Abstract
Background:
Transmission of malaria and dengue in the desert part of India is mainly caused by Anopheles stephensi and Aedes aegypti respectively. The maintenance and transmission of the pathogens that cause malaria and dengue are dependent on the physiology of the mosquito vectors. We aimed to measure the energy contents in the mosquitoes transmitting malaria and dengue in the desert part of the country.
Methods:
Immature stages of mosquitoes were collected from six different larval habitats situated in Jodhpur City of Rajasthan state, India. The immature stages of both the mosquitoes were collected once in fortnightly from each location. Quantitative estimations of the lipid, glucose, and glycogen of the laboratory-reared and field collected An. stephensi and Ae. aegypti were made by spectrophotometric method. The energy contents of the larvae, pupae, females, and males were estimated in triplicates on six different occasions.
Results:
The lipid content of laboratory-reared larvae, pupae and female mosquitoes of An. stephensi and Ae. aegypti was found to be lower than their conspecific field-collected specimens. Whereas, the glycogen content in the laboratory-reared larvae, pupae and female mosquitoes of An. stephensi and Ae. aegypti was higher than that of their conspecific field-collected specimens. The glucose content in all the stages of the laboratory-reared An. stephensi was lower than their conspecific field-collected specimens except in few cases.
Conclusion:
The higher amount of lipid in field-collected mosquitoes may be because of the availability of food in the natural habitat and adaptation of mosquitoes. Mosquitoes living in desert climate are physiologically better equipped to survive in the desert environment.
Keywords: Aedes aegypti, Anopheles stephensi, Glucose, Glycogen, Lipid
Introduction
Anopheles stephensi and Aedes aegypti are medically the two most important arthropod vectors of malaria and dengue respectively (1). Malaria and dengue cases are being reported from the desert zone due to introduction of canal irrigation for the development of agriculture. Anopheles stephensi is predominant and its presence was chronologically oldest among the entire vector species available in the desert part of India (2). The transmission of malaria in the non-irrigated part of the desert is mainly vectored by this species. It breeds in earthen pits (tanka and beri) situated in and around houses and can survive in the extremes of temperature and low humidity (3). Aedes aegypti is one of the most successful worldwide invaders, spreading from its native Africa to most tropical and subtropical regions of the world (4, 5) and is the primary vector of dengue fever, chikungunya, and Zika viruses.
Many dengue outbreaks have been recorded by this species, including several in Brazil and the Caribbean (6). Aedes aegypti, the vector of dengue is widely present in India including the Thar Desert in north-western Rajasthan (7, 8). Rajasthan is one of the dengue endemic States in India (9, 10). The maintenance and transmission of the pathogens that cause malaria and numerous viral infections are dependent on the availability of competent mosquito vectors (11).
Adult mosquitoes require carbohydrates in the form of glucose or glycogen for flight and survival during most of their adult life (12, 13). Lipid is used by the mosquitoes as energy source for long-term maintenance (14). Female mosquitoes supported by stored lipids can survive for long periods resting on the ground or in dense foliage under unfavorable nutritional and climatic conditions. In addition to energy for flight, longevity, and reproduction, nutritional status also influences the immune melanization response of Anopheles mosquitoes (15, 16) and susceptibility of Aedes mosquitoes to arbovirus (arthropod-borne virus) infection (17, 18). Newly emerged mosquitoes have different amounts of energy reserves that accumulate during their larval period (19). Some mosquitoes fail to locate blood or sugar meal after emergence and the length of the time to locate food for survival is of importance (20). Therefore, mosquitoes with higher energy reserves have a better chance of survival than the mosquitoes with lower level of the reserves.
We aimed to measure the energy contents in the mosquitoes transmitting malaria and dengue in the desert part of the country. The levels of lipid, glucose, and glycogen in field collected and laboratory-reared An. stephensi and Ae. aegypti are presented in this paper.
Materials and Methods
Study site
The desert zone of Rajasthan state is spread over 75000km2 and divided into 12 districts (2). Two seasonal rivers Luni and Mithri are present in the desert though their base is saline water. There is no perennial river flows in desert. The temperature varies from 49 °C in summer to 1 °C in winter. Immature stages of mosquitoes were collected from different larval habitat situated in Jodhpur City (Latitude: 26°18′N, Longitude: 73°04′E) of Rajasthan state, India. Immature stages were collected from the suburban and urban part of the city. The larvae were collected from Jhalamond, Kudi, Bomba Mohalla, Kheme Ka Kuan, Pratapnagar and Salawas areas of the city. The study sites were chosen on the basis of the geographic locations and availability of larval habitats.
Sources and collection of Anopheles stephensi and Aedes agypti
Larvae and pupae of An. stephensi were gathered from larval habitats such as underground water storage tanks, overhead tanks, fountains and seepage areas using the standard sampling method. The larvae and pupae of An. stephensi were collected by strainer by the larval sampling method of Service (21). However, wrigglers were collected by droppers and mugs. Aedes aegypti larvae and pupae were collected from the cattle drinking waters pots, water coolers, and temporary water storage tanks. Aedes aegypti larvae were collected from the bottom of the storage tanks and containers through a pipe. The outlet of the pipe was opened to a bucket and immature stages of mosquitoes were collected. They were transported to the insectary in ambient condition and reared (22). The immature stages of both the mosquitoes were collected once in fortnight from each location. The larvae and pupae collected at each site were combined and placed in pans in separate cages with date and location labels. Minimum 20 collections were made from each site during the study period. Preliminary identification in the field was done visually by examining the larvae during collection and species identification was confirmed in the laboratory, after the larvae had been reared to adults, using the keys by (23–27).
Maintenance of field-collected Anopheles stephensi and Aedes aegypti
The immature stages of An. stephensi and Ae. aegypti were collected from the field in all the seasons of the year. They were collected in containers along with the water and substrates where they were breeding. The field collected larvae and pupae were kept in the enamel trays after transported from the field. They were reared in the water and substrates collected from the breeding habitats. The food particles in water, as well as on the substrate were the source of their diet. Those larvae collected from field were not provided any additional food. They were maintained at an optimum photoperiod (10:14 [L/D] h), temperature (28±1 °C) and relative humidity (75±5%). Field collected pupae were kept in the cages for the adult emergence. Larvae, pupae, and adults of An. stephensi and Ae. aegypti were kept separately. Adults came out from the field collected pupae were kept in the cages.
Colonization of laboratory-reared Anopheles stephensi and Aedes aegypti
The laboratory-reared An. stephensi colony was originated from field-collected mosquitoes of urban areas of Jodhpur, India. Similarly, Ae. aegypti colony used in this study was originated from field-collected mosquitoes from water containers in Bomba Mahala of Jodhpur. Both the colonies were established in 2009. In the insectary, An. stephensi and Ae. aegypti colonies were maintained at an optimum photoperiod (10:14 [L/D] h), temperature (28±1 °C) and relative humidity (75±5%). Adults were kept in the cages (0.6m3) and allowed to mate freely. Larvae were fed on a mixture of yeast powder and dog biscuit (1: 2) as food at 24h intervals. Overall, 10mg of the above mixture was supplied to 100 larvae per day. Soaked raisins and 10% glucose solution were provided to emerging adults. The adults were kept in cyclic cages for successive generation and maintained (22).
Selection of mosquitoes for the assays
Fifty field-collected pupae of An. stephensi and Ae. aegypti were put separately along with the field water in the cages (0.6m3) for the adult emergence. Same numbers of laboratory reared pupae of both the mosquitoes were transferred to enamel bowls and kept in separate cages. Pupae of both the species were left in the cages for the adult emergence and pupae not emerged to adults were discarded on day-3. Each of ten males and females was taken for the glucose, glycogen and lipid as-says in the intervals of 24 hours. Blood meal and glucose solution was not given to the experimental female mosquitoes. Three sets of mosquitoes were taken for the tests on each occasion. Similarly, 10 larvae and pupae were taken each time and each set of experiments were conducted three times. The experiments were conducted 6 times in a year.
Quantification of lipids, glucose, and glycogen
The contents of lipids, glucose and glycogen in field collected and laboratory-reared mosquitoes were measured by using a spectrophotometric method (28). Ten males and females were collected in the test tubes from the cages for the quantification of lipids, glucose, and glycogen. These test tubes were placed in a freezer for 5min to anesthetize the mosquitoes. Each anesthetized mosquito was transferred to a 2ml microfuge vial. Two hundred μl of 2% sodium sulfate solution was dispensed to each microfuge tube containing mosquito. Wings of the adult mosquitoes were removed before grinding. Whole mosquitoes were ground in microfuge vials by pestles for homogenization of the tissue. Similarly, each larva and pupae were homogenized in a 2ml microfuge tube containing 200μl of 2% sodium sulfate solution. Homogenized mosquito was mixed with 1.5ml chloroform-methanol solution (1: 2) and vertex for 1min. The above mixture was centrifuged at 3000rpm for 1 minute. Pellet was retained for glycogen analysis. The supernatant containing glucose and lipids was taken up with a micro-pipette and added in another microfuge of 2ml. Totally, 600μl of deionized water was added to the supernatant and mixed properly. This solution (containing sugars and lipid) was centrifuged at 3000 rpm for one minute. The lipid and sugar layers were distinctly separated. The top fraction (water/methanol) was taken up for sugar analysis and a bottom portion (chloroform) for lipid analysis. The experiments were conducted in triplicates and six times a year.
Lipid determination
The solvent taken up from the bottom portion was evaporated completely in a heating block. Thereafter, 200μl sulphuric acid was added to the tubes and re-heated for 10min to convert the unsaturated lipids to water-soluble sulphonic acid derivatives (28). These developed a deep pink colour after addition of 5mL vanillin–phosphoric acid reagent, read in a microplate reader (Micro-Scan MS 5608) at 525nm. Lipid concentrations were obtained from a standard curve made with soybean oil. Soybean oil of 1mg was dissolved in 1ml of chloroform. Out of which 20, 50, 100, 200 and 400μL were put separately in tubes. These tubes were treated as explained above. The OD has been taken at 525nm. The standard curve was made from the average of three sets of ODs (standard as mentioned above) versus concentration.
Glycogen determination
The precipitate in the first microfuge containing the glycogen was washed with methanol to eliminate residual sugars. Then anthrone was filled up to 5mL level and mixed properly. The tube was heated for 17min and thereafter kept for cooling. The solution was mixed and 200μL was transferred to an untreated, flat-bottomed 96-well plate. The plate was read at 625nm against the blank in microplate reader (Micro-Scan MS 5608). Glycogen concentration was calculated for individual mosquitoes from a standard curve of absorbance of known concentration of glucose as described earlier.
Glucose determination
The top portion of the supernatant was taken up for the glucose analysis. The same was added to the tube and evaporated in a heating block till 0.1ml remained. Anthrone was filled up to 5ml level and mixed properly. The heat breaks down the body sugars into their glucose units. Anthrone binds to the glucose units and turning the mixture green. The anthrone sugar mixture was heated for 17min and left to cool (28). After cooling they were mixed properly. Overall, 200μL was transferred to an untreated, flat-bottomed 96-well plate. The plate was read at 625nm against the blank in a microplate reader (Micro-Scan MS 5608). The standard solution of glucose was prepared by dissolving 1mg of glucose in 1ml of 25% ethanol. About, 25, 50, 100, 200 and 400μl of the above glucose solution were kept in separate tubes. They were processed as mentioned above and optical densities (OD) were taken at 625nm. The standard curve was made from the average of three sets of ODs (standard as mentioned above) versus concentration. Glucose concentrations were calculated for individual mosquitoes from the standard curve of absorbance of known concentrations of glucose.
Statistical Analysis
To analyze the habitat-specific variations between the energy levels i.e. lipid, glycogen, and glucose the data the data were subjected to two-way factorial Analysis of Variance (ANOVA). The Analysis of Variance (ANOVA) was done by using the software MS Excel software.
Results
Lipid
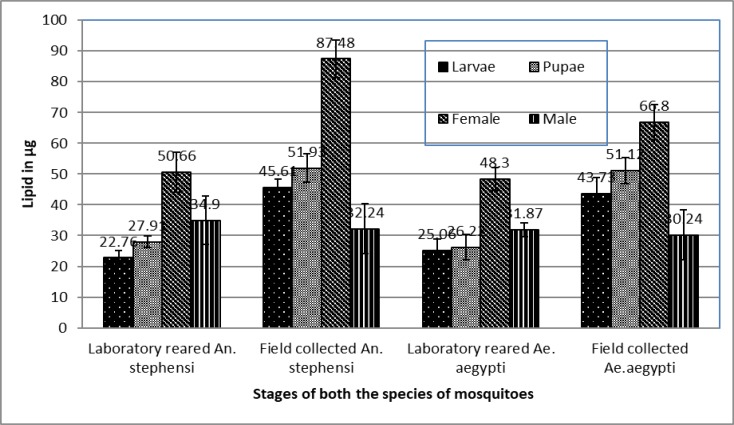
The lipid level in both fields collected and laboratory-reared larvae, pupae, and adults of An. stephensi and Ae. aegypti is shown in Fig. 1. When the lipid contents of laboratory-reared and field-collected mosquitoes of An. stephensi were compared, lipid content in field-collected larvae, pupae, and female An. stephensi was 22.85, 24.02 and 36.82μg more than the laboratory-reared mosquitoes respectively. Similarly when the lipid content was compared between the field collection and laboratory-reared Ae. aegypti, lipid content in field-collected larvae, pupae and females were found to be 18.71, 24.9 and 18.5μg higher than the laboratory-reared mosquitoes respectively. The difference in the lipid content between the laboratory-reared and field-collected mosquitoes of Ae. aegypti was found to be highly significant (ANOVA, F= 14.24, df= 1, 3, P= 0.03). The lipid content in the males of laboratory-reared An. stephensi and Ae. aegypti was 2.66 and 1.73μg higher than the filed collected males respectively.
Fig. 1.
The level of lipid (mean±SE) in different stages of the laboratory-reared and field collected Anopheles stephensi and Aedes aegypti
Glycogen
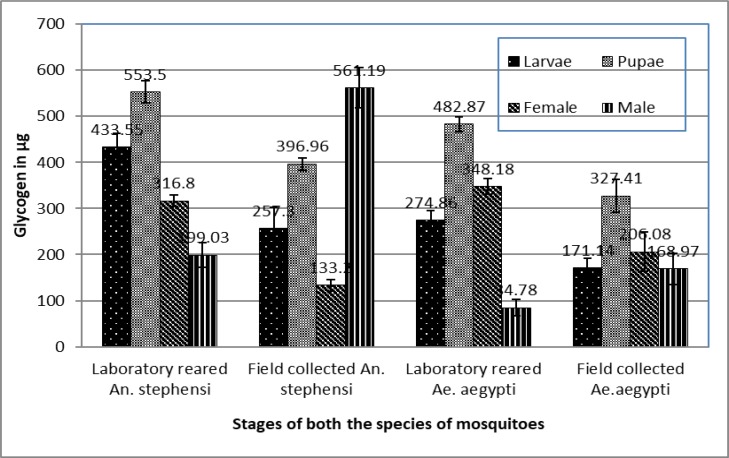
The concentration of glycogen content in different stages of laboratory-reared and field collected stages of An. stephensi and Ae. aegypti are shown in Fig. 2. When the glycogen content in the laboratory-reared and field collected An. stephensi was compared, larvae, pupae, and males of laboratory conditions were 176.25, 156.54 and 183.6μg higher than the conspecific field-collected mosquitoes (Fig. 2). Similarly, the glycogen content in the field collected larvae, pupae, and females Ae. aegypti were 103.72, 55 and 142.1 μg less than the conspecific laboratory reared Ae. aegypti. The glycogen contents in the males of laboratory-reared An. stephensi and Ae. aegypti was 362.16 and 84.19μg lower than the field collected males.
Fig. 2.
The level of glycogen (mean±SE) in different stages of the laboratory-reared and field collected Anopheles stephensi and Aedes aegypti
Glucose
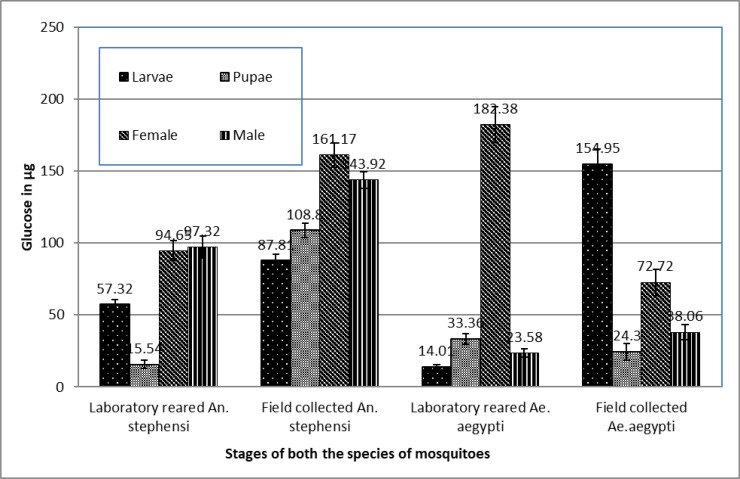
The amount of glucose in the laboratory-reared and field collected aquatic and terrestrial stages of An. stephensi and Ae. aegypti are shown in Fig. 3. When the glucose content in the laboratory-reared and field collected An. stephensi was compared, level of glucose in the field collected mosquitoes was significantly higher than the laboratory-reared mosquitoes (ANOVA, F= 14.24, df= 1, 3, P= 0.03). The level of glucose content in the field collected larvae, pupae, females, and males were 30.49, 93.26, 67.12 and 46.6μg higher than the laboratory-reared mosquitoes respectively (Fig. 3). The glucose content in the laboratory-reared larvae and males of Ae. aegypti was 140.94 and 14.43μg lower than the field-collected mosquitoes. However, the glucose content in the field collected pupae and females of Ae. aegypti were 9.3 and 110.18μg lower than the conspecific laboratory reared Ae. aegypti.
Fig. 3.
The level of glucose (mean±SE) in different stages of the laboratory-reared and field collected Anopheles stephensi and Aedes aegypti
Discussion
The benefit of the laboratory experiments was to estimate energy reserves to guide the implementation of intervention because it is essential to know how closely the physiology and behavior of laboratory maintained individual represent those from the wild. Much of the information on vector biology and vector control in India comes from the terrestrial land and laboratory experimentation. Therefore, we have recorded the energy reserves of the vectors transmitting malaria and dengue in the desert part of India. The lipid content of the field collected (66.8μg) and laboratory maintained (48.3μg) female Ae. aegypti was 196 and 38.2 μg lower in the present study than the value recorded already (29). Wherein, the glycogen (482.87μg) and glucose (182.3μg) content of laboratory maintained female Ae. aegypti was 419.07μg and 118.98μg higher in the present case than the amount presented elsewhere (29). The amount of glycogen, glucose, and lipid was measured in female Ae. aegypti after 72h of feeding on 10% sucrose solution and recorded the values as 62.4, 42.2 and 95.9μg respectively (19). However, the glycogen (482.87μg) and glucose (182.3μg) content in the laboratory-reared Ae. aegypti in the present case was 420.47 and 140μg higher than another value recorded (19) and 47μg lower lipid content was recorded in the present study. The development of insects is heavily regulated by climate and other environmental variables and also vary substantially in response to subtle difference in diet. Variations in energy reserves between the field collected and laboratory-reared mosquitoes may reveal the acquisition of resources and their subsequent assimilation by the larval stages developing in the respective larval habitats under natural conditions, occurred in the present study (30). The variations in the energy contents in the mosquitoes may be due to the habitat and adaptations of the mosquitoes. The energy reserves (glycogen, sugar, and lipid) were compared in four mosquito species in Kolkota, India and recorded higher energy level in females of Ae. aegypti than males (30). This observation is similar to the present study except in the case of glucose level in field-collected male. The glycogen level of both the sexes of Ae. aegypti (male: 0.221mg, female: 0.223 mg) and lipid level (0.038mg) of male Ae. aegypti recorded by (30) were similar to the observation made in the field collected Ae. aegypti of the present study.
The lipid, glucose and glycogen in the An. stephensi mosquitoes fed were measured with 10% glucose solution and recorded the values in laboratory-reared mosquitoes as 97.3, 101.9 and 82.2μg respectively (31). When the values are compared with the present study, lipid (50.66) and glucose (94.65) content in the present study was 46.64μg and 7.25μg lower than the values recorded by (31). The nutritional reserve of laboratory reserved and field collected male Anopheles gambiae and recorded a 5μg high lipid level in the field collected male An. gambiae than the laboratory-reared one (32). We have recorded a 2.7μg higher lipid in the laboratory-reared males (34.9 μg) than the field collected males (32.2μg). This may occurred because both the species responded in a similar fashion to the different larval diets (33). The survival and reproductive strategies of the female mosquito differ from the male in many senses, at least one of related to egg production and maturation (34). Dog biscuit is providing carbohydrates and lipid to the larvae and yeast is imparting protein to the larvae. The viability of larva and pupa does not depend upon the carbohydrates, however growth and molting of the larvae is largely dependent upon the protein source (14). The laboratory-reared larvae were provided the food routinely so they can molt from one stage to other. After the introduction of the Indira Gandhi canal in to the desert of Rajasthan, the water retention has been increased in this desert region. This leads to the establishment of mosquito colonization. Mosquitoes get breeding habitat throughout the year because of various water retention. The surrounding of the desert climate provides better habits for mosquito breeding. The field-collected mosquitoes were with more energy may be due to the presence of open water habitats that provide significantly more micro-invertebrate dietary resources for the larvae. Nutrient reserves of adult mosquitoes obtained during the larval stage plays important role in the longevity of mosquitoes. Larvae supplied with the high food amount resulted in adults with higher longevity.
The cases of malaria and dengue are increased continuously in this region after inception of the Indira Gandhi Canal; therefore, physiology of the mosquito should be investigated and compared with insecticide resistance and also with the incrimination of malaria parasite.
Conclusion
Malaria and dengue in the desert part of India are mainly transmitted by An. stephensi and Ae. aegypti respectively. Energy content of the mosquitoes was compared between two rearing habitats. The lipid content of laboratory-reared An. stephensi and Ae. aegypti was found to be lower than their conspecific field-collected specimens. The glycogen content in the laboratory-reared larvae, pupae and female mosquitoes of both the types were higher than that of their conspecific field-collected specimens. The glucose content of the laboratory-reared An. stephensi was lower than that of the conspecific field-collected specimens. The higher amount of lipid and lower amount of glycogen in field-collected mosquitoes may be because of the availability of food in the natural habitat and adaptation of mosquitoes. The higher amount of lipid in the field collected mosquitoes suggests these are physiologically better equipped to sustain in the desert ecology.
Acknowledgements
The authors are thankful to Mr SK Dhawal, Mr Rohit Prasad Joshi, Mr Trilok Kumar and Mr Mahavir Prasad for providing the technical assistance in the study. The first author is grateful to Indian Council of Medical Research, New Delhi for providing the fund in the form of Intramural-Grant. The authors declare that there is no conflict of interests.
References
- 1.Sharma G, Kapoor H, Chopra M, Kumar K, Agrawal V. (2014) Strong larvicidal potential of Artemisia annua leaf extract against malaria (Anopheles stephensi Liston) and dengue (Aedes aegypti L.) vectors and bioassay-driven isolation of the marker compounds. Parasitol Res. 113: 197–209. [DOI] [PubMed] [Google Scholar]
- 2.Tyagi BK. (2004) A review of the emergence of Plasmodium falciparum dominated malaria in irrigated areas of the Thar Desert India. Acta Trop. 89: 227–239. [DOI] [PubMed] [Google Scholar]
- 3.Tyagi BK, Chaudhary RC, Yadav SP. (1995) Epidemic malaria in Thar desert, India. Lancet. 346: 634–635. [DOI] [PubMed] [Google Scholar]
- 4.Brown JE, Evans BR, Zheng W, Obas V, Barrera-Martinez L, Egizi A, Zhao H, Caccone A, Powell JR. (2014) Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution. 68(2): 514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gloria-Soria A, Ayala D, Bheecarry A, Calderon-Arguedas O, Chadee DD, Chiappero M, Coetzee M, Elahee KB, Fernandez-Salas I, Kamal HA, Kamgang B, Khater EI, Kramer LD, Kramer V, Lopez-Solis A, Lutomiah J, Martins A, Jr, Micieli MV, Paupy C, Ponlawat A, Rahola N, Rasheed SB, Richardson JB, Saleh AA, Sanchez-Casas RM, Seixas G, Sousa CA, Tabachnick WJ, Troyo A, Powell JR. (2016) Global genetic Diversity of Aedes aegypti. Mol Ecol. 25(21): 5377–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brathwaite DO, San Martín JL, Montoya RH, delDiego J, Zambrano B, Dayan GH. (2012) The History of Dengue Outbreaks in the Americas. Am J Trop Med Hyg. 87(4): 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalra NL, Kaul SM, Rastogi RM. (1997) Prevalence of Aedes aegypti and Aedes albopictus-vectors of dengue and dengue haemorrhagic fever in north, northeast and central India. Dengue Bulletin. 21: 84–92. [Google Scholar]
- 8.Tyagi BK, Hiriyan J. (2004) Breeding of Dengue Vector Aedes aegypti (Linnaeus) in Rural Thar Desert, North-western Rajasthan, India. Dengue Bulletin. 28: 220–222. [Google Scholar]
- 9.Chouhan GS, Rodrigues FM, Shaikh BH, Ilkal MA, Khangaro SS, Mathur KN, Joshi KR, Vaidhye NK. (1990) Clinical and virological study of dengue fever outbreak in Jalore City, Rajasthan 1985. Indian J Med Res. 91: 414–418. [PubMed] [Google Scholar]
- 10.Ghosh SN, Shaikh BH. (1974) Investigations on the outbreak of dengue fever in Ajmer City, Rajasthan state in 1969. Part-II: Results of serological tests. Indian J Med Res. 62: 523–533. [PubMed] [Google Scholar]
- 11.Macdonald G. (1957) The Epidemiology and Control of Malaria. Oxford University Press, London. [Google Scholar]
- 12.Banerjee S, Mohan S, Saha N, Mohanty SP, Goutam K, Saha GK, Aditya G. (2015) Pupal productivity and nutrient reserves of Aedes mosquitoes breeding in sewage drains and other habitats of Kolkata, India: implications for habitat expansion and vector management. Indian J Med Res. (142 Suppl): S87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayar JK, Van Handel E. (1971) The fuel for sustained mosquito flight. J Insect Physiol. 17: 471–481. [DOI] [PubMed] [Google Scholar]
- 14.Clements AN. (1992) The biology of mosquitoes. Vol I, Development, Nutrition and Reproduction, Champman and Hall, London. [Google Scholar]
- 15.Suwanchaichinda C, Paskewitz SM. (1998) Effects of larval nutrition, dult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanise Sephadex beads. J Med Entomol. 35: 157–161. [DOI] [PubMed] [Google Scholar]
- 16.Koella JC, Sorensen FL. (2002) Effect of adult nutrition on the melanization immune response of Anopheles stephensi (Diptera: Culicidae). Med Vet Entomol. 16: 1–5. [DOI] [PubMed] [Google Scholar]
- 17.Grimstad PR, Haramis LD. (1984) Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. III. Enhanced oral transmission by nutrition-deprived mosquitoes. J Med Entomol. 21: 249–256. [DOI] [PubMed] [Google Scholar]
- 18.Nasci RS, Mitchell CJ. (1994) Larval diet, adult size, and susceptibility of Aedes aegypti (Diptera, Culicidae) to infection with Ross River virus. J Med Entomol. 31: 123–126. [DOI] [PubMed] [Google Scholar]
- 19.Naksathit AT, Edman JD, Scott TW. (1999) Amounts of glycogen, lipid, and sugar in adult female Aedes aegypti (Diptera: Culicidae) fed sucrose. J Med Entomol. 36: 8–12. [DOI] [PubMed] [Google Scholar]
- 20.Costero AJD, Edman JD, Clark GG, Scott TW. (1999) Survival of starved Aedes aegypti (Diptera: Culicidae) in Puert Rico and Thailand. J Med Entomol. 36: 272–6. [DOI] [PubMed] [Google Scholar]
- 21.Service MW. (1993) Mosquito Ecology Field Sampling Methods. Elsevier Applied Science, New York. [Google Scholar]
- 22.Yadav S, Singh SP, Mittal PK. (2013) Toxicity of Thevetiaperuviana (yellow oleander) against larvae of Anopheles stephensi and Aedes aegypti vectors of malaria and dengue. J Entomol Zool Stud. 1(6): 85–87. [Google Scholar]
- 23.Christophers SR. (1933) The fauna of British India: Diptera. Vol. IV Taylor and Francis, London. [Google Scholar]
- 24.Barraud PJ. (1934) The Fauna of British India: Diptera. Vol. V Taylor and Francis, London. [Google Scholar]
- 25.Belkin JN. (1962) The Mosquitoes of the South Pacific (Diptera, Culicidae). Vol. 1 University of California Press, Los Angeles. [Google Scholar]
- 26.Reuben R, Tewari SC, Hiriyan J, Akiyama J. (1994) Illustrated keys to species of Culex associated with Japanese encephalitis in Southeast Asia (Diptera: Culicidae). Mosquito Systematics. 26(2): 75–96. [Google Scholar]
- 27.Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Jones JW, Coleman RE. (2005) Illustrated keys to the mosquitoes of Thailand. II. Genera Culex and Lutzia. Southeast Asian J Trop Med Public Health. (36 Suppl 2): 1–97. [PubMed] [Google Scholar]
- 28.Kaufmann C, Brown MR. (2008) Regulation of carbohydrate metabolism and flight performance by a hypertrehalosaemic hormone in the mosquito Anopheles gambiae. J Insect Physiol. 54(2): 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day JF, Van Handel E. (1986) Differences between the nutritional reserves of laboratory-maintained and field-collected adult mosquitoes. J Am Mosq Control Assoc. 2: 154–7. [PubMed] [Google Scholar]
- 30.Mohan S, Banerjee S, Pramanik S, Banerjee S, Saha Gk, Aditya G. (2017) Comparative Account Of Energy Reserves In Four Co-Occurring Mosquito Species In Kolkata, India (Diptera: Culicidae). Polish J Entomol. 86: 49–67. [Google Scholar]
- 31.Rivero A, Ferguson HM. (2003) The energetic budget of Anopheles stephensi infected with Plasmodium chabaudi is energy depletion a mechanism for virulence. Proc Biol Sci. 270: 1365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huho BJ, Ng'habi KR, Killeen GF, Nkwengulila G, Knols BGJ, Ferguson HM. (2007) Nature beats nurture: a case study of the physiological fitness of free-living and laboratory-reared male Anopheles gambiaes.l. J Exp Biol. 210: 2939–2947. [DOI] [PubMed] [Google Scholar]
- 33.Takken W, Smallegange RC, Vigneau AJ, Johnston V, Brown M, Mordue-Luntz AJ, Billingsley PF. (2013) Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasit Vectors. 6: 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briegel H. (2003) Physiological bases of mosquito ecology. J Vector Ecol. 28(1): 1–11. [PubMed] [Google Scholar]