Abstract
Preterm birth affects approximately 1 out of every 10 births in the United States, leading to high rates of mortality and long-term negative health consequences. To investigate the mechanisms leading to preterm birth so as to develop prevention strategies, researchers have developed numerous mouse models of preterm birth. However, the lack of standard definitions for preterm birth in mice limits our field's ability to compare models and make inferences about preterm birth in humans. In this review, we discuss numerous mouse preterm birth models, propose guidelines for experiments and reporting, and suggest markers that can be used to assess whether pups are premature or mature. We argue that adoption of these recommendations will enhance the utility of mice as models for preterm birth.
Keywords: preterm birth, mouse models, pregnancy, gestation, parturition
To improve reporting of mouse models of preterm birth, a set of universal guidelines and simple assays of developmental markers are proposed to distinguish between mature and premature pups.
Introduction
Every year, approximately 15 million babies (10% of all births worldwide) are born preterm, defined as delivery before 37 weeks of gestation [1–3]. Preterm birth is the leading cause of infant mortality [4], and those born prematurely have increased lifelong risks of adverse health outcomes including cognitive impairment, cardiovascular disease, and chronic pulmonary disease [5]. In approximately one third of preterm births, labor is induced or cesarean section is performed because of maternal or fetal complications such as preeclampsia, intrauterine growth restriction, or fetal distress. However, the majority of preterm births are spontaneous, brought about by preterm labor (uterine contractions leading to cervical change and delivery) or preterm premature rupture of fetal membranes (PPROM) [6, 7]. Unfortunately, the multiple causes of preterm labor have not been fully determined, and we have limited ability to predict or prevent preterm birth [8–10].
To address these limitations, researchers have developed various mouse models of preterm birth [11]. Mice are useful for studying the timing of birth because they have a short gestation, can easily be genetically manipulated, are inexpensive, and can be studied in large numbers [12]. Additionally, key components of the labor and delivery process are conserved between mice and humans. For example, the expression levels of several proteins that activate uterine contractions including prostaglandins, oxytocin, and connexin-43 are elevated at term in both species [11, 13–15]. Finally, parturition timing is somewhat variable in both humans, in whom 90% of births occur between 37 and 42 weeks of gestation, and mice, in which gestation lengths vary between strains from 19 to 21 days [16].
Despite these similarities, three key physiological differences between human and mouse pregnancy are worth mentioning. First, whereas women typically have singleton pregnancies [17] within a single uterine cavity, mice commonly have 4 to 10 pups per litter (depending on strain) [16] within two uterine horns. This can complicate comparisons between species because women carrying multiple pregnancies are at increased risk for preterm birth [18]. Second, although progesterone is required to maintain pregnancy and is initially produced by the corpus luteum in both humans and mice, its production thereafter is regulated differently in the two species. In humans, the placenta takes over progesterone production after gestational week 7 or 8, and progesterone levels rise fairly continuously until the end of pregnancy [19, 20]. At the end of human pregnancy, a shift in progesterone receptor isoform expression and local increases in progesterone metabolism [21, 22] result in a functional withdrawal of the “pregnancy-maintaining” hormone. In mice, progesterone synthesis and levels decrease while local progesterone metabolism [23] increases dramatically in the last two days of pregnancy, facilitating the onset of parturition. Third, although estrogens inhibit uterine quiescence and promote cervical ripening after progesterone action decreases (by promoting production of uterotonins and contraction-associated proteins, including: connexin-43, oxytocin receptor, cyclooxygenase 2, and prostaglandin F receptor) in both humans and mice, estrogen is regulated differently in the two species. In humans, circulating estrogens, derived from the ovary and placenta, are high throughout gestation. In mice, estrogens are synthesized by the ovary at low levels in the first half of pregnancy and then markedly increase in the latter half of gestation.
As we detail in this review, “preterm birth” is loosely defined for mice, and our field lacks clear, accepted guidelines for conducting experiments and reporting results. Thus, we cannot easily compare mouse studies and use their results to improve our ability to predict and prevent preterm birth in women. This review seeks to fill this gap by describing mouse preterm birth models published in the literature and the ways in which they aim to represent the human condition. Additionally, we discuss experimental differences leading to inconsistent definitions of preterm birth in mice and propose criteria to provide more uniform assessment of mouse models. Finally, we suggest that investigators should assess and report the developmental status (mature or premature) of offspring. Toward this end, we describe promising fetal developmental markers that can be used to distinguish between mature and premature pups.
Mouse models of preterm birth
Here, we review the major classes of mouse preterm birth models, categorized by pathologic state. Representative examples are provided in Table 1.
Table 1.
Published preterm birth mouse models.
| Stimulant | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of model | Targeted mutation | Mouse strain | Expected gestational length (days)a | Breeding method | Defined start of pregnancyb | Treatment/ experimental manipulation | Routec and concentration | Day of injection | Preterm delivery definition | Term delivery definition in paper | Pup survival | Reference |
| Infection | ||||||||||||
| N/A | CD-1 | 19–20 | ND | Vaginal plug; dpc not defined | E. coli 2 – 10 × 105 | IU/IP | 14.5 dpc | Within 48 h | ND | None | [31] | |
| N/A | C3H/HeN x C3H/HeN C3H/HeN x B6D2F1 BALB/c x B6D2F1 | 19–20 | ND | Vaginal plug and spermatozoa in vaginal smear at 0.0 dpc | LPS serotype 055:B5 | IP - 50 or 100 μg/kg (one injection) Or 50 μg/kg (two injections) | 12, 15, or 17 dpc | <19 dpc | 19–20 dpc | None | [32] | |
| N/A | CD-1 | ND | ND | Vaginal plug at 0.0 dpc | LPS serotypes 0111:B4, 055:B5, 0127:B8*, and 0128:B12 | IU 20 μg in 25 μl | 16 dpc | 36 h postinjection | 60 ± 15 h postinjection | LPS 0111:B4 none 055:B5 80% 0127:B8 95% 0128:B12 100% | [33] | |
| TLR-4 mutant | CD-1 C3H/HeJ | 19–20 | Timed pregnant (supplier) | ND | LPS serotype L2280 (CD-1) L2880 and L4525 for TLR-4 mutant | IU 250 μg/mouse (Sigma) | 15 dpc | At least 1 pup born < 48 h after LPS admin | 19–20 dpc | None | [35] | |
| C5aR−/− | 129S4/SvJae | ND | Vaginal plug at 0 dpc | No treatment, LPS serotype 055:B5 or RU486 | 250 μg LPS intravaginally SC 150 μg RU486 dissolved in DMSO | 15 dpc | Within 48 h postinjection | 20–21 dpc | ND | [36] | ||
| N/A | CD-1 | 19–20 | ND | Vaginal plug, dpc not defined | TLR-2 ligand lipoteichoic acid, peptidoglycan, or TLR-3 ligand polyinosinic:cytidylic acid | IU and IP | 14.5 or 15.5 dpc | At least 1 pup born or in lower vagina <48 h after surgery | 19–20 dpc | None | [37] | |
| N/A | C3H/HeNCrj X Crj: B6D2F1 | 19–20 | ND | Vaginal plug and spermatozoa in the vaginal smear at 0.0 dpc | Lipoteichoic acid | IP 12.5–75 mg/kg single dose or repeated doses at 3h intervals | 15 dpc 17 dpc | <19.0 dpc | 19–20 dpc | None with doses given on 15 dpc 100% viable with doses given on 17 dpc | [38] | |
| N/A | C57BL/6 | 19.5 ± 0.5 | ND | ND | iE-DAP | IP 500, 750, or 1000 μg in 200 μl PBS | 14.5 dpc | Delivery within 24 h postinjection | ND | ND | [39] | |
| N/A | CD-1 | ND | Timed pregnant (supplier) | ND | Heat-killed GBS | IP or IU 109 in 100 μl | 14.5 dpc | Within 48 h postinjection | ND | ND | [42] | |
| N/A | C3H/HeN | 19–20 | ND | Vaginal plug at 0.0 dpc | Ureaplasmal outer membrane lipoprotein | IU 15 μg | 14.0 dpc | Within 48 h | ND | ND | [45] | |
| N/A | BALB/c (H-2d) | 19–20 | Overnight | Vaginal plug at 0 dpc | Chlamydia trachomatis mouse pneumonitis biovar (strain Nigg II) | 101 to 107 inclusion-forming units in 20 μl of 0.2 M sucrose–20 mM sodium phosphate (pH 7.2)–5 mM glutamic acid | 5 dpc | ND | 19.3 | Maternal cannibalism precluded evaluation | [47] | |
| N/A | C3H/HeJ | 20 | Timed-pregnant (supplier) | ND | E. coli Dr+ IH11128 and Dr14 | Urethral catheterization into urinary bladder | 7 dpc | On or before 18 dpc | ND | 53.6% | [50] | |
| N/A | C57Bl/6J | 20.45 | ND | Vaginal plug at 0.0 dpc | W83 strain of P. gingivalis | 108 CFU | 6 weeks before mating | 17–18.25 dpc | Delivery | ND | [55] | |
| Faah–/–; Cnr–/– | ND | ∼19.8 | ND | Vaginal plug at 1.0 dpc | LPS 0111:B4 | IP 25 μg | 16.0 dpc | Before 19 dpc | ∼19.8 dpc | None | [139] | |
| N/A | Kunming (derived from Swiss Webster) | ND | Overnight | Vaginal plug at 0.0 dpc | LPS serotype 0127 | IP 150 μg/kg | 15.5 dpc | 15.5–17 dpc | 18.0–20.0 dpc | 47%d | [140] | |
| N/A | CD-1 | 19–20 | Overnight | Vaginal plug at 0.0 dpc | LPS serotype 0111 | IU 10 μg | 16.0 dpc | 12.7 ± 7 h | ND | 14.6 ± 31% viable, but included deliveries 25–37 h after LPS | [141] | |
| N/A | C57BL/6 | 19.5 ± 0.5 | Overnight | Vaginal plug at 0.5 dpc | LPS | IP 0.5 μg | 16.5 dpc | Before 18.0 dpc | ND | 2.3 fetuses per dam, may include term deliveries | [142] | |
| N/A | C57BL/6 | 19.5 ± 0.5 | ND | Vaginal plug at 0.5 dpc | LPS serotype 0111:B4 | IA 100 ng/sac | 16.5 dpc | Before 18.0 dpc | ND | ND | [143] | |
| Uterine-specific p53 knockout | FVB/129 | ND C57BL/6-129 | Overnight | Vaginal plug at 1.0 dpc | LPS | IP 10 μg/ml | 16.0 dpc | Before 19 dpc | ND | 28% w/o LPS; 0% with LPS | [84] | |
| Inflammation | ||||||||||||
| N/A | C3H/Hel | 20–21 | Timed pregnant (supplier) | ND | Interleukin-1 | SC | 15–17 dpc | Within 24 h | 20–22 dpc | ND | [61] | |
| N/A | C57BL/6 | 19.5 ± 0.5 | Overnight | Vaginal plug at 0.5 dpc | High-mobility group box-1 | IA 9 ng | 14.5 dpc | 17.35 dpc | 19.5 ± 0.5 dpc | ∼85.4% viability; 60.9 ± 11.7% pup death by 1 week of age | [64] | |
| Cesarean | ||||||||||||
| N/A | CD-1 | 20 | Overnight | Vaginal plug at 1.0 dpc | Cesarean | NA | NA | On 18 or 19 dpc | 20 dpc | 0% (18 dpc) 87.8% (19 dpc) | [65] | |
| N/A | CD-1 | 19–20 | Timed pregnant (supplier) | ND | Cesarean | NA | NA | 1 or 2 days before term | Delivery | 100% | [144] | |
| PPROM | ||||||||||||
| N/A | C57BL/6 | 19.5 ± 0.5 | Pair mated (5 h) | ND | Fetal fibronectin | Between fetal membranes and uterine lining (100–200 μg/ml) | 17.0 dpc | <18.5 dpc | 19 dpc | 20% | [70] | |
| Biglycan and Decorin double knockout | C3H | 19.5 | Overnight | Vaginal plug at 0.0 dpc | NA | NA | NA | Before 18.0 dpc | 19.5 dpc | 0% | [67] | |
| Early progesterone withdrawal | ||||||||||||
| N/A | C3H/HeN | 19–20 | Overnight | Vaginal plug at 1.0 dpc | Mifepristone (RU486) | SC 50–250 μg | 12–14 dpc | Within 18 h | 19–20 dpc | 100% | [75] | |
| Prostaglandins | ||||||||||||
| N/A | C3H/HeN | 19–20 | Timed Pregnant (supplier) | ND | Prostaglandin F2α | IP 20 μg | 16 dpc | Within 24 h | <18 dpc | ND | [82] | |
| 15-hydroxy- prostaglandin dehydrogenase hypomorph | C57BL/6-129/SvJ | ∼19.3 | ND | Vaginal plug, dpc not defined | NA | NA | NA | Shorter gestation than control | ∼19.3 dpc | Equivalent to term | [80] | |
| Uterine quiescence | ||||||||||||
| N/A | CD-1 | 19 | Timed pregnant (supplier) | ND | Tunicamycin | IP 0–1 mg/kg | 15 dpc | 18–32 h postinjection | 19 dpc | Nonviable neonates at 16 and 17 dpc | [89] | |
| Endocannabinoid signaling | ||||||||||||
| CB1 knockout | C57BL/6J/129 | ∼20.1 | Overnight | Vaginal plug at 1.0 dpc | NA | NA | NA | Before ∼19.5 dpc | ∼20.1 dpc | Yes | [99] | |
| Hyperhomocysteinemia | ||||||||||||
| CBS knockout | C57BL/6J | 20.0 ± 0.2 | Overnight | Vaginal plug at 0.5 dpc | NA | NA | NA | At 16.6 ± 0.1 dpc | 20.0 ± 0.2 dpc | Yes | [102] | |
| Environmental effects | ||||||||||||
| N/A | C57BL/6 | 19.5 ± 0.5 | Overnight | Vaginal plug at 0.5 dpc | 2,3,7,8-tetrachlo- rodibenzo-p-dioxin (dioxin) | Mother was gavaged 10 μg/kg; no exposure as adult | In utero at 15.5 dpc, none as adult | 24 h before term | 20 dpc | Pups born preterm appeared viable at birth, died within 24 h Pups born at term survived | [105] | |
| N/A | BL6C3F1 | 20.3 ± 0.2 | Two days | First day of pairing defined as 0.0 dpc | Cigarette smoke diluted 90% | Inhalation | 2–18 dpc | At 19.6 ± 0.2 dpc | 20.3 ± 0.2 dpc | Yes | [145] | |
| Other | ||||||||||||
| N/A | CD-1 | 19–20 | Overnight | Vaginal plug at 0 dpc | L-arginine analog NG-nitro-L-arginine methyl ester (L-NAME) | SC 0 (vehicle), 40, 70, or 100 mg L-NAME/kg in 10 ml/kg body weight | 15.5 dpc and 16 dpc | Before 18 dpc | 18–19.5 dpc | Maternal cannibalism precluded evaluation | [107] | |
| N/A | CD-1 | 19–20 | Overnight | Vaginal plug at 0 dpc | Methylene Blue | SC 5, 30, 50, 60 or 85 mg/kg in 5 ml/kgb | 15.5 dpc and 16 dpc | Before 18 dpc | 18–19.5 | Appeared viable but maternal cannibalism precluded evaluation | [108] | |
| N/A | ICR (CD-1) | 19–20 | Overnight | Vaginal plug at 0.5 dpc | Surfactant protein (SP)-A | IA 3 μg in 50 μl per sac | 15 dpc | ND | 19 dpc | ND | [109] | |
| N/A | BALB/C | 20.2 ± 0.1 | Overnight | Vaginal plug at 0 dpc | Neuromedin B | IP 30, 90, or 150 μg/kg of NMB | 18 dpc and 19 dpc at 1400 and 1800 h | ND | ND | ND | [110] | |
| N/A | C57Bl/6J | 19.5 ± 0.5 | ND | Vaginal plug at 1 dpc | Alcohol | Intra-gastric 6 g/kg | 17 dpc or 18 dpc | 18.9 ± 0.1 dpc 19.5 ± 0.2 dpc | 20.1 ± 0.1 | ND | [111] | |
aAs discussed by author or determined by [16]
bdpc, days postcoital
cIA, intraamniotic; IP, intraperitoneal; IU, intruterine; SC, subcutaneous
dincludes term deliveries
NA, not applicable
ND, not discussed
LPS, Lipopolysaccharide
Infection
Multiple studies have implicated activation of inflammatory pathways in the process of normal uncomplicated labor (as reviewed in [24]). However, early initiation of the inflammatory pathway by infection contributes to between 25% and 40% of all human preterm births [25–27]. Infections can develop in two major ways. Chorioamnionitis or intrauterine infection can arise systemically, or commensal bacteria can ascend from the female genital tract. Several mouse models of infection-induced preterm birth have been developed [28, 29], most commonly using Escherichia coli or the toxic component on the surface of gram-negative bacteria, lipopolysaccharide (LPS). Other bacteria associated with increased risk of preterm birth in humans, and therefore studied in mice, include Ureaplasma, Chlamydia trachomatis, Group B streptococcus (GBS; Streptococcus agalactiae), and Porphyromonas gingivalis. To mimic local infection, investigators have performed intrauterine, intraamniotic, or intraperitoneal injections with live or heat-killed bacteria. To simulate systemic infection, they administer injections intraperitoneally, and to mimic ascending infections, they inject bacteria vaginally or via the intrauterine route [30–32].
Mice injected with live or heat-killed E. coli or LPS on 14.5–15.5 days post coitus (dpc) delivered within 7–48 h, depending on the route of administration and LPS serotype used. Specific E. coli-derived LPS serotypes differentially activated proinflammatory responses, caused mice to deliver at different times postinjection, and were associated with varying levels of offspring survival [33]. Both E. coli and LPS stimulate the Toll-like receptor (TLR)-4 in the uterus and activate an inflammatory cascade [34]. Further investigation of localized intrauterine inflammation revealed that the TLR-4 pathway stimulated platelet activating factor, an important mediator of the signal transduction pathway that led to inflammatory-induced preterm delivery [35]. In addition, LPS administered vaginally has been shown to work through the complement receptor C5a, affecting cervical remodeling by increasing metalloproteinase-9 activity and collagen degradation [36].
Intrauterine injections of lipoteichoic acid (an anionic polymer on the surface of gram-positive bacteria), peptidoglycan (also a major component on the surface of gram-positive bacteria), or polyinosinic acid (an analog of viral double-stranded RNA) caused mice to deliver preterm by mechanisms similar to that of LPS, albeit through different TLRs [37, 38]. Specifically, administration of the bacterial peptidoglycan-derived peptide γ-D-glutamyl-meso-diaminopimelic acid, an agonist of the pattern recognition receptor Nod-1, caused mouse preterm birth via maternal-fetal inflammation [39].
In humans, GBS is the leading cause of perinatal infection, and maternal GBS infection increases the risk for preterm birth [40, 41]. To model this etiology, Hirsch and colleagues injected pregnant mice (intraperitoneal or intrauterine) with 109 heat-killed GBS on 14.5 dpc [42]. In this model, approximately 86% of pregnant mice delivered within 18 h and showed signs of placental and membrane apoptosis. In another model, 75% of mice that were vaginally colonized with GBS on 16 dpc delivered preterm. However, 0% of mothers that were vaccinated with GBS before mating delivered preterm, and their pups had higher survival rates and lower levels of neonatal GBS infection than those from unvaccinated mothers [42].
Another bacterial genus that commonly infects the uterus and causes preterm birth in humans is Ureaplasma, a member of the Mycoplasmataceae family [43]. Additionally, Ureaplasma is the most frequently isolated bacterial pathogen in cases of chorioamnionitis [44]. One group reported that injecting a diacylated lipopeptide derived from Ureaplasma parvum into the uterus of pregnant mice on 15 dpc resulted in preterm delivery, although the day of delivery was not defined. This work revealed that Ureaplasma likely induced preterm birth by binding to TLR-2 and activating the NF-κB inflammatory cascade [45].
The pathogen Chlamydia trachomatis is the most prevalent sexually transmitted bacteria and is associated with preterm delivery [46]. Mice infected with 105–107 inclusion-forming units (IFU) of C. trachomatis delivered on 15.8–16.4 dpc, whereas those infected with 101–104 IFU delivered on 19.5–19.6 dpc, and those that received vehicle or no treatment delivered had a mean gestation length of 19.3 dpc [47]. Acute to severe inflammation and C. trachomatis inclusions were noted in the maternal uterine wall, endometrium, and fetal membranes but were absent in the amnion and fetal organs.
Urinary tract infections, most commonly caused by E. coli [48], have long been known to be associated with increased risk for preterm birth in humans [49]. To model this etiology, mice were infected with E. coli via urethral catheterization into the urinary bladder on 7 dpc. Nearly 90% of those infected with bacteria expressing the Dr adhesin delivered between 11 and 18 dpc [50], whereas only 10% of those infected with E. coli not expressing this Dr adhesin delivered preterm. Escherichia coli expressing Dr adhesin were able to colonize the kidneys and spleen of the mothers and transfer through the placenta to the fetuses, causing reduced fetal weight and poor organ development.
Maternal infections distant from the uterus have also been implicated in preterm birth. For example, some data suggest that maternal periodontal disease increases the risk of preterm birth by sevenfold [51]. Periodontal pathogens are capable of entering the bloodstream and spreading throughout the body and have been detected in the amniotic fluid [52, 53]. Moreover, P. gingivalis antigens have been detected in the placentas of women with chorioamnionitis [54]. In a mouse model of periodontal infection, P. gingivalis was injected into the first molar chambers of female mice 6 weeks before mating. Infected mice delivered 2 days earlier and had higher circulating levels of the proinflammatory cytokines tumor necrosis factor-alpha, interleukin (IL)-17, IL-6, and IL-1B than noninfected mice. Additionally, the bacteria were found in the placentas, and the mothers showed features of PPROM and placental abruption [55].
Inflammation
Inflammation in the absence of overt infection is a common etiology of preterm birth. In fact, sterile intra-amniotic inflammation is associated with ∼26% of all preterm deliveries and is more common in early than in late preterm deliveries [56, 57]. An important contributor in sterile intra-amniotic inflammation is the cytokine IL-1, which is expressed in the human decidua, promotes prostaglandin production, and is detected at high levels in the uterus of women who deliver preterm [58]. IL-1 stimulates preterm birth in rabbits and nonhuman primates [59, 60]. To model this in mice, researchers injected pregnant mice with IL-1 three times between 15 and 17 dpc, leading to delivery within 24 h [61]. Other important players in sterile intra-amniotic inflammation are danger signals, such as damage-associated molecular pattern molecules and alarmins, which, upon stimulation by cellular stress and necrosis, activate the innate immune system [62]. One such alarmin, high mobility group box 1 (HMGB1), is increased in women with intra-amniotic inflammation [63]. To model this etiology of preterm birth, researchers injected HMGB1 into the amniotic sacs of fetuses on 14.5 dpc; 57% of the injected mice delivered by 17.5 dpc, whereas all of the controls delivered at full term (19.5 dpc) [64].
Cesarean section
To benefit maternal or fetal health, preterm cesarean delivery may be performed after either medically indicated or spontaneous preterm labor. To investigate the effect of cesarean delivery on fetal development and mortality, researchers surgically removed mouse fetuses before the normal delivery date and found that pups of the strain CD-1 could only be resuscitated when delivered on 19 or 20 dpc (20 dpc was the normal delivery time for this strain in their colony) [65], indicating that mice cannot survive outside the uterus if birth occurs more than 1 day preterm.
Preterm premature rupture of membranes
In women, PPROM is a significant pregnancy complication. In this condition, the chorioamniotic membrane surrounding the fetus ruptures before 37 weeks of pregnancy, breaching the barrier between the extrauterine and intrauterine environment, allowing the amniotic fluid to leak out, and increasing the risk for infection and preterm birth. PPROM can be caused by infection, overdistension of the uterus and amniotic sac (such as in multiple pregnancies), trauma, or genetic disorders. For example, women with Ehlers-Danlos syndrome carry mutations in the genes encoding proteins involved in assembly of collagen and elastic fibers and are at increased risk of PPROM, cervical insufficiency, uterine rupture, and delivering an intrauterine growth-restricted fetus [66]. C3H mice with targeted mutations in genes encoding specific proteoglycans, such as biglycan and decorin, displayed an Ehlers-Danlos-like phenotype that included reduced litter size and intrauterine growth restriction. Additionally, despite lacking an inflammatory response, these mice delivered before 18.0 dpc, with 43% delivering by 17.0 dpc, and none of the fetuses survived [67].
The extracellular matrix glycoprotein fetal fibronectin (fFN) is part of the amniotic membranes, and detection of fFN in cervical and vaginal fluid has been associated with an increased risk for spontaneous preterm birth. However, few patients with a positive fFN result will actually deliver early [10, 68, 69]. In mice, injection of fFN at 17.0 dpc causes preterm delivery within 12–36 h, likely via PPROM induced by activation of matrix metalloproteinases and cyclooxygenase-2 [70]. Likewise, injecting mice with thrombin at 17.0 dpc increased levels of matrix metalloproteinases and cyclooxygenase-2 and caused delivery of non-viable pups within 24 h [70–72].
Early progesterone withdrawal
A successful pregnancy requires uterine smooth muscle quiescence before parturition/labor. During gestation, progesterone is one of the predominant hormones produced by the placenta and is responsible for keeping the myometrium in a quiescent state. Progesterone levels remain high throughout human pregnancy, but women undergo a “functional” progesterone withdrawal at the end of pregnancy. This reduction in progesterone action is mediated by alterations in the ratio of progesterone receptor A to progesterone receptor B [73], changes in cofactor expression, and/or local metabolism of progesterone, thereby allowing contractions to initiate and the cervix to ripen [74]. Consistent with this idea, the anti-progesterone and anti-glucocorticoid drug Mifepristone, also known as RU486, induces uterine contractions and cervical ripening. To model early progesterone withdrawal, investigators injected mice with RU486 on 12–14 dpc, resulting in delivery within 18 h in ∼84% of C3H/HeN mice. Although this study reported that pups were born alive, postnatal pup survival was not discussed [75]. RU486-induced premature cervical ripening in Blk6/129SvEv mice was reported to be similar to term ripening and distinct from LPS-induced premature ripening in terms of gene expression, immune cell populations, and microstructural reorganization of collagen [76, 77]. In other reports, RU486 was suggested to promote cervical remodeling by activating the complement receptor C5a [36].
Prostaglandins
At the end of human pregnancy, levels of the prostaglandins PGE2 and PGF2α increase, bind to receptors on the uterus, and promote contractions. Clinically, PGE1 (misoprostol) is used to induce both uterine contractions and cervical ripening (effacement or thinning). As an alternative to giving women exogenous prostaglandins, mechanical compression of the cervix promotes local release of endogenous prostaglandins [78, 79]. To model excess prostaglandin activity in mice, researchers created a C57BL/6-129/SvJ mouse line with a hypomorphic mutation in hydroxyprostaglandin dehydrogenase (PGDH), which hydrolyzes prostaglandins. These mice had elevated levels of PGE2 and PGF2α and delivered approximately one-half day early [80]. Consistent with these earlier studies, co-administration of PGE2 and a PGDH inhibitor on gestation day 15 induced birth within 12 to 48 h [81]. In another study, C3H/HeN inbred wild-type mice treated with 20 μg of PGF2α on 16 dpc delivered 19.3 h postinjection, whereas vehicle-injected mice delivered 53.5 ± 13.6 h postinjection [82].
Uterine senescence
One hypothesis states that gestation length is governed by the timing of placental and decidual senescence, marked by a reduction in telomere length, and that parturition is initiated when these tissues become “old” [83]. Cellular aging is promoted by signaling through mammalian target of rapamycin complex 1 (mTORC1). The tumor suppressor p53 reduces mTORC1 signaling by activating AMP kinase, thereby inhibiting the aging process. To examine the relationship between uterine senescence and parturition, mice were generated lacking p53 specifically in the uterus. Once pregnant, these mice had decreased AMP kinase activation and increased mTORC1 signaling, resulting in early decidual senescence and increased incidence of preterm delivery (before 19 dpc) in some females [84–86]. Modeling gene–environment interactions, work in this model demonstrated that low-dose LPS injection further increased the incidence of preterm birth [87].
Uterine quiescence
The protease caspase-3 helps maintain quiescence by cleaving contractile proteins during gestation [88]. Condon and colleagues hypothesized that caspase-3 is maintained in an active state during pregnancy by the endoplasmic reticulum (ER) stress response and that its activity is reduced by the unfolded protein response at the end of pregnancy. In support of this model, injecting mice with tunicamycin, which induces excessive ER stress response, on 15 dpc caused early increases in levels of the contractile proteins connexin-43, alpha actin, and gamma actin, and resulted in dose-dependent early onset of labor beginning on 16 dpc. Conversely, co-administration of 4-phenylbutrate, an inhibitor of ER stress, prevented both the increase in contractile proteins and preterm birth [89].
Endocannabinoid signaling pathway
In human pregnancy, levels of the endocannabinoid anandamide are high in early gestation, decrease during mid-gestation, and spike at the onset of labor [90, 91]. Anandamide is thought to affect parturition timing by controlling secretion of corticotropin-releasing hormone (CRH) [92], which regulates the duration of pregnancy and onset of labor [93, 94]. Anandamide and another cannabinoid, 2-arachidonoxylglycerol, signal through the G protein-coupled cannabinoid receptor CB1 [95–98]. Deletion of the CB1 gene caused pregnant mice to go into preterm labor, delivering about one-half day before term. In this model, preterm delivery was thought to occur because of early progesterone withdrawal and increased estrogen production during pregnancy. Additionally, signaling through CB1 may control the CRH/corticosterone endocrine axis, as loss of CB1 caused an early rise in CRH and high levels of corticosterone during pregnancy [99].
Hyperhomocysteinemia
In a case-control study of 651 women, elevated homocysteine in maternal blood (hyperhomocysteinemia) during pregnancy was associated with increased odds of preterm birth [100]. One cause of hyperhomocysteinemia is a mutation in the gene encoding cystathionine B-synthase (CBS), an enzyme in the trans-sulfuration pathway [101]. To test a possible mechanism linking hyperhomocysteinemia to preterm birth, mice were generated carrying a mutation in CBS. CBS−/− pregnant mice had increased blood levels of homocysteine, developed preterm uterine contractions, and delivered significantly early (16.6 dpc vs. 20.2 dpc). Further analysis of this model demonstrated that the contractions were caused by preterm expression of the oxytocin receptor and increased PGE2 synthesis by the enzyme prostaglandin endoperoxide synthase 2 [102].
Environmental effects: cigarette smoking and dioxin exposure
Women who smoke cigarettes during pregnancy are at a higher risk of delivering preterm than nonsmokers [103]. To model this etiology, Ng and Zelikoff exposed mice to cigarette smoke via inhalation between 2 and 18 dpc. The mice exposed to cigarette smoke delivered at 19.6 dpc, whereas unexposed mice delivered at 20.3 dpc; the pups survived in both groups [104]. A major toxic component of cigarettes is 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin), which is also a ubiquitous environmental contaminant, a by-product of the industrialized process, and an endocrine disruptor. Because animals and humans are most sensitive to environmental toxicant exposure during in utero development, Bruner-Tran and Osteen exposed pregnant mice to dioxin and then examined the effects on fertility and preterm birth in the next two generations [105]. They found that 36% of females in the F1 generation delivered preterm as adults. Moreover, 25% of the F2 generation (which were exposed as germ cells of the F1 mice in utero) delivered preterm. Furthermore, when F1 females were exposed to GBS, mouse parvovirus, or LPS, the rates of preterm birth increased to 83, 86, and 100%, respectively [105, 106]. This model suggests that toxicant exposure acts additively with other risk factors to cause preterm birth.
Other models of PTB
Additional mouse models of preterm birth working through mechanisms of action not discussed above include inhibition of nitric oxide synthesis by treating mice with NG-nitro-L-arginine methyl ester [107]; inhibition of soluble guanylate cyclase by treating mice with methylene blue [108]; exogenous surfactant protein-A, which initiates the NF-κB-signaling cascade in the uterus [109]; injection of neuromedin B, which acts through its receptor to induce labor onset via the RELA (NF-κB P65)/IL6-mediated pathway [110]; and alcohol-induced preterm birth, which is associated with elevated levels of uterine PGE and PGF2α and increased expression of contraction-associated proteins and is prevented by pretreatment with the prostaglandin synthesis inhibitor aspirin [14, 111].
Defining preterm birth in mouse models
Given the large number of available mouse preterm birth models, standard definitions would allow researchers to appropriately compare results between studies and make meaningful inferences for human preterm birth. We argue that two related questions should be addressed when considering available and newly developed models: (1) Is the gestational length truly shortened, or does delivery occur within the normal range for the mouse strain? (2) Does the model deliver pups that are developmentally immature at delivery? Below, we draw on representative examples listed in Table 1 to highlight variations in experimental design that complicate our ability to evaluate and compare the current mouse models of preterm birth.
Strain-specific characteristics
Researchers have used several different strains of mice to generate models of preterm birth. The mouse strain is an important consideration given the findings of Murray et al. that gestational lengths can vary by as much as 41.7 h, or a full 1.72 days [16]. For example, the FVB/NJ, C57BL/6J, 129S1/SVIMJ, and A/J strains have average gestational lengths of 450.6, 462.4, 486.3, and 492.3 h, respectively, corresponding to between 18.8 and 20.5 days [16]. Clearly, the strain background has to be considered when determining whether a preterm birth model has shortened gestation.
Approaches for timed matings
Comparing gestational lengths between studies is complicated by variations in breeding protocols. For example, whereas most investigators breed mice overnight, some breed for undefined periods of time, and others obtain timed-mated animals from commercial suppliers. Mating strategies should be standardized given that interlitter variability in fetal body weights is greater when mice are bred continuously than when they are mated for 2 h or overnight [112]. Additionally, our field should standardize the definition of the start of gestation. Investigators record the time at which they see a copulation plug as 0.0, 0.5, or 1.0 dpc. This could result in up to a 24-h difference in timing of gestational length, perhaps leading a delivery to be scored as preterm when it is within the normal variation of delivery time for that mouse strain.
Monitoring the timing of parturition
Multiple methods have been used to monitor the timing of delivery. The onset of parturition is typically defined as the time of delivery of the first pup, which can be precisely determined by using a video camera with infrared lighting to record events in the dark. In contrast, strategies such as checking the dam multiple times during the day and recording the pup number may lead to inaccuracies because newly delivered pups can be hidden in the bedding or may be cannibalized if the dam considers the offspring to be abnormal. Furthermore, mice subjected to frequent disturbances in their cage or a nearby cage may enact a “predator response” defense mechanism that can alter their parturition behavior [113]. If an investigator checks the dam only on the expected morning of delivery, he or she may not realize that offspring were already delivered and thus may report that gestation was longer than it actually was.
Measures of development
Few studies provide comprehensive fetal outcome measurements of their preterm birth models [108]. Typically, studies report survival of offspring born preterm (Table 1), yet few provide details on the pups' developmental status (immature or mature).
Proposed guidelines for defining preterm birth in mouse models
For all studies, investigators should be aware that specific animal facility characteristics, such as diets, bedding, water, animal husbandry, co-bedding with other pregnant dams, environment (conventional vs. barrier systems), and noise pollution, could affect gestation length. Thus, instead of relying on published values, investigators should measure and report term gestational length in wild-type, untreated mice in the same facility in which they will do their experiments. Additionally, we suggest the following guidelines in reporting studies of existing and novel mouse models of preterm birth.
Breeding and timing of gestational length
Because gestational length varies by mouse strain, it is extremely important to breed controls of the same genetic background when using transgenic models. We suggest two methods of breeding:
Timed breeding
One of the most accurate methods for estimating gestational length is to restrict the mating period [112]. Because hormonally receptive females usually mate within 1 h [114], pairing estrus-stage females with males for 2 to 4 h and then checking for the presence of a copulation plug allows for accurate assessment of gestational length. Alternatively, multiple breeding cages of unstaged females can be set up for a restricted time. The time at which the copulation plug is noted should be recorded as 0.0 dpc, and the following morning should be considered 0.5 dpc. We recommend caution in using timed-mated mice in preterm birth studies provided by a commercial supplier, as gestational length is likely to be affected by shipping, handling of the mice, potential quarantine, temperature changes, and alterations in light–dark cycles.
Overnight breeding
When mice cannot be bred for a restricted time period or less precision is acceptable, mice can be bred overnight. Place estrus-stage dams with stud males overnight, and check for the presence of a copulation plug before 8:00 am the next day. This method is effective because fertilization occurs around midnight, or the mid-point of the active period, in a 12-h dark/light cycle [115]. Thus, the time at which the plug is noted should be recorded as 0.5 dpc. Transgenic facilities commonly use this method and find that the majority of fertilized eggs harvested the morning after overnight mating are at the 0.5 dpc stage. This method will allow investigators to assign the time of conception (and thus the length of gestation) within a 12- to 18-h window.
Monitoring pregnancy outcomes
Timing and delivery complications
The most precise and efficient way to monitor delivery is to use an infrared video camera system. This allows an investigator to observe delivery of the first pup (which should be defined as the end of gestation), total duration of parturition, and subtle phenotypes such as dystocia, which can be ascertained by measuring the pup-to-pup interval (which averages 15 min for C57Bl/6; unpublished data). This method also allows detection of delivery even if the dam cannibalizes her litter.
Delivery outcomes
To establish a new model of preterm birth or validate an existing one, several informative characteristics should be recorded for each delivery: (1) litter size, because of the strong inverse correlation with gestational length [16, 116]; (2) offspring mortality rate, because any increase may indicate death related to prematurity; (3) pup crown-rump length [117]; and (4) pup weight. However, these characteristics are not definitive indicators of prematurity and may vary by strain. Lastly, we suggest use of reliable fetal growth markers that, preferably, are relevant to human fetal development. We describe two classes of such markers, involving skin and the lungs, in the next section.
Mouse fetal development markers
Here, we suggest two straightforward methods to assess fetal tissue maturation. The Institutional Animal Care and Use Committee at each respective institution approved all protocols performed for this review.
Skin barrier function
The skin forms a permeability barrier that regulates body temperature, prevents excess water loss, and prevents invasion by harmful pathogens. In humans, the epithelium fully develops its barrier function by late gestation (∼34 weeks) [118]. Therefore, infants born before 30 weeks' gestation are at increased risk for infection and loss of temperature and fluid [119, 120]. The barrier develops when the outer layer of the epidermis forms the stratum corneum, which is composed of a tough insoluble cornified envelope that is “glued” together by a complex extracellular lipid matrix [121]. Barrier function develops similarly in mice late in gestation, beginning at 17 dpc and developing in a dorsal-to-ventral pattern until birth, when the barrier is fully formed. Thus, assessment of barrier function can indicate developmental stage of pups.
Methods to assess skin barrier maturation
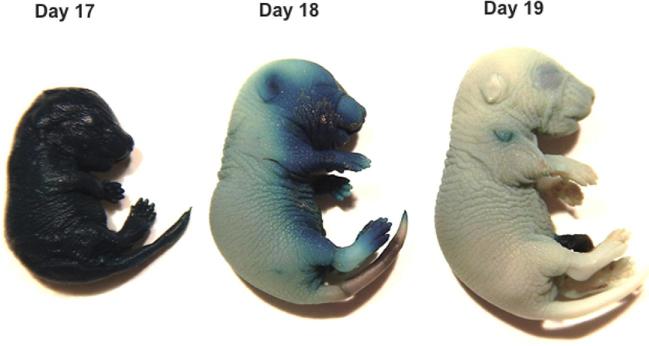
Barrier function can be assessed by performing a whole-mount skin permeability assay as previously reported [122]. To perform this assay, dissect pups from the dams on the day of delivery, place them in a solution containing the blue dye 5-bromo-4-chloro-3- indolyl-β, D-galactopyranoside (X-gal), and gently nutate for 8 h to overnight at 37°C [122]. Wash samples twice with phosphate-buffered saline, fix overnight in 4% formaldehyde at 4°C, and store in phosphate-buffered saline at 4°C. Evaluate pups for blue dye penetration. Figure 1 shows examples of pups at different gestational stages subjected to this assay. For C57Bl6/J mice at 17 dpc, the skin barrier is immature and fetuses are uniformly blue. By 18 dpc, the skin on the dorsal side starts to cornify and becomes impermeable to the dye and is thus white, while the ventral side is still permeable and thus is blue. By late 18 to 19.0 dpc, the skin barrier has fully formed and the skin is impermeable to the dye, so the pup is almost completely white.
Figure 1.
Skin permeability as a marker of maturity. In C57BL/6 mice, dye permeated mice at 17.0 dpc. By 18.0 dpc, dye was excluded on the dorsal side, but the pup remained blue on the ventral side. By 19 dpc, the skin was impermeable and faint blue stain was only noted on the ventral side. (Please see the online version for the color figure.)
Skin maturation can also be assessed histologically [123]. To perform this assay, fix fetuses in 10% formalin, dehydrate them through an ethanol series, embed them in paraffin, cut 8 μm sections, mount the sections on glass slides, and perform routine hematoxylin and eosin (H&E) staining. Examine the skin for markers of maturation including thickness, stratification of the epidermal and dermal papillary layers, and hair follicle density. As shown in Figure 2, skin samples from 17 dpc pups have thin epidermal and dermal papillary regions and contain few hair follicles. In contrast, skin samples from 19 dpc pups have thick, stratified epidermal layers and thick papillary regions and contain multiple hair follicles.
Figure 2.
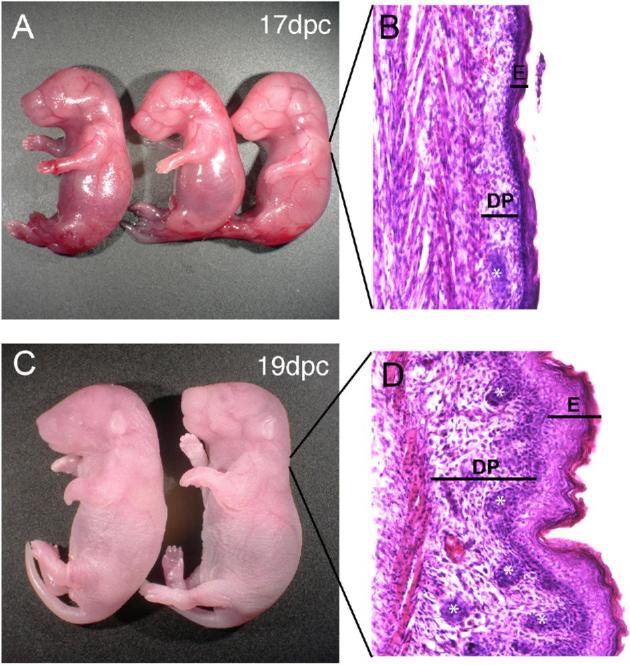
Skin histologic changes during gestation. In whole mount, the skin of 17 dpc CD-1 fetuses appeared more translucent (A) than that of 19 dpc fetuses (C). H&E-stained sections of dorsal skin from 17 dpc fetuses had thinner epidermal (E) and dermal papillary (DP) layers and contained fewer hair follicles (asterisks) than H&E-stained sections of skin from 19 dpc fetuses (B and D). (Please see the online version for the color figure.)
Lung maturity markers
Lung development proceeds through markedly similar steps in mammals [124, 125]. This process begins with branching morphogenesis, in which the embryonic tracheal lung bud reiteratively branches to form large and small airways [126]. Branching morphogenesis is complete by mid-gestation in humans and mice and is followed by a period of mesenchymal thinning and epithelial differentiation. Finally, the lungs become alveolarized, during which alveolar septation expands the gas exchange structures of the lung [127]. In humans, this process begins at 36 weeks' gestation and continues into early childhood [128, 129]. In mice, alveolarization occurs entirely postnatally, beginning at postnatal day 4–5 and becoming fully mature by postnatal day 30. We describe two methods that can be used to assess the extent of lung mesenchymal thinning and epithelial differentiation as a marker of fetal maturity in mice [130].
Morphometric assessment of lung maturation
Morphometric assessment of lung airspaces reveals the degree of mesenchymal thinning that occurs after branching morphogenesis is complete and before alveolarization initiates. To evaluate lung morphology, dissect out fetal lungs, section them, H&E stain the sections, and view the lung sections at ×40 magnification (Figure 3A and B). Avoid fields containing large vessels or airways. Use computer-assisted morphometry (e.g. Image-Pro Plus software; Media Cybernetics) to count the number of airspaces and measure airspace diameter and airspace perimeter. Calculate airspace volume density by dividing the sum of the airspace area by the total area (Figure 3C–F). In our experience, the airspace perimeter is the least reliable measure of lung maturity because airspace shape becomes more variable as the mesenchyme thins [131].
Figure 3.
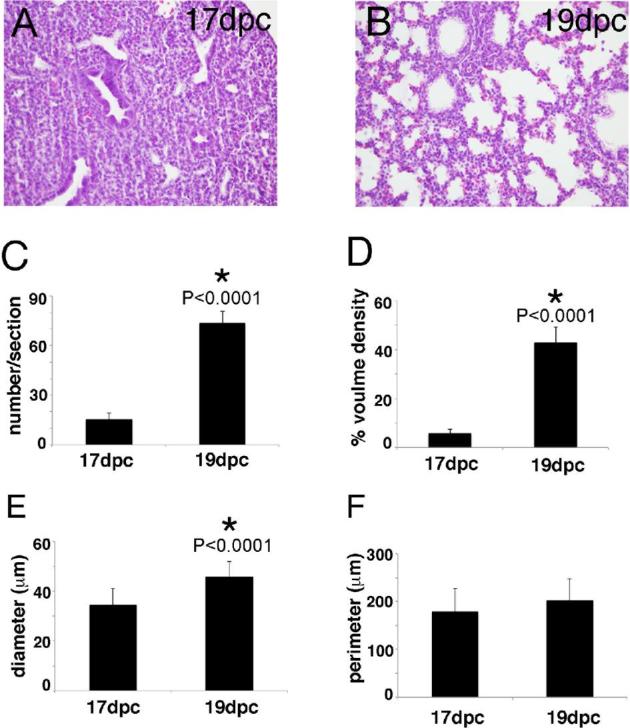
Lung morphometry as a marker of lung maturity. H&E stained lungs from 17 and 19 dpc CD-1 mouse fetuses (A, B). Lung morphometry measurements indicated that, compared to 17 dpc lungs, 19 dpc lungs contained more airspaces (C), had larger airspace volume density (airspace area divided by total lung area) (D), and had larger airspace diameter (E). However, airspace perimeter was similar in the two groups (F). n = 6 for both 17 and 19 dpc; *P-values, calculated by t-test; error bars represent SEM. (Please see the online version for the color figure.)
Protein and mRNA markers of lung epithelial differentiation
Infants born prematurely, especially at <34 weeks' gestation, have pulmonary complications due to lung immaturity, and those infants that survive are at increased risk for long-term adverse pulmonary outcomes such as asthma, reduced lung function, and chronic lung disease [2, 5]. These pulmonary deficits are due, in part, to insufficient lung surfactant, composed of lipids and proteins that are secreted by fully differentiated type 2 alveolar epithelial cells. Surfactant reduces surface tension, helps maintain normal lung volumes, and is essential for normal breathing [132]. The four major protein components of surfactant are surfactant proteins B and C (SP-B and SP-C), which interact with phospholipids to improve surfactant dispersion at the air/liquid interface and prevent alveolar collapse [130, 133], and surfactant proteins A and D (SP-A and SP-D), which act as part of the innate immune system by opsonizing bacteria for clearance by pulmonary macrophages.
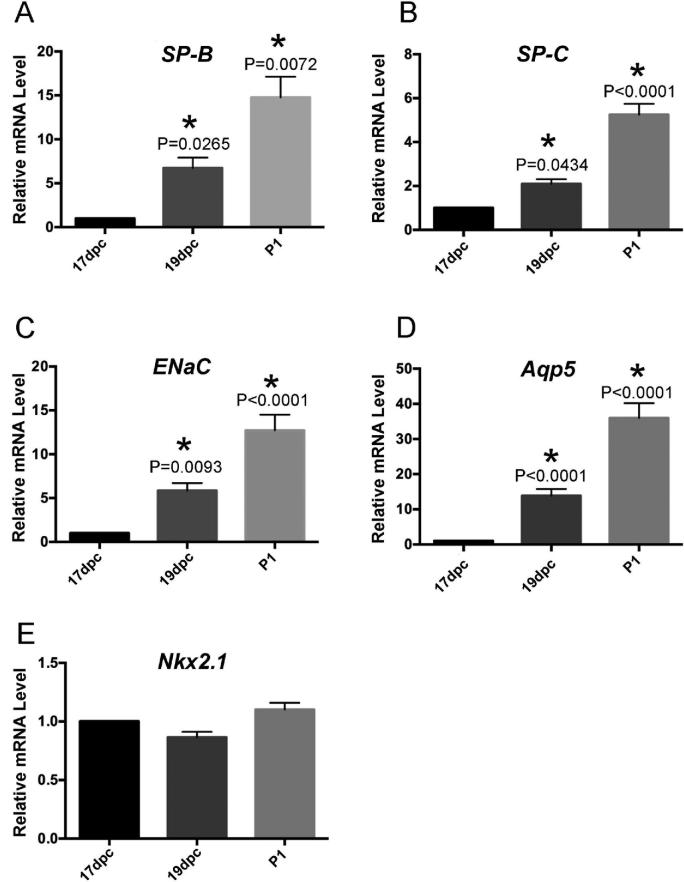
In mice, SP-A is expressed in the fetal lung late in gestation, becoming detectable in the amniotic fluid by 17.0 dpc and maximally expressed by 19.0 dpc [109]. Fetal SP-A is a particularly interesting marker of gestational lung maturity because it appears to help initiate parturition. Thus, detection of SP-A mRNA or protein in fetal lung or amniotic fluid could be used as a marker for fetal maturity in mice [109, 134]. SP-B, which is clinically administered to premature babies to treat surfactant deficiency, can likewise be used as a fetal lung marker. As shown in Figure 4A, SP-B mRNA increases in the fetal lung by up to 5-fold between 17 and 19 dpc and by up to 10-fold at postnatal day 1. SP-C, a transmembrane protein expressed exclusively in alveolar type 2 cells postnatally, can similarly be used as a marker of lung maturity (Figure 4B)[135].
Figure 4.
Gene expression as a marker of lung maturation. RNA isolated from pups of the indicated gestational ages was reverse transcribed to produce cDNA (SuperScript VILO kit; Invitrogen), and multiplex quantitative PCR reactions were performed with a StepOnePlus PCR System (Applied Biosystems) using the following FAM-labeled TaqMan Gene Expression assays (Applied Biosystems): SP-B Mm00455679_m1, ENac Mm00803386_m1, Aqp5 Mm00437578_m1, SP-C Mm00488144_m1, and Nkx2.1 Mm00447558_m1. The VIC-labeled housekeeping gene, 18S, was used as an internal control. Triplicate ΔΔCT values were generated for each sample. mRNA levels relative to dpc 17 mRNA were calculated by using the equation FC = 2−ΔΔCT. n = 4 for each timepoint; *P-values, calculated by one-way ANOVA with Dunnett post hoc analysis, denote comparisons between indicated values and 17 dpc values; error bars represent SEM.
Two other proteins that can be used as markers of lung development are the water channel Aquaporin 5, which is expressed in alveolar type 1 cells and is thought to facilitate fluid absorption in the perinatal lung [136], and the epithelial sodium ion channel (ENaC). Two of the three ENaC subunits increase sharply in the mouse fetal lung at late gestation [137]. As seen in Figure 4C and D, mRNA expression of ENaC and Aquaporin 5 increase in late gestation and can be used as markers of fetal lung maturity [135].
In addition to the above markers, panels of 50 or more genes have been evaluated as markers of fetal lung maturity in mice [130]. However, due to differences in mouse strain, detection methods, and reagents (primary antibodies, PCR primers), some of these proteins/genes may not be reproducible markers. For example, we found that NK2 Homeobox 1 (Nkx2.1), an epithelial transcription factor critical for embryonic lung morphogenesis, was expressed at similar levels in 17 dpc, 19 dpc, and postnatal day 1 lungs. Therefore, we recommend that investigators evaluate multiple lung maturation markers. In addition, absolute levels of each marker are not sufficient to assess lung maturity and should always be compared to levels in full-term pups of the appropriate genotype and strain.
Conclusion
Researchers have used several mouse models to test hypotheses regarding preterm birth. These include models of genetic, infectious, noninfectious (sterile) inflammatory, environmental toxins, and endocrine etiologies. However, comparing data between studies is challenging given the lack of uniform criteria for defining preterm birth and assessing pup maturity. A recent review by Manuel et al. encourages investigators to develop better mouse models that are more consistent with the human etiologies of preterm birth [138]. In this review, we recommend that our field adopt standardized experimental and reporting guidelines to define preterm birth and use fetal developmental markers to distinguish between premature and mature pups. In addition to the skin and lung markers proposed here, we welcome other easy-to-use developmental markers. By standardizing our methods and reporting, mice should become an even more valuable model with which to study the important problems of preterm birth and the resulting neonatal co-morbidities.
Acknowledgments
We thank Dr Deborah J. Frank and Jessica Chubiz for critical reading and editing. We also thank Dr Xiaofeng Ma for help with the images.
Footnotes
Grant support: This review was supported by NIH grants HD081121 (to JR), HD088830 (to JLH), and HL132805 (to ELS), and the March of Dimes Prematurity Research Center (to EDH, ESJ, JCF, MM, and SKE). SKE is supported by the Department of Obstetrics and Gynecology at Washington University and the March of Dimes Prematurity Research Center.
References
- 1. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinney M, Lawn J Born Too Soon Preterm Birth Action Group. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013; 10(Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lawn JE, Kinney M. Preterm birth: now the leading cause of child death worldwide. Sci Transl Med 2014; 6 (263):263ed21–263ed21. [DOI] [PubMed] [Google Scholar]
- 3. Lawn JE, Kinney MV, Belizan JM, Mason EM, McDougall L, Larson J, Lackritz E, Friberg IK, Howson CP Born Too Soon Preterm Birth Action Group. Born too soon: accelerating actions for prevention and care of 15 million newborns born too soon. Reprod Health 2013; 10(Suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet North Am Ed 2016; 388 (10063):3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raju TN, Pemberton VL, Saigal S, Blaisdell CJ, Moxey-Mims M, Buist S Adults Born Preterm Conference Speakers and Discussants. Long-term healthcare outcomes of preterm birth: an executive summary of a conference sponsored by the National Institutes of Health. J Pediatr 2017; 181:309–318.e1. [DOI] [PubMed] [Google Scholar]
- 6. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet North Am Ed 2008; 371 (9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moutquin JM. Classification and heterogeneity of preterm birth. BJOG 2003; 110(Suppl 20):30–33. [DOI] [PubMed] [Google Scholar]
- 8. Esplin MS, Elovitz MA, Iams JD, Parker CB, Wapner RJ, Grobman WA, Simhan HN, Wing DA, Haas DM, Silver RM, Hoffman MK, Peaceman AM et al. . Predictive accuracy of serial transvaginal cervical lengths and quantitative vaginal fetal fibronectin levels for spontaneous preterm birth among nulliparous women. JAMA 2017; 317 (10):1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mercer BM, Goldenberg RL, Das A, Moawad AH, Iams JD, Meis PJ, Copper RL, Johnson F, Thom E, McNellis D, Miodovnik M, Menard MK et al. . The preterm prediction study: a clinical risk assessment system. Am J Obstet Gynecol 1996; 174 (6):1885–1895; discussion 1893-1885. [DOI] [PubMed] [Google Scholar]
- 10. Son M, Miller ES. Predicting preterm birth: cervical length and fetal fibronectin. Semin Perinatol 2017; 41 (8):445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ratajczak CK, Muglia LJ. Insights into parturition biology from genetically altered mice. Pediatr Res 2008; 64 (6):581–589. [DOI] [PubMed] [Google Scholar]
- 12. Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab 2004; 15 (10):479–487. [DOI] [PubMed] [Google Scholar]
- 13. Brodt-Eppley J, Myatt L. Prostaglandin receptors in lower segment myometrium during gestation and labor. Obstet Gynecol 1999; 93:89–93. [DOI] [PubMed] [Google Scholar]
- 14. Cook JL, Zaragoza DB, Sung DH, Olson DM. Expression of myometrial activation and stimulation genes in a mouse model of preterm labor: myometrial activation, stimulation, and preterm labor. Endocrinology 2000; 141 (5):1718–1728. [DOI] [PubMed] [Google Scholar]
- 15. Fuchs AR, Fuchs F, Husslein P, Soloff MS, Fernstrom MJ. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science 1982; 215 (4538):1396–1398. [DOI] [PubMed] [Google Scholar]
- 16. Murray SA, Morgan JL, Kane C, Sharma Y, Heffner CS, Lake J, Donahue LR. Mouse gestation length is genetically determined. PLoS One 2010; 5 (8):e12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bezold KY, Karjalainen MK, Hallman M, Teramo K, Muglia LJ. The genomics of preterm birth: from animal models to human studies. Genome Med 2013; 5 (4):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. Natl Vital Stat Rep 2017; 66:1. [PubMed] [Google Scholar]
- 19. Csapo AI, Pulkkinen MO, Ruttner B, Sauvage JP, Wiest WG. The significance of the human corpus luteum in pregnancy maintenance. Am J Obstet Gynecol 1972; 112 (8):1061–1067. [DOI] [PubMed] [Google Scholar]
- 20. Cunningham FG, Williams JW. Williams Obstetrics. New York: McGraw-Hill Medical; 2010. [Google Scholar]
- 21. Andersson S, Minjarez D, Yost NP, Word RA. Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J Clin Endocrinol Metab 2008; 93 (6):2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nadeem L, Shynlova O, Mesiano S, Lye S. Progesterone via its type-A receptor promotes myometrial gap junction coupling. Sci Rep 2017; 7 (1):13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol 1999; 13:981–992. [DOI] [PubMed] [Google Scholar]
- 24. Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth 2007; 7(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galinsky R, Polglase GR, Hooper SB, Black MJ, Moss TJ. The consequences of chorioamnionitis: preterm birth and effects on development. J Pregnancy 2013; 2013:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ustun C, Kocak I, Baris S, Uzel A, Saltik F. Subclinical chorioamnionitis as an etiologic factor in preterm deliveries. Int J Gynecol Obstet 2001; 72:109–115. [DOI] [PubMed] [Google Scholar]
- 27. Higgins RD, Saade G, Polin RA, Grobman WA, Buhimschi IA, Watterberg K, Silver RM, Raju TN Chorioamnionitis Workshop Participants. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis. Obstet Gynecol 2016; 127:426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med 2012; 17:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bastek JA, Gomez LM, Elovitz MA. The role of inflammation and infection in preterm birth. Clin Perinatol 2011; 38:385–406. [DOI] [PubMed] [Google Scholar]
- 30. Akgul Y, Word RA, Ensign LM, Yamaguchi Y, Lydon J, Hanes J, Mahendroo M. Hyaluronan in cervical epithelia protects against infection-mediated preterm birth. J Clin Invest 2014; 124:5481–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hirsch E, Saotome I, Hirsh D. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol 1995; 172:1598–1603. [DOI] [PubMed] [Google Scholar]
- 32. Kaga N, Katsuki Y, Obata M, Shibutani Y. Repeated administration of low-dose lipopolysaccharide induces preterm delivery in mice: a model for human preterm parturition and for assessment of the therapeutic ability of drugs against preterm delivery. Am J Obstet Gynecol 1996; 174:754–759. [DOI] [PubMed] [Google Scholar]
- 33. Migale R, Herbert BR, Lee YS, Sykes L, Waddington SN, Peebles D, Hagberg H, Johnson MR, Bennett PR, MacIntyre DA. Specific lipopolysaccharide serotypes induce differential maternal and neonatal inflammatory responses in a murine model of preterm labor. Am J Pathol 2015; 185:2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thaxton JE, Nevers TA, Sharma S. TLR-mediated preterm birth in response to pathogenic agents. Infect Dis Obstet Gynecol 2010; 2010:8, Article ID 378472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth. Am J Pathol 2003; 163:2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol 2011; 179:838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ilievski V, Lu SJ, Hirsch E. Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci 2007; 14:315–320. [DOI] [PubMed] [Google Scholar]
- 38. Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J Pharmacol Toxicol Methods 1998; 39:147–154. [DOI] [PubMed] [Google Scholar]
- 39. Cardenas I, Mulla MJ, Myrtolli K, Sfakianaki AK, Norwitz ER, Tadesse S, Guller S, Abrahams VM. Nod1 activation by bacterial iE-DAP induces maternal-fetal inflammation and preterm labor. J Immunol 2011; 187:980–986. [DOI] [PubMed] [Google Scholar]
- 40. Berardi A, Cattelani C, Creti R, Berner R, Pietrangiolillo Z, Margarit I, Maione D, Ferrari F. Group B streptococcal infections in the newborn infant and the potential value of maternal vaccination. Expert Rev Anti Infect Ther 2015; 13:1387–1399. [DOI] [PubMed] [Google Scholar]
- 41. Bernardini R, Aufieri R, Detcheva A, Recchia S, Cicconi R, Amicosante M, Montesano C, Rossi P, Tchidjou HK, Petrunov B, Orefici G, Mattei M. Neonatal protection and preterm birth reduction following maternal group B streptococcus vaccination in a mouse model. J Matern Fetal Neonatal Med 2017; 30:2844–2850. [DOI] [PubMed] [Google Scholar]
- 42. Equils O, Moffatt-Blue C, Ishikawa TO, Simmons CF, Ilievski V, Hirsch E. Pretreatment with pancaspase inhibitor (Z-VAD-FMK) delays but does not prevent intraperitoneal heat-killed group B Streptococcus-induced preterm delivery in a pregnant mouse model. Infect Dis Obstet Gynecol 2009; 2009:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Viscardi RM. Ureaplasma species: role in diseases of prematurity. Clin Perinatol 2010; 37:393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sweeney EL, Dando SJ, Kallapur SG, Knox CL. The human ureaplasma species as causative agents of chorioamnionitis. Clin Microbiol Rev 2017; 30:349–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uchida K, Nakahira K, Mimura K, Shimizu T, De Seta F Wakimoto T, Kawai Y, Nomiyama M, Kuwano K, Guaschino S, Yanagihara I. Effects of Ureaplasma parvum lipoprotein multiple-banded antigen on pregnancy outcome in mice. J Reprod Immunol 2013; 100:118–127. [DOI] [PubMed] [Google Scholar]
- 46. Rours GI, Duijts L, Moll HA, Arends LR, de Groot R, Jaddoe VW, Hofman A, Steegers EA, Mackenbach JP, Ott A, Willemse HF, van der Zwaan EA et al. . Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population-based prospective cohort study. Eur J Epidemiol 2011; 26:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pal S, Peterson EM, De La Maza LM. A murine model for the study of Chlamydia trachomatis genital infections during pregnancy. Infect Immun 1999; 67:2607–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harris RE, Gilstrap LC 3rd. Cystitis during pregnancy: a distinct clinical entity. Obstet Gynecol 1981; 57:578–580. [PubMed] [Google Scholar]
- 49. Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 1989; 73:576–582. [PubMed] [Google Scholar]
- 50. Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun 1999; 67:5958–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, Beck J. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol 1996; 67:1103–1113. [DOI] [PubMed] [Google Scholar]
- 52. Horliana AC, Chambrone L, Foz AM, Artese HP, Rabelo Mde S, Pannuti CM, Romito GA. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS One 2014; 9:e98271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, Martinez M, Gamonal J. Detection of porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol 2007; 78:1249–1255. [DOI] [PubMed] [Google Scholar]
- 54. Katz J, Chegini N, Shiverick KT, Lamont RJ. Localization of P. gingivalis in preterm delivery placenta. J Dent Res 2009; 88:575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ao M, Miyauchi M, Furusho H, Inubushi T, Kitagawa M, Nagasaki A, Sakamoto S, Kozai K, Takata T. Dental infection of Porphyromonas gingivalis induces preterm birth in mice. PLoS One 2015; 10:e0137249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014; 345:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Yeo L. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014; 72:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heng YJ, Liong S, Permezel M, Rice GE, Di Quinzio MK, Georgiou HM. The interplay of the interleukin 1 system in pregnancy and labor. Reprod Sci 2014; 21:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bry K, Hallman M. Transforming growth factor-beta 2 prevents preterm delivery induced by interleukin-1 alpha and tumor necrosis factor-alpha in the rabbit. Am J Obstet Gynecol 1993; 168:1318–1322. [DOI] [PubMed] [Google Scholar]
- 60. Sadowsky DW, Adams KM, Gravett MG, Witkin SS, Novy MJ. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol 2006; 195:1578–1589. [DOI] [PubMed] [Google Scholar]
- 61. Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991; 165:969–971. [DOI] [PubMed] [Google Scholar]
- 62. Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010; 10:826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011; 24:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol 2016; 75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loctin J, Delost P. Mortality in premature mice at birth and during neonatal development. Reprod Nutr Dévelop 1983; 23:293–301. [DOI] [PubMed] [Google Scholar]
- 66. Barabas AP. Ehlers-Danlos syndrome: associated with prematurity and premature rupture of foetal membranes; possible increase in incidence. BMJ 1966; 2:682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Calmus ML, Macksoud EE, Tucker R, Iozzo RV, Lechner BE. A mouse model of spontaneous preterm birth based on the genetic ablation of biglycan and decorin. Reproduction 2011; 142:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peaceman AM, Andrews WW, Thorp JM, Cliver SP, Lukes A, Iams JD, Coultrip L, Eriksen N, Holbrook RH, Elliott J, Ingardia C, Pietrantoni M. Fetal fibronectin as a predictor of preterm birth in patients with symptoms: a multicenter trial. Am J Obstet Gynecol 1997; 177:13–18. [DOI] [PubMed] [Google Scholar]
- 69. Swamy GK, Simhan HN, Gammill HS, Heine RP. Clinical utility of fetal fibronectin for predicting preterm birth. J Reprod Med 2005; 50:851–856. [PubMed] [Google Scholar]
- 70. Mogami H, Kishore AH, Shi H, Keller PW, Akgul Y, Word RA. Fetal fibronectin signaling induces matrix metalloproteases and cyclooxygenase-2 (COX-2) in amnion cells and preterm birth in mice. J Biol Chem 2013; 288:1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mogami H, Keller PW, Shi H, Word RA. Effect of thrombin on human amnion mesenchymal cells, mouse fetal membranes, and preterm birth. J Biol Chem 2014; 289:13295–13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chigusa Y, Kishore AH, Mogami H, Word RA. Nrf2 activation inhibits effects of thrombin in human amnion cells and thrombin-induced preterm birth in mice. J Clin Endocrinol Metab 2016; 101:2612–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab 2012; 97:E719–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA 2003; 100:9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dudley DJ, Branch DW, Edwin SS, Mitchell MD. Induction of preterm birth in mice by RU486. Biol Reprod 1996; 55:992–995. [DOI] [PubMed] [Google Scholar]
- 76. Holt R, Timmons BC, Akgul Y, Akins ML, Mahendroo M. The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology 2011; 152:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nallasamy S, Akins M, Tetreault B, Luby-Phelps K, Mahendroo M. Distinct reorganization of collagen architecture in lipopolysaccharide - mediated premature cervical remodeling. Biol Reprod 2018; 98:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Norwitz E, Robinson J, Repke J. Obstetrics; normal and problem pregnancies. In: Gabbe SG, Niebyl JR, Simpson JL (eds.), Obstetrics: Normal and Problem Pregnancies. 4th ed New York: Churchill Livingstone; 2002: 353–394. [Google Scholar]
- 79. Timmons BC, Reese J, Socrate S, Ehinger N, Paria BC, Milne GL, Akins ML, Auchus RJ, McIntire D, House M, Mahendroo M. Prostaglandins are essential for cervical ripening in LPS-mediated preterm birth but not term or antiprogestin-driven preterm ripening. Endocrinology 2014; 155:287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Roizen JD, Asada M, Tong M, Tai HH, Muglia LJ. Preterm birth without progesterone withdrawal in 15-hydroxyprostaglandin dehydrogenase hypomorphic mice. Mol Endocrinol 2008; 22:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kishore AH, Liang H, Kanchwala M, Xing C, Ganesh T, Akgul Y, Posner B, Ready JM, Markowitz SD, Word RA. Prostaglandin dehydrogenase is a target for successful induction of cervical ripening. Proc Natl Acad Sci USA 2017; 114:E6427–E6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kurtzman JT, Spinnato JA, Goldsmith LJ, Zimmerman MJ, Klem M, Lei ZM, Rao CV. Human chorionic gonadotropin exhibits potent inhibition of preterm delivery in a small animal model. Am J Obstet Gynecol 1999; 181:853–857. [DOI] [PubMed] [Google Scholar]
- 83. Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update 2016; 22:535–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Deng W, Cha J, Yuan J, Haraguchi H, Bartos A, Leishman E, Viollet B, Bradshaw HB, Hirota Y, Dey SK. p53 coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern parturition timing. J Clin Invest 2016; 126:2941–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hirota Y, Cha J, Yoshie M, Daikoku T, Dey SK. Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice. Proc Natl Acad Sci USA 2011; 108:18073–18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest 2010; 120:803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cha J, Bartos A, Egashira M, Haraguchi H, Saito-Fujita T, Leishman E, Bradshaw H, Dey SK, Hirota Y. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J Clin Invest 2013; 123:4063–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Jeyasuria P, Wetzel J, Bradley M, Subedi K, Condon JC. Progesterone-regulated caspase 3 action in the mouse may play a role in uterine quiescence during pregnancy through fragmentation of uterine myocyte contractile proteins. Biol Reprod 2009; 80:928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kyathanahalli C, Organ K, Moreci RS, Anamthathmakula P, Hassan SS, Caritis SN, Jeyasuria P, Condon JC. Uterine endoplasmic reticulum stress-unfolded protein response regulation of gestational length is caspase-3 and -7–dependent. Proc Natl Acad Sci USA 2015; 112:14090–14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dennedy MC, Friel AM, Houlihan DD, Broderick VM, Smith T, Morrison JJ. Cannabinoids and the human uterus during pregnancy. Am J Obstet Gynecol 2004; 190:2–9; discussion 3A. [DOI] [PubMed] [Google Scholar]
- 91. Habayeb OM, Taylor AH, Evans MD, Cooke MS, Taylor DJ, Bell SC, Konje JC. Plasma levels of the endocannabinoid anandamide in women—a potential role in pregnancy maintenance and labor? J Clin Endocrinol Metab 2004; 89:5482–5487. [DOI] [PubMed] [Google Scholar]
- 92. Cota D, Steiner MA, Marsicano G, Cervino C, Herman JP, Grubler Y, Stalla J, Pasquali R, Lutz B, Stalla GK, Pagotto U. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology 2007; 148:1574–1581. [DOI] [PubMed] [Google Scholar]
- 93. McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med 1995; 1:460–463. [DOI] [PubMed] [Google Scholar]
- 94. Karalis K, Goodwin G, Majzoub JA. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med 1996; 2:556–560. [DOI] [PubMed] [Google Scholar]
- 95. Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990; 346:561–564. [DOI] [PubMed] [Google Scholar]
- 96. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993; 365:61–65. [DOI] [PubMed] [Google Scholar]
- 97. Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992; 258:1946–1949. [DOI] [PubMed] [Google Scholar]
- 98. Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 1995; 215:89–97. [DOI] [PubMed] [Google Scholar]
- 99. Wang H, Xie H, Dey SK. Loss of cannabinoid receptor CB1 induces preterm birth. PLoS One 2008; 3:e3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kramer MS, Kahn SR, Rozen R, Evans R, Platt RW, Chen MF, Goulet L, Seguin L, Dassa C, Lydon J, McNamara H, Dahhou M et al. . Vasculopathic and thrombophilic risk factors for spontaneous preterm birth. Int J Epidemiol 2009; 38:715–723. [DOI] [PubMed] [Google Scholar]
- 101. Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med 2009; 60:39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sonne SR, Bhalla VK, Barman SA, White RE, Zhu S, Newman TM, Prasad PD, Smith SB, Offermanns S, Ganapathy V. Hyperhomocysteinemia is detrimental to pregnancy in mice and is associated with preterm birth. Biochim Biophys Acta 2013; 1832:1149–1158. [DOI] [PubMed] [Google Scholar]
- 103. Wallace JL, Aland KL, Blatt K, Moore E, DeFranco EA. Modifying the risk of recurrent preterm birth: influence of trimester-specific changes in smoking behaviors. Am J Obstet Gynecol 2017; 216:310 e311-310 e318. [DOI] [PubMed] [Google Scholar]
- 104. Ng SP, Zelikoff JT. The effects of prenatal exposure of mice to cigarette smoke on offspring immune parameters. J Toxicol Environ Health A 2008; 71:445–453. [DOI] [PubMed] [Google Scholar]
- 105. Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol 2011; 31:344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ding T, Lambert LA, Aronoff DM, Osteen KG, Bruner-Tran KL. Sex-dependent influence of developmental toxicant exposure on group b Streptococcus-mediated preterm birth in a murine model. Reprod Sci 2018; 25:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Tiboni GM, Giampietro F. Inhibition of nitric oxide synthesis causes preterm delivery in the mouse. Hum Reprod 2000; 15:1838–1842. [DOI] [PubMed] [Google Scholar]
- 108. Tiboni GM, Giampietro F, Lamonaca D. The soluble guanylate cyclase inhibitor methylene blue evokes preterm delivery and fetal growth restriction in a mouse model. In Vivo 2001; 15:333–337. [PubMed] [Google Scholar]
- 109. Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA 2004; 101:4978–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang WS, Xie QS, Wu XH, Liang QH. Neuromedin B and its receptor induce labor onset and are associated with the RELA (NFKB P65)/IL6 pathway in pregnant mice. Biol Reprod 2011; 84:113–117. [DOI] [PubMed] [Google Scholar]
- 111. Cook JL, Randall CL. Early onset of parturition induced by acute alcohol exposure in C57BL/6J mice: role of uterine PGE and PGF2a. Reprod Fertil Dev 1997; 9:815–823. [DOI] [PubMed] [Google Scholar]
- 112. Endo A, Watanabe T. Interlitter variability in fetal body weight in mouse offspring from continuous, overnight, and short-period matings. Teratology 1988; 37:63–67. [DOI] [PubMed] [Google Scholar]
- 113. Beniest-Noirot E. Analyse du comportement dit maternal chez la souris. Cent Nat Rech Sci Monogr Franc Psychol. 1958; No. 1. [Google Scholar]
- 114. Wimer RE, Fuller JL. Patterns of Behavior In: Green EL. (ed.) Biology of the Laboratory Mouse. New York: Dover Publications; 1966: 629–653. [Google Scholar]
- 115. Behringer R. Manipulating the Mouse Embryo: a Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 2014. [Google Scholar]
- 116. Biggers JD, Curnow RN, Finn CA, McLaren A. Regulation of the gestation period in mice. Reproduction 1963; 6:125–138. [DOI] [PubMed] [Google Scholar]
- 117. McCoy AM, Herington JL, Stouch AN, Mukherjee AB, Lakhdari O, Blackwell TS, Prince LS. IKKβ activation in the fetal lung mesenchyme alters lung vascular development but not airway morphogenesis. Am J Pathol 2017; 187:2635–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Evans NJ, Rutter N. Development of the epidermis in the newborn. Neonatology 1986; 49:74–80. [DOI] [PubMed] [Google Scholar]
- 119. Vernon HJ, Lane AT, Wischerath LJ, Davis JM, Menegus MA. Semipermeable dressing and transepidermal water loss in premature infants. Pediatrics 1990; 86:357–362. [PubMed] [Google Scholar]
- 120. Cartlidge P. The epidermal barrier. Semin Neonatol 2000; 5:273–280. [DOI] [PubMed] [Google Scholar]
- 121. Menon GK, Cleary GW, Lane ME. The structure and function of the stratum corneum. Int J Pharm 2012; 435:3–9. [DOI] [PubMed] [Google Scholar]
- 122. Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development 1998; 125:1541–1552. [DOI] [PubMed] [Google Scholar]
- 123. Forni MF, Trombetta-Lima M, Sogayar MC. Stem cells in embryonic skin development. Biol Res 2012; 45:215–222. [DOI] [PubMed] [Google Scholar]
- 124. Pringle KC. Human fetal lung development and related animal models. Clin Obstet Gynecol 1986; 29:502–513. [PubMed] [Google Scholar]
- 125. Thurlbeck WM. Postnatal growth and development of the lung. Am Rev Respir Dis 1975; 111:803–844. [DOI] [PubMed] [Google Scholar]
- 126. Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature 2008; 453:745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res 2005; 57:38R–46R. [DOI] [PubMed] [Google Scholar]
- 128. Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol 1984; 46:617–628. [DOI] [PubMed] [Google Scholar]
- 129. Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell 2010; 18:8–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Besnard V, Wert SE, Ikegami M, Xu Y, Heffner C, Murray SA, Donahue LR, Whitsett JA. Maternal synchronization of gestational length and lung maturation. PLoS One 2011; 6:e26682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Plosa EJ, Young LR, Gulleman PM, Polosukhin VV, Zaynagetdinov R, Benjamin JT, Im AM, van der Meer R, Gleaves LA, Bulus N, Han W, Prince LS et al. . Epithelial 1 integrin is required for lung branching morphogenesis and alveolarization. Development 2014; 141:4751–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Clements JA. Pulmonary surfactant. Am Rev Respir Dis 1970; 101:984–990. [DOI] [PubMed] [Google Scholar]
- 133. Mendelson CR, Chen C, Boggaram V, Zacharias C, Snyder JM. Regulation of the synthesis of the major surfactant apoprotein in fetal rabbit lung tissue. J Biol Chem 1986; 261:9938–9943. [PubMed] [Google Scholar]
- 134. Mendelson CR, Condon JC. New insights into the molecular endocrinology of parturition. J Steroid Biochem Mol Biol 2005; 93:113–119. [DOI] [PubMed] [Google Scholar]
- 135. Shelton EL, Waleh N, Plosa EJ, Benjamin JT, Milne GL, Hooper CW, Ehinger NJ, Poole S, Brown N, Seidner S, McCurnin D, Reese JJ et al. . Effects of antenatal betamethasone on preterm human and mouse ductus arteriosus: comparison with baboon data. Pediatr Res 2018; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest 2000; 105:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Talbot CL, Bosworth DG, Briley EL, Fenstermacher DA, Boucher RC, Gabriel SE, Barker PM. Quantitation and localization of ENaC subunit expression in fetal, newborn, and adult mouse lung. Am J Respir Cell Mol Biol 1999; 20:398–406. [DOI] [PubMed] [Google Scholar]
- 138. Manuel CR, Ashby CR, Reznik SE. Discrepancies in animal models of preterm birth. Curr Pharm Des 2017; 23:6142–6148. [DOI] [PubMed] [Google Scholar]
- 139. Sun X, Deng W, Li Y, Tang S, Leishman E, Bradshaw HB, Dey SK. Sustained endocannabinoid signaling compromises decidual function and promotes inflammation-induced preterm birth. J Biol Chem 2016; 291:8231–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Guo Y, Ma Z, Kou H, Sun R, Yang H, Smith CV, Zheng J, Wang H. Synergistic effects of pyrrolizidine alkaloids and lipopolysaccharide on preterm delivery and intrauterine fetal death in mice. Toxicol Lett 2013; 221:212–218. [DOI] [PubMed] [Google Scholar]
- 141. Edey LF, O’Dea KP, Herbert BR, Hua R, Waddington SN, MacIntyre DA, Bennett PR, Takata M, Johnson MR. The local and systemic immune response to intrauterine LPS in the prepartum mouse. Biol Reprod 2016; 95:125–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chin PY, Dorian CL, Hutchinson MR, Olson DM, Rice KC, Moldenhauer LM, Robertson SA. Novel Toll-like receptor-4 antagonist (+)-naloxone protects mice from inflammation-induced preterm birth. Sci Rep 2016; 6:36112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gomez-Lopez N, Romero R, Arenas-Hernandez M, Panaitescu B, Garcia-Flores V, Mial TN, Sahi A, Hassan SS. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J Matern Fetal Neonatal Med 2018; 31:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Stelloh C, Allen KP, Mattson DL, Lerch-Gaggl A, Reddy S, El-Meanawy A. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl Res 2012; 159:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ng SP, Steinetz BG, Lasano SG, Zelikoff JT. Hormonal changes accompanying cigarette smoke-induced preterm births in a mouse model. Exp Biol Med (Maywood) 2006; 231:1403–1409. [DOI] [PubMed] [Google Scholar]