Introduction
Methotrexate (MTX) is an antirheumatic drug used worldwide in many indications of chronic inflammation and autoimmunity including psoriasis.1 In principle, it is safe if monitored carefully and has major cost-effective therapeutic benefits.2 Major adverse events for MTX are related to the folate antagonism and primarily affect highly proliferative tissues such as bone marrow and gastrointestinal mucosa.3 Life-threatening myelosuppression leading to sepsis with potential fatal outcome is the most serious complication of MTX treatment that may occur despite regular dose administration.4 By analyzing 3 cases of myelosuppression during low-dose MTX treatment with mucocutaneous manifestations, we find that impaired renal elimination to be the leading cause of MTX toxicity and identify the different durations of plasma and intracellular half-lives of MTX as the main reason for bone marrow suppression in spite of normal MTX trough levels.
Case reports
Case 1
A 60-year-old man weighing 86 kg had been treated with low-dose (15 mg/wk) MTX for psoriasis and psoriatic arthritis for more than 12 months when painful oral lesions developed. Because of joint pain, he had been taking up to 150 mg diclofenac-natrium daily. Upon developing painful gingiva necrosis, he searched advice from a dentist before presenting at the department of dermatology. Medical examination found necrotizing gingivitis, mucosal erosions (Fig 1), and purpuric skin lesions. Serum creatinine was 0.9 mg/dL (0.5-1.2 mg/dL). Leukopenia (1.2 × 109/L [normal range, 4.0-11.0 × 109/L]) with 57% neutrophilic granulocytes, and thrombocytopenia (6 × 109/L [normal range, 150-400 × 109/L]) were present.
Fig 1.
Necrotizing gingivitis and mucosal ulceration indicating bone-marrow suppression and incipient agranulocytosis during low-dose MTX treatment.
Case 2
A 78-year-old patient weighing 93 kg required systemic treatment for moderate-to-severe chronic plaque psoriasis. Serum creatinine was 1.8 mg/dL caused by hypertensive nephropathy. Because of reduced kidney function, the patient was started on a low dose of 7.5 mg/wk of MTX. After the second MTX application, the patient had monomorphic erosions of all psoriatic plaques. Serum creatinine level had increased to 2.4 mg/dL, leukocyte counts decreased from 9.3 × 109/L to 1.3 × 109/L, with neutropenia (0.71 × 109/L [1.78-6.23 × 109/L]), and thrombocytopenia (87 × 109/L). C-reactive protein in serum increased to 31.2 mg/dL (<0.5 mg/dL). MTX-induced myelosuppression was diagnosed as a consequence of MTX-induced acute renal failure (ARF) in chronic nephropathy.
Case 3
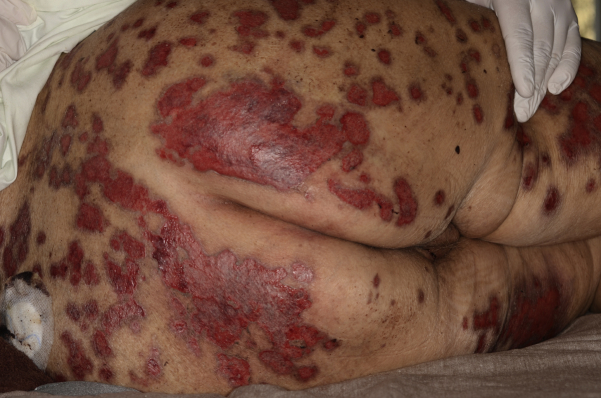
A 74-year-old woman weighing 71 kg with chronic plaque psoriasis treated with low-dose (15 mg/wk) MTX for 6 years recently had soreness of psoriatic plaques. Clinical examination found disseminated sharply demarcated monomorphic erosions with hemorrhagic crusts in all prior psoriatic skin lesions (Fig 2). Skin biopsy confirmed the presence of epidermal necrosis and dyskeratosis. MTX serum trough level was in the lower therapeutic range. Serum creatinine had increased from formerly 0.9 mg/dL (0.5-1.0 mg/dL) to 2.5 mg/dL. Laboratory abnormalities included 0.4 × 109/L leukocytes with virtually absent neutrophils and 103 × 109/L platelets (150- 400 × 109/L). Serum levels of C-reactive protein and procalcitonin were 28.5 mg/dL (<0.5 mg/dL) and 8.9 mg/dL (<0.5 mg/dL), respectively, indicating septic status. Acute nephrotoxicity from MTX was diagnosed, leading to impaired MTX elimination and severe granulocytopenia.
Fig 2.
Monomorphic erosions of all pre-existing psoriatic plaques as an early sign of MTX toxicity and myelosuppression.
Discussion
Considering the relationship between renal elimination and the different durations of plasma and intracellular MTX half-lives is essential for understanding MTX toxicity. Elimination of MTX occurs mainly via renal clearance and involves glomerular filtration and active tubular secretion. Final MTX plasma half-life for low-dose treatment is 8 to 10 hours. Intracellular MTX uptake occurs actively via the reduced folate carrier. Within the cells, MTX undergoes polyglutamation through the enzyme folylpolyglutamate synthetase. Although polyglutamation enhances MTX activity, the additional negative charge prolongs intracellular MTX retention. This determines an intracellular half-life of greater than 12 days and is responsible for intracellular MTX accumulation.5 Any condition that impairs renal elimination prolongs plasma half-life and increases intracellular MTX uptake and accumulation. Exceeding a certain intracellular threshold may then suppress replication of highly proliferating tissues. Thus, granulopoiesis, thrombopoiesis, and gastrointestinal mucosa are primary targets of the suppressive MTX effects. Agranulocytosis can occur abruptly because neutrophilic granulocytes have a lifespan of only up to 5 days in the circulation.6
Three major clinical conditions may delay MTX elimination and prolong serum half-life: pre-existing renal impairment of any cause, nephrotoxicity of MTX itself, and drug-drug interactions interfering with renal excretion of MTX. The patients reported here had several risk factors related to impaired renal MTX elimination predisposing them to increased MTX toxicity despite normal serum trough levels. The patient in case 1 was using diclofenac-natrium without medical supervision. Nonsteroidal anti-inflammatory drugs such as diclofenac are significant competitors of MTX elimination.7 The patient in case 2 had no concomitant medication competing with MTX but pre-existing nephropathy. Although MTX dosage had been adjusted for nephropathy, the nephrotoxic effects of MTX itself had obviously induced acute renal failure, which decreased MTX elimination further. The patient in case 3 had concomitant medication including acetylsalicylic acid, hydrochlorothiazide, and pantoprazole, all of which may interfere with MTX elimination and compete with plasma protein binding.8, 9, 10 Still, MTX had been tolerated despite the co-medication for several years. A sudden unanticipated nephrotoxic effect of MTX then likely promoted relative MTX overdosage in combination with drugs competing for renal MTX elimination.
Physicians treating psoriasis should be aware of the typical warning signs of incipient MTX toxicity that our cases describe, as they may indicate MTX-induced myelosuppression. Ulcerations of the oral mucosa and necrotizing gingivitis can herald pancytopenia.4 This finding may be caused by reduced mucosal proliferation and increased virulence of oral microbiota resulting from granulocytopenia.11 Epidermal necrosis in existing psoriasis plaques is a clinical symptom of MTX toxicity reserved for psoriasis patients.12 Typically, all psoriasis plaques are affected simultaneously with monomorphic erosions. Although the skin is usually not a primary target for MTX overdosage, because of a moderate proliferation rate, the increased epidermal turnover in psoriatic plaques may render keratinocytes particularly susceptible to MTX-induced necrosis.12 Although erosions of psoriasis plaques caused by MTX cannot be dismissed but may be misinterpreted, they are not mentioned in recent guidelines.8, 13
Our 3 cases illustrate that impaired renal MTX elimination of different causes is a significant reason for toxicity with therapeutic MTX doses. It increases the time of MTX exposure and cellular uptake, thereby increasing intracellular MTX concentrations and promoting toxic MTX effects caused by intracellular MTX accumulation despite normal serum MTX levels. Physicians need to be aware of the direct relationship between the duration of plasma half-life and intracellular MTX accumulation and consider any condition that impairs renal elimination of MTX as a relative contraindication for MTX treatment. Warning signs that may herald MTX toxicity with myelosuppression and agranulocytosis may occur in highly proliferative tissues and include necrotizing gingivitis and angina, purpura from thrombocytopenia, and monomorphic erosions of psoriasis plaques. Appropriate interpretation of these early clinical symptoms by both patients and physicians is vital for a timely diagnosis of myelosuppression and for preventing fatal outcomes through sepsis.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Zhai Z., Chen L., Yang H. Can pretreatment serum calcium level predict the efficacy of methotrexate in the treatment of severe plaque psoriasis? J Am Acad Dermatol. 2015;73:991–997. doi: 10.1016/j.jaad.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 2.Haustein U.F., Rytter M. Methotrexate in psoriasis: 26 years' experience with low-dose long-term treatment. J Eur Acad Dermatol Venereol. 2000;14:382–388. doi: 10.1046/j.1468-3083.2000.00058.x. [DOI] [PubMed] [Google Scholar]
- 3.Campalani E., Arenas M., Marinaki A.M. Polymorphisms in folate, pyrimidine, and purine metabolism are associated with efficacy and toxicity of methotrexate in psoriasis. J Invest Dermatol. 2007;127:1860–1867. doi: 10.1038/sj.jid.5700808. [DOI] [PubMed] [Google Scholar]
- 4.Ajmani S., Preet Singh Y., Prasad S. Methotrexate-induced pancytopenia: a case series of 46 patients. Int J Rheum Dis. 2017;20:846–851. doi: 10.1111/1756-185X.13004. [DOI] [PubMed] [Google Scholar]
- 5.Olsen E.A. The pharmacology of methotrexate. J Am Acad Dermatol. 1991;25:306–318. doi: 10.1016/0190-9622(91)70199-c. [DOI] [PubMed] [Google Scholar]
- 6.Pillay J., den Braber I., Vrisekoop N. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 7.Tracy T.S., Krohn K., Jones D.R. The effects of a salicylate, ibuprofen, and naproxen on the disposition of methotrexate in patients with rheumatoid arthritis. Eur J Clin Pharmacol. 1992;42:121–125. doi: 10.1007/BF00278469. [DOI] [PubMed] [Google Scholar]
- 8.Nast A., Gisondi P., Ormerod A.D. European S3-Guidelines on the systemic treatment of psoriasis vulgaris–Update 2015–Short version–EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29:2277–2294. doi: 10.1111/jdv.13354. [DOI] [PubMed] [Google Scholar]
- 9.Breedveld P., Zelcer N., Pluim D. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 2004;64:5804–5811. doi: 10.1158/0008-5472.CAN-03-4062. [DOI] [PubMed] [Google Scholar]
- 10.Orr L.E. Potentiation of myelosuppression from cancer chemotherapy and thiazide diuretics. Drug Intell Clin Pharm. 1981;15:967–970. doi: 10.1177/106002808101501209. [DOI] [PubMed] [Google Scholar]
- 11.Naidu M.U., Ramana G.V., Rani P.U. Chemotherapy-induced and/or radiation therapy-induced oral mucositis–complicating the treatment of cancer. Neoplasia. 2004;6:423–431. doi: 10.1593/neo.04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weidmann A., Foulkes A.C., Kirkham N. Methotrexate toxicity during treatment of chronic plaque psoriasis: a case report and review of the literature. Dermatol Ther (Heidelb) 2014;4:145–156. doi: 10.1007/s13555-014-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalb R.E., Strober B., Weinstein G. Methotrexate and psoriasis: 2009 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol. 2009;60:824–837. doi: 10.1016/j.jaad.2008.11.906. [DOI] [PubMed] [Google Scholar]