Abstract
Background
Triglyceride glucose (TyG) index is a novel marker for metabolic disorders and recently it has been reported to be associated with cardiovascular disease (CVD) risk in apparently healthy individuals. However, the prognostic value of TyG index in patients with stable coronary artery disease (CAD) is not determined.
Methods
We conducted a nested case-control study among 3,745 patients with stable CAD. Patients were followed up for 11,235 person-years. The cardiovascular events (CVEs) were defined as all-cause death, non-fatal myocardial infarction (MI), stroke and post-discharge revascularization [percutaneous coronary intervention (PCI) coronary artery bypass grafting (CABG)]. In total, 290 (7.7%) patients with CVEs and 1,450 controls were matched according to age, gender, previous history of PCI or CABG and the duration of follow-up. TyG index was calculated as formula: ln[fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2].
Results
Multivariable Cox proportional hazards models revealed that TyG index was positively associated with CVEs risk (hazard ratio: 1.364, 95% confidence interval: 1.100–1.691, P=0.005). The Kaplan-Meier analysis indicated that patients within the highest quartile of TyG index presented the lowest event-free survival (P=0.029). Moreover, a 1-standard deviation (SD) increment in TyG index was associated with 23.2% [hazard ratio (HR): 1.232, 95% confidence interval (95% CI): 1.084–1.401] higher risk of CVEs, which was superior to other triglyceride or glycemic related markers.
Conclusions
The present study, firstly, showed that TyG index was positively associated with future CVEs, suggesting that TyG may be a useful marker for predicting clinical outcomes in patients with CAD.
Keywords: Triglyceride glucose index (TyG index), stable coronary artery disease, outcome
Introduction
It has been well recognized that the development of cardiovascular disease (CVD) is driven by multiple contributing factors including glycemic abnormality and lipid disorder (1,2). Hypertriglyceridemia (HTG) is a common dyslipidemia and the association of triglyceride (TG) with CVD risk remains controversial (3,4). However, judging from a credible body of evidence, we can conclude that HTG is an independent risk factor of developing glucose metabolism disorders (5). Plasma TG levels are strongly associated with raised glucose levels because of the interactions between fat, muscle and function of pancreatic β-cells (6,7). Moreover, accumulation of TG in the liver may cause fatty liver disease, which can increase the risk of type 2 diabetes mellitus (T2DM) (8). Prospective studies have revealed that plasma TG is an independent risk factor for developing T2DM (9,10). Additionally, it has been demonstrated that lowering TG, such as fibrates do, can significantly attenuate the process of developing insulin resistance (11). Furthermore, it also has been reported that both fasting glucose and TG within the high normal range may predict CVD risk (12,13). Hence, evaluating the joint value of TG and fasting glucose in patients with stable coronary artery disease (CAD) may be clinically in need.
Triglyceride glucose (TyG) index is a novel marker, which has been demonstrated to have a high sensitivity and specificity in identifying metabolic syndrome (14). Previous studies have shown that TyG index is associated with carotid atherosclerosis, coronary artery calcification and high risk of CVD. Unfortunately, no data is currently available with regard to the effects of TyG index on clinical outcomes in patients with stable CAD (15-17). Therefore, the primary objective of the study was to investigate the prognostic role of TyG index in a large Chinese cohort with stable CAD.
Methods
Study design and population
Our study complied with the Declaration of Helsinki and was approved by the hospital’s ethical review board (Fu Wai Hospital & National Center for Cardiovascular Diseases, Beijing, China, approval number: 2013–442). Informed written consents were obtained from all patients enrolled in this study.
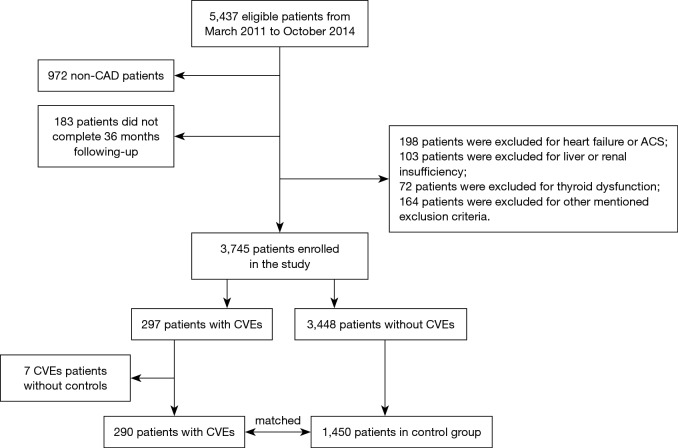
As described in Figure 1, from March 2011 to October 2014, 5,437 consecutive patients were scheduled for coronary angiography because of angina-like chest pain and/or positive treadmill exercise test or clinically suspected CAD in our division. Among these patients, 972 were excluded because they were not angiography-proven CAD. Patients with acute coronary syndrome (ACS), heart failure (left ventricular ejection fraction, LVEF <45%), severe liver and/or renal insufficiency, thyroid dysfunction, malignant disease, extreme body mass index (BMI >45 kg/m2), suspected familial HTG [plasma TG ≥500 mg/dL (5.65 mmol/L) or more than one first-degree relative with TG ≥500 mg/dL] were also excluded. Patients were prospectively followed up at 6, 12, 24, 36 months by means of interviewing directly or using telephone conducted by trained nurses or doctors who were blinded to the clinical data. The cardiovascular events (CVEs) were all-cause death, non-fatal myocardial infarction (MI), stroke and post-discharge revascularization [percutaneous coronary intervention (PCI) coronary artery bypass grafting (CABG)]. Cardiovascular death was defined as death primarily caused by acute MI, congestive heart failure, stroke, malignant arrhythmia and other structural or functional cardiac diseases. Non-fatal MI was diagnosed as positive cardiac troponins along with typical chest pain or typical electrocardiogram serial changes. Stroke was diagnosed by the presence of typical symptoms or imaging. Finally, we identified 3,745 patients with stable CAD who completed our follow-up for the present analysis. During a follow-up of per 11,235 person-years, 297 CVEs occurred and individually matched to 5 randomly selected controls on age, gender, previous history of PCI and CABG, and the duration of follow-up.
Figure 1.
Flowchart of the study. CAD, coronary artery disease; ACS, acute coronary syndrome; CVEs, cardiovascular events.
Hypertension was defined as a self-reported hypertension, currently taking anti-hypertensive drugs or recorded systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure (DBP) ≥90 mmHg for three or more consecutive times. T2DM was defined as fasting serum glucose ≥7.0 mmol/L or the 2-h serum glucose of the oral glucose tolerance test ≥11.1 mmol/L or currently using hypoglycaemic drugs or insulin. BMI was calculated as weight divided by height squared. Information of other disease, family history and current therapy of every patient was collected from self-reported medical history.
Laboratory analysis
Blood samples were obtained from each patient from the cubital vein after at least 12-h fasting. Concentrations of total cholesterol (TC), TG, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C) were measured using automatic biochemistry analyzer (Hitachi 7150, Japan) in an enzymatic assay. Non-HDL-C was calculated as TC minus HDL-C. The concentrations of glucose were measured by enzymatic hexokinase method. HbA1c was measured using Tosoh Automated Glycohemoglobin Analyser (HLC-723G8, Tokyo, Japan). TyG index was calculated as formula: ln[fasting TG (mg/dL) × fasting plasma glucose (mg/dL)/2].
Statistical analysis
The values were expressed as the mean ± standard deviation (SD) or median (Q1–Q3 quartiles) for the continuous variables and the number (percentage) for the categorical variables. The differences of clinical characteristics between groups were analyzed using Student t-test, χ2-tests, and Fisher’s exact test where appropriate. Univariate and multivariate Cox regression analyses were performed to estimate the association TyG index with CVEs. We electively included traditional risk factors [hypertension, DM, lipid, family history of CAD, smoke, BMI, hsCRP (high sensitive C-reactive protein)] and clinical factors with significant differences between CVEs and control group. A P value <0.05 was considered statistically significant. The statistical analysis was performed with SPSS version 21.0 software (SPSS Inc., Chicago, IL, USA).
Results
Cardiovascular events during follow-up
During a follow-up of per 11,235 person-years, 297 CVEs occurred. Each patient experienced CVEs was matched to 5 randomly selected controls on age (±2 years), gender, previous history of PCI and CABG, and the duration of follow-up. 7 patients with CVEs were excluded because they were without matched controls. Among 290 patients with CVEs, 35 (12.07%) died, 70 (24.14%) had stroke, 41 (14.13%) developed non-fatal MI and 144 (49.66%) underwent unplanned PCI or CABG. Patients who experienced non-fatal MI and underwent PCI or CABG were analyzed as one single event.
Baseline characteristics
As presented in Table 1, patients in CVEs group had higher levels of TyG index compared to the control group. Patients in CVEs group also showed higher proportions of hypertension (71.4% vs. 63.5%, P=0.010) and diabetes (34.5% vs. 25.4%, P=0.002), elevated concentrations of plasma glucose, TG, HbA1C but lower levels of LVEF (all P<0.05). There were no significant differences in TC, HDL-C, LDL-C, lipoprotein (a), hsCRP, the proportions of current smoking, and family history of CAD between the two groups (all P>0.05).
Table 1. Baseline characteristics of studied patients.
| Variables | Control group, N=1,450 | CVEs group, N=290 | P |
|---|---|---|---|
| Clinical factors | |||
| Age, years | 59.5±10.8 | 59.4±10.0 | 0.836 |
| Male, n (%) | 1,045 (72.1) | 209 (72.1) | 0.999 |
| BMI (kg/m2) | 25.8±3.2 | 25.5±3.3 | 0.187 |
| HT, n (%) | 921 (63.5) | 207 (71.4) | 0.010 |
| DM, n (%) | 368 (25.4) | 100 (34.5) | 0.002 |
| DM duration | 5.7±4.8 | 6.5±5.3 | 0.149 |
| Family history of CAD, n (%) | 200 (13.8) | 40 (13.8) | 0.942 |
| Current Smoker, n (%) | 770 (54.5) | 158 (53.1) | 0.667 |
| Drinking, n (%) | 417 (28.2) | 81 (28.9) | 0.807 |
| PrePCI, n (%) | 310 (21.4) | 62 (21.4) | 1 |
| PreCABG, n (%) | 25 (1.7) | 5 (1.7) | 1 |
| PreMI, n (%) | 422 (29.1) | 97 (33.4) | 0.140 |
| Laboratory factors | |||
| Glucose (mmol/L) | 5.6±1.5 | 5.9±2.0 | 0.001 |
| HbA1c (%) | 6.4±1.1 | 6.6±1.3 | <0.001 |
| ALT (IU/L) | 23.0 (17.0–33.0) | 24.0 (17.0–34.0) | 0.115 |
| AST (IU/L) | 17.0 (14.0–22.0) | 18.0 (14.0–23.0) | 0.077 |
| Creatinine (ìmol) | 75.8±16.8 | 77.3±16.4 | 0.141 |
| UA (ìmol/L) | 351.3±87.5 | 365.3±95.6 | 0.022 |
| hsCRP (ìmol/L) | 1.42 (0.72–3.13) | 1.63 (0.83–3.30) | 0.250 |
| TC (mmol/L) | 4.13±1.19 | 4.20±1.15 | 0.338 |
| HDL-C (mmol/L) | 1.06±0.28 | 1.07±0.30 | 0.825 |
| LDL-C (mmol/L) | 2.53±1.04 | 2.50±0.92 | 0.673 |
| Non-HDL-C (mmol/L) | 3.14±1.10 | 3.07±1.16 | 0.348 |
| TG (mmol/L) | 1.46 (1.09–2.03) | 1.57 (1.15–2.18) | 0.014 |
| Lp(a) (mg/L) | 158.6 (64.7–363.8) | 363.8 (77.5–431.4) | 0.369 |
| TyG index | 8.80±0.57 | 8.91±0.66 | 0.002 |
| TG/HDL-C | 1.44 (0.96–2.22) | 1.56 (1.01–2.47) | 0.046 |
| LVEF (%) | 63.7±7.6 | 61.8±9.0 | 0.001 |
| Medications, n (%) | |||
| Lipid lowering agents | 1,109 (74.7) | 218 (73.4) | 0.644 |
| ACEIs/ARBs | 205 (14.1) | 35 (12.1) | 0.351 |
| β-blockers | 410 (28.2) | 78 (26.9) | 0.396 |
| Aspirin | 1,421 (98.0) | 282 (97.2) | 0.414 |
| Antidiabetic drug, n (%) | |||
| OADs | 207 (14.3) | 52 (17.9) | 0.110 |
| Insulin | 103 (7.1) | 24 (8.3) | 0.484 |
Data were expressed as median ± SD, 25th and 75th percentile or n (%). BMI, body mass index; HT, hypertension; DM, diabetes mellitus; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UA, uric acid; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; MI, myocardial infarction; hsCRP, high sensitive C-reactive protein; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; Lp(a), lipoprotein (a); TyG index, triglyceride glucose index; LVEF, left ventricular ejection fraction; ACEIs, angiotensin-converting enzymes; ARBs, angiotensin receptor blocker.
Cardiovascular risk factors according to quartiles of TyG index
We also analyzed the distribution of cardiovascular risk factors according to quartiles of TyG index (I quart n=429, II quart n=444, III quart n=434, IV quart n=433). As shown in Table 2, TyG index was positively associated with BMI, UA, hsCRP, HDL–C, LDL-C, DM and hypertension while it was negatively related to age (all P<0.05).
Table 2. TyG index and cardiovascular risk factors.
| Variables | I quart, n=429 (<8.40) | II quart, n=444 (8.41–8.78) | III quart, n=434 (8.79–9.16) | IV quart, n=433 (>9.17) | P |
|---|---|---|---|---|---|
| Male, n (%) | 311 (72.5) | 334 (75.2) | 298 (68.7) | 311 (71.8) | 0.191 |
| Age, years | 61.2±9.4 | 60.2±9.6 | 58.2±9.9 | 57.7±10.1 | <0.001 |
| BMI (kg/m2) | 24.5±3.3 | 25.6±3.2 | 26.3±3.1 | 26.6±2.9 | <0.001 |
| Family history of CAD, n (%) | 63 (14.7) | 54 (12.2) | 66 (15.3) | 57 (13.2) | 0.753 |
| Current Smoker, n (%) | 231 (53.8) | 237 (53.4) | 219 (50.5) | 241 (55.7) | 0.489 |
| HT, n (%) | 251 (58.5) | 278 (62.6) | 276 (63.6) | 323 (74.6) | <0.001 |
| DM, n (%) | 60 (14) | 80 (18.1) | 114 (26.3) | 214 (49.7) | <0.001 |
| UA (ìmol/L) | 330.8±80.5 | 351.0±81.3 | 356.4±87.1 | 377.4±98.6 | <0.001 |
| hsCRP (mg/L) | 1.1 (0.55–2.24) | 1.3 (0.72–2.97) | 1.6 (0.78–3.37) | 1.8 (0.98–4.29) | <0.001 |
| HDL–C (mmol/L) | 1.18±0.29 | 1.09±0.27 | 1.03±0.26 | 0.95±0.23 | <0.001 |
| LDL-C (mmol/L) | 2.23±1.00 | 2.52±1.14 | 2.63±0.89 | 2.71±0.99 | <0.001 |
| Non-HDL-C (mmol/L) | 2.52±0.97 | 2.90±0.90 | 3.23±1.28 | 3.66±1.09 | <0.001 |
| LVEF (%) | 63.1±7.5 | 62.9±8.5 | 63.1±7.2 | 63.5±8.2 | 0.571 |
| PrePCI, n (%) | 99 (23.1) | 89 (20.0) | 100 (23.0) | 84 (19.4) | 0.403 |
| PreCABG, n (%) | 3 (0.7) | 8 (1.8) | 10 (2.3) | 9 (2.1) | 0.277 |
| Events, n (%) | 69 (16.1) | 57 (12.8) | 76 (17.5) | 88 (20.3) | 0.027 |
Data were expressed as mean ± SD, 25th and 75th percentile or n (%). BMI, body mass index; HT, hypertension; DM, diabetes mellitus; LVEF, left ventricular ejection fraction; UA, uric acid; hsCRP, high sensitive C-reactive protein; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
Predictive role of TyG index on cardiovascular events
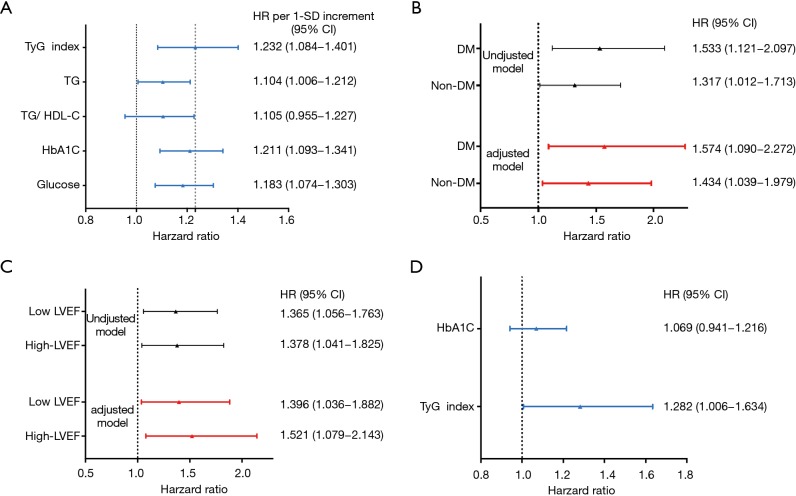
In the present study, univariate Cox proportional hazard regression analysis showed that TyG index was associated with CVEs (hazard ratio: 1.356, 95% confidence interval: 1.123–1.639, P=0.002, Table 3). Hypertension, DM and UA were also risk factors of CVEs (P<0.05) while LVEF played a protective role. In multivariate Cox proportional hazard regression analysis, we further examined the independent risk value of TyG index on CVEs (Table 3). After adjustment of BMI, LVEF, hypertension, DM, UA, smoke, hsCRP, HDL-C and LDL-C, TyG index was independently associated with CVEs [hazard ratio (HR): 1.364, 95% confidence interval (95% CI): 1.100–1.691, P=0.005]. The Kaplan–Meier analysis revealed that the patients within the highest quartile of TyG index presented the lowest event-free survival (P=0.029, Figure 2). In addition, a 1-SD increment in TyG index was associated with 23.2% (HR: 1.232, 95% CI: 1.084–1.401, P<0.05) higher risk of CVEs, which was superior to other TG or glycemic related markers [TG: HR per 1-SD increment 1.104 (95% CI: 1.006–1.212), P<0.05; TG/HDL-C: HR per 1-SD increment 1.105 (95% CI: 0.955–1.227), P>0.05; HbA1c: HR per 1-SD increment 1.211 (95% CI: 1.093–1.341), P<0.05; glucose: HR per 1-SD increment 1.183 (95% CI: 1.074–1.303), P<0.05, Figure 3A]. Furthermore, TyG index was also positively associated with CVEs in subgroup analysis according to the different status of DM and LVEF [DM group: adjusted HR 1.574 (95% CI: 1.090–2.272), P<0.05; non-DM group: 1.434 (95% CI: 1.039–1.979), P<0.05; low LVEF group: adjusted HR 1.396 (95% CI: 1.036–1.882), P<0.05; high LVEF group: adjusted HR 1.521 (95% CI: 1.079–2.143), P<0.05; Figure 3B,C]. Finally, the predictive value of TyG index remained significant after adjustment of HbA1c in the multivariate model [adjusted HR 1.282 (95% CI: 1.006–1.634), P<0.05, Figure 3D].
Table 3. Univariate and multivariate Cox proportional hazards regression analysis of the events.
| Variables | Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| BMI | 0.975 (0.940–1.012) | 0.187 | – | ||
| LVEF | 0.975 (0.962–0.987) | <0.001 | 0.976 (0.963–0.989) | <0.001 | |
| HT | 1.410 (1.092–1.812) | 0.008 | 1.317 (1.004–1.727) | 0.047 | |
| DM | 1.479 (1.161–1.885) | 0.020 | 1.350 (1.040–1.777) | 0.025 | |
| hsCRP | 1.010 (0.983–1.050) | 0.339 | – | – | |
| UA | 1.002 (1.001–1.003) | 0.013 | 1.002 (1.001–1.003) | 0.007 | |
| Smoke | 1.056 (0.838–1.331) | 0.646 | – | – | |
| HDL-C | 1.031 (0.685–1.552) | 0.882 | – | – | |
| LDL-C | 0.973 (0.866–1.092) | 0.638 | – | – | |
| TyG index | 1.356 (1.123–1.639) | 0.002 | 1.364 (1.100–1.691) | 0.005 | |
HR, hazard ratio; 95% CI, 95% confidence intervals; BMI, body mass index; LVEF, left ventricular ejection fraction; HT, hypertension; DM, diabetes mellitus; hsCRP, high sensitive C-reactive protein; UA, uric acid; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; UA, uric acid; TyG index, triglyceride glucose index.
Figure 2.
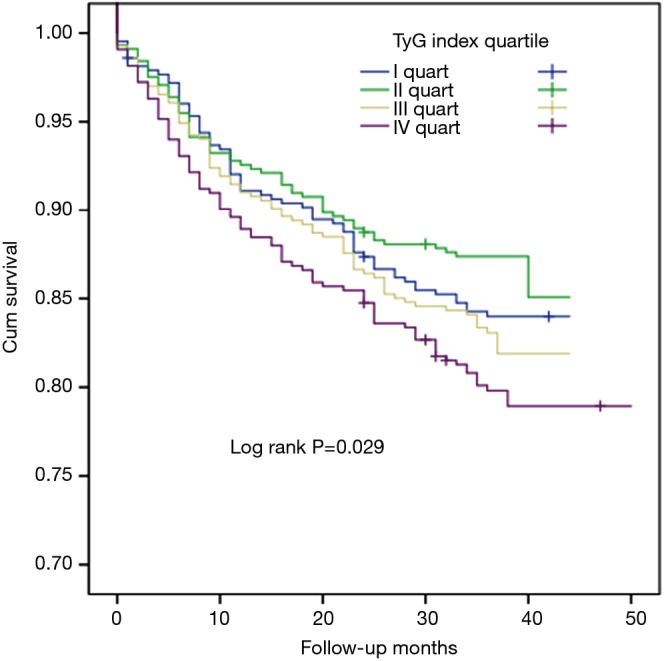
The event-free survival analysis according to the quartiles of TyG index. TyG index, triglyceride glucose index.
Figure 3.
Predictive value of TyG index for CVEs in the different models. (A) HR for cardiovascular events risk elevation associated with 1-SD increment in triglyceride or glycemic related markers; (B) predictive value of TyG index for CVEs in diabetic and non-diabetic patients; (C) predictive value of TyG index for CVEs in patients with high and low LVEF; (D) predictive value of TyG index for CVEs after adjusting for HbA1c and other confounding variables. Adjusted model included BMI, smoking, hypertension, non-LDL-C, hs-CRP, UA (age, sex, DM and LVEF when appropriate). TyGindex, triglyceride glucose index; CVEs, cardiovascular events; HR, hazard ratio; LVEF, left ventricle ejection fraction; LDL-C, low density lipoprotein cholesterol; DM, diabetes mellitus.
Discussion
TyG index has been reported to be associated with CVD risk in apparently healthy individuals (17). However, the prognostic value of TyG index in patients with stable CAD remains undetermined. Using nested case-control analysis, the data suggested that TyG index was higher in patients who experienced CVEs. In addition, TyG index was found to be positively related to cardiovascular risk factors and presented the lowest event-free survival in its top quartered group. To our knowledge, the present study firstly demonstrated that TyG index was an independent risk marker for evaluating future CVEs in patients with stable CAD.
TyG index was firstly studied as a marker of identifying insulin resistance with a high sensitivity and specificity (18-20). It was demonstrated that TyG index was a useful predictor of type 2 diabetes and metabolic syndrome which contributed to cardiometabolic risk (21,22). Subsequently, several studies were conducted and found a positive relationship between TyG index and CVD. Two of such studies demonstrated that TyG index was associated with the presence of cardiovascular risk factors (23,24). Moreover, Irace et al. evaluated the association between carotid atherosclerosis and TyG index in two different cohorts and provided consistent, positive results (15). In addition to this, a study enrolled 4,319 Korean adults also indicated that TyG index was significantly associated with the presence of coronary calcification (16). Furthermore, a study including 888 asymptomatic type 2 diabetic patients showed that the higher TyG index was associated with increased risk of coronary stenosis (25). Of the note, studies mentioned above did not evaluated the prognostic value of TyG index in CVD risk.
In fact, a few prospective studies were conducted on the link between TyG index and CVEs. Vega et al. firstly investigated the relation of TyG index to mortality from cardiovascular causes, CAD, or CVD in 39,447 men and proved that TyG index did not predict CVD mortality (26). Apparently, this study was limited by gender selection. Another study enrolled 5,014 apparently healthy individuals and identified that the higher level of TyG index was significantly associated with an increased risk of developing CVD (17). They also developed a new model containing the TyG index in addition to Framingham variables and resulted in a higher predictive efficiency in the risk of developing CVD. However, their study focused only on the healthy individuals. Consequently, determination of the prognostic role of TyG index in patients with CAD might be greatly of interest. That was the reason why we performed such study. As shown in the tables and figures, our study, for the first time, indicated that TyG index was significantly higher in patients with CVEs and had better predictive value than TG or glucose alone, suggesting that TyG index might be a simple, easy-to-use, reliable parameter in predicting the prognosis in patients with stable CAD. Moreover, we also compared the prognostic value of TyG index with HbA1c. As we well known, plasma HbA1c, the most reliable marker in evaluating long term glycemic control, had similar HR to TyG index in our study. To our knowledge, both markers were associated with insulin resistance in certain patients and only the predictive value of TyG index stayed significant when the two markers were in the same model (Figure 3D).
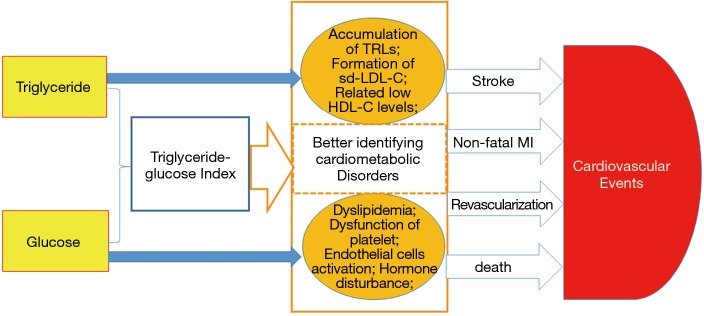
The exact mechanism underlying the relationship between the TyG index and CVEs has not been fully elucidated. The formula of TyG index is composed of TG and glucose. Although the association of TG with CVD risk is still under debate (3,4), a body of recent evidence has proved that TG and TG-rich lipoproteins are causal factors of CVD (27). Additionally, the concurrence of HTG also promotes the formation of small dense LDL particles (28). Despite the fact that most studies evaluate the CVD risk of TG only in HTG patients, a few studies have demonstrated that plasma TG within high normal range also predict CVEs. In fact, glucose disorder is another CVD risk factor frequently coexisting with HTG. Achievement of favorable goal in plasma TG by means of losing weight or drugs often helps improve glycemic control (29,30). Genetic polymorphisms affecting TG metabolism may also be associated with higher fasting plasma glucose (31). The Prospective Urban Rural Epidemiology (PURE) study demonstrated that high carbohydrate intake, which might increase plasma TG and glucose, was associated with greater risk of the total mortality (32). Therefore, using TyG index may better interpret their joint roles in CVD risk prediction. As we previously described, TyG index was also a useful marker in identifying metabolic disorder (22,23). Notably, inflammatory markers causing atherosclerotic plaque instability, including tumor necrosis factor-α, interleukins, leukocytes and fibrinogen, also played a crucial role in metabolic syndrome and related disorders (33,34). Therefore, TyG index might be a better marker of cardiometabolic risk estimation (Figure 4).
Figure 4.
Mechanism of TyG index associated with cardiovascular outcomes. TyGindex, triglyceride glucose index; TRLs, triglyceride-rich lipoproteins; sd-LDL-C, small dense LDL-C; MI, myocardial infarction.
There were several limitations in the present study. Firstly, the sample size might be not large enough and the follow-up time might be not long enough. Secondly, the measurements of TG and fasting glucose had unavoidable intra-individual biological variation and changed over time. Previous studies demonstrated that increment in TyG index over time could predict the incidence of diabetes and was positively related to the value of TyG index at first measurement (35,36). We measured TG and fasting glucose only at the baseline and did not evaluate the predictive value of the changes in TyG index for CVEs. Moreover, other confounding factors such as exercise habit, participation to cardiac rehab program and cardiorespiratory fitness were not included in the model. Finally, we did not assess the relationship between TyG index and all metabolic factors including waist circumstance due to a lack of data. Hence, larger sample and long-term studies are needed to confirm our findings.
Conclusions
Although previous studies indicated an association of TyG with the cardiovascular risk, the present study firstly reported that TyG index was associated with future CVEs in patients with stable CAD using nested case-control study.
Acknowledgements
Funding: This work was partially supported by the Capital Health Development Fund (201614035) and CAMS Major Collaborative Innovation Project (2016-I2M-1-011) awarded to Dr. Jian-Jun Li, MD, PhD.
Ethical Statement: Our study complied with the Declaration of Helsinki and was approved by the hospital’s ethical review board (Fu Wai Hospital & National Center for Cardiovascular Diseases, Beijing, China, approval number: 2013–442). Informed written consents were obtained from all patients enrolled in this study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bays HE. Adiposopathy, diabetes mellitus, and primary prevention of atherosclerotic coronary artery disease: treating sick fat through improving fat function with anti-diabetes therapies. Am J Cardiol 2012;110:4B-12B. 10.1016/j.amjcard.2012.08.029 [DOI] [PubMed] [Google Scholar]
- 2.Li XL, Guo YL, Zhu CG, et al. Relationship of high-density lipoprotein cholesterol with periprocedural myocardial injury following elective percutaneous coronary intervention in patients with low-density lipoprotein cholesterol below 70 mg/dL. J Am Heart Assoc 2015;4:e001412. 10.1161/JAHA.114.001412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miselli MA, Nora ED, Passaro A, et al. Plasma triglycerides predict ten-years all-cause mortality in outpatients with type 2 diabetes mellitus: a longitudinal observational study. Cardiovasc Diabetol 2014;13:135. 10.1186/s12933-014-0135-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993-2000. 10.1001/jama.2009.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amiri P, Jalali-Farahani S, Karimi M, et al. Factors associated with pre-diabetes in Tehranian men and women: A structural equations modeling. PLoS One 2017;12:e0188898. 10.1371/journal.pone.0188898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt MI, Duncan BB, Bang H, et al. Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities Study. Diabetes Care 2005;28:2013-8. 10.2337/diacare.28.8.2013 [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, Meigs JB, Sullivan L, et al. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068-74. 10.1001/archinte.167.10.1068 [DOI] [PubMed] [Google Scholar]
- 8.Trauner M, Arrese M, Wagner M. Fatty liver and lipotoxicity. Biochim Biophys Acta 2010;1801:299-310. 10.1016/j.bbalip.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 9.Freeman DJ, Norrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation 2001;103:357-62. 10.1161/01.CIR.103.3.357 [DOI] [PubMed] [Google Scholar]
- 10.Dotevall A, Johansson S, Wilhelmsen L, et al. Increased levels of triglycerides, BMI and blood pressure and low physical activity increase the risk of diabetes in Swedish women: a prospective 18-year follow-up of the BEDA study. Diabet Med 2004;21:615-22. 10.1111/j.1464-5491.2004.01189.x [DOI] [PubMed] [Google Scholar]
- 11.Lee MK, Miles PD, Khoursheed M, et al. Metabolic effects of troglitazone on fructose-induced insulin resistance in the rat. Diabetes 1994;43:1435-9. 10.2337/diab.43.12.1435 [DOI] [PubMed] [Google Scholar]
- 12.Miller M, Seidler A, Moalemi A, et al. Normal triglyceride levels and coronary artery disease events: the Baltimore Coronary Observational Long-Term Study. J Am Coll Cardiol 1998;31:1252-7. 10.1016/S0735-1097(98)00083-7 [DOI] [PubMed] [Google Scholar]
- 13.Shaye K, Amir T, Shlomo S, et al. Fasting glucose levels within the high normal range predict cardiovascular outcome. Am Heart J 2012;164:111-6. 10.1016/j.ahj.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angoorani P, Heshmat R, Ejtahed HS, et al. Validity of triglyceride-glucose index as an indicator for metabolic syndrome in children and adolescents: the CASPIAN-V study. Eat Weight Disord 2018. [Epub ahead of print]. 10.1007/s40519-018-0488-z [DOI] [PubMed] [Google Scholar]
- 15.Irace C, Carallo C, Scavelli FB, et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract 2013;67:665-72. 10.1111/ijcp.12124 [DOI] [PubMed] [Google Scholar]
- 16.Kim MK, Ahn CW, Kang S, et al. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol 2017;16:108. 10.1186/s12933-017-0589-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sánchez-Íñigo L, Navarro-Gonzalez D, Fernandez-Montero A, et al. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest 2016;46:189-97. 10.1111/eci.12583 [DOI] [PubMed] [Google Scholar]
- 18.Simental-Mendía LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008;6:299-304. 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 19.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010;95:3347-51. 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 20.Du T, Yuan G, Zhang M, Zhou X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol 2014;13:146. 10.1186/s12933-014-0146-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee DY, Lee ES, Kim JH, et al. Predictive Value of Triglyceride Glucose Index for the Risk of Incident Diabetes: A 4-Year Retrospective Longitudinal Study. PLoS One 2016;11:e0163465. 10.1371/journal.pone.0163465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JW, Park SH, Kim Y. The cutoff values of indirect indices for measuring insulin resistance for metabolic syndrome in Korean children and adolescents. Ann Pediatr Endocrinol Metab 2016;21:143-8. 10.6065/apem.2016.21.3.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simental-Mendía LE, Hernández-Ronquillo G, Gómez-Díaz R, et al. The triglycerides and glucose index is associated with cardiovascular risk factors in normal-weight children and adolescents. Pediatr Res 2017;82:920-5. 10.1038/pr.2017.187 [DOI] [PubMed] [Google Scholar]
- 24.Mazidi M, Katsiki N, Mikhailidis DP. The link between insulin resistance parameters and serum uric acid is mediated by adiposity. Atherosclerosis 2018;270:180-6. 10.1016/j.atherosclerosis.2017.12.033 [DOI] [PubMed] [Google Scholar]
- 25.Lee EY, Yang HK, Lee J, et al. Triglyceride glucose index, a marker of insulin resistance, is associated with coronary artery stenosis in asymptomatic subjects with type 2 diabetes. Lipids Health Dis 2016;15:155. 10.1186/s12944-016-0324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vega GL, Barlow CE, Grundy SM, et al. Triglyceride-to-high-density- lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med 2014;62:345-9. 10.2310/JIM.0000000000000044 [DOI] [PubMed] [Google Scholar]
- 27.Budoff M. Triglycerides and Triglyceride-Rich Lipoproteins in the Causal Pathway of Cardiovascular Disease. Am J Cardiol 2016;118:138-45. 10.1016/j.amjcard.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 28.Tenenbaum A, Klempfner R, Fisman EZ. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovasc Diabetol 2014;13:159. 10.1186/s12933-014-0159-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Yang C, Guo H. Treatment of coenzyme Q10 for 24 weeks improves lipid and glycemic profile in dyslipidemic individuals. J Clin Lipidol 2018;12:417-27.e5. 10.1016/j.jacl.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 30.Maharlouei N, Tabrizi R, Lankarani KB. The effects of ginger intake on weight loss and metabolic profiles among overweight and obese subjects: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr 2018. [Epub ahead of print]. 10.1080/10408398.2018.1427044 [DOI] [PubMed] [Google Scholar]
- 31.Tam CH, Ma RC, So WY, et al. Interaction effect of genetic polymorphisms in glucokinase (GCK) and glucokinase regulatory protein (GCKR) on metabolic traits in healthy Chinese adults and adolescents. Diabetes 2009;58:765-9. 10.2337/db08-1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dehghan M, Mente A, Zhang X, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050-62. 10.1016/S0140-6736(17)32252-3 [DOI] [PubMed] [Google Scholar]
- 33.González M, del Mar Bibiloni M, Pons A, et al. Inflammatory markers and metabolic syndrome among adolescents. Eur J Clin Nutr 2012;66:1141-5. 10.1038/ejcn.2012.112 [DOI] [PubMed] [Google Scholar]
- 34.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135-43. 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 35.Navarro-González D, Sánchez-Íñigo L, Fernández-Montero A, et al. TyG Index Change Is More Determinant for Forecasting Type 2 Diabetes Onset Than Weight Gain. Medicine (Baltimore) 2016;95:e3646. 10.1097/MD.0000000000003646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SH, Yang HK, Ha HS, et al. Changes in Metabolic Health Status Over Time and Risk of Developing Type 2 Diabetes: A Prospective Cohort Study. Medicine (Baltimore) 2015;94:e1705. 10.1097/MD.0000000000001705 [DOI] [PMC free article] [PubMed] [Google Scholar]