Introduction
Outcomes for patients with inoperable locally advanced non-small cell lung cancer (LA-NSCLC) are poor, with a median survival of about 2 years (1) and a 5-year survival of about 10–15% following diagnosis, even among patients with good performance status at presentation (2). Despite these poor outcomes, conventional radiation therapy (RT) with chemotherapy remains the standard of care. Immunotherapy strategies with anti-PD-1 and other immune checkpoint inhibitors have shown impressive gains compared with conventional chemotherapy for patients with metastatic NSCLC (3-6). As an immune stimulator, RT can enhance the diversity of the T-cell receptor repertoire to augment T-cell response (7). RT has also shown synergistic anti-tumor responses in mice (8,9). Immune checkpoint blockade in conjunction with RT may offer an opportunity to improve outcomes for patients with LA-NSCLC.
Because patients with LA-NSCLC often present with significant comorbid illness, treatment toxicity can mitigate potential therapeutic gains associated with combined modality therapy. Pneumonitis is a potentially life-threatening toxicity associated with both RT and immunotherapy. Among patients receiving RT for LA-NSCLC, the risk of pneumonitis is associated with the volume of normal lung within the RT field (10-14). Patients who receive a mean lung dose >20 Gy or in whom the volume of lung receiving >20 Gy exceeds 30–35% have greater than 20% risk of pneumonitis when radiation is combined with conventional chemotherapy (15). In the most recently published phase III study evaluating concurrent RT and chemotherapy, the median mean lung dose was almost 20 Gy and the median bilateral lung volume receiving 20 Gy was about 30% (1). Therefore, even with current standard treatment, the risk of pneumonitis in this population is significant.
Even in the absence of RT, pneumonitis has been associated with immune checkpoint inhibition (16). In a phase I trial of nivolumab anti-PD-1 therapy, 3/296 patients died from drug-related causes associated with pneumonitis (17-19). In patients with previously treated metastatic NSCLC who received nivolumab, approximately 7% developed reportable pulmonary toxicity, with 3/129 patients experiencing grade 3–4 pneumonitis (4). In a similar study, 6/129 patients with metastatic NSCLC developed pneumonitis with nivolumab alone (3).
Here, we utilized a well-established preclinical model of radiation pneumonitis (20,21) (CBA wild-type mice) to evaluate RT delivered in concurrently with immunotherapy. The CBA mouse model was chosen based on its closely related clinical pathogenesis to radiation-induced lung injury in humans (20,21). Combining RT and anti-PD-1 immunotherapy as an in situ vaccine has generated enthusiasm as a possible therapeutic to improve outcomes for metastatic lung cancer patients. However, data are scarce regarding the safety of this concurrent combination.
Methods
Animals
Age-matched CBA wild-type mice (purchased at 9–10 weeks old from Charles River Laboratories) had body weights between 20 and 30 g at time of irradiation. Animal experiments were approved by our Institutional Animal Care and Use Committee (IACUC). Animal husbandry details are described in the Supplementary materials.
Whole thorax lung irradiation
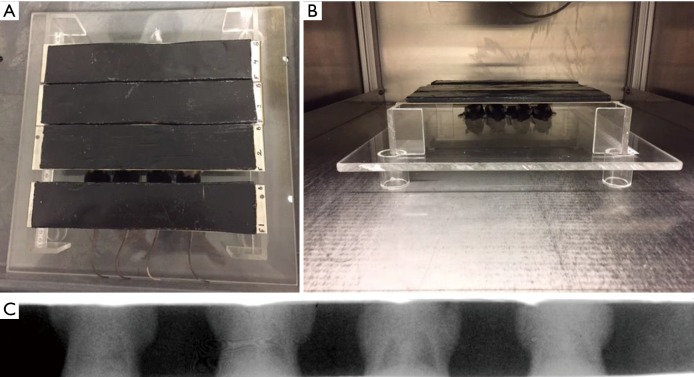
At 5–10 minutes before irradiation, mice were anesthetized with 80–100 mg/kg ketamine and 5–10 mg/kg xylazine dissolved in sterile saline. RT dose was selected based on previous studies assessing mouse strains and pneumonitis and fibrosis (20,22). RT was delivered to the thorax of prone mice through adjustable apertures with 8-mm lead shielding of the head, forelimbs, and abdomen (Figure 1). A single uniform dose of 13.5-Gy whole thorax RT was delivered to anesthetized mice using the XRAD 320 Precision X-ray system (North Branford, CT). Calibration details are described in the Supplementary materials.
Figure 1.
Whole thorax lung irradiation (WTLI) set up. (A) Positioning of mice before radiation therapy; (B) irradiation delivery; (C) Gafchromic film imaging example of whole thorax irradiation targeted to mouse lung region.
Immunotherapy
Mice received either anti-PD-1 alone or in combination with RT. Drug treatments of anti-PD-1 or isotype control were administered intraperitoneally every 3 days ×4 doses (Figure 2). Anti-PD-1 was injected intraperitoneally at 3 mg/kg (BioXCell, clone RMP1-14), with dose based on previous clinical trial studies of nivolumab targeting both NSCLC and small cell lung cancer (4,23). Control groups received isotype antibody intraperitoneally at 3 mg/kg (BioXCell, Rat IgG2a; anti Trinitrophenol, clone 2A3).
Figure 2.
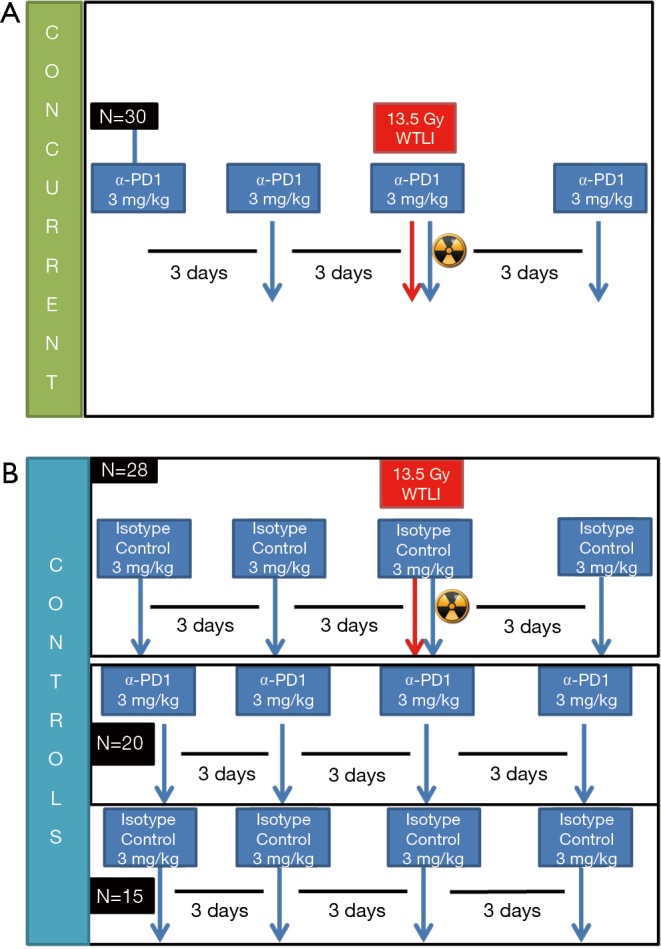
Experimental design. (A) CBA mice were grouped into treatment group (concurrent radiation + αPD-1; (B) or 1 of 3 control groups (radiation + isotype control, α-PD-1 alone, or isotype drug alone; cohorts were selected for 1-, 3-, 6-month satellite groups.
Animal observation/criterial for euthanasia
Animals were observed daily over 6 months to monitor morbidity and mortality, with body weight assessed before irradiation and twice weekly after treatment over 6 months. After death by CO2 inhalation through a regulated gas cylinder to the cage, animals underwent secondary thoracotomy. Criteria to assess individual animals included hunched/inactive posture, fur loss/piloerection, dermatitis, lethargy, and percent body weight loss. Our predetermined criteria for euthanasia outlined in the IACUC protocol included 15–25% body weight loss combined with evidence of distress, such as hunched/inactive posture or severe lethargy. Mice were assigned to 1-, 3-, and 6-month treatment cohorts to assess inflammatory pneumonitis and lung fibrosis.
Morphology and histopathology
After thoracotomy, wet lungs were first weighed (in grams), inflated through the bronchus with sterile PBS, and then processed for histologic analysis. Hematoxylin and eosin (H&E)-stained slides were evaluated and scored by a board-certified pathologist on a scale of 1 to 5 (1= minimal, 2= mild, 3= moderate, 4= marked, 5= severe) for accumulation of alveolar macrophages, alveolar septal thickening (AST), and peribronchiolar/perivascular edema. Percent area of “severe” involvement (little airspace) and the presence of lymphoid aggregates were also recorded. The pathologist was blinded regarding identity of cohorts during scoring.
Statistical analyses
Statistical plots were generated using GraphPad Prism 7.00 for Windows (GraphPad Software, La Jolla, CA, USA; www.graphpad.com). Specifically, unpaired, 2-tailed t-tests were performed for AST, edema, and lung weight analyses for the respective 1-, 3-, and 6-month cohorts. To compare edema and lung weights for the respective cohorts, we performed 2-tailed correlation analyses.
Results
In 93 total mice (Figure 2), 5 were found dead in cages, with 3 discovered as part of daily animal inspection (2 had been treated with combined RT and isotype control drugs and 1 had been concurrently treated with combined RT and anti-PD-1). These mice showed no signs of weight loss and did not meet euthanasia criteria before discovery. In addition, 5 mice were euthanized before the specified endpoint, with 2 euthanized according to the clinical endpoint protocol (1 mouse that received RT plus anti-PD-1 had >20% body weight loss and 1 mouse that received RT plus isotype control had >20% body weight loss, rapid breathing, and severe alopecia). Little behavioral and physical differences were shown for most mice across all cohorts; however, discoloration and hair loss at the thorax region were noted approximately 1.5–2 months in groups receiving a single dose of 13.5-Gy RT.
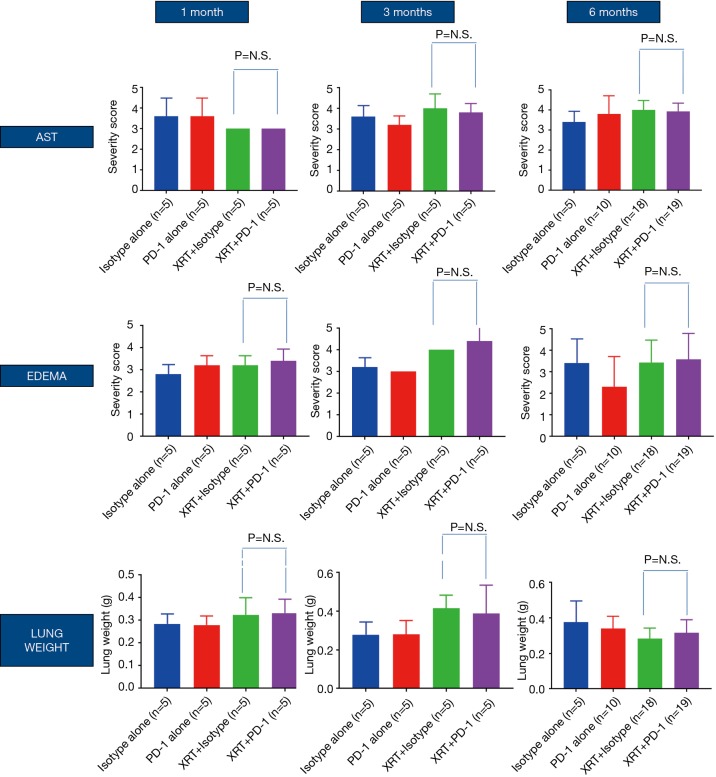
Figure 3 shows recorded wet lung weights in mice after death for all time points. Although no significant differences in lung weights were shown at the 1-month time point (Table 1), weights were significantly increased in mice with versus without RT at 3 months (P=0.0065, Table 1) and significantly decreased in mice with versus without RT at 6 months (P=0.0243, Table 1).
Figure 3.
For alveolar septal thickening (AST) and edema, mice were scored from H&E-stained slides. Wet lung weights were recorded in grams. P = not significant (NS). Lung weights at 1, 3, and 6 months.
Table 1. Statistics.
| Group comparison | Alveolar septal thickening | Edema | Lung weight | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 month | 3 months | 6 months | 1 month | 3 months | 6 months | 1 month | 3 months | 6 months | |||
| XRT + isotype vs. XRT + anti-PD-1, P values | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||
| XRT vs. no XRT, P values | 0.0372, ↑ 03 | 0.0541, ↑ 05 | NS | NS | 0.0001, ↑ 00 | 0.0259, ↑ 0 XRT | NS | 0.0065, ↑ 00 | 0.0243, ↑ 0 XRT | ||
| Anti-PD-1 alone vs. isotype alone | NS | NS | NS | NS | NS | NS | NS | NS | NS | ||
NS, not significant; XRT, irradiation.
Figure 4 shows histopathological evaluation of alveolar macrophage accumulation, AST, percent area of “severe” involvement (little airspace), and peribronchiolar/perivascular edema. No significant differences were shown between treatment groups for most of these histopathological features (data not shown), with the exception of AST, edema, and lung weights. Mice that received RT showed significantly increased AST scores at 1 (P=0.0372) and 3 months (P=0.0541) but not at 6 months (a larger evaluable cohort) compared with mice that did not receive RT (Table 1). In irradiated versus the non-irradiated groups, edema score was significantly increased at 3 months (P=0.0001; Table 1) but significantly decreased at 6 months (P=0.0259, Table 1).
Figure 4.
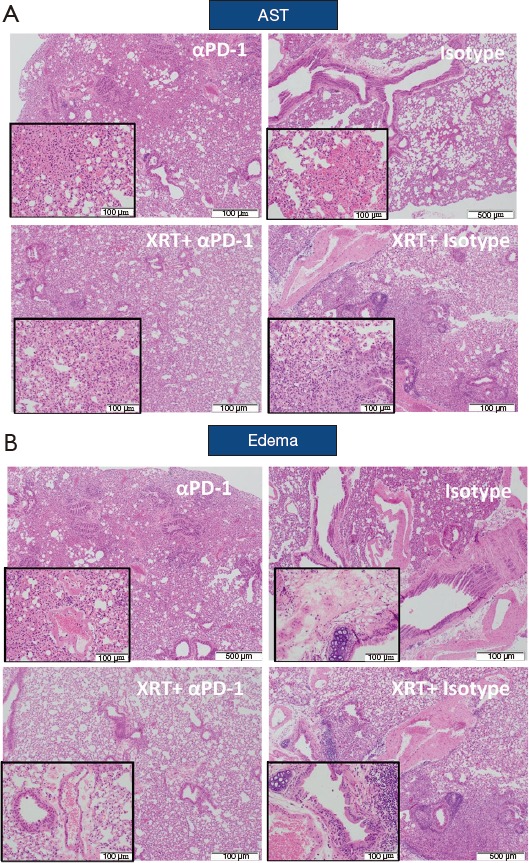
Radiation-induced lung injury and fibrosis criteria across cohorts. H&E-stained slides at ×4 magnification (inset at ×20). (A) Alveolar septal thickening (AST). (B) edema.
Discussion
We found that anti-PD-1 therapy did not significantly increase the risk of pneumonitis in our CBA mouse model, with only 3% of mice dying from concurrent anti-PD-1 plus whole thorax lung RT during our 7 month study period. No significant differences were observed in animals treated with concurrent RT plus anti-PD-1 versus other treatment groups when analyzing criteria associated with RT-induced lung injury and fibrosis toxicity. Furthermore, changes in pathology were not significantly different in mice treated with and without anti-PD-1 therapy at 1, 3, and 6 months (Figures 2 and 3). Not surprisingly, increased AST, edema, and lung weights were observed in mice that received RT versus those that did not at 1 and 3 months. The significantly reduced edema and lung weights in the irradiated cohorts at 6 months may be related to increased fibrosis and reduced pulmonary congestion.
Our study was significantly limited by the lower than expected mouse deaths due to pneumonitis and by the inherent limitations of pre-clinical models with respect to applicability to human cancer patients. Previous studies have shown differences in RT-induced lung injury based on the mouse strain being studied (20). It is also possible that we should have completed the experiments with a higher RT dose, as our study only tested a single RT and anti-PD-1 dose combination. Additional studies to evaluate other RT doses may be required to demonstrate safety with anti-PD-1 therapy.
A secondary study conducted based on the Keynote-001 human clinical trial demonstrated no significant increases in pulmonary or other toxicities among patients given RT before pembrolizumab (24). These data provide an early safety signal related to our combination; however, unlike our mouse study, RT was not given concurrently with the anti-PD-1 therapy. In the PACIFIC trial, anti-PD-L1 therapy delivered after definitive thoracic RT and chemotherapy demonstrated promising efficacy improvements with respect to progression-free survival (25). Although grade 1/2 pneumonitis was increased among patients receiving anti-PD-L1 therapy, rate of grade 3 pneumonitis remained low (~3%) in both arms. Similar to Keynote-001, immunotherapy was also delivered sequentially, at least 2 weeks after RT and chemotherapy. Another recently published human study demonstrated the safety of combined concurrent ablative RT with anti-CTLA4 treatment (26). Although the safety results regarding adding anti-PD-1 inhibitors with RT are encouraging in these and our study, results in humans are awaited regarding the safety of concurrent anti-PD-1 therapy plus large-volume, high-dose thoracic RT.
Acknowledgements
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Supplementary
Animal husbandry
Animals were housed five per cage in a vivarium barrier facility that included an irradiator room, necropsy suite, and observation rooms that were within proximity to the animal holding room. Animals were handled outside their cages in sterile laminar flow hood workstations one cage at a time.
Calibration of the irradiator
The irradiator (320 kVp, HVL 3.7 mm Cu, added filtration 1.5 mm Al + 0.25 mm Al + 0.75 mm Sn) was calibrated using a published protocol for low-energy therapeutic X-ray beams (23), which had been further adapted for mice irradiation conditions (24). The unit was first calibrated in terms of dose to small mass of water in air under the following reference conditions: 10×10 cm2 field at 50 cm source to point distance (Figure 1). A PTW Farmer chamber (model N30013, PTW, Freiburg, Germany) calibrated at the accredited laboratory in a beam of similar quality was used. Next, a reading with the same chamber was obtained with a 20×20 cm2 field in a 4×8×2.4 cm3 polystyrene phantom, approximating a mouse and positioned on the mice platform. Absolute dose to water in mice-irradiating geometry was calculated from the reference dose using the appropriate correction factors and mass energy-absorption coefficient ratios tabulated in the American Association of Physicists in Medicine protocol (23). The irradiator was further characterized in terms of the timer end effects (23), lead strip spacing output dependence, and beam flatness and symmetry (3% and 1%, respectively) (24).
Footnotes
Conflicts of Interest: BA Perez reports Bristol Myers Squibb-Advisory Board, AstraZeneca-Advisory Board outside the scope of this work. The other authors have no conflicts of interest to declare.
References
- 1.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. 10.1093/jnci/djr325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. 10.1016/S1470-2045(15)70054-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 6.Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. 10.1038/nature14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res 2015;3:345-55. 10.1158/2326-6066.CIR-14-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley JD, Hope A, El Naqa I, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys 2007;69:985-92. 10.1016/j.ijrobp.2007.04.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323-9. 10.1016/S0360-3016(99)00183-2 [DOI] [PubMed] [Google Scholar]
- 12.Kim TH, Cho KH, Pyo HR, et al. Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology 2005;235:208-15. 10.1148/radiol.2351040248 [DOI] [PubMed] [Google Scholar]
- 13.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2013;85:444-50. 10.1016/j.ijrobp.2012.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker SL, Liu HH, Liao Z, et al. Analysis of radiation pneumonitis risk using a generalized Lyman model. Int J Radiat Oncol Biol Phys 2008;72:568-74. 10.1016/j.ijrobp.2008.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70-6. 10.1016/j.ijrobp.2009.06.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino M, Sholl LM, Hodi FS, et al. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. N Engl J Med 2015;373:288-90. 10.1056/NEJMc1505197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishino M, Giobbie-Hurder A, Hatabu H, et al. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;2:1607-16. 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 19.Tanvetyanon T, Creelan BC, Antonia SJ. The safety and efficacy of nivolumab in advanced (metastatic) non-small cell lung cancer. Expert Rev Anticancer Ther 2016;16:903-10. 10.1080/14737140.2016.1220836 [DOI] [PubMed] [Google Scholar]
- 20.Jackson IL, Xu PT, Nguyen G, et al. Characterization of the dose response relationship for lung injury following acute radiation exposure in three well-established murine strains: developing an interspecies bridge to link animal models with human lung. Health Phys 2014;106:48-55. 10.1097/HP.0b013e3182a32ccf [DOI] [PubMed] [Google Scholar]
- 21.Jackson IL, Xu P, Hadley C, et al. A preclinical rodent model of radiation-induced lung injury for medical countermeasure screening in accordance with the FDA animal rule. Health Phys 2012;103:463-73. 10.1097/HP.0b013e31826386ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson IL, Vujaskovic Z, Down JD. A Further Comparison of Pathologies after Thoracic Irradiation among Different Mouse Strains: Finding the Best Preclinical Model for Evaluating Therapies Directed Against Radiation-Induced Lung Damage. Radiat Res 2011;175:510-8. 10.1667/RR2421.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonia SJ, Gettinger SN, Goldman J, et al. ORAL01.03: CheckMate 012: Safety and Efficacy of First-Line Nivolumab and Ipilimumab in Advanced NSCLC: Topic: Medical Oncology. J Thorac Oncol 2016;11:S250-1. 10.1016/j.jtho.2016.09.00827969442 [DOI] [Google Scholar]
- 24.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895-903. 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 26.Boyer MJ, Gu L, Wang X, et al. Toxicity of definitive and post-operative radiation following ipilimumab in non-small cell lung cancer. Lung Cancer 2016;98:76-8. 10.1016/j.lungcan.2016.05.014 [DOI] [PubMed] [Google Scholar]