Abstract
Background
Lung ultrasonography is increasingly used in the emergency department (ED) as a standard adjunct in the evaluation of the breathless patient. The study objective was to ascertain the diagnostic accuracy of lung and cardiac ultrasound in undifferentiated dyspneic ED patients.
Methods
We conducted this prospective observational study on patients presenting with dyspnea in the ED of a tertiary hospital. The sonographers who performed lung and cardiac ultrasound according to a locally-designed protocol were blinded to clinical and radiologic results. Ultrasonographic findings were subsequently compared with the final adjudicated diagnoses.
Results
Between February and August 2015, 231 patients were recruited. There was male predominance (63.2%) with a mean age of 67.8 years. Overall, lung ultrasonography yielded correct diagnoses in 68.3% of patients. Our protocol had likelihood ratios of 3.63 [95% confidence interval (CI): 2.44–5.40], 3.73 (95% CI: 2.50–5.57) and 6.31 (95% CI: 3.72–10.72) for positive findings; and 0.42 (95% CI: 0.29–0.63), 0.35 (95% CI: 0.25–0.50), and 0.40 (95% CI: 0.28–0.56) for negative findings in the diagnoses of pneumonia, pulmonary edema, and chronic obstructive pulmonary disease or asthma, respectively. Addition of bedside echocardiography was able to differentiate cardiogenic from nephrogenic pulmonary edema in 70% of patients.
Conclusions
Lung ultrasonography, when complemented with other tools of investigation, aids evaluation, allows for earlier treatment and more accurate disposition of undifferentiated dyspneic patients in the ED. The addition of cardiac ultrasound was not able to reliably differentiate the causes of pulmonary edema.
Keywords: Emergency department (ED), ultrasonography, dyspnea, pneumonia, pulmonary edema
Introduction
Dyspnea, which is defined as a subjective inability to breathe comfortably, is a common primary complaint among patients in the emergency department (ED) (1). Common causes include pneumonia, decompensated heart failure, chronic obstructive pulmonary disease (COPD), asthma and pulmonary embolism. The multitude of possible diagnoses makes rapid and accurate differentiation of the causes of dyspnea especially challenging. To compound the problem, there are limitations of current diagnostic tools. For example, chest radiography has moderately low sensitivity and specificity in diagnosing pulmonary edema, COPD, pleural effusion and pneumothorax (2). Arterial blood gas evaluation, though frequently sampled in patients presenting with acute dyspnea, has been shown to perform poorly in differentiating between cardiac and respiratory causes of dyspnea (3). The diagnostic value of B-type natriuretic peptide and N-terminal pro brain natriuretic peptide in heart failure is also less than perfect given its low specificity (4). Furthermore, results for these investigations require time and may be less useful in patients with acutely worsening dyspnea who require emergent therapy based on preliminary assessment (5).
In view of these limitations, lung ultrasonography has increasingly emerged as a useful point-of-care (POC) tool in evaluating these patients due to its rapid, non-invasive, repeatable and non-ionising characteristics (6). Recent studies have also demonstrated its high sensitivity and specificity in the diagnosis of decompensated heart failure (7), pneumonia (8), pneumothorax (9), pulmonary embolism (10), and pleural effusion (11). In a study conducted by Lichtenstein and colleagues, a structured lung ultrasound algorithm, known as the Bedside Lung Ultrasound in Emergency (BLUE) protocol, yielded the correct diagnosis in 90.5% of medical intensive care unit (ICU) patients with acute respiratory failure (5). The results validated in the ICU may not be generalizable as population in the ED is more heterogeneous.
This study seeks to determine the overall diagnostic accuracy of lung with the addition of cardiac ultrasonography in patients with undifferentiated dyspnea presenting to the ED.
Methods
Study design and setting
This was a prospective observational study conducted at the ED of the National University Hospital, Singapore, from February to September 2015. The hospital is a 1,100-bed tertiary academic medical center with an annual ED census of 130,000 patients. The study protocol was approved by the National Healthcare Group’s Domain Specific Review Board (2014/00332). Written informed consent from patients or their legally acceptable representatives was obtained prior to recruitment.
Participants
We enrolled participants with the following criteria: 21 years old and above, able to provide valid informed consent and presenting with a primary complaint of dyspnea. Patients who refused consent or whose dyspnea was primarily exertional in nature were excluded.
Study protocol
Initial evaluation comprising medical history, physical examination, 12-lead electrocardiogram, and standard laboratory or radiological investigations were carried out by the attending team. Eligible patients were subsequently recruited and underwent lung and cardiac ultrasonography by either a study investigator (Y Koh), who had undergone more than 20 hours of formal training in lung and cardiac ultrasonography, or a board-certified emergency physician who was not directly involved in the care of the patient. The operator of the ultrasonography was blinded to the results of the initial evaluation by the attending team. Ultrasonographic images and videos were saved, and later independently reviewed by two emergency physicians with at least 5 years of ultrasound experience, blinded to the initial diagnoses and clinical information of the patients. The patients’ final adjudicated diagnoses were ascertained by two independent emergency physicians (MT Chua and WS Kuan) who were blinded to the ultrasonographic findings, based on the history, physical examination, laboratory and radiological findings. Any discrepancy in the final diagnoses were reviewed by a third independent emergency physician.
Lung ultrasonography
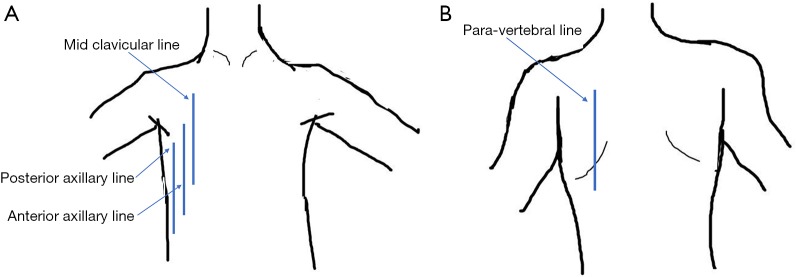
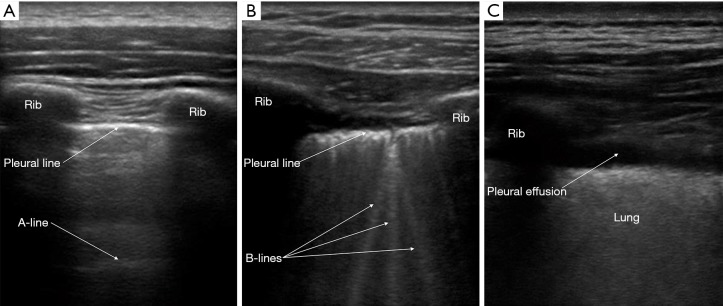
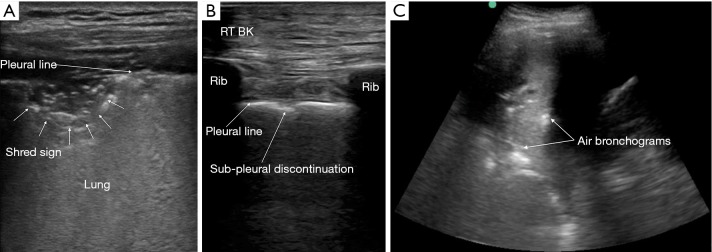
Lung ultrasonography was performed using a 13-6 MHz linear probe (Edge, FUJIFILM Sonosite Inc., Bothell, WA) with patients in erect or semi-recumbent positions depending on their severity of breathlessness. The linear probe was used in lean patients as it provides a higher resolution. If the linear probe was inadequate in depth to evaluate the lung parenchyma, the lower frequency 8-3 MHz curved probe was used instead. A total of six areas were investigated: right and left mid-clavicular, anterior axillary and paravertebral lines. On average, 2 to 3 video images per intercostal space were recorded and all intercostal spaces were evaluated vertically along the aforementioned lines. The findings on the anterior axillary line were considered as that of the lateral chest. In patients who were unable to tolerate changes in position, the posterior axillary lines were examined for posterior chest findings instead of the paravertebral lines (supplementary data Figure S1). The BLUE protocol provided the framework for the modified ultrasonography protocol in this study (12). First, the following signs were assessed on lung ultrasonography (supplementary data Table S1)—pleural, A- and B-lines, lung sliding, pleural effusion, shred sign, sub-pleural discontinuation and air bronchogram (supplementary data Figures S2,S3).
Figure S1.
Locations where lung ultrasound was performed. (A) Front; (B) back.
Table S1. Lung ultrasound signs.
| Ultrasound signs | Description |
|---|---|
| Pleural line | Located between two rib shadows and indicates the parietal pleura |
| A-line | Artifact that appears as horizontal repetitions of the pleural line generated by subpleural air that blocks ultrasound waves |
| Lung sliding | Represented by the to-and-fro movement of the pleural line with respiration. Its absence signifies that the visceral pleura is separated from the parietal pleura |
| B-line | Comet-tail artefact that arises from the pleural line and moves with lung sliding, if present. It is a hyper-echoic, long and well-defined line that erases A-lines |
| Pleural effusion | Anechoic collection that is present between the visceral and parietal pleura |
| Shred sign | Represents irregular border between consolidated and aerated lung, which signifies non-translobar consolidation |
| Sub-pleural discontinuation | Thickened, irregular pleural line that is suggestive of underlying lung consolidation |
| Air bronchogram | Linear hyper-echoic artifact within the lung parenchyma suggestive of underlying lung consolidation |
Figure S2.
Lung ultrasound signs. (A) Pleural and A-lines; (B) B-lines; (C) pleural effusion.
Figure S3.
Ultrasound findings in pneumonia. (A) Tissue-like appearance of the shred sign within the lung parenchyma; (B) sub-pleural discontinuation; (C) air bronchograms.
Cardiac ultrasonography
Cardiac ultrasonography was performed using a 5-1 MHz curvilinear probe (Edge, FUJIFILM Sonosite Inc., Bothell, WA) with the following views: parasternal long-axis, parasternal short-axis and apical four-chamber. The contractility, equality of heart chambers, and presence or absence of pericardial effusion was assessed. Additional lower limb ultrasonography was carried out to assess for deep vein compressibility in patients with suspected pulmonary embolism from cardiac ultrasound.
Lung profiles
Next, the ultrasonographic findings were grouped together to form profiles for analysis similar to the BLUE protocol, namely the A-, A’-, B-, B’-, C- and A/B-profiles (Table 1) (12). In this study, the presence of decreased cardiac contractility with B-profile is classified as pulmonary edema secondary to congestive cardiac failure with reduced ejection fraction. Conversely, normal cardiac contractility with a B-profile is assumed to be pulmonary edema secondary to renal failure, cardiac failure with preserved ejection fraction or other non-cardiogenic pulmonary edema. The B’-, C- and A/B-profiles are indicative of an underlying lung consolidation.
Table 1. Lung ultrasound profiles.
| Profile | Description |
|---|---|
| A | Depicts the presence of bilateral anterior lung sliding and antero-lateral A-lines. This is associated with severe asthma or COPD exacerbation |
| A’ | Describes presence of antero-lateral A-lines in the absence of bilateral lung sliding. This profile, together with the presence of a lung point, indicates presence of a pneumothorax |
| B | Presence of 3 or more B-lines in bilateral antero-lateral chest together with bilateral anterior lung sliding. Posterior changes were not included as they may be due to gravity alone. This profile suggests the presence of pulmonary edema |
| B’ | Comprises 3 or more B-lines in the bilateral antero-lateral chest in the absence of bilateral lung sliding |
| C | This profile is present if any of the following are demonstrated on ultrasound: air bronchograms, shred sign or subpleural discontinuation |
| A/B | Comprises an A-profile in one lung and a B-profile in another lung |
COPD, chronic obstructive pulmonary disease.
Statistical analysis
Data was analyzed using Stata 14 (StataCorp LP, College Station, TX) and Microsoft Office Excel 2013 (Microsoft Inc., Redmond, WA). Categorical variables are reported in frequency and percentages. For continuous variables, mean [standard deviation (SD)] and median [interquartile range (IQR)] are reported for normal and skewed distributions, respectively. The ultrasonographic profiles were compared with the final adjudicated diagnoses and the following were determined: accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratios of positive (PLR) and negative (NLR) tests, with their respective 95% confidence intervals. The inter-rater agreement for the adjudicated diagnoses was determined using the kappa statistic. Based on a prevalence of dyspneic patients at 60%, a sample size of 250 patients would have 80% power to detect a specificity of 60% with an α of 0.05.
Results
Baseline characteristics
Over 7 months from February to August 2015, a total of 257 patients were screened. Twenty-six patients were excluded for the following reasons: re-attendances (n=3), inaccessible electronic records (n=1), and ineligibility based on our inclusion and exclusion criteria (n=22), leaving 231 patients eligible for the study (Table 2). The majority were male (63.2%), with a mean age of 67.8 years (SD 15.5 years). The proportion of the study cohort admitted from the ED was 92.6% (214/231) and 74.5% (172/231) of patients had significant past medical history of respiratory conditions (Table 2). Other concomitant symptoms experienced included fever, cough, chest pain, orthopnea and paroxysmal nocturnal dyspnea, and hemoptysis.
Table 2. Baseline characteristics.
| Variables | Value (N=231) |
|---|---|
| Age (years), mean (SD) | 67.8 (15.5) |
| Male gender, n (%) | 146 (63.2) |
| Ethnicity, n (%) | |
| Chinese | 147 (63.6) |
| Malay | 59 (25.5) |
| Indian | 22 (9.5) |
| Others | 3 (1.3) |
| Admission, n (%) | 214 (92.6) |
| Associated symptomsa, n (%) | |
| Cough | 156 (67.5) |
| Orthopnea or paroxysmal nocturnal dyspnea | 62 (26.8) |
| Fever | 61 (26.4) |
| Chest pain | 45 (19.5) |
| Hemoptysis | 4 (1.7) |
| None | 30 (13.0) |
| Respiratory rate on presentation (breaths/min), median (interquartile range) | 24 (20 to 24) |
| Past medical historya, n (%) | |
| Respiratoryb | 172 (74.5) |
| Cardiacc | 45 (19.5) |
| Othersd | 27 (11.7) |
| None | 62 (26.8) |
| Time between ultrasound and chest X-ray (min), median (interquartile range)e | −26 (−77 to 5) |
a, total greater than 100% as some patients had more than one condition; b, past medical history of respiratory conditions include pneumonia, pulmonary embolism, pneumothorax, asthma, COPD, bronchiectasis, lung carcinoma, tuberculosis, interstitial lung disease, and previous lung surgery; c, past medical history of cardiac conditions includes fluid overload secondary to congestive cardiac failure; d, others include: renal failure, lung mass or granuloma, pulmonary hypertension, and extra-parenchymal lung disease; e, the negative value indicates that ultrasound was performed after chest X-ray. COPD, chronic obstructive pulmonary disease.
Adjudicated diagnoses
Inter-rater agreement between the two independent emergency physicians (MT Chua and WS Kuan) adjudicating the final diagnoses of the patients was excellent (κ=0.87; 95% CI: 0.81–0.92). Seven main causes of dyspnea in the recruited patients were identified—congestive cardiac failure, pneumonia, asthma, COPD, fluid overload due to non-cardiogenic causes, pneumothorax and pulmonary embolism (Table 3). Patients with asthma and COPD were grouped together as they are both airway diseases with no lung parenchyma involvement and are hence likely to manifest similar lung profiles. Similarly, patients with pulmonary edema secondary to congestive cardiac failure or non-cardiogenic causes were also analysed together. In addition, patients with uncertain diagnoses, diagnoses that were not cardiac or respiratory in nature, and non-major causes of dyspnea in the setting of the ED were not included in this analysis (Table 3).
Table 3. Adjudicated diagnoses.
| Adjudicated diagnoses | Value (N=231) |
|---|---|
| Analysis group, n (%) | |
| Congestive cardiac failure | 76 (32.9) |
| Pneumonia | 49 (21.2) |
| Asthma | 43 (18.6) |
| Chronic obstructive pulmonary disease | 19 (8.2) |
| Fluid overload secondary to renal failure | 8 (3.5) |
| Pneumothorax | 4 (1.7) |
| Pulmonary embolism | 0 (0) |
| Non-analysis group, n (%)a | |
| Others or uncertain diagnosis | 32 (13.9) |
a, other diagnoses include anemia, gastritis or gastroesophageal reflux disease, upper respiratory tract infection, thyroid goiter, panic attack, lung cancer, pleural effusion secondary to malignancy, interstitial lung disease, and bronchiectasis.
Lung ultrasonography
Overall, lung ultrasonography yielded the correct diagnoses in 68.3% (136/199) of patients included in the final analysis. The diagnostic characteristics of lung and cardiac ultrasonography for pulmonary edema, pneumonia, COPD and asthma are described in Tables 4,5.
Table 4. Number of patients with the respective lung ultrasound profiles.
| Adjudicated diagnoses | Lung ultrasound profiles | ||||||
|---|---|---|---|---|---|---|---|
| A | A’ | B | B’ | C | A/B | Total | |
| Pulmonary edema | 9 | 0 | 60 | 0 | 15 | 0 | 84 |
| Asthma or COPD | 40 | 0 | 10 | 0 | 11 | 1 | 62 |
| Pneumonia | 5 | 0 | 12 | 0 | 29 | 3 | 49 |
| Pneumothorax | 0 | 4 | 0 | 0 | 0 | 0 | 4 |
COPD, chronic obstructive pulmonary disease.
Table 5. Diagnostic performance for pulmonary edema, pneumonia, and COPD/asthma.
| Test characteristics | Pneumonia (n=49) | Pulmonary edema (n=84) | COPD/asthma (n=62) |
|---|---|---|---|
| Accuracy, % | 77.9 (71.5–83.5) | 76.9 (70.4–82.6) | 81.9 (75.6–87.0) |
| Sensitivity, % | 65.3 (50.4–78.3) | 71.4 (60.5–80.8) | 64.5 (51.3–76.3) |
| Specificity, % | 82.0 (74.9–87.8) | 80.9 (72.5–87.6) | 89.8 (83.4–94.3) |
| Positive predictive value, % | 54.2 (40.8–67.3) | 73.2 (62.2–82.4) | 74.1 (60.3–85.0) |
| Negative predictive value, % | 87.9 (81.3–92.8) | 79.5 (71.0–86.4) | 84.8 (77.9–90.2) |
| Likelihood ratio of positive test | 3.63 (2.44–5.40) | 3.73 (2.50–5.57) | 6.31 (3.72–10.72) |
| Likelihood ratio of negative test | 0.42 (0.29–0.63) | 0.35 (0.25–0.50) | 0.40 (0.28–0.56) |
Values in brackets represent the respective 95% confidence intervals. COPD, chronic obstructive pulmonary disease.
Pulmonary edema
There were 84 patients with fluid overload secondary to congestive cardiac failure or non-cardiogenic causes; 71.4% (60/84) manifested the B-profile (Table 4), indicative of pulmonary edema on lung ultrasonography.
Pneumonia
Of the 49 patients with adjudicated diagnoses of pneumonia, 29 (59.2%) had C profiles and 3 (6.1%) had A/B profiles, which corresponded with ultrasonographic characteristics suggestive of pneumonia.
COPD and asthma
Sixty-two patients were adjudicated with diagnoses of asthma or COPD; of which 40 (64.5%) patients had A-profiles.
Pneumothorax
All four patients with pneumothorax had A lines with absence of lung sliding, indicative of A’ profile.
Pulmonary embolism
There were no patients with pulmonary embolism and consequently, no lower limb ultrasonography was performed for deep vein compressibility.
Cardiac ultrasonography
In total, 41 (53.9%) out of 76 patients with the adjudicated diagnosis of congestive cardiac failure had combined B-profile and poor cardiac contractility on ultrasonography. Sixteen (21.1%) patients with B-profile had normal cardiac contractility. Among the eight patients with fluid overload secondary to renal failure, 2 (25.0%) had poor cardiac contractility with B-profile. In the presence of B-profile on lung ultrasound among patients with fluid overload state (n=60), the addition of bedside echocardiogram was able to differentiate cardiogenic pulmonary edema from non-cardiogenic causes in 42 (70.0%) patients.
Discussion
The primary aim of this study was to evaluate the diagnostic accuracy of lung and cardiac ultrasonography in patients with undifferentiated dyspnea presenting to the ED. Distinguishing between common causes of dyspnea reliably and rapidly remains one of the biggest challenges for an emergency physician as each of these diagnoses requires an entirely different management plan. Given the moderate sensitivity and specificity of clinical examination, radiological and laboratory investigations, POC ultrasonography has become an increasingly useful adjunct in evaluating such patients (2,4). Among patients with undifferentiated dyspnea in the ICU and ED, studies have demonstrated diagnostic accuracy of more than 90% (5,13). Overall, in this study, POC lung and cardiac ultrasonography yielded the correct diagnoses in 68.3% (136/199) of patients with pneumonia (29 C- and 3 A/B-profiles), pulmonary edema (60 B-profiles), COPD/asthma (40 A-profiles) and pneumothorax (4 A’-profiles).
Compared to existing literature, our study demonstrated lower sensitivities but similar specificities for various conditions for POC ultrasonography. Most studies showed specificity ranging from 80% to more than 90% with sensitivity in the similar range (8,14,15). Our results, however, had lower sensitivities compared to previous studies. This discrepancy may be expected since ultrasonography is operator dependent, despite ensuring adequate ultrasonographical training. Moreover, patient factors such as body habitus and inability to cooperate with evaluation may also be prevailing reasons for suboptimal ultrasonographic images. In addition, the cohort of ED patients is generally more heterogenous than most ICU patients, presenting with a myriad of medical conditions. In the setting of the ED, a diagnostic test with higher specificity would be of greater clinical relevance as accurate ruling-in of a diagnosis can facilitate timely and appropriate treatment.
The appearance of the B-profile on lung ultrasound, which indicates the presence of interstitial fluid, increases the post-test probability of pulmonary edema in an undifferentiated patient. However, its modest NLR of 0.35 makes it less useful in the absence of ultrasound findings. As demonstrated, 10.7% of the patients diagnosed with pulmonary edema presented with the A-profile, the lung profile seen in normal individuals and those with COPD and asthma. Although the ultrasound protocol was performed as early as possible upon patients’ arrival, it is plausible that any treatment in the out-of-hospital settings or initial stages of arrival in ED could have altered the clinical condition and thereby affected the sensitivity of the ultrasonographic findings. This highlights the importance of emergent ultrasound prior to treatment to accurately identify pulmonary edema and pneumonia in a breathless patient. In such patients, the lung ultrasound may also serve as a useful real-time tool to monitor response to nitrates and diuretic therapy, taking advantage of the dynamic nature of the B-profile (16). Additionally, the presence of B lines may also indicate low wedge pressures from conditions other than pulmonary edema (17). Hence, ultrasonography should not be used as a standalone diagnostic tool. In order to further increase the diagnostic accuracy of lung ultrasound in diagnosing heart failure, it should be used in conjunction with other biomarkers such as N-terminal pro brain natriuretic peptide as well as clinical signs and symptoms (18).
Although the accuracy of POC ultrasonography in diagnosis of pulmonary edema among undifferentiated dyspneic patients was 76.9%, the addition of cardiac ultrasound to improve accuracy in differentiating renal and cardiac causes of pulmonary edema in patients with a B profile was lower at 70%. In our study cohort, 5 out of 8 patients with renal failure had concomitant depressed cardiac contractility (2 B-profile, 3 C-profile). This may be due to co-existing cardiac dysfunction in patients with impaired renal function, with resulting poor cardiac contractility (19). Similarly, the presence of abnormal contractility on cardiac ultrasonography was not useful in diagnosing congestive cardiac failure. In total, 32.9% of patients (25/76, not shown in tables) with cardiac failure in this study had normal cardiac contractility. It is well established that approximately 50% of heart failure patients with pulmonary edema has normal ejection fraction and this is likely due to diastolic dysfunction (20). In all, these findings suggest that evaluating ejection fraction alone may not be sufficient to diagnose congestive cardiac failure and assessing diastolic heart function might be necessary for a more comprehensive and thorough ultrasonographic examination. However, evaluation of diastolic dysfunction is not usually a routine assessment for POC ultrasonography in the ED as it requires specific measurements such as E-point septal separation and annulus measurements, all of which require adequate imaging windows not easily obtainable in the acutely ill individual (21). Further studies would be needed to examine if diastolic function assessment using POC ultrasonography in the emergency setting could be readily achievable and would improve diagnostic accuracy for congestive cardiac failure.
Compared to pulmonary edema, the likelihood ratio of pneumonia in the presence of positive ultrasonographic findings is similar at 3.63 (95% CI: 2.44–5.40). The criterion of diagnosis for pneumonia was the presence of C-profile in the form of air bronchograms, consolidation (i.e., shred sign) and/or sub-pleural discontinuation (5). In this study, 17.9% (15/84) of patients with fluid overload presented with predominant ultrasound findings suggestive of pneumonia. It may be common for patients to present with ultrasonographic findings of both pneumonia and pulmonary edema as cardiac events have been shown to be associated with pneumonia due to hypoxia and physical stress (22). Additionally, the NLR of pneumonia was modest at 0.42 (95% CI: 0.29–0.63), suggesting that the absence of characteristic ultrasound findings does not adequately exclude the diagnosis of pneumonia. Indeed, patients with pneumonia may present with a normal lung profile as lung consolidation may only be evident on ultrasound if it occurs closer to the pleural surface (23). This highlights the importance of correlating ultrasonographic findings with the clinical history, physical signs and biochemical markers when evaluating an undifferentiated dyspneic patient.
In general, implementing lung ultrasound in the crowded and busy ED setting is feasible as it requires less than 5 minutes to complete the protocol and can usually be carried out by trained personnel (7). It can also be performed while other procedures and treatment are being rendered simultaneously. Moreover, it is painless, non-invasive and can be repeated to assess response to treatment, such as improvement in B-profile after diuresis. However, ultrasonographic evaluation of the posterior lung zones can be more challenging in the acutely ill and breathless patients who may be too weak physically to tolerate physical manoeuvres. Given the low sensitivities in our cohort, the diagnostic value of POC ultrasonography would be more useful in ruling-in than in ruling-out a differential diagnosis in evaluating patients with undifferentiated dyspnea.
Strengths of our study included the blinding of attending physicians and outcome assessors to the ultrasonographic findings to reduce bias. Ultrasound images were also recorded and validated for quality and accuracy in interpretation by experienced emergency physicians who perform bedside sonography daily. The pragmatic nature of our study design translates to a more modest estimate of the accuracy of ultrasound findings in dyspneic patients, unlike previous studies where patients were recruited by one or a few expert sonographers (5,13,15). However, we surmise that the diagnostic accuracy would likely be much higher if ultrasound is used as an adjunct that incorporates bedside clinical and radiological findings, in the hands of more experienced clinician-sonologists.
Limitations
There are several methodological limitations in this study. First, the sample size was below the proposed target of 250 patients after adjudication. A total of 257 patients were initially recruited but 22 were excluded as their primary complaint did not fulfil the inclusion criteria. Therefore, the results may not accurately reflect the potential of the lung and cardiac ultrasound in assessing patients with undifferentiated dyspnea in the ED.
Second, to simplify the study, only patients diagnosed with pneumonia, pneumothorax, pulmonary edema, COPD and asthma were analyzed. This study also focused primarily on the predominant ultrasonographic profile that the patient presented with. Although this may limit the generalizability of the results to the rest of the ED population, such as those with bronchiectasis, interstitial lung disease, lung cancer or multiple diagnoses, patients with the aforementioned diagnoses form the majority of cases seen in the ED (20). In this study, patients with those diagnoses formed 84.3% of the study population.
Third, the lack of a true reference diagnostic criterion for ED patients presenting with undifferentiated dyspnea remains a challenge. The study team adopted the use of two independent emergency medicine specialists in the adjudication of the final diagnosis. Despite a degree of subjectivity, the high kappa value between the two reviewers indicated that this is likely to be minimal.
Fourth, previous lung parenchyma scarring of the patient, if any, was not taken into account in our analysis. Ultrasound does not distinguish current from previous changes to the lung; hence the diagnostic accuracy of these ultrasound profiles may be affected if these were factored in. There is a need for future research to focus on these areas.
Finally, although all attempts were made to try to recruit the patients emergently, ultrasound results may have been altered by the commencement of initial treatment. This was relatively mitigated by recruitment of patients within 4 hours from ED admission.
Conclusions
In conclusion, POC ultrasonography can add value to the clinical evaluation of the undifferentiated dyspneic patient presenting to the ED. Lung ultrasonography provided moderate diagnostic accuracy while the addition of cardiac evaluation of contractility alone did not adequately distinguish the causes of pulmonary edema. Nonetheless, when complemented with other tools of investigation, this allows for earlier treatment and more accurate disposition of the patient.
Acknowledgements
We thank our colleagues at the Emergency Medicine Department, National University Hospital, Singapore, for their contributions to image acquisition and Dr. Toh Hong Chuen of Khoo Teck Puat Hospital, Singapore, for reviewing the ultrasonographic images.
Ethical Statement: The study protocol was approved by the National Healthcare Group’s Domain Specific Review Board (2014/00332). Written informed consent from patients or their legally acceptable representatives was obtained prior to recruitment.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Manning HL, Schwartzstein RM. Pathophysiology of dyspnea. N Engl J Med 1995;333:1547-53. 10.1056/NEJM199512073332307 [DOI] [PubMed] [Google Scholar]
- 2.Cardinale L, Volpicelli G, Lamorte A, et al. Revisiting signs, strengths and weaknesses of standard chest radiography in patients of acute dyspnea in the emergency department. J Thorac Dis 2012;4:398-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burri E, Potocki M, Drexler B, et al. Value of arterial blood gas analysis in patients with acute dyspnea: An observational study. Crit Care 2011;15:R145. 10.1186/cc10268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts E, Ludman AJ, Dworzynski K, et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ 2015;350:h910. 10.1136/bmj.h910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure the BLUE protocol. Chest 2008;134:117-25. 10.1378/chest.07-2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sforza A, Mancusi C, Carlino MV, et al. Diagnostic performance of multi-organ ultrasound with pocket-sized device in the management of acute dyspnea. Cardiovasc Ultrasound 2017;15:16. 10.1186/s12947-017-0105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Deeb M, Barbic S, Featherstone R, et al. Point-of-care ultrasonography for the diagnosis of acute cardiogenic pulmonary edema in patients presenting with acute dyspnea: A systematic review and meta-analysis. Acad Emerg Med 2014;21:843-52. 10.1111/acem.12435 [DOI] [PubMed] [Google Scholar]
- 8.Chavez MA, Shams N, Ellington LE. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res 2014;15:50. 10.1186/1465-9921-15-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alrajab S, Youssef AM, Akkus NI, et al. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: Review of the literature and meta-analysis. Crit Care 2013;17:R208. 10.1186/cc13016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Squizzato A, Rancan E, Dentali F, et al. Diagnostic accuracy of lung ultrasound for pulmonary embolism: A systematic review and meta-analysis. J Thromb Haemost 2013;11:1269-78. 10.1111/jth.12232 [DOI] [PubMed] [Google Scholar]
- 11.Vignon P, Chastagner C, Berkane V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med 2005;33:1757-63. 10.1097/01.CCM.0000171532.02639.08 [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care 2014;4:1. 10.1186/2110-5820-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanobetti M, Poggioni C, Pini R. Can chest ultrasonography replace standard chest radiography for evaluation of acute dyspnea in the ED? Chest 2011;139:1140-7. 10.1378/chest.10-0435 [DOI] [PubMed] [Google Scholar]
- 14.Zanobetti M, Scorpiniti M, Gigli C, et al. Point-of-Care Ultrasonography for Evaluation of Acute Dyspnea in the ED. Chest 2017;151:1295-301. 10.1016/j.chest.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 15.Russell FM, Ehrman RR, Cosby K, et al. Diagnosing acute heart failure in patients with undifferentiated dyspnea: A lung and cardiac ultrasound (LuCUS) protocol. Acad Emerg Med 2015;22:182-91. 10.1111/acem.12570 [DOI] [PubMed] [Google Scholar]
- 16.Ang SH, Andrus P. Lung ultrasound in the management of acute decompensated heart failure. Curr Cardiol Rev 2012;8:123-36. 10.2174/157340312801784907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrich CF, Mathis G, Blaivas M, et al. Lung B-line artefacts and their use. J Thorac Dis 2016;8:1356-65. 10.21037/jtd.2016.04.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): Sonographic B-lines and N-terminal Pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med 2009;16:201-10. 10.1111/j.1553-2712.2008.00347.x [DOI] [PubMed] [Google Scholar]
- 19.Fried LF, Shlipak MG, Crump C, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 2003;41:1364-72. 10.1016/S0735-1097(03)00163-3 [DOI] [PubMed] [Google Scholar]
- 20.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 2009;53:905-18. 10.1016/j.jacc.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 21.Holst JM, Kilker BA, Wright S, et al. Heart failure with preserved ejection fraction: echocardiographic VALVE protocol for emergency physicians. Eur J Emerg Med 2014;21:394-402. 10.1097/MEJ.0000000000000093 [DOI] [PubMed] [Google Scholar]
- 22.Musher DM, Rueda AM, Kaka AS, et al. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis 2007;45:158-65. 10.1086/518849 [DOI] [PubMed] [Google Scholar]
- 23.Parlamento S, Copetti R, Di Bartolomeo S. Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am J Emerg Med 2009;27:379-84. 10.1016/j.ajem.2008.03.009 [DOI] [PubMed] [Google Scholar]