ABSTRACT
Long-term use of paracetamol (at therapeutic doses) can cause the accumulation of endogenous organic pyroglutamate, resulting in metabolic acidosis with an elevated anion gap. This occurs in the presence of malnutrition, infection, antibiotic use, renal failure and pregnancy. Given the prevalence of these risk factors, this condition is thought to be relatively common in a hospitalised population but is probably significantly underdiagnosed. Prompt recognition is essential because the condition is entirely reversible if the causative agents are withdrawn.
Here we describe five cases of pyroglutamic acidosis that we have encountered in a tertiary referral hospital. Together they illustrate the common clinical risk factors and the excellent prognosis, once a diagnosis is made. We describe how a rudimentary acid-base analysis (calculation of the anion gap) usually leads to the diagnosis but how a more nuanced approach may be required in the presence of mixed acid-base disorders.
KEYWORDS: 5-oxoproline, acidosis, anion gap, paracetamol, pyroglutamate
Introduction
Pyroglutamic acid (5-oxoproline) is an endogenous organic acid that can accumulate in serum to cause anion gap metabolic acidosis. This can occur with inherited defects in enzymes that participate in the γ-glutamyl cycle or can be acquired in association with chronic therapeutic paracetamol use, malnutrition, sepsis, antibiotics and renal impairment.1 The prevalence of these risk factors, both in the hospital and the community, suggests that this diagnosis is often overlooked.
Herein we describe five cases of pyroglutamic acidosis, collected over a 7-year period in a teaching hospital. Together they illustrate the common risk factors for this condition, and demonstrate how a rudimentary acid-base analysis (calculation of the serum anion gap) can usually point towards the diagnosis. However, some of the cases also demonstrate how a nuanced approach to acid-base balance (correction for hypoalbuminaema, calculating the excess anion gap (ΔAG) and urine anion gap) is required to elicit the diagnosis when mixed acid-base disturbances are present.
Cases
Case 1
A 48-year-old female presented with 10 days of vomiting, abdominal pain and shortness of breath. Her past history included autosomal dominant polycystic kidney disease causing stage III chronic kidney disease and intermittent back pain. She had been taking paracetamol at therapeutic doses for 2 months, as well as omeprazole, thiamine and tramadol. She drank alcohol to excess. Initial clinical assessment suggested extracellular fluid volume overload and a lower respiratory tract infection; she was treated with diuretics, ceftriaxone and regular paracetamol. Despite a general improvement in her condition, over the following week she developed a progressive metabolic acidosis.
Case 2
A 67-year-old male was admitted for the management of a wound infection 1 month after open reduction and internal fixation of a right-sided ankle fracture. His history included hypertension, chronic obstructive pulmonary disease and peptic ulcer. He was taking bendroflumethiazide, lisinopril, tramadol and paracetamol (4 g daily for 1 month). He drank in excess of 30 units of alcohol per week. Wound swabs grew Staphylococcus aureus and he was treated with intravenous flucloxacillin and regular paracetamol. Over the following 2 weeks, he developed an acute kidney injury (attributed to lisinopril, hypotension and sepsis) and a disproportionate metabolic acidosis. The lisinopril was discontinued.
Case 3
A 53-year-old female was admitted having been unwell for 6 days with shortness of breath and a productive cough. She had past history of asthma and hypothyroidism. Her regular medications were oral candesartan and amitriptyline, and beclomethasone and salbutamol inhalers. She had received 3 days of amoxicillin.
Blood cultures grew S aureus. A chest radiograph showed consolidation in the right middle lobe. Trans-oesophageal echocardiography demonstrated endocarditis of a bicuspid aortic valve, with an aortic root abscess. She was treated with flucloxacillin (IV), rifampicin and paracetamol; an aortic valve replacement was performed. Her recovery was complicated by an ischaemic stroke causing left arm weakness. She had diarrhoea (three loose stools per day for a week). On day 22 of her admission, she developed hypokalaemia and on day 31 (which was 30 days after starting flucloxacillin), a metabolic acidosis.
Case 4
A 34-year-old female was transferred to the surgical ward of our hospital for management of complications of acute pancreatitis. She had been an inpatient for 4 months following her initial presentation with abdominal pain. She had chronic intra-abdominal collections, recurrent sepsis and had recovering renal function following an acute kidney injury that had required renal replacement therapy for a period of 1 month. A renal biopsy had shown mesangial immunoglobulin A deposition. She weighed 60.5 kg but was grossly oedematous. Her dry weight was estimated (from her clinical examination and recent weights) to be 52 kg.
At the time of her transfer she was receiving IV metronidazole, meropenem, vancomycin and gentamicin, subcutaneous enoxaparin and erythropoietin, and oral amlodipine, atenolol, folic acid, omeprazole, lactulose, senna, selenium, fluconazole, domperidone, ramipril, furosemide, paracetamol (1 g qds) and sodium bicarbonate (2 g qds). She was also receiving parenteral nutrition, delivering 50 mmol sodium, 30 mmol potassium and 80 mmol acetate per 24 hours. Her antimicrobials were changed to meropenem, anidulafungin, linezolid and daptomycin. An abdominal collection was drained percutaneously.
48 hours after transfer she was referred to the renal physicians for a review of a mixed acid-base disturbance. This included a respiratory alkalosis, which was investigated further by computerised tomography pulmonary angiography, demonstrating bilateral pulmonary emboli.
Case 5
A 76-year-old female was admitted with back pain. She had a past history of psoas abscess (4 years previously) and thromboembolism. She took no alcohol. Blood cultures grew S aureus. During the course of her admission she was diagnosed with a recurrent psoas abscess and discitis of the lumbar spine with an epidural abscess. She was treated with IV flucloxacillin, oral rifampicin and regular oral paracetamol (1g qds). She had poor oral intake and did not tolerate prescribed dietary supplements.
On day 43 of her admission, she was referred to the general physicians for investigation of a metabolic acidosis and hypokalaemia.
Diagnosis, treatment and outcome
Laboratory data for all cases are shown in Table 1. In all cases, the patient had taken paracetamol at a dose of 1 g four times per day for at least 7 days (and usually for several weeks) prior to the diagnosis of pyroglutamic acidosis. In all five cases there was an anion gap metabolic acidosis and the diagnosis of pyroglutamic acidosis was confirmed by a urine organic acid profile yielding a large peak of pyroglutamic acid (5-oxoproline).
Table 1.
Laboratory data
| Case | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|
| Day of hospital admission on which data were obtained | 15 | 31 | 89 | 40 | ||
| Parameter | Reference range | |||||
| Venous blood | ||||||
| Na, mM | (135–145) | 140 | 140 | 138 | 139 | 145 |
| K, mM | (3.6–5.0) | 3.0 | 3.7 | 4.3 | 2.5 | |
| HCO 3, mM | (22–30) | 11 | 9 | 14 | 15 | 14 |
| Cl, mM | (95–107) | 110 | 111 | 117 | 97 | 115 |
| Albumin, g/L | (30–45) | 20 | 24 | 18 | 14 | 17 |
| Glucose, mM | 4.7 | 5.6 | 5 | |||
| Urea, mM | (2.5–6.6) | 10.2 | 1.6 | 9.6 | 5.3 | |
| Creatinine, μM | (60–120) | 308 | 187 | 55 | 147 | 76 |
| Osmolality, mOsm | (280–296) | 311 | ||||
| L-lactate, mM | (0.6–2.4) | 1.7 | 0.7 | 1.52 | 0.9 | |
| D-lactate, μM | (<19) | 17 | ||||
| Arterial blood | ||||||
| H +, nM | (35–45) | 65 | 39.4 | 31 | ||
| PaCO 2, kPa | (4.6–6.0) | 1.9 | 3.05 | 2.13 | ||
| BE, mM | (–2 to 2) | -22 | -10.6 | |||
| Spot urine | ||||||
| Na, mM | 75 | 50 | ||||
| K, mM | 31 | 30 | 49 | |||
| Cl, mM | (0–250) | 46 | 52 | |||
| Osmolality, mOsm | (50–1200) | 403 | ||||
| Ketones | − | ++ | − | |||
| 5-oxoproline* | +++ | +++ | +++ | +++ | +++ | |
| Derived values | ||||||
| eGFR, mL/min/1.72 m 2 | 31 | >60 | 35 | >60 | ||
| AG, mM | 19 | 20 | 7 | 27 | 16 | |
| cAG, mM | (10–14) | 24 | 24 | 12.5 | 33.5 | 21.8 |
| ΔHCO 3, mM | 14 | 16 | 11 | 10 | 11 | |
| ΔAG, mM | 12 | 12 | 0.5 | 21.5 | 9.8 | |
| Δratio | (0.8–1.2) | 0.9 | 0.8 | 0.1 | 2.2 | 0.9 |
| ΔΔ, mM | (±5) | –2 | –4 | –10.5 | +11.5 | –1.2 |
| UAG, mM | 60 | 28 | ||||
| TTKG | 15.1 | |||||
| Interpretation | RALK + AGMA | AGMA | HCMA + AGMA + RC | RALK + AGMA + MALK | AGMA | |
*A large peak of pyroglutamic acid (5-oxoproline) was detected in the urine of all five patients. AG = Na–HCO3–Cl; cAG = AG+0.25×(40–albumin); ΔHCO3 = 25–HCO3; ΔAG = AG–12; Δratio = ΔAG/ΔHCO3; ΔΔ = ΔAG–ΔHCO3; UAG = Na+K–Cl; TTKG = (U.K/P.K)/(U.Osm/P.Osm).
AG = serum anion gap; AGMA = anion gap metabolic acidosis; BE = base excess; cAG = corrected serum anion gap; eGFR = estimated glomerular filtration rate; HCMA = hyperchloraemic metabolic acidosis; MALK = metabolic alkalosis; P.K = plasma concentration of potassium; P.Osm = plasma osmolality; RC = respiratory compensation; RALK = respiratory alkalosis; TTKG = transtubular potassium gradient; UAG = urine anion gap; U.K = urinary concentration of potassium; U.osm = urine osmolality.
In cases 1 to 4, blood and urine biochemistry results were taken within 48 hours of the urine sample that confirmed the presence of 5-oxoproline. In case 5, blood and urine chemistry was sent 7 and 4 days prior to the diagnostic urine sample, respectively.
Treatment was instituted either when the diagnosis of pyroglutamic acidosis was suspected (at the time of sending urine for organic acid profiling) or when the diagnosis was confirmed by this result. All cases were treated by the withdrawal of paracetamol and the administration of bicarbonate supplements (and intravenous fluid and potassium supplements if required to correct volume or potassium depletion, respectively). Cases 2, 3 and 4 received N-acetylcysteine (600 mg orally twice daily for 48 hours or until resolution of the acidosis). Depletion of intracellular glutathione is thought to be key to the pathogenesis of pyroglutamic acidosis; N-acetylcysteine has been used in other cases in an attempt to restore glutathione stores.2 Flucloxacillin may have been causative in case 3; this was therefore converted to ceftriaxone (and rifampicin) and continued so that antibiotic therapy was given for 6 weeks in total. Although flucloxacillin may also have been causative in cases 2 and 5, a clinical decision was made to continue with flucloxacillin therapy.
In cases 1, 2, 3 and 5, the acidosis resolved fully within a week. Case 4 remained acidotic for several weeks, but during that time she had recurrent episodes of sepsis and progressive renal failure, ultimately requiring multi-organ support on the intensive care unit.
Discussion
Pyroglutamic acidosis has common, well-defined risk factors
Our cases demonstrate the common risk factors for acquired pyroglutamic acidosis (Box 1).1–8 All had chronic exposure to paracetamol at therapeutic doses; all were malnourished due to either alcohol excess or prolonged illness in hospital; four were female; all had received antibiotics (flucloxacillin for Staphylococcus septicaemia in three); three had renal failure. Our cases emphasise that multiple risk factors (particularly nutritional deficiency plus chronic paracetamol use) are universally present.
Box 1.
Clinical associations of pyroglutamic acidosis
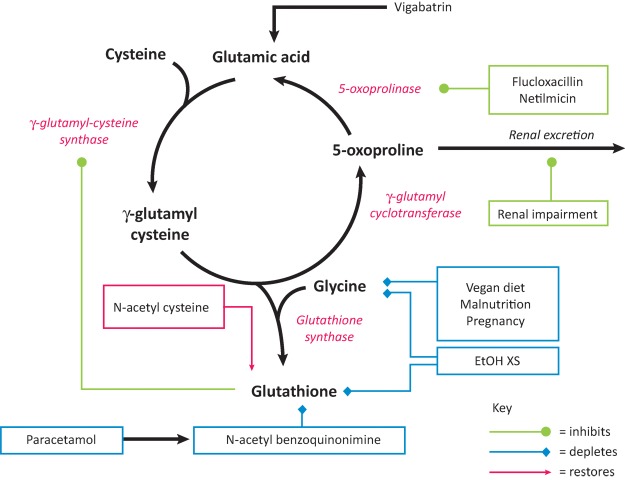
The explanation for this can be found in the mechanism underlying the pathogenesis of pyroglutamic acidosis and requires an understanding of glutathione metabolism. Glutathione is present in most mammalian cells where it acts as an anti-oxidant, detoxifying endogenous waste products and xenobiotics. It is an intermediary in the γ-glutamyl cycle – an ATP-dependant pathway that imports amino acids across the cell membrane. This cycle may be disrupted by inherited enzyme defects (in 5-oxoprolinase or glutathione synthetase) or acquired deficiencies in cellular glutathione and cysteine (Fig 1).9 When both are deficient, an ATP-depleting futile 5-oxoproline cycle ensues, resulting in the accumulation of 5-oxoproline.1 Chronic paracetamol use contributes to both glutathione and cysteine depletion and is exacerbated by nutritional deficiencies in cysteine precursors and glycine.1,2
Fig 1.
The γ-glutamyl cycle. 5-oxoprolinase operates at low capacity and, therefore, 5-oxoproline will accumulate in the plasma when its rate of production is high. Various factors act to cause accumulation of 5-oxoproline, either by depleting glutathione (blue boxes) or by inhibiting the clearance of 5-oxoproline by 5-oxoprolinase or its renal excretion (green boxes). Enzymes are shown in red italics. EtOH XS = excessive intake of ethanol. Adapted from Humphreys et al.9
The evidence that paracetamol ingestion causes pyroglutamic acidosis comes from case series demonstrating a strong clinical association, and also from an experimental rat model.10 Interestingly, the urinary excretion of pyroglutamate is increased (although not to levels that cause systemic acidosis) in children exposed to paracetamol, suggesting that asymptomatic (non-acidaemic) pyroglutamic aciduria may be a common feature of therapeutic paracetamol use.11
Pyroglutamic acidosis should be suspected and diagnosed on clinical grounds
To our knowledge, our five cases comprise the second-largest published case series (after Pitt et al).12 However, there are a growing number of isolated case reports, with ours bringing the total reported cases to at least 54.1 The true incidence is unknown, but given the prevalence of its risk factors – and the poor availability of the diagnostic assay – it is highly likely that pyroglutamic acidosis is significantly underdiagnosed both inside and outside the hospital. Whenever we have sent urine to confirm a clinically suspected case, it has returned positive. However, we have also been involved with multiple cases in recent years in which the diagnosis was made on empirical grounds (ie in a patient with risk factors and an elevated anion gap) and the acidosis resolved following withdrawal of paracetamol (with or without N-acetylcysteine). It is important to be able to achieve an empirical diagnosis because the diagnostic assay (urine organic acid profile) is not always readily available, and even when it is the result may not be returned for several days.
Below, we discuss how our cases illustrate a few key principles in the investigation of an acid-base disturbance, which are crucial to correctly diagnosing pyroglutamic acidosis (or indeed an anion gap metabolic acidosis of any cause).
General approach to metabolic acidosis
A general approach to diagnosing metabolic acidosis has been well-reviewed elsewhere.13 The first step is to identify any concurrent respiratory acid-base disorder (by calculating the expected change in PaCO2); the second, to calculate the anion gap. We, and others, prefer to omit serum potassium from this calculation (ie AG = Na–Cl–HCO3). If potassium is included (ie AG = Na+K–Cl–HCO3), then the reference range will be approximately 4 mM higher (typically 14–18).
Serum anion gap should be corrected for hypoalbuminaemia
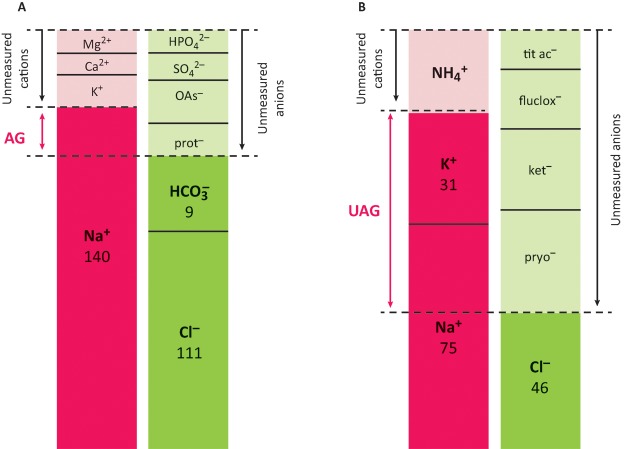
Serum proteins contribute to the unmeasured anions; thus the anion gap (unmeasured anions – unmeasured cations) is reduced in hypoalbuminaemia (Fig 2A).3,14 This effect can be corrected for using the Figge equation to calculate the corrected anion gap: cAG = AG+0.25×(40 – albumin in g/L).15–17 Failure to correct the serum anion gap for hypoalbuminaemia would have led to a significant underappreciation of the anion gap in all of our cases, and in case 3 would have resulted in the incorrect interpretation of a normal anion gap acidosis (rather than concurrent mixed normal and wide gap acidosis).
Fig 2.
Modified ‘Gamblegram’ displaying serum and urine anion gaps for case 2. A – measured and unmeasured cations (red) and anions (green) in the serum. Numbers refer to ion concentrations in mM and as the quantities of the unmeasured ions are not known, the figure is deliberately not drawn to scale. AG = Na–Cl– HCO3 = unmeasured anions. B – measured and unmeasured cations (red) and anions (green) in the urine. UAG = Na+K–Cl = unmeasured anions.
AG = serum anion gap; flucox = flucloxacillin; ket = ketones; OAs = organic acids; prot = proteins; pyro = pyroglutamate; tit ac = titratable acids; UAG = urine anion gap. Adapted from Rolleman et al3 and Gabow.14
Concurrent acid-base disturbances are common
Case 4 exhibited three concurrent acid-base disturbances. A respiratory alkalosis (which led to the diagnosis of bilateral pulmonary emboli), a metabolic alkalosis (likely the result of high-dose bicarbonate supplementation and furosemide therapy) and a wide anion gap metabolic acidosis (pyroglutamic acid). The coexistence of metabolic acidosis and alkalosis was revealed by comparison of the ΔAG and ΔHCO3 (bicarbonate deficit). Each of these terms describes the magnitude by which the AG or HCO3 differs from a normal value, so that ΔAG = AG–12 and ΔHCO3 = 25–HCO3. Some clinicians prefer to calculate the ratio between these terms (the ‘delta ratio’ or ‘Δ/Δ’ = ΔAG/ΔHCO3), where values outside the range 0.8–1.2 indicate a likely mixed acid-base disorder.10 Other clinicians calculate the absolute difference (the ‘delta-delta’ or ‘Δ–Δ’ = ΔAG–ΔHCO3), where values outside the range ±5 indicate a mixed acid-base disorder.6 For almost all purposes, either approach is valid.
In case 4, the high Δ/Δ (ie high ΔAG/ΔHCO3 ratio) in a metabolic acidosis suggests a concurrent metabolic alkalosis (Table 1).17,18
Conversely, the low Δ/Δ (ie low ΔAG/ΔHCO3 ratio) in case 3 demonstrates a concurrent hyperchloraemic metabolic acidosis, a likely result of gastrointestinal losses of HCO3 from chronic diarrhoea.
The urine anion gap can be informative in wide anion gap metabolic acidosis
In cases 2 and 3, we measured the urine anion gap and found it to be positive. This suggests unmeasured urinary anions, which were almost certainly pyroglutamate and/or flucloxacillin (as in a previous case report).3 In case 2, there will also have been a contribution from urinary ketones; small peaks of 3-OH butyrate and acetoacetate were present in the urine organic acid profile (Fig 2B).
The urine anion gap is classically used in a normal anion gap acidosis as a proxy measure of urinary NH4+ excretion, to distinguish between proximal and distal renal tubular acidosis (or extra-renal HCO3 loss). Our cases demonstrate its utility in a wide anion gap acidosis. The same principle applies in other causes of wide anion gap acidosis, eg in toluene exposure (glue-sniffing), urinary hippurate anions can manifest as a positive urine anion gap.
In appearing in the urine as non-reabsorbable anions, pyroglutamate (and flucloxacillin) may have stimulated distal K+ secretion, resulting in the hypokalaemia observed in cases 2 and 5. Hypokalaemia appears to be a common feature of flucloxacillin-associated pyroglutamic acidosis.3,4
A diagnostic ‘sieve’ for anion gap metabolic acidosis should include pyroglutamic acidosis
Metabolic acidosis is common and the differential diagnosis is wide.13,18 The common causes of an anion gap metabolic acidosis can be recalled with the aid of a mnemonic (GOLDMARK) that includes pyroglutamic acidosis (Box 2).
Box 2.
Mnemonic for causes of wide anion gap metabolic acidosis
| G – glycols (ethylene and propylene) |
| O – 5-oxoproline (pyroglutamic acid) |
| L – L-lactate |
| D – D-lactate |
| M – methanol and other toxins (ethanol, toluene, paraldehyde) |
| A – aspirin/salicylates |
| R – renal failure |
| K – ketones |
Adapted from Berend et al.13
In many cases, the underlying cause of any acidosis can be deduced from a thorough history and examination and a limited range of laboratory tests (serum electrolytes, serum anion gap and L-lactate, serum or urinary ketones). However, mixed acid-base disturbances are prevalent – particularly in patients with chronic comorbidities – and a thorough acid-base analysis (with specialist input) may be required. Measures such as the delta anion gap, delta bicarbonate, delta ratio and the urine anion gap may guide the diagnostic process, although they exhibit poor specificity in isolation and must be interpreted intelligently within the clinical context.18,19
In many cases, the cause of an anion gap metabolic acidosis is not detected by ‘routine’ laboratory tests. In approximately one third of hospital inpatients with a moderately elevated anion gap, the responsible acid was neither lactate nor ketones.20 Therefore, clinical judgement should be exercised and specific exogenous and endogenous acids sought in selected cases. Ingestion of various toxic compounds can be an exogenous source of organic acid (Box 2); pyroglutamic acid is an example of an endogenous acid, but there are others (such as Krebs cycle intermediates).21
Conclusions
Pyroglutamic acidosis is reasonably common in hospitalised patients but is almost certainly underdiagnosed. It should be suspected in patients taking long-term paracetamol at therapeutic doses if there are additional risk factors (malnutrition, infection, antibiotic use, pregnancy, renal failure). It can be detected as a metabolic acidosis with an elevated anion gap in the absence of an elevated L-lactate or ketones. Treatment involves cessation of paracetamol (and any other causative agent) and bicarbonate supplementation. N-acetyl cysteine has been used in some cases to accelerate recovery.
Key messages
Pyroglutamic acidosis is associated with therapeutic paracetamol use, malnutrition, renal failure, female sex, sepsis and antibiotic therapy.
It is diagnosed by the detection of a wide anion gap metabolic acidosis in the presence of risk factors.
The gold-standard diagnostic test (urine organic acid profile) is not always readily available.
Management comprises cessation of paracetamol and the administration of bicarbonate (and other electrolyte) supplements ± N-acetyl cysteine.
Mixed acid-base disorders are often present and specialist input may be required to reach a complete diagnosis.
Conflicts of interest
The authors have no conflicts of interests to declare.
Author contributions
RWH and JJN conceived the paper. RWH drafted the manuscript. All authors identified clinical cases and collected patient data. All authors made critical revisions of the manuscript and approved the final version.
Acknowledgements
Written consent was obtained from the patients to publish the clinical details in this article, except where the patient is deceased (case 1).
References
- 1.Emmett M. Acetaminophen toxicity and 5-oxoproline (pyroglutamic acid): a tale of two cycles, one an ATP-depleting futile cycle and the other a useful cycle. Clin J Am Soc Nephrol. 2014;9:191–200. doi: 10.2215/CJN.07730713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenves AZ. Kirkpatrick HM. Patel VV. Sweetman L. Emmett M. Increased anion gap metabolic acidosis as a result of 5-oxoproline (pyroglutamic acid): a role for acetaminophen. Clin J Am Soc Nephrol. 2006;1:441–7. doi: 10.2215/CJN.01411005. [DOI] [PubMed] [Google Scholar]
- 3.Rolleman EJ. Hoorn EJ. Didden P. Zietse R. Guilty as charged: unmeasured urinary anions in a case of pyroglutamic acidosis. Neth J Med. 2008;66:351–3. [PubMed] [Google Scholar]
- 4.Myall K. Sidney J. Marsh A. Mind the gap! An unusual metabolic acidosis. Lancet. 2011;377:526. doi: 10.1016/S0140-6736(10)61383-9. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien LMN. Hooper M. Flemmer M. Marik PE. Chronic acetaminophen ingestion resulting in severe anion gap metabolic acidosis secondary to 5-oxoproline accumulation: an under diagnosed phenomenon. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.03.2012.6020. pii: bcrbcr0320126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croal BL. Glen AC. Kelly CJ. Logan RW. Transient 5-oxoprolinuria (pyroglutamic aciduria) with systemic acidosis in an adult receiving antibiotic therapy. Clin Chem. 1998;44:336–40. [PubMed] [Google Scholar]
- 7.Bonham JR. Rattenbury JM. Meeks A. Pollitt RJ. Pyroglutamicaciduria from vigabatrin. Lancet. 1989;1:1452–3. doi: 10.1016/s0140-6736(89)90158-x. [DOI] [PubMed] [Google Scholar]
- 8.Meister A. Vigabatrin and urinary 5-oxoproline. Lancet. 1989;2:1216. doi: 10.1016/s0140-6736(89)91821-7. [DOI] [PubMed] [Google Scholar]
- 9.Humphreys BD. Forman JP. Zandi-Nejad K, et al. Acetaminophen-induced anion gap metabolic acidosis and 5-oxoprolinuria (pyroglutamic aciduria) acquired in hospital. Am J Kidney Dis. 2005;46:143–6. doi: 10.1053/j.ajkd.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Ghauri FYK. McLean AEM. Beales D, D. Wilson I. Nicholson JK. Induction of 5-oxoprolinuria in the rat following chronic feeding with N-acetyl 4-aminophenol (paracetamol) Biochem Pharmacol. 1993;46:953–7. doi: 10.1016/0006-2952(93)90506-r. [DOI] [PubMed] [Google Scholar]
- 11.Pitt J. Association between paracetamol and pyroglutamic aciduria. Clin Chem. 1990;36:173–4. [PubMed] [Google Scholar]
- 12.Pitt JJ. Hauser S. Transient 5-oxoprolinuria and high anion gap metabolic acidosis: clinical and biochemical findings in eleven subjects. Clin Chem. 1998;44:1497–503. [PubMed] [Google Scholar]
- 13.Berend K. de Vries APJ. Gans ROB. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2014;371:1434–45. doi: 10.1056/NEJMra1003327. [DOI] [PubMed] [Google Scholar]
- 14.Gabow PA. Disorders associated with an altered anion gap. Kidney Int. 1985;27:472–83. doi: 10.1038/ki.1985.34. [DOI] [PubMed] [Google Scholar]
- 15.Figge J. Jabor A. Kazda A. Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26:1807–10. doi: 10.1097/00003246-199811000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Feldman M. Soni N. Dickson B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J Lab Clin Med. 2005;146:317–20. doi: 10.1016/j.lab.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Kraut JA. Nagami GT. The serum anion gap in the evaluation of acid-base disorders: what are its limitations and can its effectiveness be improved? Clin J Am Soc Nephrol. 2013;8:2018–24. doi: 10.2215/CJN.04040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rastegar A. Use of the deltaAG/deltaHCO3- ratio in the diagnosis of mixed acid-base disorders. J Am Soc Nephrol. 2007;18:2429–31. doi: 10.1681/ASN.2006121408. [DOI] [PubMed] [Google Scholar]
- 19.Kraut JA. Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2:162–74. doi: 10.2215/CJN.03020906. [DOI] [PubMed] [Google Scholar]
- 20.Gabow PA. Kaehny WD. Fennessey PV, et al. Diagnostic importance of an increased serum anion gap. N Engl J Med. 1980;303:854–8. doi: 10.1056/NEJM198010093031505. [DOI] [PubMed] [Google Scholar]
- 21.Forni LG. McKinnon W. Lord GA, et al. Circulating anions usually associated with the Krebs cycle in patients with metabolic acidosis. Crit Care. 2005;9:R591–5. doi: 10.1186/cc3806. [DOI] [PMC free article] [PubMed] [Google Scholar]