ABSTRACT
Monoclonal antibody therapeutics have been approved for over 30 targets and diseases, most commonly cancer. Antibodies have become the new backbone of the pharmaceutical industry, which previously relied on small molecules. Compared with small molecules, monoclonal antibodies (mAbs) have exquisite target selectivity and hence less toxicity as a result of binding other targets. The clinical value of both mAbs and ligand traps has been proven. New applications of mAbs are being tested and mAbs have now been designed to target two (bi-specific, eg TNF-α and IL-17) or more targets simultaneously, augmenting their therapeutic potential. Because of space limitations and the wide ranging scope of this review there are regrettably, but inevitably, omissions and missing citations. We have chosen to highlight the first successes in inflammatory diseases and cancer, but a broader overview of approved mAbs and related molecules can be found in Table 1.
KEYWORDS: Biologic therapy, cancer, inflammation, monoclonal antibodies, traps
Introduction
There has been a revolution in therapeutics over the past 20 years, due to the introduction of protein therapeutics – often termed ‘biologics’ – into routine, mainstream medicine. Biologics are expensive and like all medicines have risks. Their advantage is exquisite specificity due to greater surface area binding, resulting in decreased ‘off-target’ effects as compared with most small molecule drugs.
New biologic therapies come in several basic forms, either growth factors and cytokines (such as erythropoietin, G-CSF, interferon, enzymes, factors that regulate coagulation) or, more commonly, monoclonal antibodies (mAbs) and related proteins such as ‘traps’ in which cytokine receptors are made soluble and fused with antibody constant regions. The latter group (mAbs and traps) have dramatically advanced the therapy of chronic inflammatory diseases and cancer. In this review, we will focus on mAbs and relatives, describing these therapeutics. The tumour necrosis factor (TNF)-blockers for autoimmune/inflammatory diseases are the most broadly deployed (with multiple products) and have engendered a revolution in therapeutic research/development, along with rather remarkable revenues. This therapeutic revolution is based on the synergy of three scientific disciplines: immunology, molecular biology and protein engineering.
Brief history
The first mAbs were produced by Milstein and Köhler (Cambridge University),1 who won the Nobel Prize in Physiology or Medicine in 1984. mAbs were initially murine proteins and hence were immunogenic in humans and not suited for chronic therapy. This limitation was solved using molecular biology and protein engineering to create more human-like mAbs with little immunogenicity. There are several versions of mAbs and traps in clinical use and trials (Fig 1, Table 1). Initial attempts replaced most murine Fc sequences with human Fc, a process referred to as ‘chimerisation’.2 This involves grafting the murine antigen binding Fab regions onto a human immunoglobulin (IgG) backbone. In ‘humanisation’, the mouse hyper-variable peptide binding loops3 are grafted onto the human IgG backbone. More recent technologies allow for generation of fully human antibodies.4
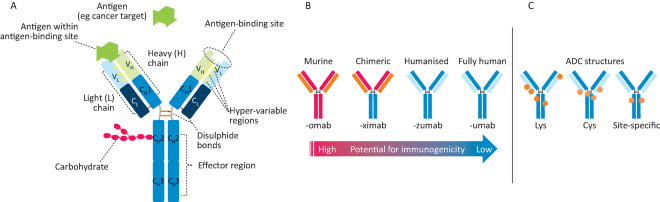
Fig 1.
Monoclonal antibodies. A – antibody structure showing light (L) and heavy (H) chains with variable (VL, VH) and constant (CL, CH) regions, which are connected by inter-chain disulphide bonds. Antigen binding (green symbol) occurs at VH/VL domains, while effector functions are mediated via the Fc (CH) portion. B – increasing the amount of human sequences in a murine antibody decreases immunogenic potential. C – on an antibody-drug conjugate (ADC, cytotoxic drugs (orange, red stars) can be linked stochastically to lysine (Lys) residues or on cysteine residues either through reduction of inter-chain disulphides (Cys) or by engineering in cysteines at select conjugation sites (site-specific).
Table 1.
Targets and approvals for monoclonal antibodies and derivatives (asterisks denote approvals in USA only)
| Target | Function | FDA-approved mAbs, Traps, Bi-specific mAbsB | Autoimmune | Malignancy | Cardiovascular | Other/comments |
|---|---|---|---|---|---|---|
| CD52 | Marker of T- and B-lymphocyte subsets | Alemtuzumab (Lemtrada) | Multiple sclerosis | Chronic lymphocytic leukemia | Trade name changed from Campath-1H (cancer) to Lemtrada (multiple sclerosis) Humanised IgG1k | |
| CD41 (glycoprotein llb/IIIa) | Platelet aggregation inhibitor | Abciximab (ReoPro) | Perisurgical anti-thrombosis | Mouse/human IgG1 | ||
| Fab fragment | ||||||
| CD25 | Alpha-chain of the IL-2 receptor | Basiliximab (Simulect) | Multiple sclerosis | Basiliximab: chimeric mouse/human IgG1k. | ||
| Daclizumab (Zenapax) | Transplant rejection | Daclizumab: humanised IgG1k. | ||||
| CD19/CD3 | Bi-specific T-cell directed killing of CD19 | Blinatumomab (Blincyto) | B-cell precursor acute lymphoblastic leukemia | |||
| CD20 | Participates in B-cell differentiation | Obinutuzumab (Gazyva)Ibritumomab tiuxetan (Zevalin) | Rheumatoid arthritis | B-cell malignancies | Obintuzumab is the first approved glycoengineered IgG1 mAb with enhanced ADCC. | |
| Tositumumab (Bexxar)* Ofatumumab (Arzerra) Rituximab (Rituxan) | Rituximab is chimeric mouse/human IgG1k Ibritumomab tiuxetan and tositumumab are radio-conjugates that can be used when tumours stop responding to the anti-CD20 mAbs. |
|||||
| Ibritumumab and tositumumab are mouse IgG2a. | ||||||
| CD30 | Member of the TNF receptor family | Brentuximab vedotin (Adcetris) | Hodgkin lymphoma, anaplastic large cell lymphoma | Second approved antibody-drug conjugate | ||
| CD38 | Cyclic ADP ribose synthetase leads to generation of adenosine in the tumour microenvironment (immunosuppression); co-stimulatory receptor; more? | Daratumumab (Darzalex) | Multiple myeloma | |||
| CD80/CD86 | Provide co-stimulatory signals necessary for T-cell activation and survival. Ligand trap prevents activation of CD28 immune checkpoint resulting in immune suppression | Nolojix Orencia (both CTLA4-Fc fusion proteins) | Rheumatoid arthritis Transplant rejection | |||
| CD279/PD-1 | Immune ‘checkpoint’ receptor that suppresses immune response after binding to PDL-1 or 2 | Nivolumab (Opdivo) Pembrolizumab (Keytruda) | Melanoma, non-small cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck squamous carcinoma | These mAbs designed not to be immune active (eg no ADCC): IgG4. This allows the mAbs to increase activity of T-cells without inducing apoptosis PDL-1 is a biomarker for pembrolizumab |
||
| CD274/PDL-1 | Tumour-expressed protein that activates the PD-1 immune suppression pathway | Atezolizumab (Tecentriq) Avelumab (Bavencio)* | Urothelial (bladder) carcinoma, non-small cell lung cancer merkel cell carcinoma | Atezolizumab: IgG1k mAb engineered to lack ADCC activity PDL-1 is biomarker (Avelumab: intact IgG1k)** Active IgG1 targets tumour cells. Approval likely | ||
| CTLA4/CD152 | When activated through binding CD80/86 this receptor turns down cytotoxic T-cells | Ipilimumb (Yervoy) | Metastatic melanoma, mesothelioma | This is the first of the immuno-oncology mAbs to be approved (IgG1k). Largely due to efforts of J Allison (ref PMID 16730267) | ||
| Glycolipid GD2 | Disialoganglioside preferentially expressed on tumours of neuroectodermal origin | Dinutuximab (Unituxin) | Neuroblastoma | Mouse/human chimeric IgG1k. Induces severe pain managed by morphine | ||
| HER2 | A growth factor-like receptor tyrosine kinase without a known ligand | Trastuzumab (Herceptin) Pertuzumab (Perjeta) Ado-trastuzumab emtansine (Kadcyla) | Breast, gastric adenocarcinoma | Two monoclonal antibodies (T,P) and one ADC (ado-trastuzumab) Overexpression of HER2 leads to spontaneous signaling Crosslinking with mAb leads to internalization First commercially successful ADC (Lewis et al, PMID 19010901)Biomarker driven therapy |
||
| EGFR (receptor for multiple growth factors) | Many tumours are addicted to the EGFR signalling pathway | Cetuximab (Erbitux) Panitumumab (Vectibix) | Squamous cell carcinoma of the head and neck, colorectal cancer, non-small cell lung cancer | Biomarker: usually requires EGFR IHC positivity; always requires test for normal ras oncogene Cetuximab is mouse/human chimeric IgG1; Vectibix is human IgG2 from recombinant mice |
||
| TNF-alpha | Inflammatory cytokine that drives multiple autoimmune diseases* | Adalimumab (Humira) Certolizumab pegol (Cimzia) Golimumab (Simponi) Infliximab (Remicade) Etanercept (Enbrel) There are more than 20 anti-TNF biosimilars in various stages of development. Already approved are infliximab biosimilars (Remsina, Inflectra, Flexiabi), etanercept biosimilars (Erelzi, Benepali), adalimumab biosimilars (Amjevita). This field will change very rapidly as many dossiers are now under regulatory scrutiny. Biosimilars are given a suffix, eg etanercept-szzs (Erlezi) | Crohn's disease, ulcerative colitis, RA, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, hidradentis suppurtiva, uveitis | Not all TNF blockers are approved for all indications Drugs in italics are biosimilars: adalimumab, infliximab, infliximab, inflectra, adalimumab-atto are IgG1k mAbs; certolizumab pegol is a pegylated Fab fragment; etanercept is a soluble Fc: TNF receptor ‘trap’ that binds TNF (there is one biosimilar) At least four biosimilar TNF-blockers have been approved in the EU (two each for infliximab and etanercept) |
||
| VEGF | Cytokine that stimulatesvasculogenesis and angiogenesis. Overproduced in some inflammatory disorders and tumours to induce increased blood supply. | Bevacizumab (Avastin) Ramucirumab (Cyramza)Aflibercept (Eylea/Zaltrap) Ranibizumab (Lucentis) | Age-related macular degeneration, macular oedema, diabetic macular oedema, diabetic retinopathy | Colorectal cancer, Non-squamous NSCLC, breast cancer, glioblastoma, renal cell carcinoma, gastric cancer or gastro-esophageal junction adenocarcinoma | Bevacizumab, ramucirumab are IgG1 mAbs for cancer therapy (ramucirumab was derived from phage display); ranibizumab is a Fab fragment (single arm binder). It has a short half-life if administered intravenously, but it is stable and effective when locally injected into the eye Aflibercept is a ligand trap with optical (Eylea) and cancer (Zaltrap) applications |
|
| IgE | Binds to mast cells, basophils, other cells that express Fc-epsilon receptor, and induces release of inflammatory cytokines | Omalizumab (Xolair) | Asthma | IgG1k mAb also used off-label to treat IgE-related conditions (allergic rhinitis, drug allergies, other) | ||
| Alpha-4 integrin | Alpha-4 integrin facilitates exit of inflammatory cells from blood into intestine or across the blood brain barrier | Vedolizumab (Entyvio) Natalizumab (Tysabri) |
Multiple sclerosis, Crohn's disease and ulcerative colitis | IgG4 natalizumab therapy has been associated with PML caused by John Cunningham virus in immunocompromised patients. IgG1k vedolizumab MAY not be associated with PML. | ||
| Complement C5 | Inhibits complement cascade | Eculizumab (Solaris) | Prevents destruction of red blood cells by activated complement (paroxysmal nocturnal hemoglobinuria) | IgG2/4 mAbMost expensive drug in the world ($409,500 annually) | ||
| Interleukin-1 | Mutations in cryopyrin lead to overproduction of IL-1 and inflammatory disease; IL-1 also drives other inflammatory diseases | Canakinumab (Ilaris)Rilonacept (Arcalyst)* | Rare inflammatory syndromes, active juvenile arthritis, gouty arthritis | Canakinumab is an IgG1k mAb; rilonacept is an IL-1 ‘trap’ designed from the IL-1R fused with human mAb Fc region. | ||
| P40 subunit of IL-12 and IL-23 | Prevents cytokine (inflammatory) activation of cellular receptors | Ustekinumab (Stelara) | Plaque and psoriatic arthritis, Crohn's disease | IgG1k human mAb from recombinant mice | ||
| Receptor activator of NF kappa-B ligand (RANKL) | Activates osteoclastic bone resorption | Denosumab (Xgeva) | Can be used to prevent bone mass loss in cancer patients | Human IgG2 mAb inhibits bone resorption and enhances bone mass (due to multiple causes) | ||
| Interleukin-6 | Overexpression of IL-6 is associated with multiple malignancies | Siltuximab (Sylvant) | Pseudo-malignancy: Castleman's disease (similar to lymphoma) | Murine/human chimeric IgG1κ | ||
| IL-6R (Interleukin 6 receptor) | Current approvals based upon role of IL-6 in promoting inflammatory autoimmune disease | Tocilizumab (Actemra) | Rheumatoid arthritis, polyarticular juvenile arthritis, juvenile idiopathic arthritis | Human/mouse chimeric mAb with initial approval for efficacy vs RA after failure of TNF blocker | ||
| BAFF (tumour necrosis factor superfamily member 13b) | Role in proliferation and differentiation of B-cells | Belimumab (Benlysta) | Systemic lupus erythematosus | IgG1-gamma/lambda | ||
| Bacillus anthraces (anthrax) toxin | Recognises the protective antigen portion of the toxin produced by Bacillus anthracis and prevents the toxin from entering cells and killing them | Obiltoxaximab (Anthim)* | Chimeric human/mouse chimeric mAb to anthrax toxin for prevention and treatment | |||
| PCSK9 | PCSK9 binds to LDLR, causing it to be degraded. With the result of higher blood levels of LDL mAb vs PCSK9 prevents degradation of LDLR and thereby increases LDL removal from blood. Therefore, blocking PCSK9 can lower blood cholesterol levels | Evolocumab (Repatha) Alirocumab (Praluent) |
Second line treatment for high cholesterol for adults whose cholesterol is not controlled by diet and statin treatment. Perhaps most important for hereditary high cholesterol | Evolocumab: IgG2 for low ADCC Alirocumab is IgG1k | ||
| SLAMF7/CD319 | SLAM7 triggers the activation and differentiation of a wide variety of immune cells (innate and adaptive immune response) perhaps primarily mediated by natural killer cells and myeloma cells | Elotuzumab (Empliciti) | Multiple myeloma | This IgG1k mAb is thought to activate SLAM7 receptor and to have a secondary mechanism of mediating ADCC vs multiple myeloma cells | ||
| Dabigatran | Anti-coagulant | Idarucizumab (Praxbind) | This Fab fragment sequesters dabigatran and clears it from blood, reversing anti-coagulation | |||
| IL-5 | Induces differentiation and survival of eosinophils | Reslizumab (Cinqair) Mepolizumab (Nucala) | Asthma | Both IgG1k mAbs | ||
| IL-17A | Inflammatory cytokine | Ixekizumab (Taltz) Secukinumab (Cosentyx) | Plaque psoriasis, ankylosing spondylitis | Ixekizumab is an IgG4; secukinumab is an IgG1k | ||
| Clostridium | Reduce recurrence of Clostridium difficile recurrence | Bezlotoxumab (Zinplava) Actoxumab | Meant to be used for the prevention of recurring clostridial disease. Actoxumab is only approved in the UK. | |||
| PDGF | Inhibit association of the PDGF with its receptor | Olaratumab (Lartruvo) | Soft tissue sarcoma | Human IgG1k that prevents interaction of PDGF with its receptor(s) |
An actively updated summary of MAb approvals can be found at www.antibodysociety.org.
MMabs can be murine, chimeric (human Fc region), humanised or human; Ttraps are derived from receptors and compete with natural receptor for binding target; Bbi-specific mAbs are engineered to bind to two different targets simultaneously (usually to bring immune cell into contact with target cell, thereby triggering target cell killing). Antibody drug conjugate (X): toxin or radioisotope attached to mAb to increase efficacy. Asterisks denote approvals in USA only; others are approved in both USA and UK.
ADC = antibody-drug conjugate; ADCC = antibody-dependent cell-mediated cytotoxicity; ADP = adenosine diphosphate; BAFF = B-cell activating factor; CTLA-4 = cytotoxic T-cell lymphocyte associated protein-4; EGFR = epidermal growth factor receptor; HER2 = human EGFR-2; IHC = immunohistochemistry; Ig = immunoglobulin; IL = interleukin; LDL = low density lipoprotein; LDR = low density lipoprotein receptor; NSCLC = non-small cell lung cancer; PCSK9 = proprotein convertase subtilisin/kexin type 9; PDGF = platelet-derived growth factor; PD-1 = programmed cell death protein 1; PDL-1 = programmed cell death ligand 1; PML = progressive multifocal leukoencephalopathy; RA = rheumatoid arthritis; SLAMF7 = signalling lymphocyte activation molecule; TNF = tumour necrosis factor; VEGF = vascular endothelial growth factor
Improved therapy for inflammatory/autoimmune disease
The concept of autoimmune diseases, pioneered by Sir Frank McFarlane Burnet5 (Nobel Laureate 1960), was validated by the detection of serum autoantibodies in many diseases in the 1960s. This suggested a role for B-lymphocytes, and human leukocyte antigen (HLA) associations documented from the 1970s indicated the role of T-cells when the peptide presenting function of HLA was understood. However, these advances did not lead to new treatments at the time because of the lack of specific therapeutics.
With the advent of mAbs, the role of T-cells was tested in rheumatoid arthritis patients using lytic antibodies to CD4, without clinical benefit.6 Nor did small trials of several other anti-T-cell antibodies, such as anti-CD7. OKT3, a mouse mAb recognising CD37 given to reduce acute rejection in patients with organ transplants was the first monoclonal antibody approved for use in humans although it was later withdrawn.
The success of anti-TNF therapy
The targeting of a single pro-inflammatory cytokine, TNF, to treat a complex disease in rheumatoid arthritis (RA) where multiple pro-inflammatory cytokines were upregulated was based on work using human disease tissue by Feldmann and Maini8 and Brennan et al.9 They analysed cytokine production from joints and cytokine regulation in cultures of rheumatoid synovium in which the majority of the cells survived, producing the mediators generated in vivo. In these cultures, blocking TNF-α reduced the production of many other inflammatory cytokines (IL-1, IL-6, GM-CSF, IL-8 etc),9 thus defining a ‘TNF-dependent cytokine cascade’. The dramatic clinical success of TNF blockade, demonstrated first in late stage RA then in earlier stage disease, also validated this concept.8 Noteworthy was the fact that tissue (bone and cartilage) damage was controlled. But also striking was the heterogeneity of the clinical response, with some individuals close to a cure and others virtually unimproved. The reasons for this are not yet clear, despite much work to try to elucidate the reasons. Genetic differences were an obvious possibility although never established, and recent clinical data demonstrating that non-responders may respond subsequently to anti-TNF has excluded it. Although anti-TNF in humans is relatively safe,10 more infections in patients occur, eg with intracellular organisms, especially tuberculosis. Many large patient registers have documented the long-term benefits of anti-TNF therapy, reducing some complications and maintaining a favourable benefit/risk ratio.11
Currently, anti-TNF is used in RA, Crohn's disease, ulcerative colitis, psoriasis, psoriatic arthritis, ankylosing spondylitis and juvenile RA; its use is now being explored in other indications (Table 1). Anti-TNF antibodies are the most successful and widely used antibody-based therapeutic. It is noteworthy that if used together with methotrexate early in the course of RA, over 50%12 of patients can be taken off infliximab and remain virtually disease-free, even with reduced dosage of methotrexate,12 and some patients can be taken off all medication.13
In the wake of anti-TNF's success, other mAbs have also proven useful in inflammatory diseases. The antibody to the IL-6 receptor, tocilizumab (Actemra®),14 pioneered by Professor Tadamitsu Kishimoto, has been approved for RA and for juvenile idiopathic arthritis. Currently, other anti-cytokine antibodies to IL-6, GM-CSF and the GM-CSF receptor have been approved (siltuximab (Sylvant®) – anti-IL-6) or are in late stage clinical trials after successful phase II studies.
Rituximab, a chimeric mAb to CD20,15 (an antigen in most B-cells although not plasma cells), was first to treat B-cell-driven cancers such as non-Hodgkin lymphoma. It was pioneered by Jo Edwards for RA and subsequently approved, but was not successful in systemic lupus erythematosus (SLE) trials. Anti-CD52 (alemtuzumab) is a first generation humanised antibody, now used in multiple sclerosis. There are other antibodies approved, eg belimumab (also known as Benlysta) is an anti-BLys mAb approved for SLE,16 ustekinumab (also known as Stelara) is an antibody to the shared p40 subunit of IL-12 and IL-23 approved for psoriasis and psoriatic arthritis and potentially for Crohn's disease,17 and secukinamab (Cosentyx) is an anti-IL17A mAb approved for severe psoriasis and ankylosing spondylitis.18
Receptor IgG fusion proteins (‘ligand traps’) have also been generated. TNF receptor 2-Fc (etanercept, Enbrel®) is effective and has been approved.19 Other ligand traps include rilonacept (Arcalyst – an IL-1 Trap) and the anti-angiogenesis vascular endothelial growth factor trap (aflibercept (Eylea®) for retinopathies and ziv-aflibercept (Zaltrap®) for colorectal cancer) (Table 1). Abatacept (Orencia®) and belatacept (Nolojix®) are CTLA4-Fc fusion proteins that inhibit antigen presentation to T-cells and are effective in RA.
Monoclonal antibodies in cancer therapy
mAb discovery: a case study of trastuzumab (Herceptin)
Modern mAb therapy of solid tumours was initiated by the humanised human epidermal growth factor receptor 2 (HER2) mAb trastuzumab (Herceptin®).20 The rigorous science that laid the foundation for this breakthrough mAb also initiated personalised/biomarker driven drug discovery and treatment in oncology.
Trastuzumab, the first successful monoclonal anti-cancer antibody to be successful against solid tumours, is well tolerated in patients. The pathway leading to TNF-resistance of most tumour cell lines21 was unraveled by collaboration between the Shepard (Genentech) and Schreiber laboratories (Chicago), which revealed that macrophages kill tumour cells largely by secreting TNF.22 They hypothesised that if tumour resistance to macrophages could be reversed, the tumour would become sensitive to killing by host defence. Macrophage (or TNF)-resistant tumour cells implanted into syngeneic mice formed aggressive tumours, while their TNF-sensitive parental cells regressed.21
The mechanism of TNF-resistance of tumours needed to be widespread since most tumour cell lines were resistant. Sporn and Todaro's autocrine growth factor hypothesis23 of malignant transformation involving autocrine stimulation by transforming growth factors seemed plausible. Various growth factors were combined with TNF on TNF-sensitive tumour cell lines and growth factors that activated receptor tyrosine kinases converted TNF-sensitive tumour cells to TNF-resistant cells.24 Host defence was completely subverted and the growth inhibitor (TNF) even became a growth factor.25
Slamon et al26,27 and Zhou et al28 reported that overexpression of the receptor tyrosine kinase HER2 in breast and ovarian cancers was associated with more aggressive disease contemporaneously with Shepard's demonstration that overexpression of HER2 induced resistance to TNF29 and that mAbs to HER2 inhibited growth of HER2-overexpressing tumours in vitro and in vivo.30–32 The lead antibody, MuMAb4D5 – the murine partent of trastuzumab,24was ineffective on normal cells or tumour cells not overexpressing HER2.26,33 Anti-HER2 also validated the importance of stability of the target itself as HER2 is indispensable in driving disease in subsets of breast, gastric and ovarian cancers. These findings laid the foundation for the success of trastuzumab and subsequently for pertuzumab,34 which binds a separate epitope on HER2, and then trastuzumab emtansine, an antibody drug conjugate.35 The clinical success of trastuzumab required the co-development of two novel companion diagnostic approaches: immunohistochemistry and fluorescence in situ hybridisation for selecting patients most likely to respond.36 This was a new idea at the time, but is now a routine part of most cancer drug discovery and therapeutic programmes. Finally, this mAb showed that antibody therapy was feasible in solid tumours, overcoming problems such as high internal tumour pressure, which causes vascular collapse and reverse flow of fluid in solid tumours.37
MuMAb4D5 was humanised using a consensus human variable domain sequence from the Kabat database, together with human immunoglobulin constant domain (IgG1, kappa) sequences.38 This resulted in the synthesis of a recombinant antibody, non-existent in nature, with almost 95% human sequence, reducing concerns related to immunogenicity. The humanised antibody has three mechanisms of action dependent upon upregulated HER2:
growth inhibition
antibody-dependent cell-mediated cytotoxicity (ADCC)
induction of TNF sensitivity.20,30
The strategy employed in the development of trastuzumab is now widely employed in cancer drug discovery (Fig 2). Very recently in clinical trials, a combination of trastuzumab and another anti-HER2 mAb (pertuzumab30) generated a complete pathological response in approximately 50% of HER2-positive patients,39 which is a previously unheard of therapeutic advance in the treatment of breast cancer. The HER2 programme proved that mAbs have a place in therapy of solid tumours and that tyrosine kinase oncogenes were viable targets in cancer.
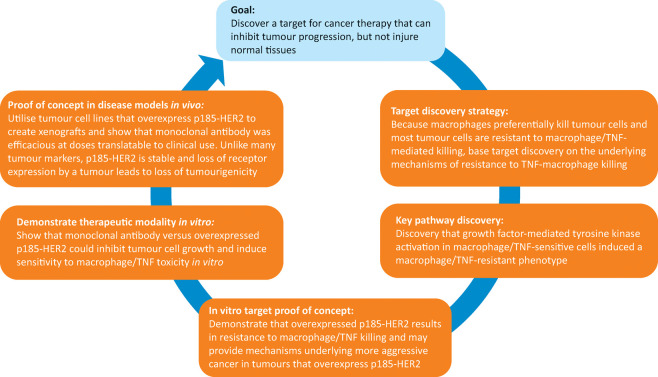
Fig 2.
Strategy and key steps for development of trastuzumab, from target discovery to in vitro and in vivo proof of concept studies. HER2 = human epidermal growth factor receptor 2; TNF = tumour necrosis factor
Not all successful mAb therapeutics followed this pathway of discovery. The first approved mAbs targeted membrane proteins shared between haematologic malignancies and their precursor immune cell subsets (eg alemtuzumab (Campath®) – an anti-CD52, rituximab (Rituxan®) – an anti-CD20). These mAbs ablate both normal and cancer cells. Alemtuzumab was the first humanised mAb therapeutic, depleting lymphocytes, monocytes and dendritic cells, useful in diseases such as chronic lymphocytic leukemia. Similarly, rituximab depletes CD20-expressing B-cells. Resistance to these mAb therapeutics occurs with the loss of the target as neither CD52 nor CD20 is essential for malignant growth. These mAbs have toxicity issues due to massive depletion of immune cells, potentially causing ‘cytokine release syndrome’ or ‘tumour lysis syndrome’ – a ‘cytokine storm’ resulting from aberrant immune activation attributable to cellular debris activating ‘danger receptors’. Despite these issues, both rituximab and alemtuzumab are successful drugs and often lead to disease regression.
More recently, Jim Allison and other cancer researchers discovered the utility of mAbs targeting ‘immune checkpoints’. By inhibiting these pathways (eg programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) or cytotoxic T-cell lymphocyte associated protein-4), tumour-associated immune suppression can be reversed with clinically effective immune cell attack on tumours in about 20% cases. Some patients have had dramatic improvements, notably in metastatic melanoma, and there is no doubt that this is a major step forward. Not unexpectedly there are immune-mediated diseases in a large fraction (about 30%) of treated patients, but this field of mAb therapy is growing very rapidly. CTLA-4 expressed on regulatory T-cells exerts immune suppressive activity. Anti-CTLA-4 prevents CTLA-4 from inhibiting immunity.40 PD-1 is present on ‘exhausted’ T-cells; anti-PD-1 reactivates them. The first approvals of this new class of anti-cancer mAbs were in melanoma with ipilimumab (anti-CTLA-4, Yervoy®) and pembrolizumab (anti-PD1, Keytruda®) in 2015, followed by atezolizumab (anti-PD-L1, Tecentriq®) for non-small cell lung cancer and bladder cancer. Checkpoint inhibitor mAbs, such as anti-OX40, can also be agonistic.40
Other antibody-related approaches and mechanisms
Therapeutic antibodies: structural marvels that mediate multiple functional effects
Antibodies have evolved over the past 400 million years to provide broad ranging host defence in all vertebrates. Thus, it is not surprising that engineered antibodies have emerged as one of the main sources of new medicines. Antibodies evolved to possess several important functional properties exploited by recombinant mAb therapeutics, such as exquisite specificity and affinity toward their antigen targets, recruiting immune system effector components and a long serum half-life.
Antibodies possess a unique tertiary domain structure, folding into a complex quaternary structure. Human serum immunoglobulin is approximately 80% IgG, as are most approved therapeutic antibodies. These complex proteins (approximately 150 kDa) comprise four polypeptide chains – 2 heavy and 2 light chains – with disulphide bonds providing significant rigidity. The heavy chain consists of a variable domain (VH) and three constant domains (CH1, CH2 and CH3). The light chain consists of a variable domain (VL) and a constant domain (CL) (Fig 1A). IgG antibodies are bivalent and have post-translational modifications. Glycosylation in the Fc domain also helps to provide stability and modulates properties such as binding to Fc receptors.41
The antibody/antigen interaction
Several technologies are used to identify novel antibodies: hybridomas, genetically modified mice with human immunoglobulin sequences and phage or yeast display. B-cells from a mouse injected with an antigen are fused with immortalised B-cell myeloma cells to make hybridomas. To improve their efficacy in patients, protein engineers created chimeric antibodies containing human constant domains and murine variable domains, which reduce immunogenicity and activate effector functions (Fig 1). Approved chimeric antibodies include Remicade® (infliximab), Erbitux® (cetuximab) and Rituxan® (rituximab). As an example of a ‘humanised’ antibody Herceptin® (trastuzumab) was isolated from a murine hybridoma and then ‘humanised’ where all but the binding site to the HER2 antigen was changed to a human sequence (Fig 1). Genetically engineered mice have been developed that can make fully human antibodies, eg Vectibix® (panitumumab) – anti-epidermal growth factor receptor. Another important approach employs synthetic (human) antibody libraries that are displayed on the surface of yeast or phage, which is useful for less immunogenic targets. There are advantages and limitations to each of these methods; therefore, antibody discovery groups will attempt multiple approaches in parallel against a particular target.
Antibody effector function
Immunoglobulins can stimulate immune defence mechanisms through ADCC, antibody-dependent cellular phagocytosis and complement-dependent cytotoxicity. During ADCC, immune cells – usually natural killer (NK) cells – lyse a target cell with an IgG bound to a cell surface target. CD16 Fc receptors expressed on NK cells bind to the Fc region of the IgG, forming a lytic synapse between the NK cell and the target cell, destroying the target cell. During complement-dependent cytotoxicity, complement is recruited to the IgG-bound surface pathogen through C1q binding to the antibody Fc domain, triggering proteolytic events, yielding a membrane attack complex that disrupts cellular membranes with cell death. Many therapeutic antibodies use ADCC as a key mechanism of action, eg trastuzumab and rituximab. Methods have been developed for increasing or decreasing antibody effector function, the number of epitope binding sites or attaching cytotoxic drugs. Some of these strategies are shown in Fig 1.
Antibody pharmacokinetics
Antibodies have a long serum half-life, typically lasting 2–4 weeks in circulation. Antibody binding the Fc domain of the neonatal Fc receptor, FcRn, recycles the antibody, protecting it from other clearance mechanisms. This long half-life with less frequent dosing is often more favourable for patients when extended drug function is needed. Antibody dosing is typically intravenous or subcutaneous. Oral administration is not feasible because of rapid degradation of the antibody in the gut. In addition, therapeutic antibodies cannot pass in appreciable quantities through the intact blood-brain barrier.
Antibody-drug conjugates: using antibodies for tumour-selective delivery of cytotoxics
The therapeutic use of antibody-drug conjugates (ADCs) is based on antibody-mediated tumour-selective delivery of potent cytotoxic compounds. This relies on antibody-induced receptor internalisation, followed by trafficking of the ADC to the lysosomes where the cytotoxic drug is released from the ADC. The free drug (inside the target cell) initiates its anti-tumour activity. Stability of the linker that joins the antibody and cytotoxic agent is key to both efficacy and safety. The linker must be stable in the circulation to prevent premature release of the cytotoxic agent, yet allow release of the cytotoxic drug within the tumour cell. The most common linkers are cleavable linkers such as peptides, cleaved by proteases, or disulphide linkers, which undergo reduction to release the drug within the lysosome. ADCs can also utilise a stable or non-cleavable linker, the free drug is released in the lysosome after lysosomal degradation of the entire ADC molecule.
Despite decades of intense research and development, only three ADCs have gained marketing approval. The first, gemtuzumab ozogamicin (Mylotarg®), was approved then taken off the market. Significant advances were subsequently made in ADC linker design and conjugation technologies, yielding two additional approved ADCs, one for CD30-positive Hodgkin lymphoma and anaplastic large cell lymphoma (brentuximab vedotin (Adcentris®), and one for metastatic breast tumours overexpressing HER2/neu trastuzumab emtansine (Kadcyla®). The anti-CD30 antibody component of brentuximab vedotin is linked to the tubulin polymerisation inhibitor mono-methyl auristatin E through a protease cleavable linker, valine-citrulline. Trastuzumab emtansine is comprised of trastuzumab covalently linked to the maytansinoid derivative, DM1, through a stable, non-cleavable linker. Current research suggests that more efficacious and better tolerated ADCs will be developed, which will provide greater clinical benefit to cancer patients.
Emerging antibody technologies
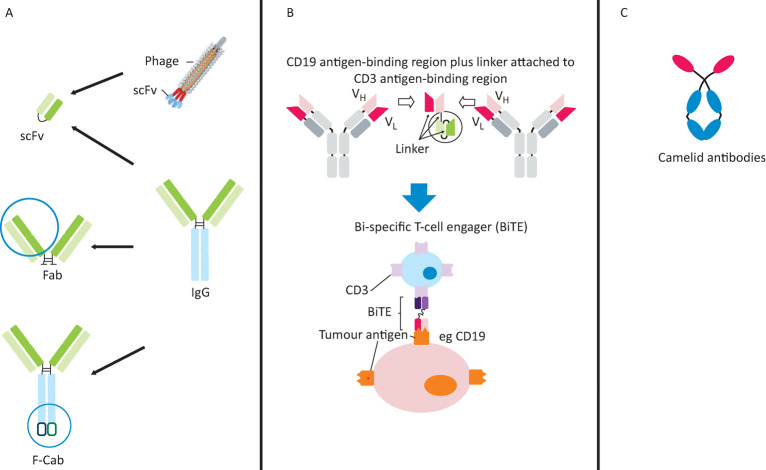
The Fab fragment (Fig 3A) used in ranibizumab (Lucentis)42 can be manufactured in Escherichia coli. The short half-life of Fabs can be remedied by polyethylene glycol used in certilizumab pegol, Cimzia®.43
Fig 3.
Emerging antibody technologies. A – derivatives of classical mAbs: scFv, Fab, and F-Cab antibody formats; B – engineered bi-specific mAbs: structure and mechanism of a bi-specific T-cell engaging (BiTE) antibody; C – novel mAb frameworks: unique structure of camelid antibodies. mAb = monoclonal antibody
Until recently, it has been difficult to synthesise bi-specific mAbs. Now various types are possible. Bi-specific T-cell engaging (BiTE) antibodies consist only of the variable regions from distinct antibodies (Fig 3B) and are the format of the first approved bi-specific antibody, catumaxomab (Removab®), for malignant ascites.
Antibody-based cellular immunotherapies
A new class of antibody-based therapeutics called ‘chimeric antigen receptor T-cells’ are emerging in cancer. An antibody-like antigen binding region is fused with signalling components of the T-cell receptor and the chimeric gene is inserted into the patient's own T-cells. When the T-cells recognise their target on tumour cells, the T-cell cytotoxic response is activated, lysing the cancer cells.43 Using CTL019, a chimeric antigen receptor T-cell engineered to target cancer cells that express the CD19 protein present on acute lymphoblastic leukemia cells, 19 of 22 paediatric patients with lethal acute lymphoblastic leukaemia experienced remission.44 However, this treatment upregulates inflammatory cytokines, with consequent toxicity.
Conclusions
mAb therapeutics have been approved for 33 targets in more than 37 distinct diseases (Table 1), most commonly for cancer (27 approvals). The clinical value of both mAbs and ligand traps has been proven. The most targeted pathway is the TNF pathway, now employed to treat nine different indications and likely to expand as TNF is found to be a driver of additional diseases and conditions, including postoperative cognitive decline45 and fibrosis.46 New applications of mAbs are being tested, with approvals in bone metabolism47 and hypercholesterolemia.48
Antibodies have become the new backbone of the pharmaceutical industry, which previously relied on huge libraries of small molecules. Compared with small molecules, mAbs have an exquisite target selectivity and, therefore, less toxicity attributable to binding to other targets. mAbs have now been designed to target two or more targets simultaneously, augmenting the therapeutic potential. We are only just at the beginning of the mAb therapeutic era.
Conflicts of interest
GLP is an employee of Genentech and owns Roche stock. CT and HMS are employees of Halozyme and own Halozyme stock. MF is one of the inventors of anti-TNF therapy and receives royalties on combination therapy.
Author contributions
All authors wrote part of the review, read all of it and approved the final version.
References
- 1.Köhler G. Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976;6:511–9. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- 2.Morrison SL. Johnson MJ. Herzenberg LA. Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci USA. 1984:;81:6851–2. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones PT. Dear PH. Foote J. Neuberger MS. Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986;321:522–5. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- 4.An Z. Monoclonal antibodies – a proven and rapidly expanding therapeutic modality for human diseases. Protein Cell. 2010;1:319–30. doi: 10.1007/s13238-010-0052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnet FM. Immunological recognition of self. Science. 1961;133:307–11. doi: 10.1126/science.133.3449.307. [DOI] [PubMed] [Google Scholar]
- 6.Choy EH. Kingsley GH. Panayi GS. Anti-CD4 monoclonal antibodies in rheumatoid arthritis. Springer Semin Immunopathol. 1998;20:261–73. doi: 10.1007/BF00832011. [DOI] [PubMed] [Google Scholar]
- 7.Van Wauwe JP. De Mey JR. Goossens JG. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980;124:2708–13. [PubMed] [Google Scholar]
- 8.Feldmann M. Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nature Medicine. 2003;9:1245–50. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 9.Brennan FM. Chantry D. Jackson A. Maini RN. Feldmann M. Inhibitory effect of TNFα antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2:244–7. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 10.Leombruno JP. Einarson TR. Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis. 2009;68:1136–45. doi: 10.1136/ard.2008.091025. [DOI] [PubMed] [Google Scholar]
- 11.Kievit W. Fransen J. Adang EM, et al. Long term effectiveness and safety of TNF-blocking agents in daily clinical practice: results from the Dutch Rheumatoid Arthritis Monitoring register. Rheumatol. 2011;50:196–203. doi: 10.1093/rheumatology/keq325. [DOI] [PubMed] [Google Scholar]
- 12.van der Bijl AE. Goekoop-Ruiterman YP. de Vries-Bouwstra JK, et al. Infliximab and methotrexate as induction therapy in patients with early rheumatoid arthritis. Arthr Rheum. 2007;56:2129–34. doi: 10.1002/art.22718. [DOI] [PubMed] [Google Scholar]
- 13.Quinn MA. Conaghan PG. O'Connor PJ, et al. Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal. Results from a 12-month randomized, double-blind, placebo-controlled trial. Arthr Rheum. 2005;52:27–35. doi: 10.1002/art.20712. [DOI] [PubMed] [Google Scholar]
- 14.Kang S. Tanaka T. Kishimoto T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol. 2015;27:21–9. doi: 10.1093/intimm/dxu081. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MD. Keystone E. Rituximab for rheumatoid arthritis. Rheumatol Ther. 2015;2:99–111. doi: 10.1007/s40744-015-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn BH. Belimumab for systemic lupus erythematosus. New Engl J Med. 2013;368:1528–35. doi: 10.1056/NEJMct1207259. [DOI] [PubMed] [Google Scholar]
- 17.Feagan BG. Sandborn WJ. Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn's Disease. New Engl J Med. 2016;375:1946–60. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 18.Baeten D. Sieper J. Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in Ankylosing Spondylitis. New Engl J Med. 2015;373:2534–48. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 19.Weinblatt ME. Moreland LW. Westhovens R, et al. Safety of abatacept administered intravenously in treatment of rheumatoid arthritis: integrated analyses of up to 8 years of treatment from the abatacept clinical trial program. J Rheumatol. 2013;40:787–97. doi: 10.3899/jrheum.120906. [DOI] [PubMed] [Google Scholar]
- 20.Carter P. Presta L. Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugarman BJM. Aggarwal BB. Hass PE, et al. Recombinant human tumour necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985;230:943–5. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- 22.Urban JL. Shepard HM. Rothstein JL. Sugarman BJ. Schreiber H. Tumor necrosis factor: a potent effect molecule for tumor cell killing by activated macrophages. Proc Natl Acad Sci USA. 1986;83:5233–7. doi: 10.1073/pnas.83.14.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sporn MB. Todaro GJ. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980;303:878–80. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- 24.Sugarman BJ. Lewis GD. Eessalu TE. Aggarwal BB. Shepard HM. Effects of growth factors on the anti proliferative activity of tumor necrosis factors. Cancer Res. 1987;47:780–6. [PubMed] [Google Scholar]
- 25.Lewis GD. Aggarwal BB. Eessalu TE. Sugarman BJ. Shepard HM. Modulation of the growth of transformed cells by human tumor necrosis factor-alpha and interferon-gamma. Cancer Res. 1987;47:5382–5. [PubMed] [Google Scholar]
- 26.Slamon DJ. Clark GM. Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 27.Slamon DJ. Godolphin W. Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 28.Zhou D. Battifora H. Yokota J. Yamamoto T. Cline MJ. Association of multiple copies of the c-erbB-2 oncogene with spread of breast cancer. Cancer Res. 1987;47:6123–5. [PubMed] [Google Scholar]
- 29.Hudziak RM. Lewis GD. Shalaby MR, et al. Amplified expression of the HER2/ERBB2 oncogene induces resistance to tumor necrosis factor alpha in HIH 3T3 cells. Proc Natl Acad Sci USA. 1988:;85:5102–6. doi: 10.1073/pnas.85.14.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis GD. Figari I. Fendly B, et al. Differential responses of human tumor cell lines to anti-p185HER2 monoclonal antibodies. Cancer Immunol Immunother. 1993;37:255–63. doi: 10.1007/BF01518520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceran C. Cokol M. Cingoz S, et al. Novel anti-HER2 monoclonal antibodies: synergy and antagonism with tumor necrosis factor-alpha. BMC Cancer. 2012;12:450. doi: 10.1186/1471-2407-12-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobol RE. Astarita RW. Hofeditz C, et al. Epidermal growth factor receptor expression in human lung carcinomas defined by a monoclonal antibody. J Natl Cancer Inst. 1987:;79:403–7. [PubMed] [Google Scholar]
- 33.Varley JM. Swallow JE. Brammer WJ. Whittaker JL. Walker RA. Alternations to either c-erbB-2(neu) or c-myc proto-oncogenes in breast carcinomas correlate with poor short-term prognosis. Oncogene. 1987;1:423–30. [PubMed] [Google Scholar]
- 34.Adams CW. Allison DE. Flagella K, et al. Humanization of a recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother. 2006;55:717–27. doi: 10.1007/s00262-005-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis Phillips GD. Li G. Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 36.Lebeau A. Deimling D. Kaltz C, et al. Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001;19:354–63. doi: 10.1200/JCO.2001.19.2.354. [DOI] [PubMed] [Google Scholar]
- 37.Jain RK. Vascular and interstitial barriers to delivery of therapeutic agents in tumors. Cancer Metastasis Rev. 1990;9:253–66. doi: 10.1007/BF00046364. [DOI] [PubMed] [Google Scholar]
- 38.Johnson G. Wu TT. Kabat database and its applications: 30 years after the first variability plot. Nucleic Acids Res. 2000;28:214–8. doi: 10.1093/nar/28.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiwari SR. Mishra P. Raska P, et al. Retrospective study of the efficacy and safety of neoadjuvant docetaxel, carboplatin, trastuzumab/pertuzumab (TCH-P) in nonmetastatic HER2-positive breast cancer. Breast Cancer Res Treat. 2016;258:189–93. doi: 10.1007/s10549-016-3866-0. [DOI] [PubMed] [Google Scholar]
- 40.Mahoney KM. Rennert PD. Freeman GJ. Combinatino cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov. 2015;14:561–84. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- 41.Bournazos S. Ravetch JV. Fcγ receptor pathways during active and passive immunization. Immunol Rev. 2015;268:88–103. doi: 10.1111/imr.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrara N. Damico L. Shams N. Lowman H. Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–70. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 43.Choy EH. Hazleman B. Smith M, et al. Efficacy of a novel PEGylated humanized anti-TNF fragment (CDP870) in patients with rheumatoid arthritis: a phase II double-blinded, randomized, dose-escalating trial. Rheumatology. 2002;41:1133–7. doi: 10.1093/rheumatology/41.10.1133. [DOI] [PubMed] [Google Scholar]
- 44.Maude SL. Frey N. Shaw PA. Aplenc R. Barrett DM. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terrando N. Monaco C. Ma D, et al. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci USA. 2010;107:20518–22. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glass GE. Chan JK. Freidin A, et al. TNFα promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci USA. 2010;108:1585–90. doi: 10.1073/pnas.1018501108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pageau SC. Denosumab. MAbs. 2009;1:210–5. doi: 10.4161/mabs.1.3.8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Everett BM. Smith RJ. Hiatt WR. Reducing LDL with PCSK9 inhibitors - the clinical benefit of lipid drugs. N Engl J Med. 2015;373:1588–91. doi: 10.1056/NEJMp1508120. [DOI] [PubMed] [Google Scholar]