25 | 125 | 125 | 31.25 | 62.5 | 3.90 | 62.5 | 3.90 | 62.5 | NIL |
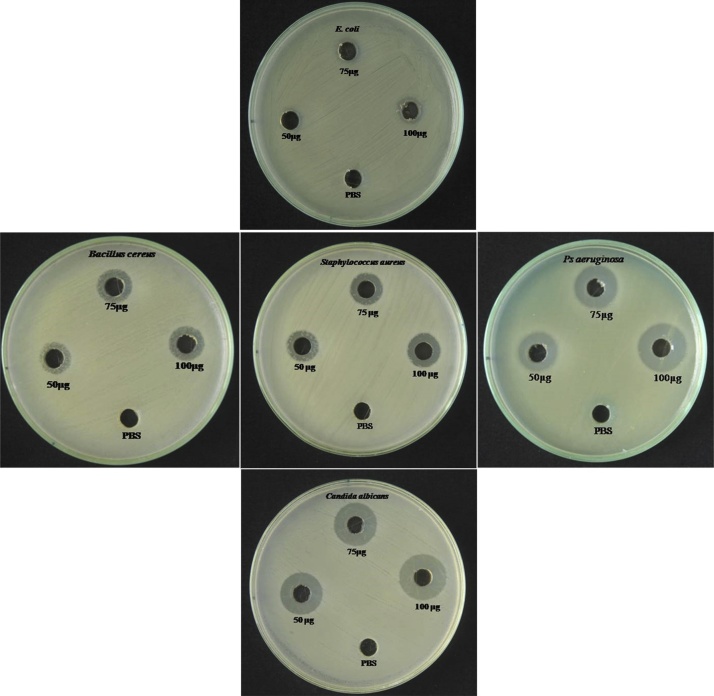
3.8. Antimicrobial activity
To overcome the antibiotics resistant bacteria, an alternative drug invention is in necessity. Usage of silver nanoparticles (AgNPs) could be a one of the alternatives against various microbial pathogens. The silver nanoparticles have been grown predominantly in pharmaceuticals and other drug industries, because of a great impact on antibacterial properties due to a different size variation [43]. In our study, the green synthesized AgNPs using A. reticulata extract showed an excellent antimicrobial activity against tested microorganisms as shown in Fig. 7. The silver nanoparticles produced a potent zone of inhibition in the respective well at 100 μg/mL of concentration and the zone of inhibition is 17 mm for B. cereus (gram + ve), 18 mm for the S. aureus (gram + ve), 22 mm for Ps. aeruginosa (gram - ve), 12 mm for Escherichia coli (gram - ve) and 21 mm for yeast pathogen Candida albicans was obtained as described in Table 3. The silver nanoparticles elicited the antimicrobial activity against almost similar zone of inhibition for B. cereus and S. aureus than that of ps. aeruginosa and Candida albicans, it might be due to cell wall permeability dependency [44]. This study is in agreement with earlier studies carried out by Gogoi et al. [45] and these mechanisms of its activity are still unknown and moreover the direct contact with cellular DNA and protein was not occurred by silver nanoparticles. The only possible mechanisms of antimicrobial activity is due to by free radicals generation, where the silver nanoparticles was interacted with bacteria, consequently release the silver ions inside of the cell and contents of the cells leaked out, eventually, it leads to the protein denaturation [46]. Hence, based on our results, we assume that, the A. reticulata mediated synthesized silver nanoparticles also could mediate the antimicrobial activity in this same way. Henceforth, the A. reticulata meditated synthesized silver nanoparticles would considered as better agent to treat the infectious disease causes by tested pathogens.
Fig. 7.
Antimicrobial activity of synthesized Ag-NPs evaluated by well diffusion method against Escherichia coli, Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans.
Table 3.
Antimicrobial activity of synthesized silver nanoparticles against Staphylococcus aureus (St. a); Bacillus cereus (B. c); Escherichia coli (Es. c); Pseudomonas aeruginosa (Ps. a); Candida albicans (C. a).
| Concentration μg/mL | Zone of inhibition (mm) |
||||
|---|---|---|---|---|---|
| Ar-AgNP | Gram-positive bacteria |
Gram-negative bacteria |
Yeast pathogen | ||
| St. a | B. c | Es. c | Ps. a | C. a | |
| 50 | 14 | 13 | – | 16 | 18 |
| 75 | 15 | 15 | – | 18 | 19 |
| 100 | 18 | 17 | 12 | 22 | 21 |
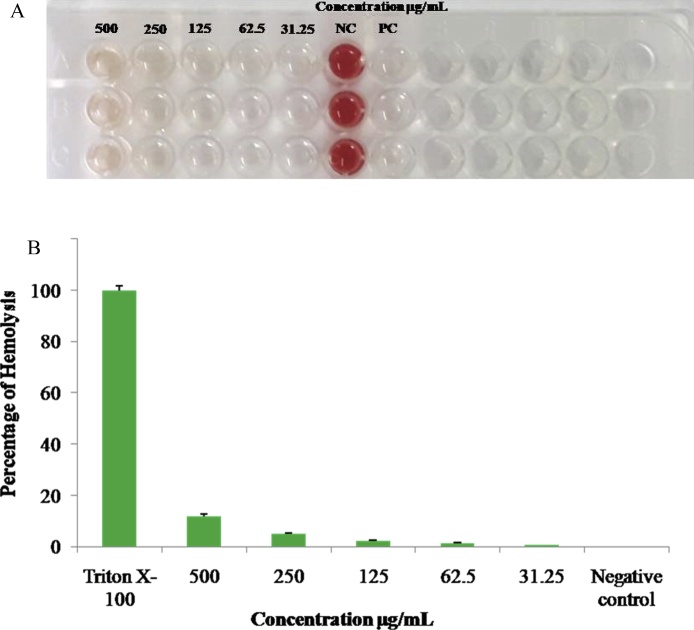
3.9. Toxicology assessment of Red blood cell
The toxicity of AgNPs was determined using hemolytic assessment is very essential for human welfare oriented clinical applications due to its efficient drug delivering capacity. The investigation on blood compatibility of AgNPs is much important at the nanotoxicity level, because the blood cells are being directly or indirectly affected by such a kind of nanoparticles especially erythrocytes are circulating to various organs through cardiovascular system and may lead to considerable damages such as cell membrane injury, DNA damage and congenital malformations [47]. In this circumstance, biocompatibility of silver nanoparticles rupturing and releasing the contents of erythrocytes is gaining attention to determine toxicity level of nanoproducts. In this connection, the present study was evaluated to study the toxicity level of the average sized 8 nm green synthesized AgNPs against human erythrocyte belonging to ‘O’ positive blood group. As shown in Fig. 8A and B the percentage of lysis was obtained only about 13.8 ± 1.72 to 0.87 ± 0.28 at 500 to 31.25 μg/ml concentration respectively. This result proved that these nanoparticles are safe and can be carried out for further investigations in multidiscipline of biomedical studies. In addition to these findings, this investigation shows significant outcomes compared to previous reports such as the hemolytic activity of green synthesized silver nanoparticles from Cathranthus roseus caused hemolysis about 6 to 92% even at minimum concentration of 5 to 50 μg/mL tested [48]. Similarly, Tahira et al. [49], who also reported that toxicity was occurrence to cells by causing lysis even minimum concentrations tested. From this result we obtained, the A. reticulata mediated synthesized silver nanoparticles is would be considered as safe to human for the pharmacological purpose in future.
Fig. 8.
Hemolytic activities of AgNPs treated against human O(+ve) RBCs. (A) Photographs showing RBC treated with various concentrations (500 to 31.25 μg/mL) of AgNPs and Negative control-1% TritonX-100 and Positive control- PBS pH 7.4 for 2 h. (B) shows a graphical representation percentage of hemolytic.
4. Conclusion
The biosynthesized of silver nanoparticles from A. reticulata aqueous extract using alkaline pH 9.8 has produced small sized nanoparticles with the spherical shapes and crystalline in nature. It was shown to have potent larvicidal activity without harming the non-target aquatic insect and moreover the green synthesized silver nanoparticles also exhibit antimicrobial activity potentially with biocompatibility to the red blood cells against O + ve erythrocytes regarding human welfare concern. Hence, overall the biocompatibility of these silver nanoparticles is to be considered as nontoxic and in these coming circumstances, it can be used as pest management and drug delivery to the human affected from infectious pathogens.
Conflicts of interest
None.
Acknowledgements
This work was supported by a grant from the University Grants Commission, New Delhi, under University Potential for Excellence II- Herbal Science Research Program (No. 2013/PFEP/C3/199) through the University of Madras. We are grateful to IIT Madras for the HR-TEM analysis.
References
- 1.Benelli G., Iacono A.L., Canale A., Mehlhorn H. Mosquito vectors and the spread of cancer: an overlooked connection. Parasitol. Res. 2016;115(6):2131–2137. doi: 10.1007/s00436-016-5037-y. [DOI] [PubMed] [Google Scholar]
- 2.Benelli G. Commentary: data analysis in bionanoscience – issues to watch for. J. Clust. Sci. 2017 doi: 10.1007/s10876-016-1143-3. [DOI] [Google Scholar]
- 3.Fauci A.S., Morens D.M. Zika virus in the Americas - yet another arbovirus threat. N. Engl. J. Med. 2016;374(7):601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 4.Directorate General of Health Services; 2017. National Vector Borne Disease Control Programme. [Google Scholar]
- 5.Marques A.M., Kaplan M.A.C. Active metabolites of the genus Piper against Aedes aegypti: Natural alternative sources for dengue vector control. Univ. Sci. Bogota. 2015;20:61–82. [Google Scholar]
- 6.Mabona U., Viljoen A., Shikanga E. Antimicrobial activity of Southern African medicinal plants with dermatological relevance: from an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J. Ethnopharmacol. 2013;148(1):45–55. doi: 10.1016/j.jep.2013.03.056. [DOI] [PubMed] [Google Scholar]
- 7.Schmid G. VHC Press; New York: 1995. Colloids and Clusters. [Google Scholar]
- 8.Santhosh S.B., Ragavendran C., Natarajan D. Spectral and HRTEM analyses of Annona muricata leaf extract mediated silver nanoparticles and its Larvicidal efficacy against three mosquito vectors Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti. J. Photochem. Photobiol. B: Biol. 2015;153:184–190. doi: 10.1016/j.jphotobiol.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Benelli G., Mehlhorn H. Review: declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol. Res. 2016;115(5):1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- 10.Panneerselvam C., Murugan K., Roni M., Aziz A.T., Suresh U., Rajaganesh R., Madhiyazhagan P., Subramaniam J., Dinesh D., Nicoletti M., Higuchi A., Alarfaj A.A., Munusamy M.A., Kumar S., Desneux N., Benelli G. Fern-synthesized nanoparticles in the fight against malaria: LC/MS analysis of Pteridium aquilinum leaf extract and biosynthesis of silver nanoparticles with high mosquitocidal and antiplasmodial activity. Parasitol. Res. 2016;115(3):997–1013. doi: 10.1007/s00436-015-4828-x. [DOI] [PubMed] [Google Scholar]
- 11.Ishwarya R., Vaseeharana B., Anuradhaa R., Rekhaa R., Govindarajan M., Alharbi N.S., Kadaikunnanc S., Khaledc J.M., Benelli G. Eco-friendly fabrication of Ag nanostructures using the seed extract of Pedalium murex, an ancient Indian medicinal plant: Histopathological effects on the Zika virus vector Aedes aegypti and inhibition of biofilm-forming pathogenic bacteria. J. Photo Photo, B: Biol. 2017;174:133–143. doi: 10.1016/j.jphotobiol.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 12.Elango G., Roopan S.M., Al-Dhabi N.A., Arasu M.V., Dhamodaran K.I., Elumalai K. Coir mediated instant synthesis of Ni-Pd nanoparticles and its significance over larvicidal, pesticidal and ovicidal activities. J. Mol. Liq. 2016;223:1249–1255. [Google Scholar]
- 13.Orhan B., Ozcelik T., Karaoglu B., Sener Z., Naturforsch C. J. Biosci. 2007;62:19. doi: 10.1515/znc-2007-1-204. [DOI] [PubMed] [Google Scholar]
- 14.Miyazawa M., Yoshio K., Ishikawa Y., Kameoka H. Insecticidal Alkaloids against Drosophila melanogaster from Nuphar japonicum DC. J. Agric. Food Chem. 1998;46(3):1059–1063. [Google Scholar]
- 15.Jae Yong S., Soo K.B. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009;32:79–84. doi: 10.1007/s00449-008-0224-6. [DOI] [PubMed] [Google Scholar]
- 16.Sukirtha R., Priyanka K., Antony J.J., Kamalakkannan S., Thangam R., Gunasekaran P. Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Proc. Biol. 2012;47(2):273–279. [Google Scholar]
- 17.World Health Organization . WHO; Geneva: 1996. Report, CTD/WHO PES/IC/96.1; p. 69. [Google Scholar]
- 18.Parthiban E., Ramanibai R. Entomotoxicity Properties of Eco-Friendly Crude Protein Extract FromManilkara Zapota Seed against Asian Tiger Vector Aedes Aegypti. SO J. Vet. Sci. 2017;3(1):1–6. [Google Scholar]
- 19.Zhu Q.Y., Holt R.R., Lazarus S.A., Orozco T.J., Keen C.L. Inhibitory effects of cocoa flavanols and procyanidin oligomers on free radical-induced erythrocyte haemolysis. Exp. Biol. Med. 2002;227(5):321–329. doi: 10.1177/153537020222700504. [DOI] [PubMed] [Google Scholar]
- 20.Magaldi S., Mata-Essayag S., Hartung de Capriles C., Perez C., Colella M.T., Olaizolaa Carolina, Ontiveros Yudith. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004;8(1):39–45. doi: 10.1016/j.ijid.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Magaldi S., Mata-Essayag C., Hartung de C. Well diffusion for antifungal susceptibility S.testing. Int. J. Infect. Dis. 2004;8(1):39–45. doi: 10.1016/j.ijid.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Mishraa A., Kaushikb N.K., Sardara M., Sahalb D. Evaluation of antiplasmodial activity of green synthesized silver nanoparticles. Colloids Surf. B Biointerfaces. 2013;111:713–718. doi: 10.1016/j.colsurfb.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Aparajita V., Singh M.M. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2016;9:109–115. [Google Scholar]
- 24.Viveka R., Thangama R., Muthuchelian K., Gunasekaran P., Kaveri K., Kannan S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Biol. Sci. 2012;47(12):2405–2410. [Google Scholar]
- 25.Bulut E., Mahmut O. Rapid facile synthesis of silver nanostructure using hydrolysable tannin. Ind. Eng. Chem. Res. 2009;48(12):5686–5690. [Google Scholar]
- 26.Shivakumar singh P., Vidyasagar G.M. Biosynthesis, characterization, and antidermatophytic activity of silver nanoparticles using Raamphal plant (Annona reticulata) aqueous leaves extract. Int. J. Mater. Sci. 2015;412452:5. [Google Scholar]
- 27.Verma A., Mehata M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Rad. Res. Appl. Sci. 2016;9(1):109–115. [Google Scholar]
- 28.Santhosh S.B., Yuvarajan R., Natarajan D. Spectral and HRTEM analyses of Annona muricata leaf extract mediated silver nanoparticles and its Larvicidal efficacy against three mosquito vectors Anopheles stephensi, Culex quinquefasciatus, and Aedes aegypti. J. Photo Photo, B: Biol. 2015;153:184–190. doi: 10.1016/j.jphotobiol.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 29.Asmathunisha N., Kathiresan K., Nabeel Anburaj M.A. Synthesis of antimicrobial silver nanoparticles by callus leaf extracts from saltmarsh plant Sesuvium portulacastrum L. Colloids Surf. B Biointerfaces. 2010;79:488–493. doi: 10.1016/j.colsurfb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Suriyakalaa U., Antony J.J., Suganya S., Siva D., Sukirtha R., Kamalakkannan S., Pichiah T., Achiraman S. Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf B. 2013;102:189–194. doi: 10.1016/j.colsurfb.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 31.Lu X., Hanrath T.K., Johnton P., Kargel B.A. Growth of single crystal silicon nanowires in supercritical solution from tethered gold particles on a silicon substrate. Nano Lett. 2003;3(1):93–99. [Google Scholar]
- 32.Khan M.A., Kumar S.M., Ahamed M., Alrokayan S.A., Alsalhi M.S. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nano Res. Lett. 2011;6(1):434. doi: 10.1186/1556-276X-6-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suganya G., Karthi S., Muthugounder S., Shivakumar M.S. Larvicidal potential of silver nanoparticles synthesized from Leucas aspera leaf extracts against dengue vector Aedes aegypti. Par Res. 2013;113(3):875–880. doi: 10.1007/s00436-013-3718-3. [DOI] [PubMed] [Google Scholar]
- 34.Anil Kumar V., Ammani K., Jobina R., Parasuraman P., Siddhardha B. Larvicidal activity of green synthesized silver nanoparticles using Excoecaria agallocha L. (Euphorbiaceae) leaf extract against Aedes aegypti. IET Nanobiotechnol. 2016;10(6):1–7. doi: 10.1049/iet-nbt.2015.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarah S.J., Pillai R.K., Chandramohanakumar N., Balagopalan M. Larvicidal potential of biologically synthesised silver nanoparticles againstAedes Albopictus. Res. J. Recent Sci. 2012;1:52–56. [Google Scholar]
- 36.Cortelezzi A., Paggi A.C., Rodríguez M., Rodrigues A.C. Taxonomic and non taxonomic responses to ecological changes in an urban lowland stream through the use of Chironomidae (Diptera) larvae. Sci. Total Environ. 2011;409(7):1344–1350. doi: 10.1016/j.scitotenv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Patil C.D., Borase H.P., Patil S.V., Salunkhe R.B., Salunke B.K. Larvicidal activity of silver nanoparticles synthesized using Pergularia daemia plant latex against Aedes aegypti and Anopheles stephensi and nontarget fish Poecillia reticulate. Parasitol. Res. 2012;111(2):555–562. doi: 10.1007/s00436-012-2867-0. [DOI] [PubMed] [Google Scholar]
- 38.Subarani S., Sabhanayakam S., Kamaraj C. Studies on the impact of biosynthesized silver nanoparticles (AgNPs) in relation to malaria and filariasis vector control againstAnopheles stephensi Liston and Culex quinquefasciatus Say (Diptera: Culicidae) Parasitol. Res. 2013;112(2):487–499. doi: 10.1007/s00436-012-3158-5. [DOI] [PubMed] [Google Scholar]
- 39.Govindarajan M., Benelli G. Facile biosynthesis of silver nanoparticles using Barleria cristata: mosquitocidal potential and biotoxicity on three non-target aquatic organisms. Parasitol. Res. 2015;115(3):925–935. doi: 10.1007/s00436-015-4817-0. [DOI] [PubMed] [Google Scholar]
- 40.Abalkhil T.A., Alharbi S.A., Salmen S.H., Wainwright M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biol. Biotechnol. Equp. 2017;31(2):411–417. [Google Scholar]
- 41.Song H., Ko K., Oh I., Lee B. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur. Cells Mater. 2006;11(1):58–59. [Google Scholar]
- 42.Panacek A., Kvitek L., Prucek R. Silver colloid nanoparticles: synthesis, characterization and their antibacterial activity. J. Phys. Chem. B. 2006;110(33):16248–16253. doi: 10.1021/jp063826h. [DOI] [PubMed] [Google Scholar]
- 43.Shrivastava S., Bera T., Roy A., Singh G., Ramachandrarao P., Dash D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nano Technol. 2007;18 doi: 10.1088/0957-4484/18/22/225103. 225–103. [DOI] [PubMed] [Google Scholar]
- 44.Abalkhil T.A., Alharbi S.A., Salmen S.H., Wainwright M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Bio Biotechnol. Equp. 2017;31(2):411–417. [Google Scholar]
- 45.Gogoi S.K., Gopinath P., Paul A. Green fluorescent protein- expressing Es- coli as a model system for investigating the antimicrobial activities of silver nanoparticles. Langmuir. 2006;22(22):9322–9328. doi: 10.1021/la060661v. [DOI] [PubMed] [Google Scholar]
- 46.Cui J., Liang Y., Yang D., Liu Y. Facile fabrication of rice husk based silicon dioxide nanospheres loaded with silver nanoparticles as a rice antibacterial agent. Sci. Rep. 2016;6:214–223. doi: 10.1038/srep21423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asha Rani P.V., Mun G.L.K., Hande M.P., Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3(2):279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 48.Raja A., Salique S.M., Gajalakshmi P., James A. Antibacterial and hemolytic activity of green silver nanoparticles from Cathranthus roseus. IJPSN. 2016;9(1):3112–3117. [Google Scholar]
- 49.Tahira K., Nazirb S., Ahmada A., Li B., Shaha S.A.A., Khana A.U., Khand G.M., Khana Q.U., Khanc Z.U.H., Khana F.U. Biodirected Synthesis of Palladium nanoparticles using Phoenix dactylifera leaves extract and their size dependent biomedical and catalytic applications. RSC Adv. 2016;6(89):1–39. doi: 10.1039/C6RA11409A. [DOI] [Google Scholar]