ABSTRACT
Disorders of mast cell activation can be classified as primary (mastocytosis), secondary (reactive) or idiopathic. This article discusses how to recognise and approach the diagnosis of patients suspected to have symptoms of abnormal mast cell activation. Given the highly varied and often complex symptomatology of such patients, we advocate applying a logical step-wise approach to investigating these patients to ensure the correct diagnosis is made. Treatments of mast cell activation disorders are discussed, dividing them into those that ameliorate the effects of mast cell mediators and those that act to stabilise the mast cell.
KEYWORDS: Activation, disorder, mast cell, mast cell disorders, mastocytosis, tryptase
Key points
Symptoms of mast cell activation can be highly varied
Mast cell activation disorders can be classified as primary (clonal), secondary (reactive) or idiopathic
An elevated serum tryptase is a useful initial marker of a mast cell activation disorder
Specific diagnostic criteria must be met to diagnose both mastocytosis and mast cell activation syndrome
Antihistamines are a helpful first-line treatment in all mast cell activation disorders
Introduction
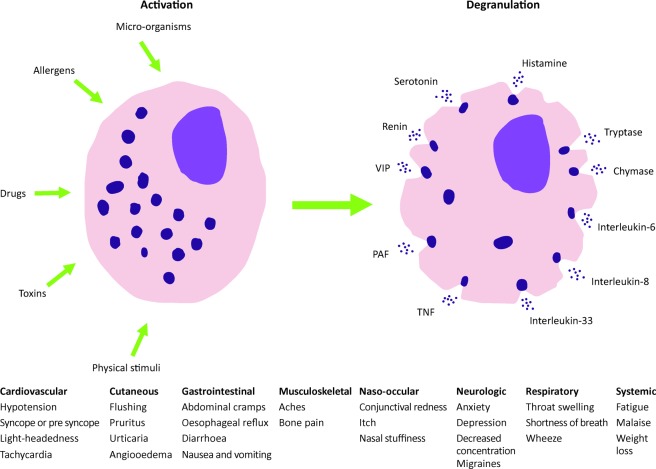
The mast cell is a tissue resident granulocyte, active in the allergic response but also playing a vital role in immune tolerance, wound healing, angiogenesis and the innate immune response. It may be activated by the binding of allergens to receptor-bound specific IgE or by multiple other non-specific stimuli. Once activated, it releases histamine and other pro-inflammatory mediators (Fig 1). Disorders of mast cell activation can be highly varied in their presentation. The symptoms the patient experiences are caused by the excessive release of mast cell mediators, most notably histamine. The symptoms experienced depend on the affected organ, eg wheals, redness and itching in the skin or cramps and diarrhoea in the gastrointestinal tract. Sometimes patients will present with an isolated finding, such as the rash of cutaneous mastocytosis; other patients will present with a broad constellation of symptoms, eg flushing, pre-syncope, diarrhoea and cramps. The symptoms may be vague, subtle recurrent episodes or more immediate presentations, such as anaphylaxis or life threatening hypotension (Table 1). This article will discuss how to approach the diagnosis and treatment of a patient you suspect to have symptoms linked to abnormal mast cell activation.
Fig 1.
Possible symptoms of mast cell activation. Adapted from Akin1 and Akin et al.10
Table 1.
WHO diagnostic criteria for systemic mastocytosis
| Major criterion | Minor criteria |
|---|---|
| Multifocal, dense aggregates of mast cells (15 or more) detected in sections of bone marrow and confirmed by tryptase immunohistochemistry or other special stains |
|
| Diagnosis may be made if one major plus one minor, or three minor criteria are fulfilled | |
Reproduced from Valent et al.2
In order to encompass the wide range of conditions that may present with symptoms of mast cell activation, the umbrella term mast cell activation disorders (MCAD) has been proposed. Within this heterogeneous group of conditions, Akin1 proposes stratifying them into primary (clonal), secondary (or reactive) and idiopathic (of unknown cause).
Primary
The primary mast cell activation disorder, mastocytosis, is caused by clonal proliferation of mast cells, with an abnormal accumulation of these cells in tissues including the skin, bone marrow and gastrointestinal tract. The World Health Organization classification of mastocytosis includes seven subtypes and their diagnostic criteria (Box 1, Table 1).2
Box 1.
WHO classification of mastocytosis
|
- Reproduced from Valent et al.2
Mast cell regulation, proliferation and survival are driven by stem cell factor receptor on the mast cell surface (CD117). Activating mutations in KIT (a proto-oncogene that encodes the transmembrane receptor tyrosine kinase KIT) have been identified in nearly all adult patients with systemic mastocytosis.3 Other markers of a clonal mast cell population include positive co-staining for CD2 and CD25 with CD117, tryptase or both on immunohistochemistry.
Although mastocytosis is associated with an increased number of mast cells, its presentation is not necessarily accompanied by symptoms of mast cell activation. The most common presentation of mastocytosis is in the skin. Often a rash is the only presenting complaint. Mastocytosis in the skin has several different patterns: urticaria pigmentosa (UP; also known as maculopapular cutaneous mastocytosis (Fig 2)), diffuse cutaneous mastocytosis, or isolated mastocytoma. The red brown macules, papules or nodules of UP usually urticate if rubbed gently (Darier's sign) although adult UP may not react.
Fig 2.
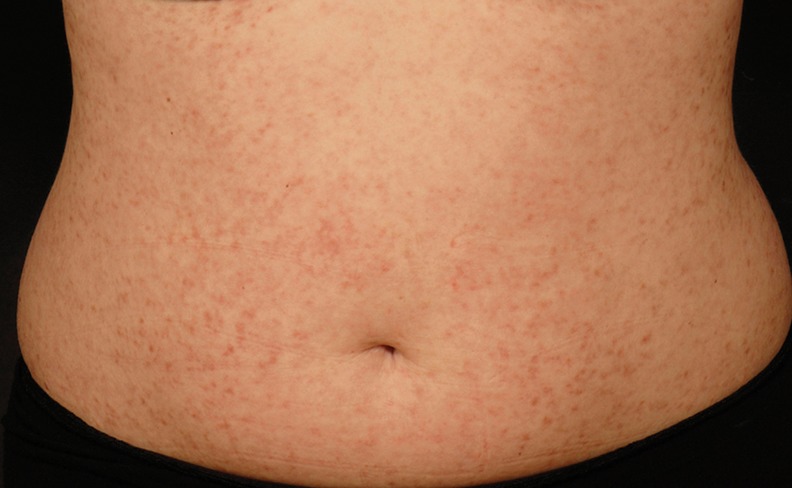
Urticaria pigmentosa (maculopapular cutaneous mastocytosis) on the abdomen.
UP presenting in childhood is usually benign and self-limiting, improving or resolving by puberty. Any child presenting with UP should be examined for palpable hepatosplenomegaly, lymphadenopathy and asked about other symptoms of abnormal mast cell activation, ie syncope, flushing, diarrhoea or anaphylaxis to hymenoptera (wasp and bee) venom. In the absence of these findings and the presence of a normal full blood count and tryptase, the likelihood of clinically significant systemic involvement is very small. Approximately 80% of childhood UP will have a KIT mutation but only 40% will have the common auto-activating D816V mutation at codon 8164 present in most adults with systemic mastocytosis.5
Approximately 20% of adult mastocytosis patients with no skin involvement may present with symptoms of mast cell activation, such as recurrent unexplained anaphylaxis or diarrhoea and abdominal cramps if there is infiltration of the abnormal mast cell population into the gastrointestinal tract. If there is a convincing history of recurrent unexplained episodes of mast cell activation, a baseline serum tryptase level should be checked and if >20 ng/mL, further investigations should be considered, including bone marrow biopsy with mutational analysis for D816V on bone marrow aspirate or biopsy of another affected organ (such as endoscopic biopsy the gastrointestinal tract). Screening peripheral blood for the KIT D861V mutation is a much less invasive next step in the diagnostic algorithm where this facility is available6–8 although lack of the mutation in peripheral blood does not rule out mastocytosis because of the lower sensitivity of the test compared with use of bone marrow aspirate. An alternative KIT mutation may also be present in D816V mutation negative patients. A bone marrow biopsy can be used to diagnose systemic mastocytosis, assess the degree of bone marrow involvement and check for an associated blood disorder, such as a myelodysplastic syndrome.
The life expectancy of indolent systemic mastocytosis is near normal, whereas aggressive systemic mastocytosis and mast cell leukaemia are relatively refractory to treatment and carry a high mortality.
Secondary or reactive
The most commonly encountered mast cell activation disorders are secondary or reactive. These include specific IgE-mediated allergic reactions, including anaphylaxis, but also chronic autoimmune urticaria, the physical urticarias, some adverse drug reactions and underlying inflammatory disease involving mast cells (eg allergic rhinitis, atopic dermatitis). The key to diagnosing a secondary or reactive cause for mast cell activation comes from the patient's history but investigations to exclude a possible secondary cause of mast cell activation should be included in the assessment of all MCAD patients – ie skin prick tests, specific IgEs and a thorough medical assessment to rule out an alternative underlying chronic disease. It should be remembered that in patients reporting an anaphylactic reaction to hymenoptera venom, associated with a raised specific IgE, there may still be an underlying primary clonal mast cell disorder and investigations such as a baseline tryptase level should be undertaken to exclude this.9
Idiopathic mast cell disorders
Once a primary or secondary mast cell activation disorder has been excluded, a final assessment should be made as to whether there is sufficient evidence to diagnose an idiopathic disorder of mast cell activation in the context of a suggestive history. The potential scope of this group of conditions is heterogeneous, far reaching and ill defined.
Emerging from the literature in an effort to define this subgroup more stringently is the concept of mast cell activation syndrome (MCAS). There have been efforts recently for expert consensus to define the condition and apply diagnostic criteria.1,10 The following questions have been proposed to elicit if there is sufficient evidence of mast cell activation dysfunction:
Are there episodic symptoms consistent with release of mast cell mediators affecting ≥2 organ systems (eg respiratory, cardiovascular, gastrointestinal, skin)?
-
Is there a transient (>20% from baseline or >2 ng/mL) increase in a mast cell mediator following an episode?
Currently, the most available marker is serum tryptase, measured up to 4 hours after the onset of symptoms. Other mediators that can be measured include urinary histamine metabolites.
Do these symptoms respond to treatments that target the effects of mast cell mediators, eg H1 anti-histamine or anti-leukotriene agents?
All three criteria should be met to diagnose MCAS. Once a primary or secondary cause for the symptoms has been ruled out, the question ‘is there another condition that could be responsible for these symptoms?’ should be asked and fully investigated. Chronic spontaneous urticaria and idiopathic anaphylaxis are mast cell activation disorders,1 but are distinct clinical diagnoses that should not be included under MCAS.
The remaining patients with evidence of systemic mast cell activation (after other causes have been ruled out) then fall under the umbrella of MCAS. The pathogenesis of this condition remains poorly understood. While the understanding of this condition remains limited, the focus of the physician should remain on therapies that are of benefit to the patient without causing harm.
Treatment of mast cell activation disorders
Although the cause of the abnormal mast cell activation may be varied, the same initial treatment principles apply to all three MCADs.
When clinicians approach the treatment of MCAD, they should aim primarily to reduce the patient's symptom burden; this can be done with drugs that modulate the effects of mast cell mediator release or drugs that regulate mast cell activity.
Drugs that modulate the symptoms of mast cell activation
Antihistamines are the first line of treatment in MCAD. Non-sedating H1 antihistamines, eg cetirizine, loratadine, fexofenadine, are often preferred. H2 receptor antagonists, eg ranitidine, may be particularly useful for gastrointestinal symptoms of heartburn and epigastric pain. The leukotriene receptor antagonist montelukast can also be added to antihistamines.
In all cases of MCAD where a consistent trigger of symptoms can be identified, this trigger should be avoided. In patients with a history of anaphylaxis, at least one adrenaline autoinjector should be prescribed. Clinicians should also prescribe such devices for patients with systemic mastocytosis with no history of anaphylaxis as they are at a greater risk of hymenoptera sting-induced anaphylaxis with or without IgE sensitisation or multifactorial anaphylaxis.
The lesions of cutaneous mastocytosis prove quite refractory to treatment. Twice daily application of a very potent topical steroid (eg 0.05% clobetasol propionate) to a localised area of disease on the body (but not face, neck or flexures) for a continuous 6-week period may improve the appearance, but often this treatment approach is limited by the extent of cutaneous involvement and should be done in stages to minimise the risk of percutaneous steroid absorption. Narrow band ultraviolet B and psoralen combined with ultraviolet A phototherapy have been shown to improve the appearance of cutaneous mastocytosis and may reduce pruritus in the short term, but the lesions gradually return after cessation of therapy.
Drugs that interfere with mast cell activation
Sodium cromoglicate is considered a mast cell stabiliser but it is most effective in controlling gastrointestinal symptoms with little effect on systemic symptoms because it is absorbed poorly from the bowel and other mucosal surfaces.
Omalizumab, a monoclonal anti-IgE antibody, has proven very effective in its approved indication for the treatment of chronic spontaneous urticaria refractory to treatment with H1 antihistamines by reducing IgE and IgE receptor density on mast cells and basophils.11 There is also emerging anecdotal evidence for its use in recurrent anaphylaxis in patients with clonal and non-clonal mast cell proliferations.12,13
Drugs that interfere with mast cell proliferation and survival
Although many patients with indolent systemic mastocytosis can often be managed with monitoring and symptomatic relief alone, specialist mast cell cytoreductive therapy may be required if the disease becomes aggressive.
Conclusion
Given the broad symptomatology of mast cell activation disorders it is important try to diagnose each patient with a logical stepwise approach. Drawing on the classifications discussed above, the following questions should be asked:
Is there a clear pattern emerging from the patient history of a recurrent trigger or exposure to suggest an allergic (reactive) cause? Consider specific IgEs or skin prick tests to confirm this.
Is there another illness responsible for these symptoms and what do I need to do to exclude this?
Do I have sufficient evidence to support a clonal mast cell disorder? For example, elevated serum tryptase, biopsy of an affected organ, or D861V mutational analysis.
Does this patient fulfil the diagnostic criteria for mast cell activation syndrome?
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Akin C. Mast cell activation disorders. J Allergy Clin Immunol Pract. 2014;2:252–7. doi: 10.1016/j.jaip.2014.03.007. e1. [DOI] [PubMed] [Google Scholar]
- 2.Valent P. Horny HP. Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res. 2001;25:603–25. doi: 10.1016/s0145-2126(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 3.Cruse G. Metcalfe DD. Olivera A. Functional deregulation of KIT: link to mast cell proliferative diseases and other neoplasms. Immunol Allergy Clin North Am. 2014;34:219–37. doi: 10.1016/j.iac.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodemer C. Hermine O. Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804–15. doi: 10.1038/jid.2009.281. [DOI] [PubMed] [Google Scholar]
- 5.Furitsu T. Tsujimura T. Tono T, et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest. 1993;92:1736–44. doi: 10.1172/JCI116761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erben P. Schwaab J. Metzgeroth G, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol. 2014;93:81–8. doi: 10.1007/s00277-013-1964-1. [DOI] [PubMed] [Google Scholar]
- 7.Ingelfinger JR. Theoharides TC. Valent P. Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:163–72. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 8.Valent P. Escribano L. Broesby-Olsen S, et al. Proposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on Mastocytosis. Allergy. 2014;69:1267–74. doi: 10.1111/all.12436. [DOI] [PubMed] [Google Scholar]
- 9.Brockow K. Jofer C. Behrendt H. Ring J. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy. 2008;63:226–32. doi: 10.1111/j.1398-9995.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 10.Akin C. Valent P. Metcalf DD. Mast cell activation syndrome: proposed diagnostic criteria. J Allergy Clin Immunol. 2010;126:1099–104. doi: 10.1016/j.jaci.2010.08.035. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer M. Rosén K. Hsieh H-J, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–35. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- 12.Carter MC. Robyn JA. Bressler PB, et al. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. 2007;119:1550–1. doi: 10.1016/j.jaci.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 13.Bell MC. Jackson DJ. Prevention of anaphylaxis related to mast cell activation syndrome with omalizumab. Ann Allergy Asthma Immunol. 2012;108:383–4. doi: 10.1016/j.anai.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]