ABSTRACT
Prescribing is the most important tool used by physicians to cure illness, relieve symptoms and prevent future disease. It is also a complex intellectual task that requires formulation of an appropriate treatment regimen from the many thousands available, taking into account the infinite variation in the patients they encounter. Unfortunately, the selection of a medicine and dosage regimen is sometimes suboptimal, leading to poor patient outcomes (eg treatment failure, avoidable adverse reactions). This article will highlight some of the common prescribing errors and will develop a rational approach that includes making a diagnosis, estimating prognosis, establishing the goals of therapy, selecting the most appropriate treatment and monitoring the effects of the treatment.
KEYWORDS: Drug selection, interindividual variation, monitoring therapy, personalised medicine, prescribing, rational prescribing
Key points
Prescribing is a complex task that requires interpretation of evidence from clinical trials in light of individual patient factors.
Rational prescribing describes a logical approach that includes making a (differential) diagnosis, estimating prognosis, establishing the goals of therapy, selecting the most appropriate treatment and monitoring the effects of that treatment.
Patients should be involved in several of these stages and their beliefs, expectations and attitudes to risk will contribute to rational prescribing decisions.
Pharmacogenetics will help to individualise prescribing choices but will not replace the need for an understanding of the clinical pharmacology underpinning the selection of the most appropriate drug and treatment regimen.
Introduction
Prescribing is the most important tool used by physicians to cure illness, relieve symptoms and prevent future disease. Prescribing is also a complex task that requires diagnostic skills, knowledge of common medicines, understanding of the principles of clinical pharmacology, communication skills, and the ability to make decisions based on judgments of potential benefit and risks, having taken into account available evidence and specific factors relating to the patient being treated.
The progressive accumulation of clinical trial data concerning medicines in common usage might be expected to provide sufficient evidence to support most prescribing decisions when, in fact, clinicians prescribe in varied circumstances, often in the absence of any directly related evidence. Rational prescribing decisions are often based on evidence that must be interpreted in the context of many other factors not encountered in any clinical trial.1
Rational prescribing
Rational prescribers should attempt to:
maximise clinical effectiveness
minimise harms
avoid wasting scarce healthcare resources
respect patient choice.
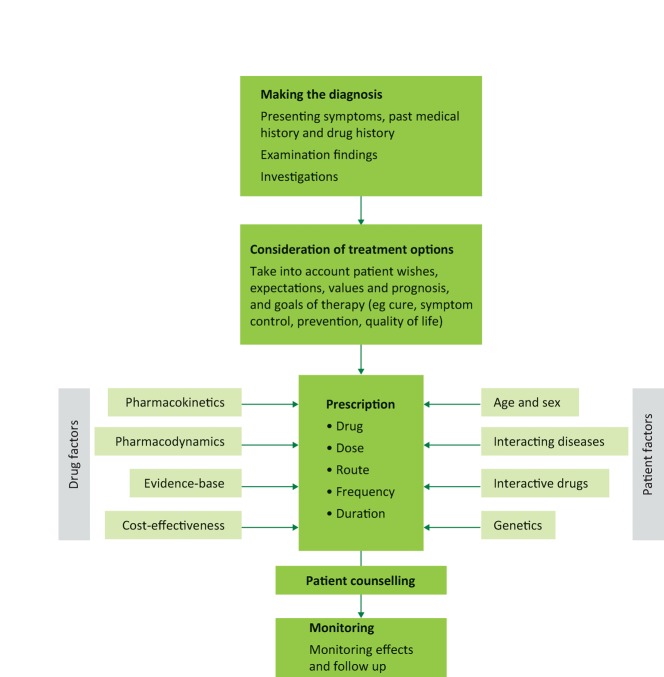
Rational prescribing normally follows a logical sequence from diagnosis to follow up (Fig 1).2–5
Fig 1.
The process of rational prescribing.
Diagnosis
Prescribing decisions should be based on the primary diagnosis and relevant secondary diagnoses. Ideally, these should have been made or confirmed by the prescriber who will take responsibility for the effects of treatment. Appreciating that diagnoses are made with varying degrees of uncertainty is important when assessing the benefit-to-harm balance of treatment. For instance, antibiotics are often prescribed on the basis of presumed antibacterial sensitivity with the expectation of significant benefit. However, subsequent antibiotic sensitivity results might show that the prescription exposed the recipient to harm without the prospect of cure.
Prognosis
The prognoses of the primary and secondary diagnoses will affect rational treatment choices. A secondary diagnosis with a poor prognosis, such as lung cancer, will severely limit the benefits of treating a primary one, such as hypercholesterolaemia, where the benefits are only likely to accrue over several years. On the other hand, the excellent prognosis of influenza in a healthy adult limits the potential benefits of antiviral therapy.
Goals of therapy
Goals of therapy may include:
curing a disease (eg breast cancer, chest infection)
relieving symptoms without affecting the underlying condition (eg headache, diarrhoea)
combining both of the above goals (eg inflammatory bowel disease, rheumatoid arthritis)
long-term prevention (eg hypertension, osteoporosis)
replacing deficiencies (eg hypothyroidism)
addressing lifestyle wishes (eg hormonal contraception)
and, occasionally,
therapeutic trials to aid diagnosis (eg edrophonium to diagnose myasthenia gravis).
Ideally, patients should be fully informed about the goal of treatment before commencing it, especially for preventative therapy where there is no prospect of immediate improvement in quality of life and any benefits will only be delivered by adherence to treatment over many years.6,7
Treatment selection
Prescribers are commonly faced with more than one choice of treatment, including non-pharmacological therapies or no treatment. For example, the management of arthritis might include reassurance, simple analgesia, physiotherapy, non-steroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, intra-articular steroids or surgery.8
Monitoring
Each prescription constitutes an experiment, the outcome of which is never certain. It is therefore important to monitor the effects of treatment, re-evaluate the benefit-harm balance and, if indicated, withdraw the drug or change the dose.9 The most appropriate endpoint will be objective assessment of the clinical outcome (eg recovery from pneumonia) but assessment may be subjective (eg pain relief, improved quality of life). Patient satisfaction is also important. Sometimes the outcome is difficult to measure (eg in management of epilepsy) or requires long-term follow-up (eg preservation of health in HIV infection). In such cases, validated surrogate markers (eg serum anticonvulsant concentration, CD4 cell count) may guide therapy. Adverse events can also be monitored in different ways.
Partnership with patients
Patients make important contributions to rational prescribing decisions.6,7 Their beliefs and expectations affect the goals of therapy and help in judging the acceptable benefit-harm balance when selecting treatments. They will often play a key role in monitoring treatment, not least by providing early warning of adverse events. Patients involved in clear communication with prescribers concerning reasons for drug selection, goals, duration of treatment and potential adverse effects are more likely to have improved adherence, more confidence in prescribers and greater satisfaction with healthcare services.10 Thus, whenever possible, patients should be fully informed about their medicines (Table 1).
Table 1.
What patients need to know about their medicines
| Knowledge | Comment and examples |
|---|---|
| The reason for taking the medicine | This will provide justification for adhering to the treatment regimen and enable the patient to explain the indications for therapy to other clinicians |
| How the medicine works | Many patients will be interested in how a medicine works and this may provide additional justification and confidence in the prescribing decision |
| How to take the medicine | This may be important for maximising effectiveness (eg metered-dose inhalers) and safety (eg bisphosphonate tablets) |
| What benefits to expect | This will help to affirm the benefits of continued adherence to the medicine if it is working and allow more rapid reconsideration of the prescription if it is not |
| What adverse effects might occur | |
| • common | This may reduce anxiety and distress, especially if there are unpleasant but short-lived symptoms (eg nitrate-induced headache) |
| • serious | This may influence the initial decision to accept treatment but also allows potentially serious adverse outcomes to be recognised at an early stage and avoided |
| Precautions that improve safety | |
| • symptoms to report | Those suggestive of emerging adverse effects might allow early discontinuation of therapy (eg sore throat related to bone marrow toxicity) |
| • monitoring required | The importance of any monitoring regimen should be emphasised (eg measurement of renal function after prescription of nephrotoxic drugs, plasma drug levels of anti-epileptic drugs) |
| • potential drug-drug interactions | The possibility of important drug interactions should be highlighted (eg warfarin) |
| • altered behaviour | Some patients might need to alter behaviour when exposed to drugs (eg photosensitivity caused by amiodarone, abstinence from alcohol with metronidazole) |
| When to return for review | The need to assess the impact of a prescription will often necessitate a review and patients should know when this will be |
Drug and dose selection
Having considered diagnosis, prognosis and goals of therapy, prescribers often select from several pharmacological options. The best choice should maximise the benefit-harm balance based on drug and patient factors, taking into account restrictions based on availability and costs (Table 2).
Table 2.
Factors that influence rational drug and dosage selection
| Factor | Comment |
|---|---|
| Diagnosis | Primary – condition to be treated |
| Secondary – other conditions that may influence the benefit-to-harm balance | |
| Prognosis | Influences the likely duration of benefits and harms of treatment |
| Drug factors | |
| Pharmacokinetic | Frequency of dosing – influences adherence
Bioavailability – if consistent, makes drug response more predictable Tissue distribution – influences the likelihood of adverse effects at sites other than those targeted Routes of metabolism/excretion – influences the variability of response in the presence of renal or hepatic disease Drug interactions – influences response for patients who are (or may be) subjected to polypharmacy |
| Pharmacodynamic | Target specificity and selectivity – influences the likelihood of adverse effects
Dose-response characteristics – influences ease of dose titration Therapeutic index – influences ease of dose selection |
| Efficacy | Beneficial impact on important outcomes such as cure, symptom relief, disease progression, morbidity, hospitalisation and mortality |
| Safety | Frequency of adverse effects
Seriousness of adverse effects (eg allergy, idiosyncratic reactions) Ease with which adverse effects can be predicted, monitored and prevented |
| Cost-effectiveness | Availability of alternatives with similar efficacy and safety but lower cost |
| Patient factors | Health beliefs and attitude to risk
History of previous adverse drug reactions Vulnerability to adverse effects (eg organ damage, reduced physiological reserve) Current drug therapy, including interacting drugs Likely adherence to therapy or follow-up monitoring |
| Prescriber factors | Familiarity with prescribing choices
Ease of follow up may depend on resources |
Drug factors influencing drug selection
Pharmacokinetics
Drugs in the same class (or different formulations of the same drug) may have different bioavailability, dose-concentration curves and half-lives. These factors will determine the dosing schedule. Once-daily dosing is convenient and encourages adherence. Pharmacokinetic characteristics may also influence interindividual variability in dosage requirements. For example, some drugs:
differ with respect to their specificity for the target organ (eg atenolol versus propranolol when used to treat heart disease)
reach tissues to cause adverse effects (eg the capacity of different antimuscarinic medicines to cross the blood-brain barrier to cause confusion in elderly patients with overactive bladder)11
are metabolised in the liver or excreted – important in patients with hepatic or renal disease (eg fentanyl versus morphine when used as an analgesic in patients with renal impairment)
are more likely to cause drug interactions through cytochrome P450 inhibition (eg macrolide antibiotic inhibition of the metabolism of simvastatin versus pravastatin).12
Pharmacodynamics
A drug with a low therapeutic index (the ratio between the dose required to cause adverse effects and that required for efficacy) is less favourable if alternatives exist. Similarly, the steepness of the dose-response curve will influence the ease with which the dose can be optimally titrated. Selectivity for a receptor subtype may be relevant when choosing drugs that avoid predictable adverse effects. Some drugs require more complex monitoring, which can affect costs and patient time (eg oral anticoagulation with warfarin requires regular INR monitoring to assess efficiency and safety while the newer oral anticoagulant apixaban does not).
Therapeutic efficacy and safety
A drug may be more efficacious in relieving symptoms, improving surrogate markers or preventing clinical events such as morbidity, mortality and hospitalisation (eg atorvastatin is more efficacious at lowering cholesterol and preventing cardiovascular disease than pravastatin) or have fewer and less serious adverse effects (eg bisoprolol has fewer serious adverse effects when used to control atrial fibrilation than amiodarone). Patients will often find it difficult to understand these comparisons because they are often expressed as relative or absolute risks. The use of the concepts of numbers needed to treat (NNT) or harm (NNH), derived from the comparison of absolute risk, will often make these comparisons of efficacy and safety more accessible.13 For example, if a drug reduces the rate of myocardial infarction over 10 years from 20% to 15% for a specific treatment group this can be expressed as an absolute risk reduction of (ARR) of 5%, a relative risk reduction (RRR) of 25% or an NNT of 20. It is the latter which, for many patients, most easily illustrates their likelihood of personally being a beneficiary of 10 years of treatment.
Large randomised controlled trials (RCTs) are considered the optimal sources of evidence. However, extrapolating the results of RCTs to prescribing decisions in the real world requires caution because RCTs usually recruit highly selected participants (eg based on age or disease severity) without comorbidities or who are not receiving interacting drugs. Such additional factors can influence efficacy or adverse outcomes, potentially reducing the former and enhancing the latter, thus limiting the external validity of RCTs.14 It is also important to recognise that some drugs have more accumulated RCT evidence than other similar drugs in the same class and this might also be a relevant factor when prescribing (eg the thiazide diuretic bendroflumethiazide has many years of accumulated experience and clinical trials data making it a more familiar choice than cyclopenthiazide for most prescribers).
Cost-effectiveness
All healthcare systems have limited resources. The rapidly increasing cost of medicines forces all prescribers to consider cost-effectiveness as a factor in drug selection.15 This is taken into account when devising local formularies and in the guidelines produced by the National Institute for Health and Care Excellence in the UK. Perhaps the most widespread example of cost-effective prescribing is selecting a generic rather than a branded drug from the same class. However, considerations of cost may be outweighed by other factors, notably significant differences in efficacy or safety.
Patient factors influencing drug selection
Previous adverse drug reactions
Knowledge of previous adverse reactions will affect drug or dose selection but this is reliant on taking a careful drug history. This is particularly important in the case of allergic reactions to drugs (eg beta-lactam antibiotics).
Vulnerability to adverse effects
Some patients will have organ damage that may affect drug choices. For instance, beta-adrenoceptor antagonists for angina may be undesirable in patients with peripheral vascular disease or asthma (because they precipitate vasoconstriction and bronchoconstriction) but attractive in those with heart failure (for which they are also indicated). Older patients are more vulnerable to the adverse effects of many drugs (eg anticholinergics, central nervous system depressants, vasoactive drugs) because of age-related reduction in the function of vital organs (eg the central nervous and cardiovascular system) and this may necessitate dosage reductions.16
Current drug therapy
Any current drug therapy may affect drug or dosage selection, mainly because of potential drug interactions. For example, the dose of simvastatin should not be increased beyond 20 mg at night in patients also taking amiodarone or verapamil because of the increased risk of muscle toxicity.
Other patient factors
The likelihood that patients will adhere to therapy or follow-up monitoring is important for drugs such as warfarin and insulin, which have a low therapeutic index, and where alternatives are less effective. However, patients who cannot meet these arrangements will be more vulnerable to serious adverse outcomes.
Health beliefs and attitude to risk can influence the initial decision to prescribe or the choice of medicine. This is particularly obvious in long-term preventive therapy when benefits may be imperceptible. About half of patients adhere poorly to such treatments, emphasising the role of patient partnership in making rational prescribing decisions with which the patient agrees.
Some patients may not be able to administer the medicine correctly, leading to unintentional non-adherence (eg lacking the manual dexterity and timing to use metered-dose inhalers). For others, swallowing tablets may present difficulties so liquid formulations would be more suitable.
Prescriber factors influencing drug selection
Familiarity
If a prescriber lacks familiarity with medicines, it increases the chance of adverse outcomes; continuing professional development is required. However, lack of experience should not impede the introduction of new, more rational prescribing practices when they become available.
Ease of follow up
Some medicines require careful review and monitoring to ensure that safety is maximised or dose titration is optimal. If the appropriate supervision cannot be guaranteed then alternative treatment options (or no treatment) might be preferred.
Examples of irrational prescribing
Rational prescribing aims to ensure that selection is not a simple formulaic linkage of drugs and doses to particular diagnoses but involves individualising prescriptions as far as possible, taking account of the variables discussed above. Table 3 offers some simple examples of irrational prescribing. They are illustrative only and do not acknowledge the complexity of real prescribing decisions. Prescribers commonly make probabilistic judgements that involve interpreting trial evidence in the light of specific circumstances, such as patients' wishes, availability of resources and previous adverse events. For instance, more expensive but equivalent medications may be justified if others have caused adverse effects or patients have lost confidence in them. Higher risk medicines may be acceptable if the potential benefit is estimated to be greater for an individual patient (eg chemotherapy for cancer).
Table 3.
Examples of irrational prescribing
| Reason | Example |
|---|---|
| Low chance of benefit (compared with harm) | |
| Short-term conditions with good prognosis | Antiviral drugs for influenza in healthy adults |
| Preventive therapy in patients with poor
prognosis conditions/poor quality of life |
Statin therapy in patients with a malignancy |
| Drugs used beyond the evidence base | Statin therapy for very young or very old patients |
| Dose too low | ACEIs for chronic heart failure |
| Wrong diagnosis | Anti-anginal drugs for patients with GORD
Antibiotics for viral illnesses |
| Increased risk of harm (compared with benefit) | |
| Vulnerability to adverse effects | Prescribing psychoactive medicines for elderly patients; NSAIDs for patients with
impaired renal function; thromboprophylaxis in patients at risk of serious bleeding due to factors such as thrombocytopenia, peptic ulcer disease, coagulopathies, intracranial disease |
| Drug clearance altered | Wrong doses in patients with renal or hepatic disease |
| Drug interactions likely | Enzyme-inhibiting drugs (eg macrolide antibiotics) in patients taking warfarin |
| Dose too high | Thiazide diuretics prescribed in chronic heart failure dosage to treat hypertension
Aspirin prescribed in analgesic dosage for the prevention of cardiovascular disease |
| Reduced adherence likely | |
| Too many medicines (polypharmacy) in patients with multiple conditions | Prescribing all evidence-based therapies in older patients with chronic airways disease, hypertension, chronic heart failure, osteoporosis, GORD and diabetes |
| Poor communication | Antihypertensive drugs in young patients unclear about or unimpressed with the extent of the likely benefit |
| Unnecessary cost | |
| Expensive drugs with no evidence of superior outcomes | Prescribing branded rather than generic statins in primary prevention when these are more expensive |
| Expensive drugs that offer slightly better outcomes at enormously increased cost | Some new therapies for cancer |
| Drugs for adverse drug reactions | |
| Drugs prescribed to counteract the adverse effects of other medicines that could be replaced with suitable alternatives | Laxatives for verapamil-induced constipation
Salbutamol for beta-adrenoceptor antagonist-induced bronchospasm Loop diuretics for amlodipine-induced ankle oedema |
ACEI = angiotensin-converting enzyme inhibitor; GORD = gastro-oesophageal reflux disease; NSAID = non-steroidal anti-inflammatory drug.
Personalised medicines: the future?
This article has discussed the traditional approach to prescribing in which individualised drug selection is based on clinical trial evidence gathered from groups of similar patients mixed with best-guess judgements about the variability introduced by specific patient and drug factors. Unfortunately, even with the best care, a significant proportion of patients treated for common illnesses fail to respond or suffer intolerable adverse effects. Therapeutics is now entering a new era of ‘personalised’ or ‘precision’ medicine in which therapeutic choices will be individualised based on genetic variables affecting drug handling and action, allowing more specific prediction of outcomes.17 Indeed, pharmacogenetics is already being used to distinguish responders from non-responders (eg prescribing trastuzumab for HER2-overexpressing breast cancer) and to avoid adverse effects (eg HLA B*5701 for abacavir hypersensitivity).18 However, the impact of this approach may be limited because many of the variables outlined in Table 2 are not affected by genetics. This suggests that rational prescribing will continue to be based on a firm grounding in the principles of clinical pharmacology.
Conflicts of interests
The author declared no conflicts of interest.
Note
This article is an update of a previously published CME article: Maxwell S. Rational prescribing: the principles of drug selection. Clin Med 2009;9:481–5.
References
- 1.Maxwell S. Evidence based prescribing. BMJ. 2005;331:247–8. doi: 10.1136/bmj.331.7511.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maxwell SRJ. Therapeutics and good prescribing. In: Walker B, editor; Colledge N, editor; Ralston S, editor; Penman I, editor. Davidson’s principles and practice of medicine. 22nd Edn. Edinburgh:: Churchill Livingstone Elsevier; 2012. pp. 17–40. [Google Scholar]
- 3.British Pharmacological Society Ten principles of good prescribing. London:: British Pharmacological Society; 2010. Available online at www.bps.ac.uk/BPSMemberPortal/media/BPSWebsite/Assets/BPSPrescribingStatement03Feb2010.pdf. [Accessed 15 July 2016]. [Google Scholar]
- 4.de Vries TPGM. Henning RH. Hogerzeil HV. Fresle DA. Guide to good prescribing: a practical manual. Geneva:: World Health Organization; 1994. [Google Scholar]
- 5.Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62:629–32. doi: 10.1111/j.1365-2125.2006.02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elwyn G. Coulter A. Laitner S. Walker E. Watson P. Thomson R. Implementing shared decision making in the NHS. BMJ. 2010;341:c5146. doi: 10.1136/bmj.c5146. [DOI] [PubMed] [Google Scholar]
- 7.Agoritsas T. Heen AF. Brandt L, et al. Decision aids that really promote shared decision making: the pace quickens. BMJ. 2015;350:g7624. doi: 10.1136/bmj.g7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence Rheumatoid arthritis in adults: management. NICE clinical guideline No 79. London:: NICE; 2009. [Google Scholar]
- 9.Hitchings AW. Monitoring drug therapy. Medicine. 2016;44:427–31. [Google Scholar]
- 10.Osterberg L. Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 11.Staskin DR. Zoltan E. Anticholinergics and central nervous system effects. Rev Urol. 2007;9:191–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson TA. Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors. Am J Cardiol. 2004;94:1140–6. doi: 10.1016/j.amjcard.2004.07.080. [DOI] [PubMed] [Google Scholar]
- 13.Sedgwick P. What is number needed to treat (NNT)? BMJ. 2013;347:f4605. [Google Scholar]
- 14.Rothwell PM. Factors That Can Affect the External Validity of Randomised Controlled Trials. PLoS Clin Trials. 2006;1:e9. doi: 10.1371/journal.pctr.0010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafferty J. Powell J. Health technology assessment in the UK. Lancet. 2013;382:1278–85. doi: 10.1016/S0140-6736(13)61724-9. [DOI] [PubMed] [Google Scholar]
- 16.Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly. Arch Intern Med. 1997;157:1531–6. [PubMed] [Google Scholar]
- 17.Francis S. Collins FS. Varmus H. A new Initiative on precision medicine. N Engl J Med. 2015;372:793–5. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration Table of pharmacogenomic biomarkers in drug labeling. Silver Spring, MD:: US FDA; 2016. Available online at www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm. [Accessed 15 July 2016]. [Google Scholar]