Abstract
Low molecular weight heparin (LMWH) is being investigated as a potential preventative therapy against preeclampsia. There is evidence suggesting that LMWH may prevent preeclampsia through anticoagulation-independent mechanisms. In this study, we compared the in vitro placental, endothelial, and anti-inflammatory effects of an LMWH (dalteparin) with a nonanticoagulant, glycol-split heparin derivative (gsHep). In contrast with dalteparin, gsHep did not interact with antithrombin III, possess significant anti-Factor Xa activity, or significantly prolong in vitro plasma clotting time. However, dalteparin and gsHep were otherwise mechanistically similar, both interacting with soluble fms-like tyrosine kinase-1 (sFlt1) and promoting release of the pro-angiogenic protein placental growth factor, but not the antiangiogenic sFlt1, from healthy placental villous explants. Placental explant media pretreated with dalteparin or gsHep significantly stimulated endothelial cell tube formation compared to untreated explants. Lastly, dalteparin and gsHep both significantly suppressed inflammation by inhibiting complement activation and leukocyte adhesion to endothelial cells that were activated using serum from preeclamptic women. Our data suggest that nonanticoagulant heparin derivatives may be utilized as a tool to distinguish the anticoagulation-independent mechanisms of LMWH, and provide insight into the role of anticoagulation in the prevention of preeclampsia.
Keywords: preeclampsia, placenta, angiogenesis, immunology, molecular biology
Nonanticoagulant derivatives of heparin may be useful for investigating the role of anticoagulation-independent functions of heparin for the prevention of preeclampsia.
Introduction
Preeclampsia impacts 3–7% of all pregnancies and is characterized by new-onset hypertension after 20 weeks gestation with evidence of organ damage [1]. Preeclampsia is often associated with fetal growth restriction, which may necessitate iatrogenic preterm delivery [2]. The pathogenesis of most cases of severe early-onset preeclampsia (clinical presentation <34 weeks) is hypothesized to be initiated by poor spiral artery remodeling, resulting in reduced perfusion of the placenta that creates a local state of low oxygen tension, high oxidative stress, increased inflammation of the placental villi, and suppression of the secretion of the pro-angiogenic protein placenta growth factor (PlGF) into maternal blood [3]. The damaged placenta in women with severe preeclampsia subsequently secretes large amounts of antiangiogenic molecules, such as soluble fms-like tyrosine kinase-1 (sFlt1), and disrupts the function of pro-angiogenic molecules such as PlGF [4]. Hemodynamic data suggest that this antiangiogenic state mediates the development of maternal hypertension through widespread systemic maternal endothelial dysfunction that contributes to systemic vasoconstriction and impaired vital organ perfusion in women with severe early-onset preeclampsia [5].
Currently, there are limited preventative therapies for pregnant women at high-risk of preeclampsia. Low-dose aspirin can significantly reduce the incidence of preeclampsia; however, the effect is modest and benefits only approximately half of all cases [6]. Several clinical trials suggest that low molecular weight heparin (LMWH), which is often prescribed to pregnant women at elevated risk of pregnancy-related venous thromboembolism, may further reduce the rate or severity of severe preeclampsia and improve fetal outcomes [7], but more recent larger trials demonstrated no significant effect of LMWH [8, 9]. Although conflicting, these clinical trials have determined that LMWH is not effective for women at risk of all types of preeclampsia, and may only be effective for a subtype [10]. Understanding the precise molecular mechanisms that mediate the beneficial effects of LMWH in preeclampsia is therefore of critical importance, but these mechanisms are thus far not well understood. Despite its general use as an anticoagulant, there is some evidence suggesting that the potential preventive effects of LMWH in preeclampsia are mediated by nonanticoagulant mechanisms. A multicenter randomized controlled clinical trial conducted with 114 women demonstrated that LMWH reduced the recurrence of preeclampsia in high-risk pregnant women who were not at risk of thrombophilia [11]. In addition, daily unfractionated heparin therapy used at doses for venous thromboembolism prophylaxis does not significantly reduce placental thrombosis [12]. Therefore, the nonanticoagulant mechanisms of LMWH may mediate the prevention of preeclampsia, but the relevant nonanticoagulant mechanisms have not been thoroughly investigated.
LMWH possesses various nonanticoagulant properties that may be relevant in the prevention of preeclampsia. In vitro studies show that LMWH may promote extravillous trophoblast invasion and cytotrophoblast proliferation [13, 14], and promote endothelial angiogenesis [15]. Acute administration of LMWH to pregnant women at high-risk of preeclampsia improves endothelium-dependent vasodilation [16]. In addition, LMWH treatment improves angiogenesis in the presence of antiphospholipid (aPL) antibodies [17], and prevents aPL antibody-mediated fetal loss in a mouse model by suppressing inflammation [18]. Prophylactic LMWH therapy could exert beneficial effects on the pathogenic features of preeclampsia which often includes poor placental development, maternal systemic endothelial dysfunction, and inflammation.
An established chemical process called “glycol-splitting” renders heparin nonanticoagulant by disrupting the antithrombin-binding region (ATBR) that is required for anticoagulation [19]. The objective of the current study is to compare the LMWH dalteparin to a nonanticoagulant LMWH compound in their ability to modulate pathways relevant to preeclampsia pathogenesis in vitro, specifically the placental release of angiogenic factors, endothelial angiogenesis, and inflammatory pathways.
Materials and methods
Low molecular weight heparins
Nonanticoagulant heparin (glycol-split heparin, gsHep) was provided by Dilafor (Stockholm, Sweden), chemically generated by periodate oxidation of pig intestinal mucosa heparin, followed by alkaline β-oxidation of the product as previously described [20]. The average molecular weight of gsHep was approximately 6 kDa as determined by high performance gel permeation chromatography according to manufacturer's data. This is similar to the average molecular weight distribution of dalteparin [21] (Supplementary Figure S1), which is being used as the comparison for this study to allow direct comparisons at the molar basis. Concentrations of dalteparin and gsHep used for functional experiments fall within the clinical range used for dalteparin prophylaxis and acute therapy (2–10 μg/mL, equivalent to 0.3–1.5 anti-Factor Xa IU/mL).
Tissue culture
Human umbilical vein endothelial cells (HUVECs; Lonza, Walkersville, MD, USA) were maintained in EGM-2 growth media (CC-3162). Where appropriate for experiments, fetal bovine serum (Wisent Inc, St-Bruno, QC, Canada) was replaced with 5% patient serum and the heparin that was included in the growth media kit was replaced with dalteparin or gsHep. Cells were passaged at 90% confluency. Experiments performed on HUVECs utilized cells that were less than passage 8. THP-1 monocytes (ATCC, Manassas, VA, USA) were cultured in RPMI-1640 media (Thermo Fisher Scientific, Burlington, ON, Canada) containing 10% FBS and 0.05 mM β-mercaptoethanol as described by the manufacturer, and maintained at a density no greater than 1.0 × 106 cells/mL.
Anticoagulant assays
The ability of the heparins to inhibit Factor Xa, the major coagulation protease target of LMWHs, was assessed using the Biophen Heparin Anti-Xa 2-stages kit (Aniara Diagnostica, West Chester, OH) according to manufacturer instructions for assessing purified heparin activity, using dalteparin of known concentrations as the standard. Activated partial thromboplastin time (aPTT) assay was performed using the Pacific Hemostasis APTT-XL kit (Thermo Fisher Scientific) according to manufacturer's instructions.
Differential scanning fluorimetry
Differential scanning fluorimetry assesses the thermal stability of proteins in the presence or absence of nonpeptide small molecules, where a melting curve shift indicates a positive interaction [22]. Twenty microliter reaction mixtures consisting of 1.2 μg of protein, heparin (in molar excess), and 5× SYPRO Orange (Thermo Fisher Scientific) were loaded into Biorad hard-shell 96-well PCR plates (Mississauga, ON, Canada). Samples were heat denatured using a Biorad CFX96 Real-Time PCR system using a ramp configuration starting at 25°C and increasing at 1°C/minute (min) to 95°C. Fluorescence was measured every 30 s using the HEX filter configuration. Data for each curve were fitted against a four-parameter logistic curve to determine the melting temperature of the protein, defined as the temperature at half-maximal fluorescence of the curve. Recombinant sFlt1 was from BioLegend (San Diego, CA, USA), and recombinant antithrombin III (ATIII) was from Haematologic Technologies (Essex Junction, VT, USA).
Placental villous tissue explant model
Healthy first trimester placental tissue was obtained from elective terminations via the Mount Sinai Hospital Biobank with research ethics board approval and written consent from the patient (REB #11-0248-E). The placental villous explant model was employed as previously described [13]. In brief, villous trees from placenta samples of 8–12 weeks of gestation were dissected in cold sterile PBS and cultured in a 24-well plate containing DMEM Ham F12 media supplemented with 1× insulin-transferrin-selenium, 1× penicillin-streptomycin-glutamine, 0.25 μg/mL fungizone, and 500 μg/mL gentamicin (all from Thermo Fisher Scientific). Explants were acclimated overnight in a humidified 37°C incubator kept at 8% O2/5% CO2 before treating with heparin compounds prepared in fresh media for 24 h. The treated tissues and conditioned media were stored at –80°C until further use. sFlt1 and PlGF ELISAs (R&D Systems, Minneapolis, MN, USA) were used to determine protein release into the conditioned media from control and treated explants as per manufacturer's instructions.
Quantitative polymerase chain reaction
RNA extraction and qRT-PCR was performed as described [16]. Primers used in this study are provided in Supplementary Table S1. Gene expression was normalized to the geometric mean of the housekeeping genes YWHAZ, TOP1, and HPRT.
Endothelial tube formation assay
Fifty microliter of growth factor-reduced Matrigel was added to a 96-well tissue culture plate and allowed to polymerize at 37°C for 20 min. A total of 17 000 HUVEC cells/well were seeded on top of the Matrigel in the presence of 50% conditioned media from untreated and treated placental explants diluted in endothelial basal media. Cells were incubated in a humidified 37°C incubator at 20% O2/5% CO2 for 16 h before imaging with light microscopy and manual analysis by ImageJ Software assessing total tube length in the field of view.
Serum samples
Serum samples from preeclamptic patients were collected upon admission for delivery at Mount Sinai Hospital (Toronto, ON) upon informed consent (REB #11-0248-E). All samples were collected from patients diagnosed with early-onset preeclampsia at 26–28 weeks with fetal growth restriction. Specific patient characteristics are provided in Supplementary Table S2. Whole blood was collected into BD Vacutainer SST tubes (#367988), centrifuged at 2 000 g for 15 min, and then the separated serum was stored at –80°C until use.
Complement-mediated hemolysis assay and ELISA
Washed 5% sheep erythrocytes (Cedarlane, Burlington, ON, Canada), in HEPES-buffered saline, which are sensitive to lysis by activation of the classical pathway of complement [23], were mixed, in order, with 1:100 dilution of antisheep erythrocyte IgM (Cedarlane), 1 mM CaCl2, 1 mM MgCl2, heparin, and 3.5% patient serum to induce complement-mediated hemolysis. The reaction was incubated for 30 min at 37°C and stopped with 330 mM EDTA. Unlysed cells were pelleted by centrifuging at 600 g for 5 min, and 100 μL of the supernatant was mixed with 100 μL water in duplicates. The absorbance of this mixture at A405nm was used to determine the hemoglobin release into the supernatant via lysis of the erythrocytes. In the absence of treatment, the reaction conditions resulted in 70% lysis of erythrocytes compared to total lysis with water. For treatment effects, lysis was relative to the control conditions. An aliquot of the hemolysis reaction supernatant for C5a measurements was stored at –80°C until use. A C5a ELISA kit (BioLegend) was used to measure C5a generation.
Leukocyte adhesion assay
HUVECs were seeded onto 96-well plates at a density of 50 000 cells/well and allowed to adhere overnight in the absence of heparin, and then stimulated with 5% preeclamptic serum in EGM-2 media without FBS for 2 h. Following treatment, cells were washed two times with PBS and then incubated with heparin compounds in EGM-2 media for 1 h. Meanwhile, THP-1 cells were labeled with calcein-AM according to manufacturer instructions and resuspended in EGM-2 media. Following heparin incubation, labeled THP-1 cells were added directly on top of the activated HUVECs and allowed to adhere for 45 min. Nonadherent cells were removed through a series of three washes with PBS, and adhered cells were quantified by measuring the fluorescence intensity at 480 nm ex/520 nm em using the Tecan Infinite M200 microplate reader (Mannedorf, Switzerland). Values were normalized to FBS control in growth media.
Statistical analyses
All experiments were performed as technical triplicates unless otherwise stated. Data are presented as mean ± standard error. One-way ANOVA with Bonferroni's post hoc was used to compare melting temperature changes in the absence or presence of treatment. Two-way ANOVA with Bonferroni's post hoc was used to compare dose-dependent differences between dalteparin and gsHep. Repeated measures ANOVA with Bonferroni's post hoc was used to compare the dose-dependent effect of treatment to the untreated condition. GraphPad Prism 5.0 software (La Jolla, CA, USA) was used for analyses and P ≤ 0.05 was considered statistically significant.
Results
Glycol-split heparin has negligible anti-FXa and anticoagulant activity
The lack of anticoagulant properties of gsHep was verified using clinically relevant assays for coagulant activity. Using a commercially available anti-Xa activity assay, gsHep showed negligible anti-FXa activity in comparison with dalteparin (P < 0.001; Figure 1A). In addition, gsHep showed significantly reduced ability to prolong aPTT fibrin clot formation, in comparison with dalteparin at concentrations greater than 1 μg/mL (P < 0.001; Figure 1A). These results confirm that gsHep has substantially reduced anticoagulant activity.
Figure 1.
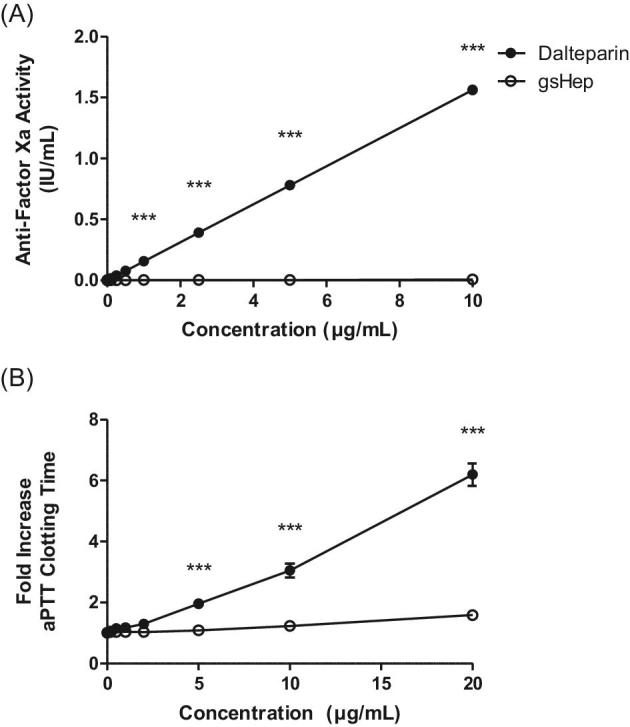
Anticoagulant properties of dalteparin and gsHep. (A) Glycol-split heparin had negligible anti-Factor Xa activity compared to dalteparin at all concentrations tested. (B) Glycol-split heparin had significantly reduced ability to prolong aPTT clotting time compared to dalteparin at concentrations beyond 5 μg/mL. ***P < 0.0001 compared to dalteparin at equivalent concentration. aPTT, activated partial thromboplastin time. n = 3 independent experiments.
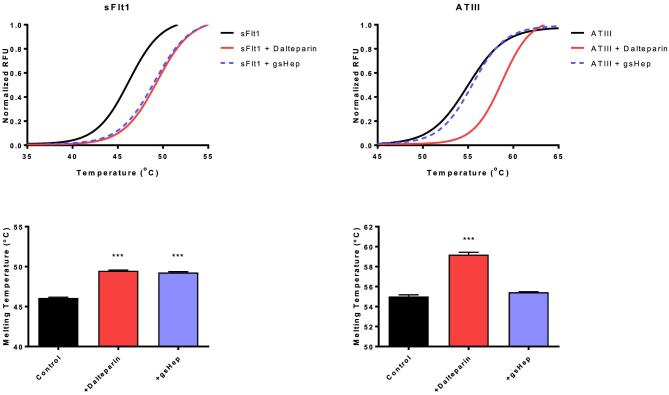
Glycol-split heparin interacts with sFlt1 but not ATIII
Differential scanning fluorimetry was used to assess the ability of dalteparin and gsHep to interact with various proteins (Figure 2). Dalteparin and gsHep both induced a significant shift in the melting curve of sFlt1, indicative of a positive interaction (P < 0.001). The magnitude of the shift was not significantly different between dalteparin and gsHep (P > 0.05). Other classic heparin-binding growth factors, such as FGF1 and FGF2, also interact with gsHep (Supplementary Figure S2). While dalteparin induced a curve shift for ATIII, no significant shift was observed in the presence of gsHep. This confirms that gsHep does not bind ATIII.
Figure 2.
Differential scanning fluorimetry for sFlt1 and ATIII interactions with dalteparin and gsHep. Both dalteparin and gsHep induced a melting curve shift for sFlt1, indicating a positive interaction. However, only dalteparin and not gsHep shifted the melting curve for ATIII, indicating the inability for gsHep to interact with ATIII. ***P < 0.0001 compared to no heparin control. n = 3 independent experiments. RFU, relative fluorescence units; sFlt1, soluble fms-like tyrosine kinase-1; ATIII, antithrombin III.
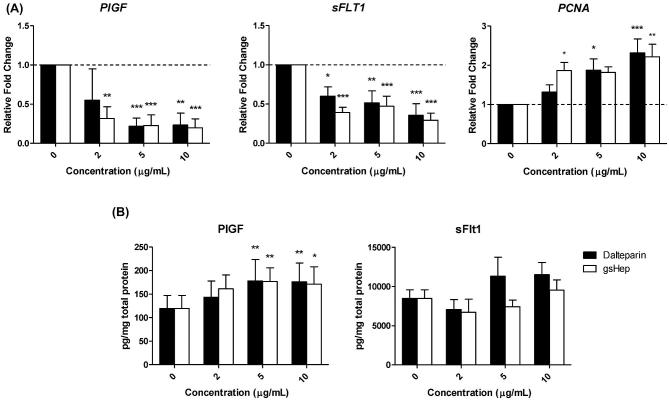
Glycol-split heparin and dalteparin elicit similar gene expression and protein release from first trimester placental villous explants
Healthy first trimester placental explants were treated with dalteparin and gsHep to evaluate gene expression and protein release. Dalteparin and gsHep dose-dependently downregulated placental explant PlGF and sFLT1 mRNA (ρ < 0.001 and ρ < 0.0001, respectively) compared to untreated controls (Figure 3A). mRNA of the proliferation gene PCNA was increased with both treatments (ρ < 0.01), suggesting the absence of toxicity. The effects between dalteparin and gsHep were not significantly different (P > 0.05). Conditioned media from 5- and 10 μg/mL dalteparin and gsHep-treated placental explants had elevated PlGF protein levels (ρ < 0.01), but not sFlt1 protein levels compared to media from untreated explants (Figure 3B). No differences in effects between dalteparin and gsHep were observed. These results indicate that gsHep and dalteparin elicit similar angiogenic regulator mRNA level changes and protein release from placental villous explants.
Figure 3.
Dalteparin and gsHep elicit similar gene expression and protein release from first trimester placental villous explants. (A) Both treatments dose-dependently suppressed mRNA levels of PlGF and sFLT1 while increasing mRNA levels of the proliferation gene PCNA. (B) Glycol-split heparin and dalteparin increased PlGF release into the conditioned media without significantly affecting sFlt1 release. *P < 0.05, **P < 0.01, ***P < 0.001 compared to no heparin controls. n = 6 placenta and media samples. PlGF, placental growth factor; sFlt1, soluble fms-like tyrosine kinase-1; PCNA, proliferating cell nuclear antigen.
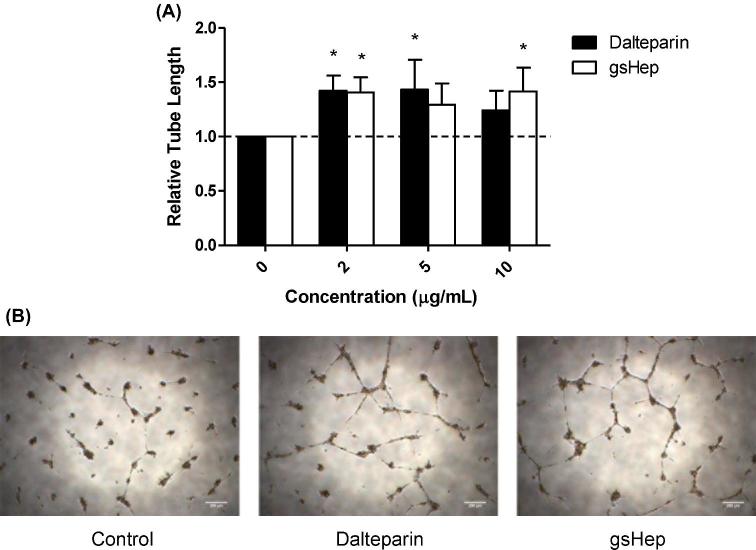
Endothelial tube formation is stimulated by media conditioned by gsHep-treated placenta explants
Endothelial tube formation assays were performed to evaluate the angiogenic effect of the conditioned media from dalteparin and gsHep treated explants. Media from 2 μg/mL dalteparin and gsHep treated placental explants stimulated HUVEC tube formation on Matrigel compared to media from untreated explants, increasing the total lengths of tubes formed by 1.42 ± 0.14 and 1.41 ± 0.14 fold, respectively (ρ < 0.05; Figure 4A). No significant difference was observed between dalteparin and gsHep treatments. Representative images of the tube formation experiments are shown in Figure 4B. These data suggest that gsHep can also promote endothelial function similarly to dalteparin.
Figure 4.
Angiogenic effects of placenta conditioned media from dalteparin and gsHep-treated placental explants. (A) Placenta-conditioned media from dalteparin- or gsHep-treated explants stimulated tube formation from HUVECs on growth factor-reduced Matrigel compared to media from untreated explants. (B) Representative images of tube formation were taken from HUVECs treated with media conditioned with 2 μg/mL treatment. *P < 0.05 compared to no heparin controls. n = 5 media samples. Scale bars = 200 μm.
Glycol-split heparin inhibits complement activation
Dalteparin and gsHep were evaluated for the ability to inhibit complement activation. Both dalteparin and gsHep dose-dependently inhibited complement-mediated hemolysis of sheep erythrocytes, using serum from preeclamptic patients as the source of complement. No significant difference was observed between dalteparin and gsHep treatments. The concentration at which dalteparin and gsHep inhibited 50% of lytic activity was achieved at 10 μg/mL treatment (P < 0.0001; Figure 5A). To determine whether dalteparin and gsHep also reduced the generation of the C5a pro-inflammatory anaphylatoxin, a C5a ELISA was performed on the hemolytic reaction supernatant. There was a dose-dependent decrease in the generation of C5a for both treatments, also achieving approximately 50% inhibition at 10 μg/mL treatment concentration (P < 0.0001; Figure 5B). No significant difference between dalteparin and gsHep was observed. These results suggest that both dalteparin and gsHep may impact complement activation in a comparable manner.
Figure 5.
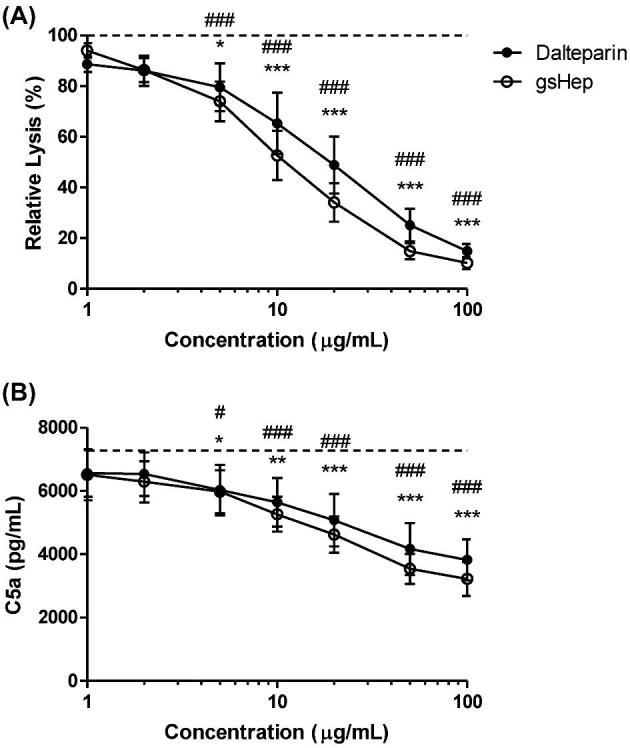
Anticomplement activity of dalteparin and gsHep. (A) Inclusion of dalteparin or gsHep in an assay of complement-mediated hemolysis of red blood cells dose-dependently suppressed lysis. (B) ELISA analyses of the reaction supernatant also showed a reduction in the generation of the active C5a pro-inflammatory mediator. Dotted line represents no heparin control. * = dalteparin; # = gsHep. *,#P < 0.05, **,##P < 0.01, ***,###P < 0.001 compared to no heparin control. n = 3 serum samples.
Glycol-split heparin prevents leukocyte adhesion to preeclamptic serum-activated endothelial cells
The leukocyte adhesion assay was used to investigate if the heparins can reduce the inflammatory effects of endothelial activation. As previously reported, preeclamptic serum activated cultured endothelial cells and increased THP-1 monocyte adhesion to the endothelial monolayer [24]. Both dalteparin and gsHep interfered with the adherence of THP-1 monocytes onto activated endothelial cells when treatment was incubated with the cells following activation but prior to seeding of leukocytes (P < 0.01; Figure 6). No significant difference between dalteparin and gsHep was observed. These data suggest that gsHep can also inhibit inflammation mediated by leukocyte adhesion to activated endothelial cells in preeclampsia.
Figure 6.
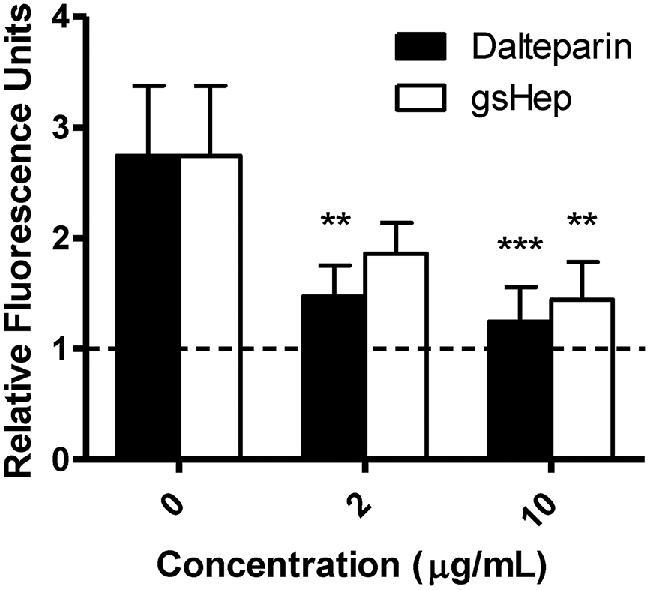
Inhibition of leukocyte adhesion onto activated endothelial cells by dalteparin and gsHep. Preeclamptic serum stimulated THP-1 monocyte adhesion to HUVECs compared to FBS control (dashed line), and both dalteparin and gsHep can interfere with this reaction. **P < 0.01, ***P < 0.001 compared to no heparin control. n = 8 serum samples.
Discussion
Despite differences in anticoagulant properties, we demonstrate that the LMWH dalteparin and the nonanticoagulant gsHep exert similar in vitro placental, endothelial, and anti-inflammatory effects. Both dalteparin and gsHep stimulate PlGF release from first trimester placental explants, promote endothelial tube formation in the presence of placenta conditioned media, inhibit complement activation, and inhibit leukocyte adhesion to endothelial cells activated by preeclamptic serum. Overall, these collective actions suggest that both heparins evaluated may be capable of suppressing the various pathways that could contribute to the development of severe preeclampsia.
Placental ischemic injury is a hallmark of severe preeclampsia [3], and therefore a drug strategy that is designed to support a more normal physiologic trajectory of placental development is an attractive approach to preventing the most severe forms of preeclampsia. In accordance with our previous study [13], both dalteparin and gsHep increased gene expression of PCNA, a proliferation antigen, in whole first trimester placental explants. Restoration of the physiologic turnover of the villous trophoblast compartment may be an effective pathway to prevent preeclampsia, as healthy syncytiotrophoblast secretes PlGF into the maternal circulation [25], which is hypothesized to be protective against this disease [26, 27]. We determined that, similar to dalteparin, gsHep increased the angiogenic potential of the conditioned media derived from treated explants and improved tube formation of cultured endothelial cells on Matrigel compared to media from untreated explants. This was accompanied by an increase in PlGF levels in the conditioned media; while the mechanism has not been well-explored, heparin could potentially elevate PlGF release by promoting de novo synthesis, stimulating release of intracellular stores, or displacing cell-surface heparin-binding PlGF isoforms, such as PlGF-2 or PlGF-4. Previous investigators have also shown that LMWH enhances trophoblast differentiation and survival [28, 29] and promotes extravillous trophoblast invasion [30]. Collectively, these data support the hypothesis that LMWH favorably influences placental development and normalizes angiogenic protein release, which may subsequently alleviate the systemic endothelial dysfunction evident in women with preeclampsia.
sFlt1 is associated with the extracellular matrix, and LMWH can bind to and displace sFlt1 from endothelial cell surfaces [31, 32]. Although the in vivo implications of this effect remains unclear, we speculate that this interaction could disrupt the decoy action of sFlt1 at the VEGFR2 endothelial cell surface, and contribute to the clearance of excess sFlt1 [31]. Here, we show that, in the absence of a functional antithrombin-binding region, gsHep can also bind to sFlt1. Therefore, a nonanticoagulant heparin may, in theory, be used to facilitate the efficient removal of sFlt1 from the blood. This mechanism may be equivalent to apheresis of sFlt1 using a heparin-binding column [33], yet could be safer, more feasible, and cost-effective.
Widespread host inflammation and complement activation is present in women with preeclampsia. Activated complement fragments are observable in biological fluids [34–36] and on the placenta, which can play a role in placental damage [37, 38]. Excess complement activation also occurs in HELLP (hemolysis, elevated liver enzymes, and low platelet) syndrome, inhibition of which could lead to disease resolution [39]. Elevated serum levels of the complement anaphylatoxins such as C5a can also contribute to endothelial dysfunction [40]. In this study, we showed that both dalteparin and gsHep can suppress complement activation, leading to reduced lytic damage to sheep erythrocytes and reduced generation of C5a. Clinically, relevant doses of LMWH have also been shown to suppress complement activation in vivo [41]. Suppression of complement-mediated inflammation is therefore a potential mechanism by which LMWH can confer protection against preeclampsia.
The premise of LMWH therapy for the prevention of preeclampsia has traditionally been to prevent thrombosis-mediated placental damage. However, the strength of the association between placental thrombotic disease and preeclampsia is controversial [42], with recent data suggesting only a 25% contribution to disease burden [43]. A wide variety of other factors, including placental dysfunction, cardiovascular status, and inflammation, also contributes to increased preeclampsia risk, which in turn may be blocked by nonanticoagulant actions [11]. Interestingly, a recent meta-analysis suggests that LMWH may be most beneficial for at-risk women without thrombophilia [44], suggesting that LMWH confers protection against preeclampsia via nonanticoagulant pathways. This concept has not been directly investigated. Our validation of gsHep in this study as an LMWH mimetic without anticoagulant properties serves as a justification for in vivo experiments to determine specifically whether the nonanticoagulant effects are therapeutically relevant. A hypothetical advantage of a nonanticoagulant therapeutic is that it may permit the use of such heparin derivatives in pregnant women for the prevention of preeclampsia without incurring significant risk of bleeding complications.
There are limitations to the interpretation of results related to this investigation. First, a major limitation is the inherent disadvantages of in vitro studies, the results of which do not necessarily translate to the in vivo settings. The current study provides a strong basis for further investigation into the in vivo relevance of the observed effects and the anticoagulation-independent actions of heparin for the prevention of preeclampsia. Second, while gsHep is a very close approximation to native heparin in biochemical structure, there may be minor differences that may or may not impact its nonanticoagulant functions. The process of glycol-splitting that yields gsHep specifically modifies nonsulfated uronic acid residues, which mainly localizes to the ATBR but may be found scattered throughout the molecule [19]. It is not known whether these modifications outside of the ATBR will also impact nonanticoagulant properties not assessed in this study. Furthermore, disruption of the ATBR prevents the inactivation of the major coagulation enzymes Factor Xa and thrombin by heparin-antithrombin complexes. However, these enzymes may also contribute to additional anticoagulation-independent effects such as by activating protease-activated receptors on cell surfaces or regulating the activity of other proteins through proteolytic cleavage, among other effects [45]. Thus, these nonanticoagulant effects may not be fully evaluated by gsHep.
In summary, we have characterized several nonanticoagulant actions of a modified form of LMWH with minimal ATIII binding activity. This drug demonstrated promotion of PlGF release by placental villi, enhanced endothelial cell tube formation exposed to placenta-conditioned media, inhibition of complement activation, and suppression of leukocyte adhesion to activated endothelial cells. These results warrant further investigation and testing of anticoagulant and nonanticoagulant heparin derivatives in appropriate animal models of preeclampsia to evaluate the relevance of nonanticoagulant pathways modulated by LMWH for the prevention of preeclampsia.
Supplementary Material
Acknowledgments
The authors thank the donors, the Research Centre for Women's and Infants’ Health BioBank program, the Lunenfeld-Tanenbaum Research Institute, and the MSH/University Health Network Department of Obstetrics & Gynaecology for the human specimens used in this study.
Notes
Edited by Dr. Romana Nowak, PhD, University of Illinois Urbana-Champaign
Footnotes
Grant Support: This work was funded by Canadian Institutes of Health Research Operating Grant.
Supplementary data
Supplementary Figure S1. Molecular weight distribution of different heparin compounds compared to gsHep. Fifty microgram of unfractionated heparin, enoxaparin, dalteparin, and gsHep were separated by gel electrophoresis in a 10% tris-borate-EDTA gel and stained with a 1% solution of alcian blue in 3% acetic acid to visualize sulfated glycosaminoglycans. Dalteparin and gsHep exhibited a similar size distribution compared to enoxaparin, which has a lower average molecular weight.
Supplementary Figure S2. Differential scanning fluorimetry showed melting curve shifts of the bonafide heparin-binding growth factors FGF1 and FGF2 in the presence of either dalteparin or gsHep compared to protein-only curve, indicating a positive interaction.
Supplementary Table S1. List of primers used for qRT-PCR studies.
Supplementary Table S2. Patient characteristics.
References
- 1. American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 2. Mitani M, Matsuda Y, Makino Y, Akizawa Y, Ohta H. Clinical features of fetal growth restriction complicated later by preeclampsia. J Obstet Gynaecol Res 2009; 35:882–887. [DOI] [PubMed] [Google Scholar]
- 3. Jim B, Karumanchi SA. Preeclampsia: pathogenesis, prevention, and long-term complications. Semin Nephrol 2017; 37:386–397. [DOI] [PubMed] [Google Scholar]
- 4. Maynard S, Min J, Merchan J, Lim K, Li J, Mondal S, Libermann T, Morgan L, Sellke F, Stillman I, Epstein F, Sukhatme V et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doherty A, Carvalho JCA, Drewlo S, El-Khuffash A, Downey K, Dodds M, Kingdom J. Altered hemodynamics and hyperuricemia accompany an elevated sFlt-1/PlGF ratio before the onset of early severe preeclampsia. J Obstet Gynaecol Can 2014; 36:692–700. [DOI] [PubMed] [Google Scholar]
- 6. Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol 2017; 216:110–120.e6.e6. [DOI] [PubMed] [Google Scholar]
- 7. Rodger MA, Carrier M, Le Gal G, Martinelli I, Perna A, Rey E, de Vries JIP, Gris JC, Low-Molecular-Weight Heparin for Placenta-Mediated Pregnancy Complications Study Group, Meta-analysis of low-molecular-weight heparin to prevent recurrent placenta-mediated pregnancy complications. Blood 2014; 123:822–828. [DOI] [PubMed] [Google Scholar]
- 8. Haddad B, Winer N, Chitrit Y, Houfflin-Debarge V, Chauleur C, Bages K, Tsatsaris V, Benachi A, Bretelle F, Gris JC, Bastuji-Garin S. Enoxaparin and aspirin compared with aspirin alone to prevent placenta-mediated pregnancy complications. Obstet Gynecol 2016; 128:1053–1063. [DOI] [PubMed] [Google Scholar]
- 9. Groom KM, McCowan LM, Mackay LK, Lee AC, Said JM, Kane SC, Walker SP, van Mens TE, Hannan NJ, Tong S, Chamley LW, Stone PR et al. Enoxaparin for the prevention of preeclampsia and intrauterine growth restriction in women with a history: a randomized trial. Am J Obstet Gynecol 2017; 216:296.e1–296.e14. [DOI] [PubMed] [Google Scholar]
- 10. McLaughlin K, Scholten RR, Parker JD, Ferrazzi E, Kingdom JCP. Low molecular weight heparin for the prevention of severe preeclampsia: where next? Br J Clin Pharmacol 2018; 84:673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, Morin F, Demers C, Kahn SR, Magee LA, Rodger M. Dalteparin for the prevention of recurrence of placental-mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. J Thromb Haemost 2009; 7:58–64. [DOI] [PubMed] [Google Scholar]
- 12. D’Souza R, Keating S, Walker M, Drewlo S, Kingdom J. Unfractionated heparin and placental pathology in high-risk pregnancies: secondary analysis of a pilot randomized controlled trial. Placenta 2014; 35:816–823. [DOI] [PubMed] [Google Scholar]
- 13. Drewlo S, Levytska K, Sobel M, Baczyk D, Lye SJ, Kingdom JCP. Heparin promotes soluble VEGF receptor expression in human placental villi to impair endothelial VEGF signaling. J Thromb Haemost 2011; 9:2486–2497. [DOI] [PubMed] [Google Scholar]
- 14. Di Simone N, Di Nicuolo F, Sanguinetti M, Ferrazzani S, D’Alessio MC, Castellani R, Bompiani A, Caruso A. Low-molecular weight heparin induces in vitro trophoblast invasiveness: role of matrix metalloproteinases and tissue inhibitors. Placenta 2007; 28:298–304. [DOI] [PubMed] [Google Scholar]
- 15. Sobel ML, Kingdom J, Drewlo S. Angiogenic response of placental villi to heparin. Obstet Gynecol 2011; 117:1375–1383. [DOI] [PubMed] [Google Scholar]
- 16. McLaughlin K, Baczyk D, Potts A, Hladunewich M, Parker JD, Kingdom JCP. Low molecular weight heparin improves endothelial function in pregnant women at high risk of preeclampsia: novelty and significance. Hypertension 2017; 69:180–188. [DOI] [PubMed] [Google Scholar]
- 17. D’Ippolito S, Marana R, Di Nicuolo F, Castellani R, Veglia M, Stinson J, Scambia G, Di Simone N. Effect of low molecular weight heparins (LMWHs) on antiphospholipid antibodies (aPL) - mediated inhibition of endometrial angiogenesis. PLoS One 2012; 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med 2004; 10:1222–1226. [DOI] [PubMed] [Google Scholar]
- 19. Casu B, Vlodavsky I, Sanderson RD. Non-anticoagulant heparins and inhibition of cancer. Pathophysiol Haemos Thromb 2007; 36:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fransson LÅ, Lewis W. Relationship between anticoagulant activity of heparin and susceptibility to periodate oxidation. FEBS Lett 1979; 97:119–123. [DOI] [PubMed] [Google Scholar]
- 21. Fragmin (dalteparin sodium) [product monograph]. In Kirkland, Canada: Pfizer Canada Inc.; 2017. [Google Scholar]
- 22. Niesen FH, Berglund H, Vedadi M. The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2007; 2:2212–2221. [DOI] [PubMed] [Google Scholar]
- 23. Morgan BP. Complement Methods and Protocols [Internet]. Vol. 150 New Jersey: Humana Press; 2000:61–71. [Google Scholar]
- 24. Ryu S, Huppmann AR, Sambangi N, Takacs P, Kauma SW. Increased leukocyte adhesion to vascular endothelium in preeclampsia is inhibited by antioxidants. Am J Obstet Gynecol 2007; 196:400.e1–400.e8. [DOI] [PubMed] [Google Scholar]
- 25. Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle MJ, Weich H, Ahmed A. Localisation of placenta growth factor (PlGF) in human term placenta. Growth Factors 1996; 13:243–250. [DOI] [PubMed] [Google Scholar]
- 26. Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC, Redman CWG, Shennan AH. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: A prospective multicenter study. Circulation 2013; 128:2121–2131. [DOI] [PubMed] [Google Scholar]
- 27. Zhu M, Ren Z, Possomato-Vieira JS, Khalil RA. Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy. Am J Physiol Regul Integr Comp Physiol 2016; 311:R505–R521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bolnick AD, Bolnick JM, Kohan-Ghadr HR, Kilburn BA, Pasalodos OJ, Singhal PK, Dai J, Diamond MP, Armant DR, Drewlo S. Enhancement of trophoblast differentiation and survival by low molecular weight heparin requires heparin-binding EGF-like growth factor. Hum Reprod 2017; 32:1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hills FA, Abrahams VM, González-Timón B, Francis J, Cloke B, Hinkson L, Rai R, Mor G, Regan L, Sullivan M, Lam EWF, Brosens JJ. Heparin prevents programmed cell death in human trophoblast. Mol Hum Reprod 2006; 12:237–243. [DOI] [PubMed] [Google Scholar]
- 30. Tersigni C, Marana R, Santamarìa A, Castellani R, Scambia G, Di Simone N. In vitro evidences of heparin's effects on embryo implantation and trophoblast development. Reprod Sci 2012; 19:454–462. [DOI] [PubMed] [Google Scholar]
- 31. Hagmann H, Bossung V, Belaidi AA, Fridman A, Karumanchi SA, Thadhani R, Schermer B, Mallmann P, Schwarz G, Benzing T, Brinkkoetter PT. Low-molecular weight heparin increases circulating sFlt-1 levels and enhances urinary elimination. PLoS One 2014; 9:e85258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Searle J, Mockel M, Gwosc S, Datwyler SA, Qadri F, Albert GI, Holert F, Isbruch A, Klug L, Muller DN, Dechend R, Muller R et al. Heparin strongly induces soluble fms-like tyrosine kinase 1 release in vivo and in vitro–brief report. Arterioscler Thromb Vasc Biol 2011; 31:2972–2974. [DOI] [PubMed] [Google Scholar]
- 33. Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, Wenger J, Lucchesi KJ, Tamez H, Lindner T, Fridman A, Thome U et al. Removal of soluble Fms-like tyrosine kinase-1 by dextran sulfate apheresis in preeclampsia. J Am Soc Nephrol 2016; 27:903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol 2008; 198:385.e1–385.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Derzsy Z, Prohászka Z, Rigó J, Füst G, Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol 2010; 47:1500–1506. [DOI] [PubMed] [Google Scholar]
- 36. Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB. Urinary excretion of C5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension 2013; 62:1040–1045. [DOI] [PubMed] [Google Scholar]
- 37. Rampersad R, Barton A, Sadovsky Y, Nelson DM. The C5b-9 membrane attack complex of complement activation localizes to villous trophoblast injury in vivo and modulates human trophoblast function in vitro. Placenta 2008; 29:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buurma A, Cohen D, Veraar K, Schonkeren D, Claas FH, Bruijn JA, Bloemenkamp KW, Baelde HJ. Preeclampsia is characterized by placental complement dysregulation. Hypertension 2012; 60:1332–1337. [DOI] [PubMed] [Google Scholar]
- 39. Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta 2013; 34:201–203. [DOI] [PubMed] [Google Scholar]
- 40. Lillegard KE, Loeks-Johnson AC, Opacich JW, Peterson JM, Bauer AJ, Elmquist BJ, Regal RR, Gilbert JS, Regal JF. Differential effects of complement activation products c3a and c5a on cardiovascular function in hypertensive pregnant rats. J Pharmacol Exp Ther 2014; 351:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oberkersch R, Attorresi AI, Calabrese GC. Low-molecular-weight heparin inhibition in classical complement activaton pathway during pregnancy. Thromb Res 2010; 125:e240–e245. [DOI] [PubMed] [Google Scholar]
- 42. de Maat MPM, de Groot CJM. Thrombophilia and pre-eclampsia. Semin Thromb Hemost 2011; 37:106–110. [DOI] [PubMed] [Google Scholar]
- 43. Wright E, Audette MC, Ye XY, Keating S, Hoffman B, Lye SJ, Shah PS, Kingdom JC. Maternal vascular malperfusion and adverse perinatal outcomes in low-risk nulliparous women. Obstet Gynecol 2017; 130:1112–1120. [DOI] [PubMed] [Google Scholar]
- 44. Rodger MA, Gris JC, de Vries JIP, Martinelli I, Rey É, Schleussner E, Middeldorp S, Kaaja R, Langlois NJ, Ramsay T, Mallick R, Bates SM et al. Low-molecular-weight heparin and recurrent placenta-mediated pregnancy complications: a meta-analysis of individual patient data from randomised controlled trials. Lancet North Am Ed 2016; 388:2629–2641. [DOI] [PubMed] [Google Scholar]
- 45. Ebrahimi S, Rahmani F, Behnam-Rassouli R, Hoseinkhani F, Parizadeh MR, Keramati MR, Khazaie M, Avan A, Hassanian SM. Proinflammatory signaling functions of thrombin in cancer. J Cell Physiol 2017; 232:2323–2329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.