Abstract
Obesity hypoventilation syndrome (OHS) is a condition in which an individual with a body mass index >30 kg/m2 develops daytime alveolar hypoventilation (defined as a resting PaCO2 >45 mmHg) that cannot be attributed to other pathologies. It is a condition with increasing prevalence and rising cost to healthcare systems worldwide. Right heart failure and pulmonary hypertension are well-known complications of this syndrome. Here, we present the case of a female patient with OHS who presented to our centre with severe pulmonary hypertension, which resolved with appropriate treatment. We also review this clinical condition and its diagnosis and management.
Keywords: obesity hypoventilation syndrome, Pickwickian syndrome, pulmonary hypertension, obstructive sleep apnoea
Introduction
Obesity hypoventilation syndrome (OHS), also known as Pickwickian Syndrome, is an under-recognised condition that can cause respiratory failure, right heart failure and severe pulmonary hypertension (PH). Individuals with OHS develop daytime alveolar hypoventilation, resulting in hypercapnia and hypoxaemia, which cannot be attributed to other pathologies (Table 1).1 Of patients with OHS, 90% also have obstructive sleep apnoea (OSA), while the remaining 10% experience nocturnal hypoventilation but without frequent episodes of apnoea.2 In addition to respiratory impairment, OHS is also a known cause of PH (Table 2).
Table 1.
Diagnostic criteria for obesity hypoventilation syndrome
| Criteria | Description |
|---|---|
| Obesity | Body mass index >30 kg/m2 |
| Hypoventilation | Awake arterial hypercapnia (PaCO2 >45 mmHg) |
| Sleep-disordered breathing | Polysomnography reveals sleep hypoventilation with nocturnal hypercapnia with or without obstructive apnea/hypopnea events |
| Rule out other causes of hypoventilation | Severe chronic obstructive pulmonary disease, severe interstitial lung disease, chest wall disorders, thyroid disorders and congenital hypoventilation syndromes |
Table 2.
Clinical classification of pulmonary hypertension
| Type of pulmonary hypertension | Causes |
|---|---|
| Grade 1 | |
| Pulmonary arterial hypertension | Idiopathic |
| Heritable: (eg BMPR, ALK1, mutations) | |
| Drugs and toxins | |
| Connective tissue diseases | |
| HIV infection | |
| Portal hypertension | |
| Congenital heart disease | |
| Schistosomiasis | |
| Persistent pulmonary hypertension of the newborn | |
| Pulmonary veno-occlusive disease and/or pulmonary capillary haemangiomatosis | |
| Grade 2 | |
| Pulmonary hypertension resulting from left heart disease | LV systolic dysfunction |
| LV diastolic dysfunction | |
| Valvular heart disease | |
| Grade 3 | |
| Pulmonary hypertension resulting from lung diseases and/or hypoxia | Chronic obstructive pulmonary disease |
| Interstitial lung disease | |
| Other pulmonary diseases with mixed restrictive and obstructive patterns | |
| Sleep-disordered breathing | |
| Alveolar hypoventilation disorders | |
| Chronic exposure to high altitude | |
| Developmental abnormalities | |
| Grade 4 | |
| Chronic thromboembolic pulmonary hypertension | |
| Grade 5 | |
| Pulmonary hypertension with unclear and/or multifactorial mechanisms | Haematological disorders (eg myeloproliferative disorders, splenectomy) |
| Systemic disorders (eg sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangioleiomyomatosis, neurofibromatosis, vasculitis) | |
| Metabolic disorders (eg glycogen storage disease, Gaucher's disease, thyroid disorders) | |
| Others: tumoural obstruction, fibrosing mediastinitis, chronic renal failure |
ALK = anaplastic lymphoma kinase; BMPR = bone morphogenetic protein receptor; LV = left ventricular
In recent years, the prevalence of OHS has markedly increased; it is estimated that 0.3–0.4% of the general population and up to 30% of inpatients with a body mass index (BMI) >35 kg/m2 might be affected.3 Here, we present a recent case from our institute in which a young patient presenting with breathlessness was found to have severe PH and respiratory failure; after receiving a diagnosis of OHS and appropriate management, the patient now is well with no PH.
Lesson
A 39-year-old Fijian woman presented to the cardiology clinic with a chronic history of exertional dyspnoea, with an acute worsening over the past 3 months. Her exercise tolerance was 50 yards (approximately 46 m). She denied chest pains or palpitations but complained of disturbed sleep and of having to sleep in a chair. Her family also mentioned loud snoring at night and daytime somnolence.
On examination, her weight and height were 132 kg and 167 cm, respectively (BMI 47 kg/m2). Her resting blood pressure was 123/75 mmHg, her pulse was 100 beats per minute, with a resting oxygen saturation of 70% breathing room air. She had no audible murmurs but did have a raised jugular venous pressure (JVP) with bilateral pitting oedema. She mobilised no more than 10 m to undergo electrocardiography (ECG; which revealed sinus tachycardia with partial right bundle branch block) and became acutely more breathless, with her oxygen saturation dropping to 60%. An urgent arterial blood gas sample revealed type 2 respiratory failure (pH 7.41, PaCO2 7.8 kPa, PaO2 4.2 kPa and HCO3– 36.6 mmol/L) with a markedly elevated bicarbonate level, implying chronic CO2 retention. Transthoracic echocardiography (TTE) revealed an enlarged right heart with an estimated pulmonary arterial systolic pressure >90 mmHg, consistent with severe PH.
She was urgently admitted to the coronary care unit and treated with intravenous diuretics and low-flow oxygen. Unfortunately, her condition deteriorated, requiring transfer to the intensive care unit for non-invasive ventilation (NIV). Urgent CT pulmonary angiography revealed neither acute nor chronic pulmonary emboli.
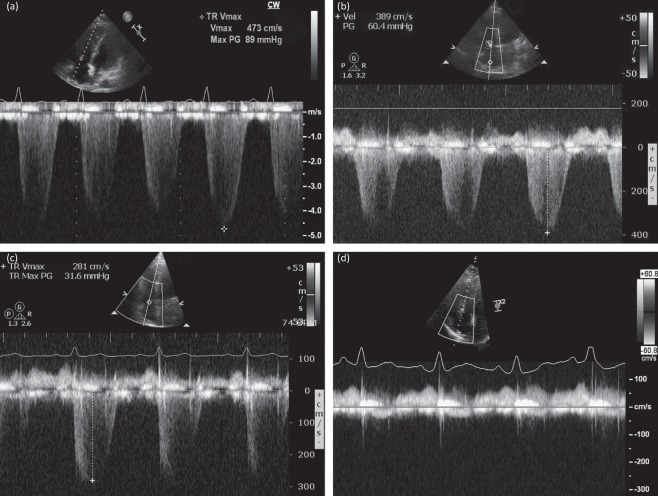
Following bi-level positive airways pressure (BiPAP) ventilation, repeat echocardiography 24 h after admission showed a reduction in her estimated right heart pressures from 90 mmHg to 60 mmHg. Given that the patient's pulmonary pressures had improved with oxygen, it was felt that idiopathic PH was unlikely. A clinical diagnosis of OHS with chronic (untreated) OSA was made. The patient's symptoms improved with respiratory support and diuretics, and she made an excellent recovery, being discharged home 13 days after admission with nocturnal NIV. Repeat echocardiography before discharge showed further reduction of her right heart pressure to 31 mmHg. She was seen in the outpatient clinic 4 months later, at which point she appeared very well and had no symptoms. Repeat echocardiography now showed undetectable right heart pressures, indicating normalisation of the pulmonary pressures (Fig 1). She continues with nocturnal NIV and no longer has poor sleep or daytime somnolence.
Fig 1.
Doppler echocardiographic recordings taken across the tricuspid valve, using the velocity of the tricuspid regurgitant (TR) jet to estimate right ventricular systolic pressure (RVSP). On first presentation, a severely elevated RVSP of 89 mmHg + right atrial (RA) pressure was recorded (a). After 24 h of treatment, this improved to 60 mmHg + RA pressure (b). Before discharge from hospital, the RVSP had fallen further to 31 mmHg + RA pressure (c) and, when the patient was seen several months after discharge, there was no detectable TR jet and no evidence of pulmonary hypertension (d).
Discussion
OHS was first described in 1956, at which time the condition was referred to as ‘Pickwickian syndrome’ after the character Joe, an overweight red-faced boy in the novel The Pickwick Papers by Charles Dickens.4 OHS can present with a range of symptoms, from complaints of snoring and daytime somnolence by family members to breathlessness, fatigue, mood disorders and morning headaches. The past medical history can reveal conditions that provide alternative explanations for CO2 retention, including mechanical disorders, such as lung diseases and kyphoscoliosis, neuropathies, and central nervous system abnormalities, such as prior stroke and severe hypothyroidism. A careful social history is required to screen for potential respiratory suppressants, such as alcohol and narcotic drugs. Physical examination invariably reveals significant obesity and frequently an elevated resting respiratory rate, and can show features of right heart failure, such as peripheral oedema. Several initial investigations are warranted; a full blood count can reveal erythrocytosis and thyroid function should also be checked. ECG frequently reveals sinus tachycardia, can show right bundle branch block, and often right heart abnormalities, including elevated pulmonary arterial pressures. Arterial blood gas analysis should be performed and usually reveals a compensated respiratory acidosis, with elevated PaCO2, reduced PaO2 and elevated serum bicarbonate levels. OHS is associated with Grade 3 pulmonary hypertension.7 When diagnosing PH, the gold standard investigation is right heart catheterisation (RHC); however, TTE is commonly used for screening and monitoring of the disease because it is non-invasive, cheaper, more widely available and has good diagnostic accuracy.8
Treatment aims for patients with OHS are based on minimising morbidity and mortality by normalising arterial CO2 levels. Other goals include the prevention of oxygen desaturation, reversal of erythrocytosis, cor pulmonale and symptomatic relief of daytime somnolence.9 These goals are achieved through a multidisciplinary approach, with the mainstay treatment being long-term lifestyle changes focused on weight loss.10 Weight loss of at least 10 kg results in a significant improvement in vital capacity and maximum voluntary ventilation and a significant reduction in daytime PaCO2. In cases where lifestyle changes prove to be ineffective or if the patient fails to tolerate NIV, bariatric surgery has been considered.2,9 A randomised control trial evaluating the treatment impact between BiPAP and continuous positive airway pressure (CPAP) showed that, after 3 months, both methods resulted in improvements in symptoms, quality of life and respiratory failure, with no statistically significant difference between the two.8
Conclusion
With an increasing prevalence of OHS, it is important that medical professionals both in primary and secondary care are aware of this obesity-related condition. With aggressive early lifestyle intervention, it might be possible to avoid the development of OHS and related PH in a bid to reduce hospitalisation rates, morbidity and mortality.
Conflicts of interest
The authors have no conflicts of interest to declare.
Author contributions
FW and ZI wrote the first draft of the manuscript. BNS revised and critically appraised the manuscript. BNS is the guarantor.
References
- 1.Kaw R. Obesity and pulmonary hypertension. What’s the link? Br J Med Pract. 2009;2:4–5. [Google Scholar]
- 2.Egea-Santaolalla C. Javaheri S. Obesity hypoventilation syndrome. Curr Sleep Med Rep. 2016;2:12–9. [Google Scholar]
- 3.Hart N. Mandal S. Manuel A, et al. Obesity hypoventilation syndrome: does the current definition need revisiting? Thorax. 2014;69:83–4. doi: 10.1136/thoraxjnl-2013-204298. [DOI] [PubMed] [Google Scholar]
- 4.Olsen A. Zwillich C. The obesity hypoventilation syndrome. Am J Med. 2005;118:948–56. doi: 10.1016/j.amjmed.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Tulaimat A. Littleton S. Defining obesity hypoventilation syndrome. Thorax. 2014;69:491. doi: 10.1136/thoraxjnl-2013-204992. [DOI] [PubMed] [Google Scholar]
- 6.Naeije R. Pulmonary hypertension in hypoventilation syndromes. Eur Resp J. 2014;43:12–5. doi: 10.1183/09031936.00185213. [DOI] [PubMed] [Google Scholar]
- 7.Galie N. Humbert M. Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 8.Kauppert C A. Dvorak I. Kollert F, et al. Pulmonary Hypertension in obesity-hypoventilation syndrome. Resp Med. 2013;107:2061–70. doi: 10.1016/j.rmed.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Brown LK. Obesity hypoventilation syndrome. Curr Sleep Med Rep. 2015;1:241–50. [Google Scholar]
- 10.Nolte JES. Koehler U. Sohrabi AK, et al. Reversible severe pulmonary hypertension in obesity hypoventilation and Mohr Syndrome. Resp Med. 2011;4:30–2. [Google Scholar]