ABSTRACT
In this article, we report on a 22-year-old patient with myocardial infarction, which was the initial manifestation of polycythaemia vera. The awareness of myeloproliferative disorders as possible underlying disease – especially in young patients presenting with myocardial infarction – is crucial for clinical management, as a missed diagnosis can worsen the patient's further prognosis.
KEYWORDS: Acute coronary syndrome, myeloproliferative disorder, myocardial infarction, polycythaemia vera
Case presentation
A 22-year-old male patient was admitted in a haemodynamically stable condition because of recurrent chest pain for 2 days. There were discrete ST-segment elevations in the anterior leads (V1-V4) on the electrocardiogram (ECG). His heart rate was 110 bpm. Cardiac markers were significantly elevated with a maximum level of CK-MB of 92 U/L (7–25 U/L) and a high sensitive troponin T level of 0.73 ng/mL (0–0.014 ng/mL) at admission. The blood count showed a markedly elevated haematocrit (53.4% (42–50%) and haemoglobin 19.8 g/dL (14–17 g/dL), as well as leukocytosis (17.22 g/L (4–10 g/L)) and thrombocytosis (666 g/L (150–400 g/L)). On echocardiography a severe hypokinesia of the left ventricular anterior wall was found. Abdominal ultrasound revealed a splenomegaly (14.3 cm). The chest X-ray was unremarkable. Besides a body mass index of 38, no cardiovascular risk factors were detected.
Differential diagnosis
Because of all of the collected clinical information and the diagnostic findings with a discrete ST-segment elevation on the ECG and significantly elevated high sensitive troponin T levels, the patient was diagnosed with an ST-elevation myocardial infarction.
Most common and likely differential diagnoses, like pulmonary embolism, acute aortic dissection and myocarditis, could be ruled out because of atypical clinical findings, normal D-dimer levels and a lack of indirect signs for these diseases on transthoracic echocardiography. Cocaine use, which may cause a similar clinical presentation owing to coronary spasm, was also ruled out.
Initial management
Standard medical treatment for ST-elevation myocardial infarction, including dual antiplatelet therapy, beta-blockade and low molecular weight heparin, was initiated according to current guidelines.1 Coronary angiography was performed within 30 minutes of admission and revealed an occlusion of the left anterior descending artery at its origin with visible collaterals from the circumflex and right coronary artery, indicating sub-acute occlusion. Because of the proximity to the left main stem, a percutaneous coronary intervention would have involved the distal main stem and the proximal circumflex artery; as a result, revascularisation was delayed and a heart team discussion was planned for the next day.
Case progression and outcome
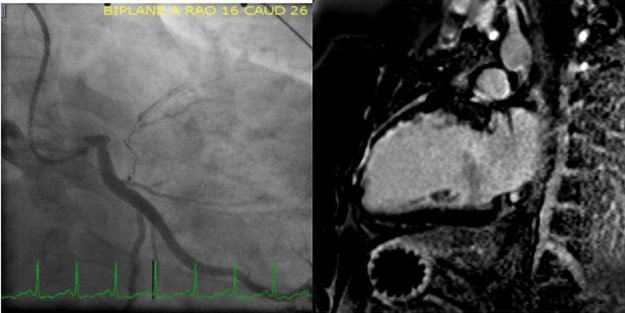
Complementary cardiac magnetic resonance imaging demonstrated subendocardial late gadolinium enhancement located in the anterior and anteroseptal wall segments of the left ventricle (Fig 1). As more than 50% of the myocardium was viable, coronary revascularisation was mandatory, in our opinion. Considering the patient’s age and coronary anatomy, elective single coronary artery bypass grafting of the left anterior descending artery was successfully performed.
Fig 1.
A – coronary angiogram of the left coronary artery showing a proximal occlusion of the left anterior descending coronary artery with visible collaterals from the normal circumflex; B – magnetic resonance imaging reveals subendocardial late gadolinium enhancement in the apical and mid anterior and anteroseptal wall segments of the left ventricle with more than 50% of viable myocardium.
As we did not expect such a finding in a patient of such young age, we thoroughly investigated the patient's past medical history. Thereby, we found out that our patient had already been admitted to another local hospital 1 year previously because of similar complaints with a normal ECG and a significantly elevated high sensitive troponin T level. At that time, a coronary angiography was performed and did not show any evidence of coronary stenoses. The patient was discharged with the diagnosis of myocarditis and was set on oral medical treatment with non-steroidal anti-inflammatory drugs, according to current guidelines.2
Because of obvious overproduction of all blood cell lines, the patient was tested for a mutation in the Janus kinase 2 gene (JAK2 V617F); he tested positive. With elevated haemoglobin and a low erythropoietin level of 3 mU/mL (4.3–29 mU/mL), polycythaemia vera (PV) was diagnosed according to the World Health Organization guidelines.3 A subsequent bone marrow biopsy ruled out myelofibrosis. Besides ongoing antiplatelet therapy, recurrent phlebotomy to achieve a haematocrit <45% was started. After recovery from surgery, additional treatment with hydroxyurea for PV was initiated.
Discussion
A 22-year-old patient suffered myocardial infarction due to proximal occlusion of the left anterior descending coronary artery. In this case, myocardial infarction was the initial manifestation of PV, which was suspected by careful interpretation of the complete blood count and confirmed by genetic testing.
PV, a myeloproliferative disorder caused predominantly by mutations in the JAK2 gene, is characterised by an overproduction of all blood cell lines leading to various complications, including thromboembolic events (in 40% of patients).3,4 In some cases, PV is a possible underlying cause of acute coronary syndromes in young patients. Normally, the average age at time of diagnosis is about 60 years – with only 7% of patients being younger than 40 years.3,5
Hyperviscosity due to the increased haematocrit and intimal proliferation, as well as increased platelet stimulation and aggregation, have been discussed as potential causes for the development of coronary ischaemia.3
The combined treatment of PV includes repeated phlebotomy and antiplatelet therapy amended by cytoreductive therapy with hydroxyurea or interferon-A.3
Awareness of myeloproliferative disorders as possible underlying disease – especially in young patients presenting with myocardial infarction – is crucial for clinical management, as a missed diagnosis can worsen the patient's further prognosis.5
Key learning points
Thromboembolic events are an important complication of polycythaemia vera.
The presence of polycythaemia in the complete blood count can lead to the diagnosis of PV.
In young patients with acute coronary syndromes, a careful interpretation of the complete blood count is important.
A missed diagnosis of PV can worsen the patient's outcome.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgements
Consent was obtained from the patient to publish the clinical details and images in this article.
References
- 1.Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Steg PG. James SK, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569–619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 2.Maisch B. Seferović PM. Ristić AD, et al. Task Force on the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology. Guidelines on the diagnosis and management of pericardial diseases executive summary. Eur Heart J. 2004;25:587–610. doi: 10.1016/j.ehj.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A. Thiele J. Orazi A, et al. Proposals and rationale for revision of the World Health Organization diagnostic criteria for polycythemia vera, essential thrombocythemia, and primary myelofibrosis: recommendations from an ad hoc international expert panel. Blood. 2007;110:1092–7. doi: 10.1182/blood-2007-04-083501. [DOI] [PubMed] [Google Scholar]
- 4.Passamonti F. Elena C. Schnittger S, et al. Molecular and clinical features of the myeloproliferative neoplasm associated with JAK2 exon 12 mutations. Blood. 2011;117:2813–6. doi: 10.1182/blood-2010-11-316810. [DOI] [PubMed] [Google Scholar]
- 5.Rattarittamrong E. Norasetthada L. Tantiworawit A, et al. Acute non-atherosclerotic ST-segment elevation myocardial infarction in an adolescent with concurrent hemoglobin H-constant spring disease and polycythemia vera. Hematol Rep. 2015;7:5941. doi: 10.4081/hr.2015.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]