Abstract
In this article, using human pancreatic cancer cell lines and tumor specimens, we analyze the expression and localization of the invadopodia-related proteins TKS5 and Cortactin. Specifically, we present data on: a) TKS5 expression and localization by immunofluorescence in human pancreatic tumors, b) Cortactin expression by western blotting in various human pancreatic adenocarcinoma cell lines, c) TKS5 and Cortactin localization at invadopodia in BxPC-3 pancreatic adenocarcinoma cells, and d) TKS5 and Cortactin localization by co-immunofluorescence in human pancreatic cancer specimens. Data presented here is related to and supportive of the research article by Chen et al., “TKS5-positive invadopodia-like structures in human tumor surgical specimens” (Chen et al., 2019), where interpretation of the research data presented here is available.
Specifications table
| Subject area | Biology |
| More specific subject area | Cancer and Tumor Biology |
| Type of data | Fluorescence images and film scans |
| How data was acquired | Fluorescence microscope and western blotting followed by chemiluminescence. |
| Data format | Analyzed |
| Experimental factors | Pancreatic cell lines were cultured in standard conditions; human tumor surgical specimens were formalin-fixed and paraffin embedded. |
| Experimental features | Protein lysates were obtained from pancreatic cell lines and processed for western blotting with a Cortactin antibody. BxPC-3 cells were grown in gelatin and processed for immunofluorescence with TKS5 and Cortactin antibodies. Human tumor sections were processed for immunofluorescence stained for TKS5 and Cortactin or F-actin. |
| Data source location | Los Angeles Biomedical Research Institute, Torrance, California, USA. |
| Data accessibility | Data is with this article. |
| Related research article | Yu-Chuan Chen, Matthew Baik, Joshua T. Byers, Kathryn T. Chen, Samuel W. French and Begoña Díaz. TKS5-positive Invadopodia-like Structures in Human Tumor Surgical Specimens. (2019) [1] |
Value of the data
-
•
TKS5 expression pattern in pancreatic centriacinar cells might be relevant for further histological and pathological studies.
-
•
Comparison of total Cortactin protein expression in a set of six commonly used pancreatic adenocarcinoma cell lines will be useful for further studies on the relevance of Cortactin in pancreatic cancer.
-
•
Co-staining of TKS5 and Cortactin in human pancreatic surgical specimens will be a valuable reference for the identification of human invadopodia and podosomes in a variety of paraffin-embedded archived tissue samples.
1. Data
Here we report experimental data on Cortactin expression in human pancreatic adenocarcinoma cells, and on TKS5 expression and co-localization with Cortactin in human pancreatic cancer cells and tumors. Experimental analysis of TKS5 immunofluorescence revealed strong TKS5 cytoplasmic staining in pancreatic solid pseudopapillary tumors (Fig. 1A), and strong TKS5 positivity in centriacinar cells within preserved acini structures in ampullary invasive adenocarcinomas (Fig. 1B). Cortactin expression data by western blotting in the pancreatic adenocarcinoma cell lines PANC-1, BxPC-3, MiaPaCa-2, SU.86.86, L3.6pl and Capan-1 is shown in Fig. 2. Fluorescence microscopy images of TKS5 and Cortactin co-staining at invadopodia in BxPC-3 cells are shown in Fig. 3. Data corresponding to co-staining of TKS5 and Cortactin, or TKS5 and F-actin in invasive ampullary adenocarcinoma cases is shown in Fig. 4.
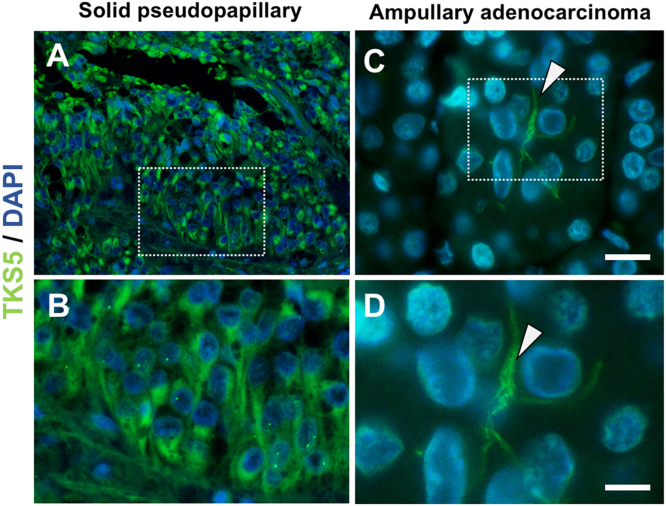
Fig. 1.
TKS5 staining in human pancreatic tumors. (A, B) Human pancreatic pseudopapillary tumor stained with TKS5 and DAPI displays intense TKS5 positivity in pseudopapillary tumor cells. (C, D) Ampullary invasive adenocarcinoma stained with TKS5 and DAPI reveals TKS5 positive centriacinar cells (arrowhead). The squares in A and C represent the area magnified in B and D respectively. Panels A, B correspond to specimen 3 and panels C, D to specimen 8 (Table 1 in Ref. [1]). Bar, 20 μm in A, C and 10 μm in B, D.
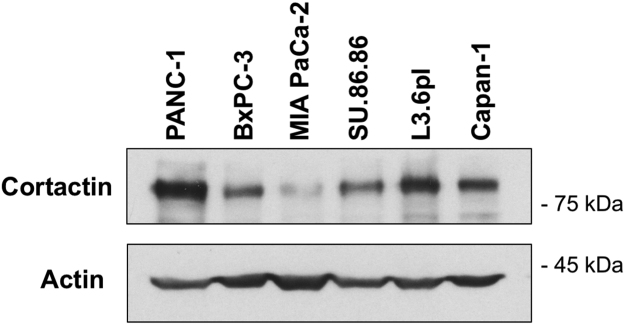
Fig. 2.
Cortactin protein expression in pancreatic adenocarcinoma cells. Protein lysates from the indicated pancreatic adenocarcinoma cell lines were subjected to electrophoresis followed by immunoblotting with the Cortactin antibody 4F11. Actin was used as loading control.
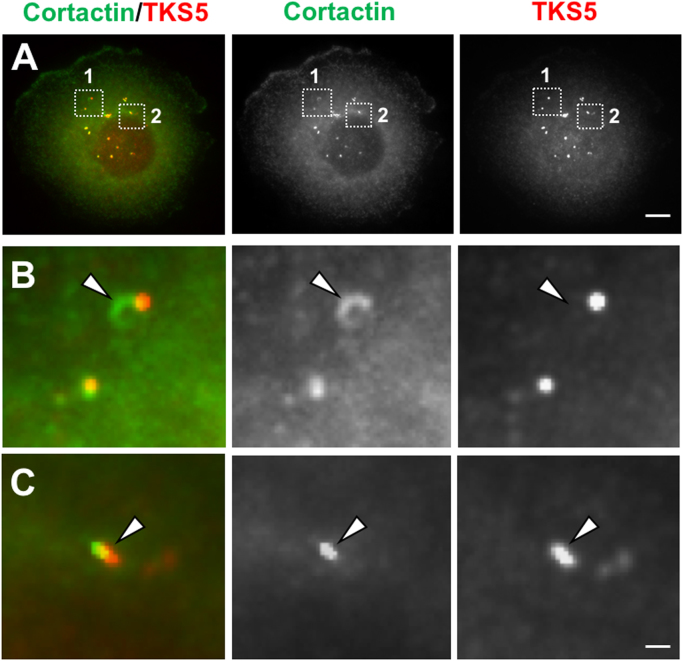
Fig. 3.
TKS5 and Cortactin co-staining in BxPC-3 pancreatic adenocarcinoma cells. (A) BxPC-3 cells were co-stained for TKS5 (red) and Cortactin (green). Some TKS5 and Cortactin positive puncta show both proteins in close apposition. Regions 1 and 2 indicated by the squares in A are magnified in B and C respectively. Arrowheads, invadopodia with TKS5 and Cortactin signals in apposition. Bar, 0.8 μm in A, and 0.05 μm in B, C.
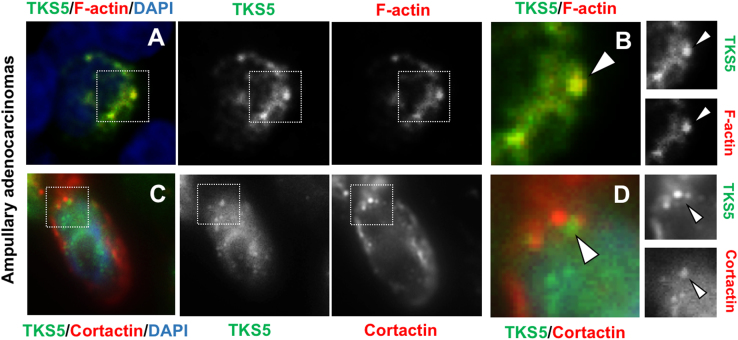
Fig. 4.
TKS5-positive puncta containing F-actin and Cortactin in human pancreatic tumor specimens. (A, B) Invasive ampullary adenocarcinoma (specimen 9, Table 1 in Ref. [1]) was stained with anti-TKS5 antibody (green) and Phalloidin (red), and DAPI (blue). Square in (A) shows the region magnified in (B). Arrowheads, F-actin and TKS5-positive puncta. (C, D) Invasive ampullary adenocarcinoma (specimen 8, Table 1 in Ref. [1]) stained with a TKS5 antibody (green) and Cortactin antibody (red), and DAPI (blue). Square in (C) shows the region magnified in (D). Arrowheads, TKS5 and Cortactin-positive puncta. Bar, 0.5 μm in A, C, and 0.05 μm in B, D.
2. Experimental design, materials and methods
2.1. Immunofluorescence analysis of TKS5 and Cortactin in BxPC-3 cells
BxPC-3 cells were grown on gelatin B-coated coverslips for 24 h to induce invadopodia formation. Coverslips were prepared using a method similar to the one we have described previously for fluorescently labeled gelatin [2], [3]. Cells were subsequently fixed with 4% paraformaldehyde, blocked in PBS containing 0.01% Triton X100 and 3% BSA and stained with a polyclonal antibody raised against mouse Tks5 (a gift from Dr. Sara Courtneidge) [4], along with a mouse monoclonal cortactin antibody (4F11, MilliporeSigma). Tks5 and cortactin antibodies were diluted at 1:500 and 1:1000 respectively. Signal was developed using anti-mouse or rabbit IgG antibodies conjugated with Alexa 488 or Alexa 594 (Thermo Scientific) diluted at 1:500. Samples were mounted in vectashield containing DAPI (Vector Labs) and visualized on a Zeiss AxioImager A1 equipped with a PRIOR Lumen 200 illumination system. Image acquisition was performed under 63x objective using an Axiocam 503 mono camera and ZEN2 software (Zeiss). Images were transferred as tiff files, and Photoshop Software was used to prepare the Figures.
2.2. Immunofluorescence analysis of invadopodia-associated proteins in human tumor surgical specimens
Archived paraffin-embedded blocks from patients treated at Harbor-UCLA Medical Center (Torrance, California, USA), who underwent surgical resections for varying pancreatic tumor pathologies were used. The study was reviewed and approved by Los Angeles Biomedical Research Institute Human Subjects Committee (IRB Protocol number 31363). Paraffin sections were subjected to antigen retrieval for 15 minutes in a microwave using antigen retrieval solution (HK086 from Biogenex). After blocking in 5% normal serum, samples were incubated with anti-TKS5 antibody from LSBio (LS-C312027) diluted 1:50 overnight at 4 °C. Signal was developed with anti-rabbit IgG coupled with Alexa 488 (Jackson Immuno Research) at 1:100. For TKS5 co-staining with F-actin, Phalloidin coupled with Alexa 568 (Thermo Scientific) was included along with the secondary antibody. For TKS5 co-staining with Cortactin, samples were sequentially stained with TKS5 as indicated above, and then with the Cortactin antibody 4F11 diluted at 1:50 overnight at 4 °C. Signal was developed with anti-mouse IgG coupled with Alexa 594 (Jackson Immuno Research) at 1:200 dilution. Images were obtained using 63x or 100x oil-immersion objective in a Nikon eclipse E400 microscope equipped with an Excelitas X-Cite 120 fluorescence illuminator, a Nikon Y-IDP Double port, a Nikon DS-Fi2 camera head, and Nikon NIS elements D imaging software. Images were transferred as tiff files, and Photoshop Software was used to prepare the Figures.
2.3. Immunoblotting
Cells were lysed in lysis buffer containing 1% NP40. A total of 30 micrograms of protein were run per sample on an 8% acrylamide gel. After electrotransfer, membranes were blocked in 5% milk in PBS and incubated overnight at 4 °C in primary antibody (4F11) diluted 1:1,000 in PBS containing 0.1% Tween20 and 0.3% BSA. Membrane was washed and incubated with HRP-conjugated anti-mouse (GE Healthcare) and imaged using enhanced chemiluminescence (Supersignal West Pico PLUS, ThermoFisher Scientific) and a film developer. Immunoblotting with Anti-β actin antibody (D6A8) from Cell Signaling Technology was used as a loading control. Films were scanned to digital files.
Acknowledgements
We are grateful to Barbara French and Isabel Mejia for excellent technical assistance. We thank Dr. Sara Courtneidge (OHSU) for sharing the Tks5 polyclonal antibody 1737.
This work has been supported by Los Angeles Biomedical Research Institute Grant, and by NIH National Center for Advancing Translational Sciences (NCATS) UCLA CTSI Grant number UL1TR001881.
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.11.138.
Transparency document. Supplementary material
Supplementary material
.
References
- 1.Y. Chen, M. Baik, J.T. Byers, K.T. Chen, S.W. French, B. Diaz, TKS5-positive invadopodia-like structures in human tumor surgical specimens, Exp. Mol. Pathol. (106) Feb 2019, 17-26. 10.1016/j.yexmp.2018.11.005 [DOI] [PMC free article] [PubMed]
- 2.Diaz B. Invadopodia detection and gelatin degradation assay. Bio Protoc. 2013;3(24) doi: 10.21769/BioProtoc.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.B. Diaz, et al., Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia, J Cell Biol. 201 (2), (2013), 279–292. 10.1083/jcb.201209151 [DOI] [PMC free article] [PubMed]
- 4.Lock P. A new method for isolating tyrosine kinase substrates used to identify fish, an SH3 and PX domain-containing protein, and Src substrate. EMBO J. 1998;17(15):4346–4357. doi: 10.1093/emboj/17.15.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material