ABSTRACT
Hypercalcaemia is a common disorder normally caused by primary hyperparathyroidism (PHPT) or malignancy. A proportion of cases present as an emergency, which carries a significant mortality. Emergency management of hypercalcaemia is based on intravenous rehydration with normal saline but when this is inadequate, bisphosphonate therapy is used; more recently the novel anti-resorbtive agent denosumab has been shown to have a useful role in treatment. It is estimated that up to 10% of all cases of PHPT presenting under the age of 45 years have an underlying genetic predisposition; nine potentially causative genes are now recognised and may be screened in routine clinical practice. Although parathyroidectomy is the only curative treatment for PHPT, this is indicated in a minority of cases. Many cases can be adequately managed conservatively and guidance from the 4th international workshop on the management of asymptomatic PHPT has recently been updated in a consensus statement.
Key points
Nine potentially causative genes are now implicated in primary hyperparathyroidism (PHPT)
Guidance on management of asymptomatic PHPT was recently updated in the 4th international consensus guidelines
Cinacalcet is licensed for PHPT and its role is recognised in the 4th international consensus guidelines
Denosumab is recognised to have a role in the emergency management of hypercalcaemia
Pre-operative parathyroid imaging is increasingly being performed by SPECT CT
Introduction
Hypercalcaemia is a common disorder, accounting for approximately 0.6% of all acute medical admissions.1 Its prevalence in the general population is up to 1/1,000. It is most commonly due to primary hyperparathyroidism or malignancy although there are numerous other causes.2 The classical symptomatic presentation of hypercalcaemia is seen relatively rarely in the developed world, the commonest presentation being asymptomatic detection on biochemical testing. Management of hypercalcaemia depends on the presentation and underlying cause; while severe hypercalcaemia (>3.5 mmol/L) requires emergency management,3 a significant proportion of asymptomatic mild hypercalcaemia is due to primary hyperparathyroidism (PHPT) and is amenable to conservative management.
Presentation
The commonest presentation is detection of a raised serum calcium concentration on a biochemical screen. If symptoms are present, classically they are ‘moans, bones, stones and groans’ referring to depressed mood, musculoskeletal pain, renal colic and abdominal pain related to constipation or peptic ulceration. Symptomatic patients may also experience polyuria and polydipsia secondary to nephrogenic diabetes insipidus and intellectual obtundation. As the severity of hypercalcaemia progresses, nausea, vomiting, QT shortening potentially leading to ventricular fibrillation arrest, confusion and coma may occur. Physical signs of hypercalcaemia are relatively few but include band keratopathy, although this is very rare, and signs related to the aetiology of the hypercalcaemia. Classical radiographic features of PHPT, although rarely seen, include resorption of the distal ends of the clavicles, sub periosteal erosions on the radial borders of the middle or terminal phalanges, brown tumours and ‘pepperpot skull’.
Differential diagnosis
The two commonest causes of hypercalcaemia are PHPT and malignancy. Other causes include tertiary hyperparathyroidism, granulomatous disorders (principally sarcoidosis), thyrotoxicosis, drugs (principally thiazides but poorly monitored therapy with alfacalcidol and calcitriol are important causes of hypercalcaemia, and lithium therapy may also be associated with hypercalcaemia due to Li-induced PHPT), excessive ingestion of calcium carbonate (‘milk alkali syndrome’), vitamin D toxicity, vitamin A intoxication, immobilisation, familial hypocalciuric hypercalcaemia (FHH) and untreated Addison's disease.
Tertiary hyperparathyroidism occurs in the context of long-standing secondary hyperparathyroidism where some degree of autonomy of parathyroid function arises and thus the calcium level rises inappropriately. FHH is a rare inherited disorder of calcium metabolism in which the set point for serum calcium concentration is elevated, usually due to an inactivating mutation of the calcium sensing receptor gene,4 leading to lifelong and usually asymptomatic elevation of serum calcium with normal or slightly elevated PTH levels and reduced urinary calcium excretion.
Primary hyperparathyroidism
Approximately 85% of all PHPT arises because of a solitary parathyroid adenoma, 1% are due to parathyroid carcinoma and the remainder are due to multiple adenomata or multi-gland hyperplasia.5 Although the majority of PHPT is sporadic, a proportion arises on the background of a familial predisposition and abnormalities in a number of potential genes are now recognised (Table 1).6 Thus, during clinical assessment of PHPT, a family history should always be sought and the physician should remain vigilant for other endocrinopathies as may be seen in, for example, multiple endocrine neoplasia (MEN)1. Occurrence of PHPT at a young age (approximately <45 years) and/or presence of multi-gland disease should also prompt consideration of this possibility.
Table 1.
Genes potentially implicated in primary hyperparathyroidism
| Disorder | Gene |
|---|---|
| MEN1 | MEN1 |
| MEN2 | RET |
| MEN4 | CDKN1B |
| HPT-JT | CDC73 |
| FIHPT | MEN1, CDC73, CASR, CDKN1A, CDKN2B, CDKN2C |
| NSPHPT | CASR |
| nsPHPT | PTH |
MEN1/2/4 = multiple endocrine neoplasia type 1/2/4; HPT-JT = hyperparathyroidism jaw tumour syndrome; FIHPT = familial isolated hyperparathyroidism; NSPHPT = neonatal severe primary hyperparathyroidism; nsPHPT = non syndromic primary hyperparathyroidism.
Malignancy
Up to 20% of all cases of carcinoma will be affected by hypercalcaemia at some point during their clinical course.2 Several mechanisms account for hypercalcaemia due to malignancy:
Humoral hypercalcaemia of malignancy arises because of secretion of PTHrP.
Osteolytic metastases.
A limited range of neoplasms express 1 alpha hydroxylase and are able to activate vitamin D to the active 1,25 dihydroxylated form and drive hypercalcaemia. Lymphomas are the commonest tumour in this category.
True ectopic production of PTH is very rare. Of course, a diagnosis of carcinoma does not rule out the simultaneous presence of PHPT, which is common.
Investigations
It is assumed throughout that calcium levels are adjusted for the albumin concentration according to the widely used formula:
Adjusted calcium = Total calcium – 0.02 (40–Albumin)
where calcium is measured in mmol/L and albumin in g/L. There is, however, some debate as to whether this formula is universally appropriate or whether individual labs should derive local adjustment formulae.7
Broadly speaking, if PTH is detectable in the presence of hypercalcaemia then the cause of the hypercalcaemia is usually primary (sometimes tertiary) hyperparathyroidism. Conversely, if the PTH level is suppressed, then the cause is likely malignancy, although consideration of other PTH-independent pathologies should be undertaken. However, two caveats to this approach should be considered. Firstly, while most laboratories will quote a reference range for PTH in the region of 1.6–6.9 pmol/L, the lower limit of this reference range is very close to the lower limit of detection of the assay and therefore results at the lower end of the range require careful interpretation, specifically asking if the result is appropriate for the clinical presentation. When in doubt, repeating the assay, discussing with the local laboratory and proceeding with other investigations to rule out other possible pathology are all reasonable approaches. Secondly, FHH and PHPT may have indistinguishable serum biochemistry and conventionally, the only way to separate these two conditions is by urinary biochemistry – specifically, the calcium to creatinine clearance ratio (FHH<0.01 versus PHPT>0.01). This is calculated from simultaneous measurements of urine and plasma calcium and creatinine concentrations using the following formula:
CaCl/CrCl = (urine calcium × plasma creatinine)/(plasma calcium × urine creatinine)
The plasma creatinine concentration needs to be converted to the same units as the other parameters (mmol/L).
The 25 OH vitamin D level should be measured when investigating hypercalcaemia. This allows the detection of vitamin D toxicity and, more importantly, vitamin D deficiency, which is common in PHPT and should be corrected as part of the management.
Other investigations should be performed depending on the results of the initial investigations, as follows:
Parathyroid localisation should be carried out if surgical management is appropriate, but it should be noted that the purpose of such imaging is not to confirm the diagnosis of PHPT but rather to localise the target gland pre-operatively. Conventional practice has been to perform ultrasound and Sesta-MIBI nuclear imaging, although the literature gives wide ranges for sensitivity for these, with typical values often in the region of 50–70%,8 and concordance between these modalities is suboptimal. When scans are concordant, parathyroidectomy will often be performed as a day case procedure, as minimal neck dissection is required. Where there is non-concordance or complete failure of localisation, more extensive neck dissection will be required, with removal of any enlarged or abnormal-appearing glands. However, there is increasingly a move towards Single photon emission computerised tomography (SPECT CT) scanning, which combines both functional and cross sectional imaging into a single investigation (Fig 1). This is a more efficient approach and provides sensitivity of up to 88%.9 It should be noted that in genetic forms of PHPT pre-operative localisation is not usually performed prior to a first neck exploration.
If PHPT (and FHH) have been excluded then further tests will be required to elucidate whether the cause of hypercalcaemia is associated with malignancy. In this scenario, PTH levels will be suppressed/undetectable and further tests should include erythrocyte sedimentation rate (ESR), immunoglobulins, protein electrophoresis, urinary Bence Jones proteins and/or serum free light chains, as well as CXR, liver function tests (LFTs), bone scans, abdominal ultrasound etc as indicated by presenting symptoms and signs. Although many cases of malignancy-associated hypercalcaemia are due to tumoural PTHrP secretion, it is rarely necessary to assay PTHrP levels and indeed this test is not widely available. Conversely, if the clinical presentation raises the possibility of sarcoidosis then chest X-ray, serum angiotensin-converting enzyme, 1,25 (OH)2 vitamin D levels and biopsy of appropriate tissue will usually be indicated. If other causes are suspected then presenting clinical features should alert the physician to these possibilities and appropriate further investigations should be arranged.
Increasingly, genetic testing is becoming a routine part of the investigation of a case of hypercalcaemia, specifically where primary hyperparathyroidism is suspected to be part of an inherited endocrinopathy or where the diagnosis is suspected to be FHH. It is estimated that up to 10% of cases of PHPT presenting below the age of 45 arise on the background of a germline mutation and there are now nine genes recognised to predispose to PHPT (Table 1).
Fig 1.
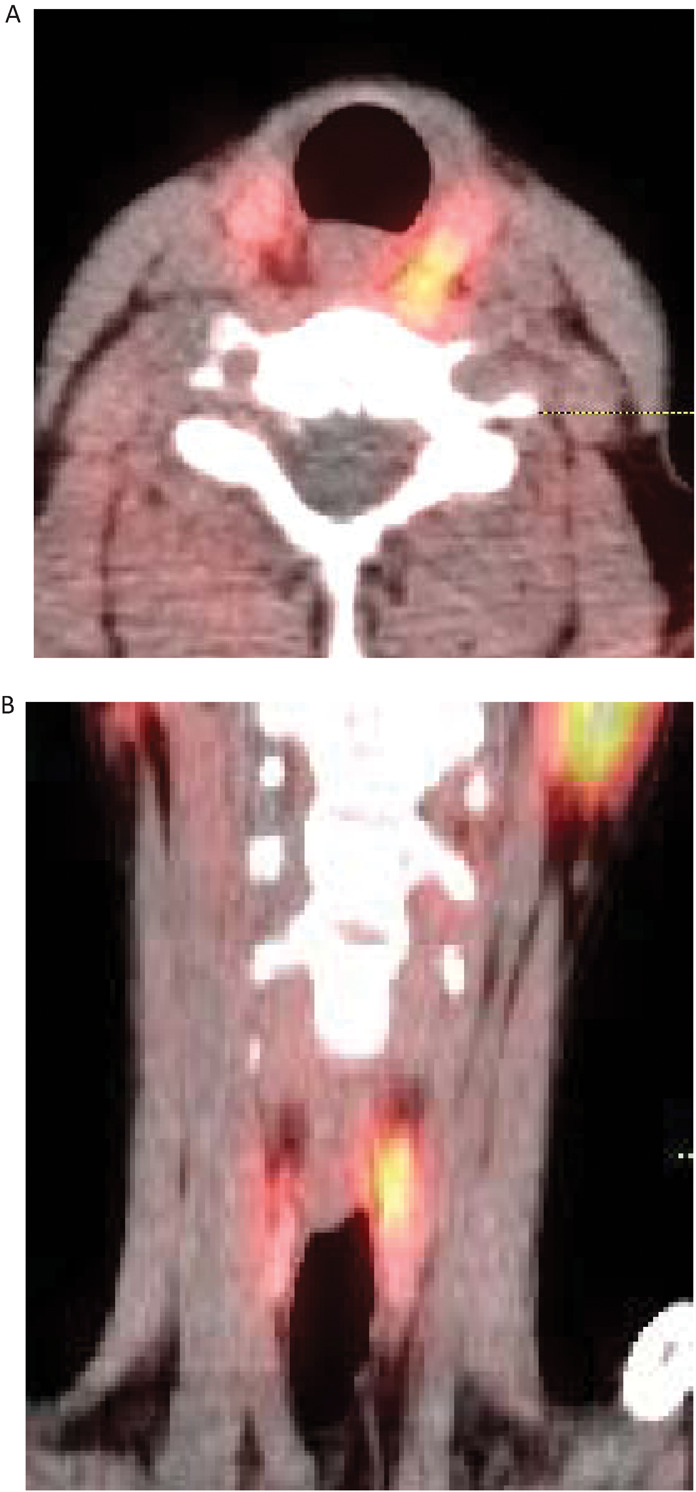
SPECT CT images of parathyroid adenoma. Fused (A) axial and (B) coronal SPECT CT images showing a superior left parathyroid adenoma adjacent to the oesophagus posterior to the left lobe of thyroid. Physiological uptake in the parotid glands is also seen. SPECT = single photon emission computerised tomography
Consideration of genetic testing is appropriate where a family history of hypercalcaemia is apparent, where other features of one of the above syndromes are present or where the age of presentation is unusually low thereby raising the prior probability of an abnormality in one of the causal genes. While the calcium sensing receptor gene (CASR) is recognised as one of the causal genes for FIHPT, it is also the causal gene for FHH1, wherein inactivating mutations lead to an elevated set point for adjusted serum calcium levels. Two other FHH variants and their causal genes are also now recognised: FHH2 and FHH3, respectively arising because of mutations in the GNA11 and AP2S1 genes.10
Management
Emergency management
Intravenous hydration with normal saline to restore calciuresis should be the mainstay of management. In cases where this is inadequate even after full and thorough rehydration (which can take 24 hours or more), where there is no prospect of identification and definitive treatment of the underlying cause or where attempts at definitive treatment would not be appropriate, then the next step is usually intravenous bisphosphonate therapy (pamidronate or zoledronate).3 Traditionally, other options for the emergency management of hypercalcaemia have included calcitonin, dialysis and mithramycin. However, more recently the anti-resorbtive agent denosumab (licensed for treatment of osteoporosis) is recognised as a useful adjunct in the emergency management of hypercalcaemia.11,12
PHPT
The only curative treatment of hypercalcaemia due to hyperparathyroidism is parathyroidectomy, and where patients are unequivocally symptomatic or markedly hypercalcaemic there is usually little debate about proceeding to surgery. However, in cases of asymptomatic PHPT, where the hypercalcaemia is mild or where the patient may not have long life expectancy or is unfit for surgery, other options including conservative and medical management should be considered.
There is now good guidance on when to operate and when to consider conservative management in the consensus statement from the fourth international workshop on management of asymptomatic primary hyperparathyroidism.13 These are summarised in Table 2. There is also now an evidence base on conservative management of primary hyperparathyroidism14. In brief, this shows that the calcimimetic cinacalcet works in mild to moderate hypercalcaemia to normalise serum calcium in approximately 70% of cases. It should be noted that it has no beneficial effect on bone mineral density, although it does reduce urinary calcium excretion. The oral bisphosphonates have good evidence for protecting bone mineral density but no evidence for fracture risk reduction in PHPT and no consistent evidence for reduction of hypercalcaemia in PHPT.
Table 2.
Recommended thresholds for consideration of parathryoidectomy in management of asymptomatic PHPT
| Parameter | Threshold for recommending surgery |
|---|---|
| Serum adjusted calcium | 0.25 mmol/L > upper limit of reference range |
| Bone mineral density | T score < –2.5 at lumbar spine, total hip, femoral neck or distal 1/3 radius |
| Vertebral morphometry | Presence of vertebral fracture detected by X-ray, CT, MRI or VFA |
| Creatinine clearance | eGFR <60 mL/min/1.73m 2 |
| 24-hour urine calcium excretion | >10 mmol/day by 24 hour urine analysis and increased stone risk by biochemical stone risk analysis |
| Renal imaging | Presence of nephrolithiasis or nephrocalcinosis on X-ray, USS or CT |
| Age | <50 years |
Thresholds given are according to consensus statement of fourth international workshop on management of asymptomatic primary hyperparathyroidism.13 Patients need only meet one of the above criteria for parathyroidectomy to be indicated. CT = computerised tomography; eGFR = estimated glomerular filtration rate; MRI = magnetic resonance imaging; USS = ultrasound scan; VFA = vertebral fracture assessment.
Similarly, conservative management has also been shown to be a relatively safe option so long as some degree of medical vigilance is maintained, although data now show that durations of conservative management in excess of 15 years pose some risk with up to one third of cases progressing to display overt features of the disease.15 Thus, monitoring for conservative management should include serum calcium measurement annually, periodic DEXA scans, imaging if there are symptoms (eg X-ray if height loss/back pain is experienced), eGFR annually and, if stones are suspected, renal imaging. With respect to DEXA scanning, the distal forearm may be considered to be the most informative site for serial T-scores to be measured as the predominantly cortical bone at this site is most at risk of loss in PHPT.
One other consideration in the management of hypercalcaemia due to PHPT is vitamin D repletion: as there is a very small risk of exacerbating hypercalcaemia and hypercalciura and relatively little evidence of benefit, on balance expert opinion is therefore to do so cautiously.
Finally, although there are associations between PHPT and cardiovascular risk and between PHPT and low quality of life, impaired cognition and other subtle neuro-cognitive symptoms, the presence of these features should not be used as indication for surgery until more definitive evidence of benefit is available.
Conflicts of interest
The author has no conflicts of interest to declare.
References
- 1.Dent DM. Miller JL. Klaff L. Barron J. The incidence and causes of hypercalcaemia. Postgrad Med J. 1987;63:745–50. doi: 10.1136/pgmj.63.743.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goltzman D. Approach to Hypercalcemia. In: De Groot LJ, editor; Chrousos G, editor; Dungan K, et al., editors. Approach to Hypercalcemia. South Dartmouth (MA): MDText.com, Inc.; 2000. [Google Scholar]
- 3.Walsh J. Gittoes N. Selby P. Emergency management of acute hypercalcaemia in adult patients. Endocr Connect. 2016;5:G9–G11. doi: 10.1530/EC-16-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce SH. Trump D. Wooding C, et al. Calcium-sensing receptor mutations in familial benign hypercalcemia and neonatal hyperparathyroidism. J Clin Invest. 1995;96:2683–92. doi: 10.1172/JCI118335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcocci C. Cetani F. Primary hyperparathyroidism. N Engl J Med. 2011;365:2389–97. doi: 10.1056/NEJMcp1106636. [DOI] [PubMed] [Google Scholar]
- 6.Eastell R. Brandi ML. Costa AG, et al. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the Fourth International Workshop. J Clin Endocrinol Metab. 2014;99:3570–9. doi: 10.1210/jc.2014-1414. [DOI] [PubMed] [Google Scholar]
- 7.Hughes D. Doery JCG. Choy KW. Flatman R. Calculated chemistry parameters – do they need to be harmonised? Clin Biochem Rev. 2016;37:131–4. [PMC free article] [PubMed] [Google Scholar]
- 8.Purcell GP. Dirbas FM. Jeffrey RB, et al. Parathyroid localization with high-resolution ultrasound and technetium Tc 99m sestamibi. Arch Surg. 1999;134:824–8. doi: 10.1001/archsurg.134.8.824. [DOI] [PubMed] [Google Scholar]
- 9.Treglia G. Sadeghi R. Schalin-Jäntti C, et al. Detection rate of (99m) Tc-MIBI single photon emission computed tomography (SPECT)/CT in preoperative planning for patients with primary hyperparathyroidism: a meta-analysis. Head Neck. 2016;38(Suppl 1):E2159–72. doi: 10.1002/hed.24027. [DOI] [PubMed] [Google Scholar]
- 10.Nesbit MA. Hannan FM. Howles SA, et al. Mutations affecting G-protein subunit α11 in hypercalcemia and hypocalcemia. N Engl J Med. 2013;368:2476–86. doi: 10.1056/NEJMoa1300253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karuppiah D. Thanabalasingham G. Shine B, et al. Refractory hypercalcaemia secondary to parathyroid carcinoma: response to high-dose denosumab. Eur J Endocrinol. 2014;171:K1–5. doi: 10.1530/EJE-14-0166. [DOI] [PubMed] [Google Scholar]
- 12.Adhikaree J. Newby Y. Sundar S. Denosumab should be the treatment of choice for bisphosphonate refractory hypercalcaemia of malignancy. BMJ Case Reports. 2014 doi: 10.1136/bcr-2013-202861. bcr2013202861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilezikian JP. Brandi ML. Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the fourth international workshop. J Clin Endocr Metab. 2014;99:3561–9. doi: 10.1210/jc.2014-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marcocci C. Bollerslev J. Khan AA. Shoback DM. Medical management of primary hyperparathyroidism: Proceedings of the Fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism. J Clin Endocrinol Metab. 2014;99:3607–18. doi: 10.1210/jc.2014-1417. [DOI] [PubMed] [Google Scholar]
- 15.Singh Ospina N. Maraka S. Rodriguez-Gutierrez R, et al. Comparative efficacy of parathyroidectomy and active surveillance in patients with mild primary hyperparathyroidism: a systematic review and meta-analysis. Osteoporos Int. 2016;27:3395–407. doi: 10.1007/s00198-016-3715-3. [DOI] [PubMed] [Google Scholar]


