Abstract
Osteocalcin is no longer regarded as a molecule exclusive to bone remodeling and osteogenesis, but as a hormone with manifold functions. The discovery of the interaction of osteocalcin with the G protein-coupled receptor family C group 6-member A (GPRC6A) receptor has accompanied the characterization of several roles that this peptide serves in body regulation and homeostasis. These roles include the modulation of memory in the brain, fertility in the testis, fat accumulation in the liver, incretins release in the intestine and adaptation to exercise in muscle, in addition to the well-known effects on β-cell proliferation, insulin release and adiponectin secretion. The aim of the present review was to provide a practical update of the multi-organ effects that osteocalcin exerts through its interaction with GPRC6A and the clinical implications of this.
Keywords: osteocalcin, GPRC6A, glycaemia, diabetes, brain, vascular, liver, muscle, testis, intestine
1. Bone produces osteocalcin
Bone is a dynamic tissue in constant remodeling (resorption and formation) and with a high capacity to regenerate. In addition to providing support to the body, protection for certain organs and enabling locomotion, bone produces molecules that act in an autocrine, paracrine and endocrine manner (1). One such molecule is osteocalcin, the endocrine function of which was discovered 10 years ago. Due to its extensive secretion during bone mineralization, osteocalcin was suspected to be exclusive to bone physiology. However, through studies performed in mice, the role of osteocalcin in metabolic modulation was elucidated (2,3).
Osteocalcin is a small protein (49 amino acids) encoded by the BGLAP gene synthesized by osteoblasts, and is present in two forms: Carboxylated (cOC) and undercarboxylated (ucOC). Only ucOC can signal as a hormone while cOC cannot (2,3).
ucOC and cOC can be measured in plasma separately or as the total osteocalcin (tOC), which includes the two forms independently of their degree of carboxylation, as well as recognizable fragments released when bone resorption occurs. Only 10–30% of the secreted osteocalcin reaches systemic circulation, while the remaining fraction is incorporated into bone matrix. In bone, cOC represents 15% of the non-collagen proteins of the matrix and contains three γ-carboxyglutamic acid residues. On the other hand, ucOC represents one third of tOC. The serum concentration of tOC has been considered a biochemical marker of osteogenesis that reflects the number and activity of osteoblasts (4,5).
2. Identification of the endocrine effect of osteocalcin and its action through GPRC6A
In 2007, Lee et al (6) demonstrated that ucOC increases the insulin secretion and proliferation of pancreatic β-cells, as well as adiponectin secretion from adipose tissue, thereby improving insulin sensitivity in mice. They also demonstrated that osteocalcin reduces fat mass and increases energy expenditure by increasing the expression of genes involved in β-oxidation (Pparα and Foxa2) and in the electron transport chain (Atp5a1, Atp5b, Mt-nd2, Cox and Cyc1). This was the first irrefutable evidence of the participation of osteocalcin in carbohydrate, lipid and energy metabolism (6).
ucOC acts through binding to G protein-coupled receptor family C group 6-member A (GPRC6A). In fact, ucOC and testosterone are the only ligands of Gprc6a that have been validated using genetics in vivo, despite other ligands having been discovered in vitro (7–9). Although well stablished functions of OC through GPRC6A and GPR158 are presented later, a brief description of the expression, localization and function of GPRC6A in human cells and tissues is displayed next.
GPRC6A is expressed in several human, chimpanzee and small species tissues, including brain, lung, liver, heart, kidney, pancreas, skeletal muscle, placenta, spleen, ovary, testis, leukocytes, monocytes and adipocytes. However, the human ortholog GPRC6A is mostly retained intracellularly, in contrast to the cell-surface-expressed murine and goldfish ortholog (9,10).
This intracellular retention occurs in carriers of an insertion/deletion in exon 2 (SNP rs6907580 A/G/T) that eventually leads to a stop-codon early in the receptor sequence at amino acid position 57 (located in the third intracellular loop of GPRC6A), resulting in a non-functional receptor as reported by Jørgensen et al (10). According to this author, the functional variant is much more prevalent in the African population than in European and Asian populations, but further studies are required to elucidate the clinical significance of this allele variation among different populations (10).
As three mRNA isoforms for Gprc6a have been identified (1365, 853 and 1165 bp), the functionality of the GPRC6A receptor may be dependent on a tissue-specific regulation mechanism, which is also the case for other receptors whose function and tissue-specific expression is regulated by alternative splicing (11). GPRC6A mRNA isoform 1 is highly expressed in the brain, skeletal muscle, testis and leucocytes; moderately expressed in the liver, heart, kidney and spleen; and lowly expressed in the lung, pancreas, adipocytes, placenta and ovary. Isoforms 2 and 3 are less abundant and are possibly naturally occurring splice variants (12). Therefore, although the pancreas and adipocytes express low levels of GPRC6A mRNA at the transcriptional level, these are the main organs of ucOC action, suggesting a different mechanism of regulation at other levels (translational and post-translational) or the existence of an ortholog receptor that also partially mediates the action of ucOC.
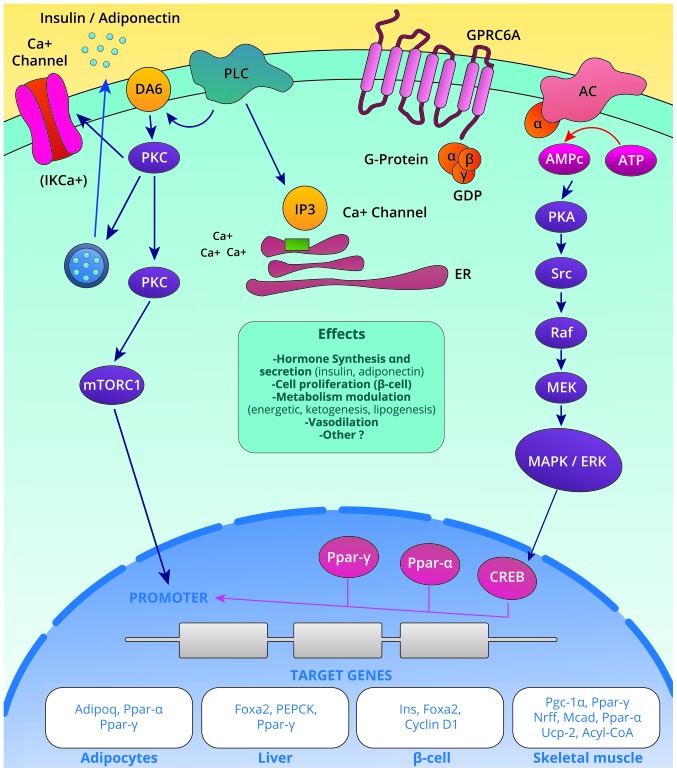
3. Downstream signaling pathways activated by the osteocalcin-GPRC6A interaction
At least two signaling pathways activated by the osteocalcin-GPRC6A interactions have been identified (Fig. 1): i) The IP3-Ca+2 pathway activated by the action of phospholipase C (PLC) that yields the secretion of insulin, adiponectin and possibly other hormones; and ii) the adenylyl cyclase-cAMP-PKA pathway that leads to the activation of the Mek-Erk cascade, thereby promoting functions in cellular proliferation, differentiation and modulation of insulin sensitivity (13).
Figure 1.
Signal transduction triggered by osteocalcin binding to GPRC6A and their final target genes and biological effects. GPRC6A, G protein-coupled receptor family C group 6-member A.
The extracellular signal-regulated kinases (Erk) induce phosphorylation of CREB, which in turn binds to the cAMP response element (CRE) in the Pparγ gene. The Pparγ gene consequently leads to the transactivation of the adiponectin gene (Adipoq) by linking the Pparγ-Rxr heterodimer to the promoter region of the Adipoq gene, resulting in the synthesis of adiponectin (14). Signaling pathways are depicted in Fig. 1. In the pancreas, the binding of osteocalcin to GPRC6A also induces Erk phosphorylation and increases insulin synthesis (7). In Leydig cells, ucOC activates the ERK1/2 signaling pathway, increasing the intracellular calcium content and promoting the production of 25-OH Vitamin D (8).
Furthermore, osteocalcin promotes the nuclear translocation of activated Nrf2, while inhibiting the activation of JNK in the liver; these are two well-described pathways in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) (15).
4. Osteocalcin target tissues
Independently of the tissues where GPRC6A is expressed or is functionally active and, of the discoveries of signaling pathways that it activates, there are clear effects of ucOC in certain tissues and cells that are summarized in Fig. 2 and are described in the following paragraphs.
Figure 2.
Target organs of OC action, and the effects of high and low serum or expression levels. OC, osteocalcin; GLP-1, glucagon-like peptide type 1.
Pi et al (16) reported that knockout mice for the GPRC6A receptor (−/−) and that those that do not express the GPRC6A gene in pancreatic islets (GPRC6Aβ−cell-cko) have a smaller pancreatic islet size, lower insulin content, lower pancreatic weight, lower number of islets, lower insulin mRNA expression and lower insulin secretion in response to osteocalcin. Furthermore, these mice exhibit glucose intolerance with a non-altered sensitivity to insulin. In this way, it was established that the direct activation of GPRC6A by osteocalcin modifies β-cell proliferation and insulin secretion (16). These findings were confirmed by Wei et al (17), who demonstrated that osteocalcin promotes the proliferation of pancreatic β-cells during development and adulthood through GPRC6A in mice (14,17).
In rodent-derived cultured adipocytes, Otani et al (18) demonstrated that ucOC increases the expression of adiponectin by increasing cAMP through GPRC6A activation. This occurs thanks to intracellular ERK signaling, which leads to the expression of Pparγ and the subsequent production of this insulin-sensitizing hormone (14,18).
In skeletal muscle, osteocalcin binds to GPRC6A, favoring the uptake and catabolism of glucose and fatty acids during exercise. In line with this, it stimulates the release of interleukin-6 by the muscle, a molecule that modulates the secretion of ucOC in the bone, increases the hepatic production of glucose and stimulates the release of fatty acids from the adipocyte. During aerobic exercise, circulating levels of ucOC doubled at the time insulin reaches its lowest point. By contract, in aged mice, osteocalcin is required and sufficient to maintain muscle mass. Therefore, osteocalcin participates in the body's adaptation to exercise and aids in maintaining muscle mass (19,20).
In the liver of mice, osteocalcin has been proven to be a deterrent of NAFLD. Following intermittent and continuous intraperitoneal infusion, osteocalcin upregulates the expression of antioxidant genes, including Cat, Sod and Gpx, which encode catalase, superoxide dismutase and glutathione peroxidase. Osteocalcin also decreases the content of triglycerides and reverses the histological damage in the liver of mice with NAFLD (15,20).
A potential interaction of ucOC with GPRC6A in blood vessels is possible but requires clarification, since identification of GPRC6A receptors in the aortic rings of rats and its activation by their agonist ornithine leads to the modulation of ion channels, including the intermediate-conductance Ca2+-dependent K+ (IKCa) channel, which in turn generates myocyte hyperpolarization, which may indicate a potential vasodilatory effect in vivo (17). As this is only an initial observation, vascular actions should be investigated in depth.
In the testicle, ucOC binds to GPRC6A receptors in Leydig cells to induce testosterone biosynthesis. Furthermore, osteocalcin acts through a pancreas-bone-testis axis that regulates male reproductive functions by promoting testosterone production independently of and in parallel with the hypothalamus-pituitary-testis axis (21). Additionally, knockout mice for osteocalcin exhibit low testosterone serum levels and secretion by Leydig cells, in addition to microanatomical and functional abnormalities in the testis, epididymis, seminal vesicles and sperm count (22–24).
GPRC6A receptors have also been observed in the basolateral membranes of intestinal endocrine cells. Here, oral, intraperitoneal and intravenous osteocalcin exerts its action by binding to GPRC6A, which in turn increases the secretion of glucagon-like peptide type 1 (GLP-1) in vitro and its serum levels in mice (25,26). In fact, the concept of a bone-intestine axis is plausible since, in addition to the effect of ucOC on GLP-1 release, certain animal and human studies have demonstrated that GLP-1 stimulates bone formation (the stage where osteocalcin release occurs at a high rate) and reduce bone resorption (25,26). In addition, certain osteoblastic cell lines express the GLP-1 receptor, which is regulated in accordance to the glycemic level (27). Despite this, limitations include the few in vivo studies on this intestine-bone communication and the fact that osteocalcin (−/−) mice exhibit no decrease in serum GLP-1 levels (28).
In the brain, ucOC has several direct and indirect effects, including increasing growth hormone synthesis (29). ucOC serves an important role in memory increasing and decreasing anxiety by binding to the newly identified GPR158 receptor, as described by Prof. Karsenty's group (30,31). Additionally, regions of the brain involved in spatial learning and memory processing exhibit an intense accumulation of osteocalcin. Notably, the brains of mice with low levels of osteocalcin are consistently smaller than those where normal levels of osteocalcin are observed. Additionally, mice with defective production of osteocalcin exhibit higher rates of anxiety-like behavior and cognitive decline, and the two symptoms are fully corrected when osteocalcin is injected into the test subjects (32).
5. Importance of biological experimental findings regarding osteocalcin in human health and disease
There is abundant evidence of the pleiotropic effects of osteocalcin in animal and cellular models; however, replicating these findings in humans is paramount to embarking on meaningful translational research that will elucidate the therapeutic and/or prognostic value of this hormone. For this purpose, several studies have been conducted to assess and support the role of osteocalcin in human health and disease.
Recently, it was reported that the rs2274911 polymorphism in the GPRC6A gene is associated with insulin resistance in healthy weight and obese subjects independently of body mass index (BMI). Carriers of the risk allele A exhibited higher levels of fasting insulin, fasting plasma glucose, HOMA-IR and triglycerides, following correction for sex, age and ucOC levels (33). Furthermore, Oury et al (23) analyzed a cohort of patients with primary testicular failure and identified 2 individuals harboring the same heterozygous missense variant (SNP rs2274911; F464Y) in one of the transmembrane domains of GPRC6A, which prevented the receptor from localizing to the cell membrane. These patients exhibit glucose intolerance, insulin resistance and increased BMI (23). Therefore, the A risk allele of this variant predisposes to metabolic abnormalities and provides evidence of the importance of GPRC6A in human energetic metabolism, as suggested by seven studies (34–40) published in the last 10 years, comparing serum concentrations of osteocalcin among people with type-2 diabetes mellitus (T2DM) and the non-diabetic population. It is clear that lower levels of osteocalcin occur more frequently in T2DM when compared with healthy subjects (Table I). In fact, a recent meta-analysis also suggested that serum tOC levels may be lower among people with T2DM (41). Furthermore, patients with metabolic syndrome also have lower levels of serum tOC than healthy individuals, and an increase in serum tOC levels is associated with a significant mean increase in HOMA-B and a mean reduction of HbA1c, fasting plasma glucose levels, HOMA-IR and BMI (42). Additionally, a significant correlation between tOC and ucOC serum levels exists with markers of glycemic status and other cardio-metabolic parameters (43–50). Table II describes the correlation between tOC and ucOC and these glycemic and cardio-metabolic variables.
Table I.
Comparison of total OC and ucOC concentrations between healthy and diabetic subjects.
| A, Total OC (ng/ml) | ||||
|---|---|---|---|---|
| Author, year | Diabetes | HS | P-value | (Refs.) |
| Pietschmann and Schernthaner, 1988 | 5.2 | 6.6 | 0.03 | (34) |
| Rosato et al, 1998 | 2.5 | 4.4 | 0.0006 | (35) |
| Akin et al, 2003 | 4.44 | 8.82 | 0.05 | (36) |
| Achemlal et al, 2005 | 15.3 | 18.3 | 0.012 | (37) |
| B, ucOC (ng/ml) | ||||
| Author, year | Diabetes | HS | P-value | (Refs.) |
| Sanchez-Enriquez et al, 2017 | 1.5±1.4 | 2.3±1.8 | <0.05 | (38) |
| Díaz-López et al, 2013 | 3.57 | 4.45 | 0.009 | (39) |
| Razny et al, 2016 | 3.04±0.28 | 4.48±0.57 | 0.025 | (40) |
OC, osteocalcin; ucOC, undercarboxylated osteocalcin; HS, healthy subjects.
Table II.
Correlation coefficients of total OC and ucOC serum levels with glycemia, HbA1c, insulin and HOMA-IR, blood pressure, lipids and cIMT.
| Total OC | Undercarboxylated OC | |||||
|---|---|---|---|---|---|---|
| Variable | r | P-value | (Refs.) | r | P-value | (Refs.) |
| Glucose | −0.213 | 0.009 | (45) | −0.283 | 0.006 | (38) |
| −0.085 | 0.025 | (44) | −0.220 | NS | (59) | |
| HbA1c | −0.140 | 0.208 | (46) | −0.228 | (60) | |
| −0.023 | 0.573 | (44) | <0.001 | |||
| Insulin | −0.243 | 0.003 | (45) | −0.108 | <0.001 | (60) |
| HOMA-IR | −0.005 | NS | (47) | −0.349 | <0.05 | (61) |
| −0.144 | <0.001 | (60) | ||||
| SBP | −0.068 | 0.001 | (48) | 0.277 | 0.049 | (38) |
| DBP | −0.077 | <0.001 | (48) | 0.450 | 0.003 | (38) |
| % body fat | −0.240 | <0.05 | (49) | −0.311 | 0.048 | (38) |
| BMI | −0.258 | <0.001 | (48) | −0.310 | 0.046 | (38) |
| c-HDL | 0.097 | <0.001 | (48) | 0.150 | 0.030 | (50) |
| Apo-B/Apo A-1 | −0.01 | 0.850 | (50) | −0.160 | 0.020 | (50) |
| cIMT | −0.145 | 0.041 | (43) | −0.33 | <0.01 | (59) |
OC, osteocalcin; ucOC, undercarboxylated osteocalcin; HS, healthy subjects; HbA1c, glycated hemoglobin; HOMA-IR, homeostasis model assessment-insulin resistance; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; c-HDL, cholesterol contained in High Density Lipoproteins; NS, non-significant; cIMT, carotid intima-media thickness.
Other studies have evaluated the association between osteocalcin serum levels and parameters of atherosclerosis. The majority of studies have reported a significant association between osteocalcin serum levels and determinations of carotid intima-media thickness (cIMT), brachial-ankle wave pulse velocity and carotid plaques in patients with diabetes and healthy subjects (43,44,51–55).
A recent observational study evaluated the association between osteocalcin serum levels and cognitive performance in healthy adults, demonstrating that they were positively correlated with measures of executive functioning and global cognition in older women. The authors reported that lower serum osteocalcin concentrations were associated with brain microstructural changes in the putamen, thalamus and caudate, as well as with poorer cognitive performance (56). These findings have therefore broadened the functions undertaken by osteocalcin to include the brain and neural processing.
Finally, two previous studies evaluated the association between osteocalcin and NAFLD in children and adolescents with and without obesity. Patients with NAFLD exhibited lower serum osteocalcin levels than those in the control group and the osteocalcin concentration were inversely correlated with liver enzymes and the severity of NAFLD. In addition, a serum osteocalcin level below 44.5 ng/ml was revealed to be a good predictor of hepatic steatosis severity with a sensitivity and specificity of 80% (57,58). Furthermore, normoglycemic postmenopausal women with NAFLD exhibited significantly lower serum osteocalcin levels than controls and the serum osteocalcin levels exhibited a negative correlation with the fatty liver index values, even following adjusting for confounding factors (51). In males, NAFLD is negatively associated with serum osteocalcin (53). These observations highlighted the role of osteocalcin as a potential protector against NAFLD development and deterioration, as well as a marker of its progression.
In conclusion, the increasing volume of evidence regarding the multi-organ effect of ucOC, supported by in vivo and in vitro findings, indicates the requirement for deeper approaches to clarify its participation in human health and disease, as well as to test its therapeutic potential. On the other hand, the validation of ucOC as a prognostic or pathogenic marker for metabolic-endocrine disorders remains to be fully elucidated since no universal standardized method for its measurements, nor any reference values, have been established. Therefore, the medical-scientific community must continue to advance efforts to clarify the participation of ucOC in human health and disease and the clinical implications of this.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- BGLAP
gene for osteocalcin
- GPRC6A
G protein-coupled receptor family C group 6-member A
- ucOC
undercarboxylated osteocalcin
Funding
No funding was received.
Availability of data and materials
The datasets used during this review are available from the corresponding author on reasonable request.
Authors' contributions
MCD-F and RF-DL performed the literature search, interpreted the results and wrote the manuscript. JRV-B conceived the review, performed the literature search, interpreted the results, wrote the manuscript and gave final approval of the version to be published.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Moore KL, Dalley AF. Clinically oriented anatomy. Lipp Williams Wilkins. 2013 [Google Scholar]
- 2.Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villafán-Bernal JR, Sánchez-Enríquez S, Muñoz-Valle JF. Molecular modulation of osteocalcin and its relevance in diabetes (Review) Int J Mol Med. 2011;28:283–293. doi: 10.3892/ijmm.2011.706. [DOI] [PubMed] [Google Scholar]
- 4.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 5.Hernández-Gil IFT, Gracia MAA, Pingarrón MDC, Jerez LB. Bases fisiológicas de la regeneración ósea I. Histología y fisiología del tejido óseo. Med Oral Patol Oral Cir Bucal. 2006;11:47–51. [Google Scholar]
- 6.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in β-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26:1680–1683. doi: 10.1002/jbmr.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Toni L, De Filippis V, Tescari S, Ferigo M, Ferlin A, Scattolini V, Avogaro A, Vettor R, Foresta C. Uncarboxylated osteocalcin stimulates 25-hydroxy vitamin D production in Leydig cell line through a GPRC6a-dependent pathway. Endocrinology. 2014;155:4266–4274. doi: 10.1210/en.2014-1283. [DOI] [PubMed] [Google Scholar]
- 9.Clemmensen C, Smajilovic S, Wellendorph P, Bräuner-Osborne H. The GPCR class C, group 6, subtype A (GPRC6A) receptor: Fom cloning to physiological function. Br J Pharmacol. 2014;171:1129–1141. doi: 10.1111/bph.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jørgensen S, Have CT, Underwood CR, Johansen LD, Wellendorph P, Gjesing AP, Jørgensen CV, Quan S, Rui G, Inoue A, et al. Genetic variations in the human G protein-coupled receptor class C, group 6, member A (GPRC6A) control cell surface expression and function. J Biol Chem. 2017;292:1524–1534. doi: 10.1074/jbc.M116.756577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold KA, Eichelbaum M, Burk O. Alternative splicing affects the function and tissue-specific expression of the human constitutive androstane receptor. Nucl Recept. 2004;2:1. doi: 10.1186/1478-1336-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wellendorph P, Bräuner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Ozaki KI, Awazu M, Tamiya M, Iwasaki Y, Harada A, Kugisaki S, Tanimura S, Kohno M. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am J Physiol Endocrinol Metab. 2016;310:E643–E651. doi: 10.1152/ajpendo.00445.2015. [DOI] [PubMed] [Google Scholar]
- 14.Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. 2016;5:1042–1047. doi: 10.1016/j.molmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du J, Zhang M, Lu J, Zhang X, Xiong Q, Xu Y, Bao Y, Jia W. Osteocalcin improves nonalcoholic fatty liver disease in mice through activation of Nrf2 and inhibition of JNK. Endocrine. 2016;53:701–709. doi: 10.1007/s12020-016-0926-5. [DOI] [PubMed] [Google Scholar]
- 16.Pi M, Kapoor K, Ye R, Nishimoto SK, Smith JC, Baudry J, Quarles LD. Evidence for osteocalcin binding and activation of GPRC6A in β-cells. Endocrinology. 2016;157:1866–1880. doi: 10.1210/en.2015-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei J, Hanna T, Suda N, Karsenty G, Ducy P. Osteocalcin promotes β-cell proliferation during development and adulthood through Gprc6a. Diabetes. 2014;63:1021–1031. doi: 10.2337/db13-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otani T, Mizokami A, Hayashi Y, Gao J, Mori Y, Nakamura S, Takeuchi H, Hirata M. Signaling pathway for adiponectin expression in adipocytes by osteocalcin. Cell Signal. 2015;27:532–544. doi: 10.1016/j.cellsig.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galán-Díez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016;23:1078–1092. doi: 10.1016/j.cmet.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–575. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karsenty G, Oury F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol Cell Endocrinol. 2014;382:521–526. doi: 10.1016/j.mce.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le B, Chen H, Zirkin B, Burnett A. New targets for increasing endogenous testosterone production: Clinical implications and review of the literature. Andrology. 2014;2:484–490. doi: 10.1111/j.2047-2927.2014.00225.x. [DOI] [PubMed] [Google Scholar]
- 23.Oury F, Ferron M, Huizhen W, Confavreux C, Wei J, Galán-Díez M, Lacampagne A, Mitchell SJ, Mattison JA, Chen Y, et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J Clin Invest. 2013;123:2421–2433. doi: 10.1172/JCI65952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith LB, Saunders PT. The skeleton: The new controller of male fertility? Cell. 2011;144:642–643. doi: 10.1016/j.cell.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Mizokami A, Yasutake Y, Higashi S, Kawakubo-Yasukochi T, Chishaki S, Takahashi I, Takeuchi H, Hirata M. Oral administration of osteocalcin improves glucose utilization by stimulating glucagon-like peptide-1 secretion. Bone. 2014;69:68–79. doi: 10.1016/j.bone.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Mizokami A, Yasutake Y, Gao J, Matsuda M, Takahashi I, Takeuchi H, Hirata M. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS One. 2013;8:e57375. doi: 10.1371/journal.pone.0057375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao C, Liang J, Yang Y, Yu M, Qu X. The impact of glucagon-like peptide-1 on bone metabolism and its possible mechanisms. Front Endocrinol (Lausanne) 2017;8:98. doi: 10.3389/fendo.2017.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Li K. Osteocalcin induces growth hormone/insulin-like growth factor-1 system by promoting testosterone synthesis in male mice. Horm Metab Res. 2014;46:768–773. doi: 10.1055/s-0034-1371869. [DOI] [PubMed] [Google Scholar]
- 30.Khrimian L, Obri A, Karsenty G. Modulation of cognition and anxiety-like behavior by bone remodeling. Mol Metab. 2017;6:1610–1615. doi: 10.1016/j.molmet.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khrimian L, Obri A, Ramos-Brossier M, Rousseaud A, Moriceau S, Nicot AS, Mera P, Kosmidis S, Karnavas T, Saudou F, et al. Gpr158 mediates osteocalcin's regulation of cognition. J Exp Med. 2017;214:2859–2873. doi: 10.1084/jem.20171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Obri A, Khrimian L, Karsenty G, Oury F. Osteocalcin in the brain: From embryonic development to age-related decline in cognition. Nat Rev Endocrinol. 2018;14:174–182. doi: 10.1038/nrendo.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Nisio A, Rocca MS, Fadini GP, De Toni L, Marcuzzo G, Marescotti MC, Sanna M, Plebani M, Vettor R, Avogaro A, Foresta C. The rs2274911 polymorphism in GPRC6A gene is associated with insulin resistance in normal weight and obese subjects. Clin Endocrinol (Oxf) 2017;86:185–191. doi: 10.1111/cen.13248. [DOI] [PubMed] [Google Scholar]
- 34.Pietschmann P, Schernthaner G. The effect of pirenzepine on growth hormone and blood glucose levels in type I diabetes mellitus. A controlled study in patients on basal bolus insulin treatment. Acta Endocrinol (Copenh) 1988;117:315–319. doi: 10.1530/acta.0.1170315. [DOI] [PubMed] [Google Scholar]
- 35.Rosato MT, Schneider SH, Shapses SA. Bone turnover and insulin-like growth factor I levels increase after improved glycemic control in noninsulin-dependent diabetes mellitus. Calcif Tissue Int. 1998;63:107–111. doi: 10.1007/s002239900498. [DOI] [PubMed] [Google Scholar]
- 36.Akin O, Göl K, Aktürk M, Erkaya S. Evaluation of bone turnover in postmenopausal patients with type 2 diabetes mellitus using biochemical markers and bone mineral density measurements. Gynecol Endocrinol. 2003;17:19–29. doi: 10.1080/713603182. [DOI] [PubMed] [Google Scholar]
- 37.Achemlal L, Tellal S, Rkiouak F, Nouijai A, Bezza A, Derouiche el M, Ghafir D, El Maghraoui A. Bone metabolism in male patients with type 2 diabetes. Clin Rheumatol. 2005;24:493–496. doi: 10.1007/s10067-004-1070-9. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Enriquez S, Ballesteros-Gonzalez IT, Villafán-Bernal JR, Pascoe-Gonzalez S, Rivera-Leon EA, Bastidas-Ramirez BE, Rivas-Carrillo JD, Alcala-Zermeno JL, Armendariz-Borunda J, Llamas-Covarrubias IM, Zepeda-Moreno A. Serum levels of undercarboxylated osteocalcin are related to cardiovascular risk factors in patients with type 2 diabetes mellitus and healthy subjects. World J Diabetes. 2017;8:11–17. doi: 10.4239/wjd.v8.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Díaz-López A, Bulló M, Juanola-Falgarona M, Martínez-González MA, Estruch R, Covas MI, Arós F, Salas-Salvadó J. Reduced serum concentrations of carboxylated and undercarboxylated osteocalcin are associated with risk of developing type 2 diabetes mellitus in a high cardiovascular risk population: A nested case-control study. J Clin Endocrinol Metab. 2013;98:4524–4531. doi: 10.1210/jc.2013-2472. [DOI] [PubMed] [Google Scholar]
- 40.Razny U, Fedak D, Kiec-Wilk B, Goralska J, Gruca A, Zdzienicka A, Kiec-Klimczak M, Solnica B, Hubalewska-Dydejczyk A, Malczewska-Malec M. Carboxylated and undercarboxylated osteocalcin in metabolic complications of human obesity and prediabetes. Diabetes Metab Res Rev. 2017;33 doi: 10.1002/dmrr.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu C, Wo J, Zhao Q, Wang Y, Wang B, Zhao W. Association between serum total osteocalcin level and type 2 diabetes mellitus: A systematic review and meta-analysis. Horm Metab Res. 2015;47:813–819. doi: 10.1055/s-0035-1564134. [DOI] [PubMed] [Google Scholar]
- 42.Kunutsor SK, Apekey TA, Laukkanen JA. Association of serum total osteocalcin with type 2 diabetes and intermediate metabolic phenotypes: Systematic review and meta-analysis of observational evidence. Eur J Epidemiol. 2015;30:599–614. doi: 10.1007/s10654-015-0058-x. [DOI] [PubMed] [Google Scholar]
- 43.Luo Y, Ma X, Hao Y, Xiong Q, Xu Y, Pan X, Bao Y, Jia W. Relationship between serum osteocalcin level and carotid intima-media thickness in a metabolically healthy Chinese population. Cardiovasc Diabetol. 2015;14:82. doi: 10.1186/s12933-015-0245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheng L, Cao W, Cha B, Chen Z, Wang F, Liu J. Serum osteocalcin level and its association with carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2013;12:22. doi: 10.1186/1475-2840-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pirilä S, Taskinen M, Turanlahti M, Kajosaari M, Mäkitie O, Saarinen-Pihkala UM, Viljakainen H. Bone health and risk factors of cardiovascular disease-a cross-sectional study in healthy young adults. PLoS One. 2014;9:e108040. doi: 10.1371/journal.pone.0108040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maser RE, Lenhard MJ, Sneider MB, Pohlig RT. Osteoprotegerin is a better serum biomarker of coronary artery calcification than osteocalcin in type 2 diabetes. Endocr Pract. 2015;21:14–22. doi: 10.4158/EP14229.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giudici KV, PhD, Fisberg RM, Marchioni DML, Peters BSE, Martini LA. Crosstalk between bone and fat tissue: Associations between vitamin D, osteocalcin, adipokines, and markers of glucose metabolism among adolescents. J Am Coll Nutr. 2017;36:273–280. doi: 10.1080/07315724.2016.1274923. [DOI] [PubMed] [Google Scholar]
- 48.Tan A, Gao Y, Yang X, Zhang H, Qin X, Mo L, Peng T, Xia N, Mo Z. Low serum osteocalcin level is a potential marker for metabolic syndrome: Results from a Chinese male population survey. Metabolism. 2011;60:1186–1192. doi: 10.1016/j.metabol.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Wang JW, Tang QY, Ruan HJ, Cai W. Relation between serum osteocalcin levels and body composition in obese children. J Pediatr Gastroenterol Nutr. 2014;58:729–732. doi: 10.1097/MPG.0000000000000243. [DOI] [PubMed] [Google Scholar]
- 50.Alfadda AA, Masood A, Shaik SA, Dekhil H, Goran M. Association between osteocalcin, metabolic syndrome, and cardiovascular risk factors: Role of total and undercarboxylated osteocalcin in patients with type 2 diabetes. Int J Endocrinol. 2013;2013:197519. doi: 10.1155/2013/197519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magni P, Macchi C, Sirtori CR, Corsi Romanelli MM. Osteocalcin as a potential risk biomarker for cardiovascular and metabolic diseases. Clin Chem Lab Med. 2016;54:1579–1587. doi: 10.1515/cclm-2015-0953. [DOI] [PubMed] [Google Scholar]
- 52.Ma H, Lin H, Hu Y, Li X, He W, Jin X, Gao J, Zhao N, Gao X. Serum levels of osteocalcin in relation to glucose metabolism and carotid atherosclerosis in Chinese middle-aged and elderly male adults: The Shanghai Changfeng Study. Eur J Intern Med. 2014;25:259–264. doi: 10.1016/j.ejim.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 53.Yang R, Ma X, Dou J, Wang F, Luo Y, Li D, Zhu J, Bao Y, Jia W. Relationship between serum osteocalcin levels and carotid intima-media thickness in Chinese postmenopausal women. Menopause. 2013;20:1194–1199. doi: 10.1097/GME.0b013e31828aa32d. [DOI] [PubMed] [Google Scholar]
- 54.Reyes G, arcía R, Rozas Moreno P, Muñoz-Torres M. Osteocalcin and atherosclerosis: A complex relationship. Diabetes Res Clin Pract. 2011;92:405–406. doi: 10.1016/j.diabres.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 55.Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, Sugimoto T. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:45–49. doi: 10.1210/jc.2008-1455. [DOI] [PubMed] [Google Scholar]
- 56.Bradburn S, McPhee JS, Bagley L, Sipila S, Stenroth L, Narici MV, Pääsuke M, Gapeyeva H, Osborne G, Sassano L, et al. Association between osteocalcin and cognitive performance in healthy older adults. Age Ageing. 2016;45:844–849. doi: 10.1093/ageing/afw137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amin S, El Amrousy D, Elrifaey S, Gamal R, Hodeib H. Serum osteocalcin levels in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2018;66:117–121. doi: 10.1097/MPG.0000000000001768. [DOI] [PubMed] [Google Scholar]
- 58.Abd-Allah Ebrahim HT, El-Behery EG. Osteocalcin: A new biomarker for non alcoholic fatty liver disease (NAFLD) in children and adolescents. Clin Med Biochem. 2017;3:133. [Google Scholar]
- 59.Zhang Q, Riddle RC, Clemens TL. Bone and the regulation of global energy balance. J Intern Med. 2015;277:681–689. doi: 10.1111/joim.12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iki M, Tamaki J, Fujita Y, et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos Int. 2012;23:761–770. doi: 10.1007/s00198-011-1600-7. [DOI] [PubMed] [Google Scholar]
- 61.Villafán-Bernal JR, Llamas-Covarrubias MA, Muñoz-Valle JF, et al. A cut-point value of uncarboxylated to carboxylated index is associated with glycemic status markers in type 2 diabetes. J Investig Med. 2014;62:33–36. doi: 10.2310/JIM.0000000000000015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during this review are available from the corresponding author on reasonable request.