Abstract
Visfatin, an adipocytokine and cytosolic enzyme with nicotinamide phosphoribosyltransferase (Nampt) activity, is involved in the pathogenesis of numerous metabolic disorders. In addition, the nuclear receptor peroxisome proliferator-activated receptor-γ (PPAR-γ) serves important roles in anti-inflammatory reactions and regulates glucose and lipid metabolism. The aim of the present study was to investigate the effect of interleukin-6 (IL-6) on the expression and secretion of visfatin in BeWo cells, and to determine whether the PPAR-γ pathway is involved in the regulation of visfatin by IL-6. Therefore, BeWo cells were stimulated with serial concentrations of IL-6 or pioglitazone, and the expression levels of visfatin and PPAR-γ were determined by reverse transcription-quantitative polymerase chain reaction and western blotting. The results of the present study demonstrated that IL-6 downregulated the mRNA levels of visfatin and PPAR-γ, which were strongly associated. Activation of PPAR-γ by pioglitazone resulted in significantly increased expression of visfatin, which abrogated the inhibitory effect of IL-6 on visfatin in BeWo cells. Furthermore, treatment using pioglitazone alone increased the expression and secretion of the visfatin protein, compared with the control or IL-6 alone group. In summary, the findings of the present study suggested that IL-6 inhibited the expression of visfatin and PPAR-γ at the transcriptional level; in addition, activation of PPAR-γ upregulated visfatin at the mRNA and protein expression levels. Therefore, the PPAR-γ signaling pathway may be involved in the regulation of visfatin by IL-6 in BeWo cells. These results may provide novel insight into the roles of visfatin in trophoblastic cells. Furthermore, thiazolidinedione pioglitazone, by upregulating visfatin expression, may promote the energy metabolism of trophoblastic cells, maintain the function of the placenta and improve the outcome of pregnancy.
Keywords: visfatin, Nampt, PPAR-γ, IL-6, inflammation
Introduction
Visfatin, additionally termed pre-B-cell colony-enhancing factor or nicotinamide phosphoribosyltransferase (Nampt), is an adipocytokine that is predominantly produced in visceral adipose tissue (1–3); however, during pregnancy, visfatin is additionally expressed and secreted by the placental tissue (4,5). Visfatin is involved in the pathogenesis of various metabolic disorders; increased plasma concentrations of visfatin have been reported in individual with obesity, as well as in patients with gestational diabetes mellitus (GDM) or metabolic syndromes (6–8).
Visfatin functions as an immunomodulatory cytokine involved in the inflammatory responses, and is a factor associated with obesity, inflammation and insulin resistance (3,6). Upregulation of inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6), serves important roles in the induction of insulin resistance (9). Numerous previous studies indicated that visfatin was associated with inflammation (10–12); however, the underlying molecular mechanisms remain unknown. Furthermore, visfatin functions as the rate-limiting enzyme of NAD+ biosynthesis and converts nicotinamide into nicotinamide mononucleotide, a key NAD+ intermediate (13). In addition, visfatin-mediated NAD+ biosynthesis in adipocytes is an important physiological regulator of the metabolic function of adipose tissue and whole-body (13). Although still controversial, visfatin was reported to exert insulin mimetic or sensitizing effects; similar to insulin, visfatin increases lipogenesis, enhances glucose uptake of myocytes and adipocytes, and inhibits hepatocyte glucose release, consequently contributing to glucose and lipid metabolism (12,14,15). Previous studies identified the upregulation of IL-6 in the placenta of patients with GDM (16,17). In addition, it was indicated that the expression of visfatin in placenta was unchanged in patients with GDM (18). IL-6 (16,17) and visfatin (8,9) are associated with GDM. Kralisch et al (19) observed that IL-6 suppressed the mRNA synthesis of visfatin in 3T3-L1 adipocytes; however, in human adipocytes and amniotic epithelial cells, IL-6 significantly promoted visfatin protein expression (20,21). Therefore, the present study hypothesized that the modulation of visfatin expression by IL-6 may be tissue specific; however, how pro-inflammatory IL-6 regulates the expression of visfatin in placenta remains unknown.
Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a nuclear receptor that is abundantly expressed in adipocytes, where it regulates adipogenesis and lipid homeostasis (22). PPAR-γ functions in other tissues by promoting anti-immune responses and lipid metabolism in macrophages, increasing lipid storage in the liver and enhancing glucose-stimulated insulin secretion in pancreatic β-cells (22,23). PPAR-γ is involved in the pathophysiology of GDM and serves key roles in the regulation of glucose metabolism, lipid homeostasis and anti-inflammatory processes (22,23). Previous studies identified the downregulation of PPAR-γ in the trophoblast cells of patients with GDM (24–26). Mayi et al (12) observed that synthetic PPAR-γ ligands upregulated the expression of visfatin gene in a PPAR-γ-dependent manner in resting primary human macrophages and adipose tissue macrophages, but not in adipocytes; however, whether PPAR-γ ligands induce the gene expression of visfatin in placenta remains unknown.
BeWo cells possess characteristics of the syncytiotrophoblast and extravillous trophoblast, and serve as an in vitro model to investigate trophoblast fusion (27,28). BeWo cells are frequently used to investigate the regulation of transplacental transport and cellular activities and uptake mechanisms (28). The aim of the present study was to determine the effect of IL-6 on the expression and secretion of visfatin in BeWo cells, and to determine whether the PPAR-γ pathway is involved in the regulation of visfatin by IL-6.
Materials and methods
Cell culture and treatment
All materials were purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA) unless otherwise stated. BeWo cells were obtained from the National Infrastructure of Cell Line Resource (Beijing, China). The cells were cultured in Ham's F12 medium supplemented with 10% fetal bovine serum (ScienCell Research Laboratories, Inc., San Diego, CA, USA), 1% L-glutamine, 1% penicillin and 1% streptomycin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) at 37°C in a humidified atmosphere of 5% CO2. BeWo cells were seeded at a density of 2×106 cells/ml in six-well plates or 25 cm2 culture flasks (Corning Incorporated, Corning, NY, USA). Cells in the logarithmic growth phase were incubated at 37°C for 12, 24, 48 and 72 h with recombinant human IL-6 (cat. no. 200-06; PeproTech, Inc.) at serial concentrations (0, 1, 5 and 10 ng/ml). Additionally, BeWo cells were treated with the PPAR-γ ligand pioglitazone (cat. no. HY-14601; MedChem Express Co., Ltd., Monmouth Junction, NJ, USA) at serial concentrations (0.1, 1 and 10 µM) or dimethyl sulfoxide as a control at 37°C for 48 h. Subsequently, BeWo cells were incubated with recombinant human IL-6 (5 ng/ml) and/or the PPAR-γ agonist pioglitazone (10 µM), or dimethyl sulfoxide as a control at 37°C for 48 h. The supernatants were collected, and levels of secreted visfatin were determined using an ELISA kit (cat. no. KE1720; ImmunoWay Biotechnology Company, Plano, TX, USA) according to the manufacturer's protocols. Additionally, BeWo cells were treated with recombinant human visfatin (cat. no. 130-09; PeproTech, Inc., Rocky Hill, NJ, USA) at serial concentrations (0, 10, 50 and 100 ng/ml) at 37°C for 48 h. The secretion levels of IL-6 and TNF-α were determined using an IL-6 ELISA kit (cat. no. EHC007; Xinbosheng Biotechnology Co., Ltd., Beijing, China) and TNF-α ELISA kit (cat. no. EHC103a; Xinbosheng Biotechnology Co., Ltd.) respectively, according to the manufacturer's protocol.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from BeWo cells using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was reverse transcribed into cDNA using the Easy Script First Strand cDNA Synthesis Super Mix kit (Beijing Transgen Biotech Co., Ltd., Beijing, China) according to the manufacturer's protocol. RT was performed with a total of 20 µl reaction mixture at 42°C for 15 min and 95°C for 3 min. Specific primers designed for the amplification of visfatin, PPAR-γ, and GAPDH were verified by NCBI BLAST (http://blast.ncbi.nlm.gov/Blast.cgi). The sequences were as follows: Human visfatin, forward, 5′-GCCAGCAGGGAATTTTGTTA-3′ and reverse, 5′-TGATGTGCTGCTTCCAGTTC-3′; human PPAR-γ, forward, 5′-GCCCTTCACTACTGTTGACTTCT-3′ and reverse, 5′-CAGGCTCCACTTTGATTGC-3′; and human GAPDH, forward, 5′-TGAACGGGAAGCTCACTG-3′ and reverse, 5′-GCTTCACCACCTTCTTGATG-3′. Reactions were performed on an ABI PRISM 7300 PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) using PowerUp™ SYBR Green Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR was performed with a total of 20 µl reaction mixture at 50°C for 2 min and 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Gene expression levels were analyzed in triplicate and normalized to GAPDH using the 2−∆∆Cq method (29).
Western blot analysis
Treated BeWo cells were washed twice using ice-cold PBS, and incubated using lysis buffer [1% NP-40, 150 mM NaCl, 50 mMTris (pH 8.0), 0.1% aprotinin, 0.1% leupeptin, 0.035% pepstatin A and 100 µg/ml PMSF] supplemented with protease and phosphatase inhibitor cocktail (Beijing Solarbio Science & Technology Co., Ltd.) at 4°C for 30 min. The samples were centrifuged at 11,600 × g at 4°C for 30 min, and protein concentrations were determined using a bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.). The protein samples were subsequently denatured in SDS sample buffer (125 mM Tris-HCl, pH 6.8, 50% glycerol, 2% SDS, 5% β-mercaptoethanol and 0.01% bromophenol blue) at 100°C for 10 min. Equal amounts of protein (30 µg/lane) were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Subsequently, the membranes were blocked in Tris-buffered saline with 5% non-fat milk at room temperature for 4 h. The membranes were incubated with rabbit anti-human primary antibodies raised against visfatin (1:250; cat. no. ab45890; Abcam, Cambridge, MA, USA) and GAPDH (1:5,000; cat. no. AP0063; Bioworld Technology, Inc., Louis Park, MN, USA) overnight at 4°C, followed by incubation with a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G secondary antibody (1:5,000; cat. no. L3012-2; Signalway Antibody, LLC, College Park, MD, USA) at room temperature for 2 h. The bands were visualized using an enhanced chemiluminescence detection kit (GE Healthcare, Chicago, IL, USA). Band densities were quantified using Bio-Rad ChemiDoc™ XRS+System with Image Lab™ software version 4.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The experiments were repeated at least three times.
Statistical analysis
The experiments were repeated at least three times. Data were presented as the mean ± standard deviation and analyzed using SPSS 21.0 (IBM Corp., Armonk, NY, USA). Groups were compared by one-way analysis of variance and a Student-Newman-Keuls post-hoc test for normally distributed data. Correlation analysis was performed using Spearman's or Pearson's correlation coefficients. P<0.05 was considered to indicate a statistically significant difference.
Results
IL-6 reduces the expression of visfatin gene in BeWo cells
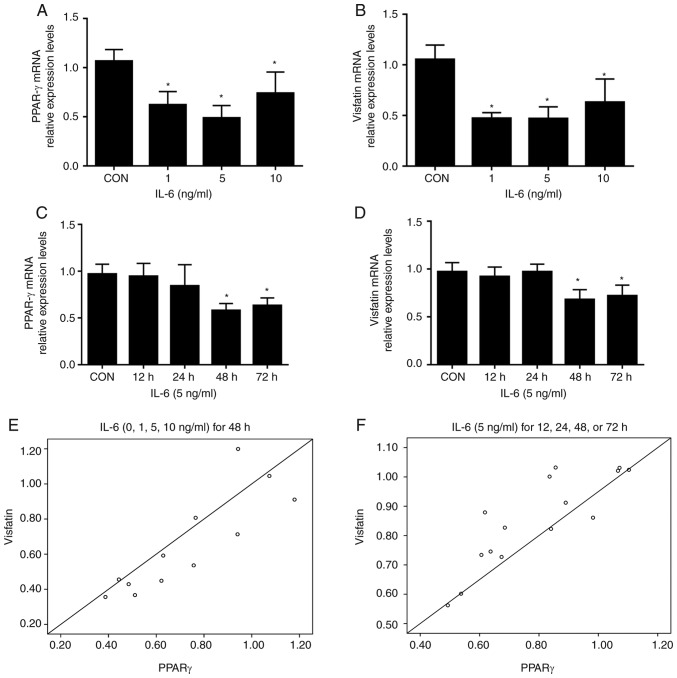
Treatment of BeWo cells with increasing concentrations of IL-6 (1, 5, and 10 ng/ml) resulted in significantly reduced levels of PPAR-γ and visfatin mRNA compared with the control; no further decrease was detected with 10 ng/ml IL-6 (Fig. 1A and B). Time course experiments demonstrated that the inhibitory effects of IL-6 (5 ng/ml) on visfatin and PPAR-γ mRNA peaked at 48 h; no further reductions were observed at 72 h (Fig. 1C and D). Pearson correlation analysis demonstrated that the mRNA levels of visfatin were positively correlated with the expression of PPAR-γ (r=0.857, P<0.05; r=0.854, P<0.001, Fig. 1E and F, respectively). In summary, the data of the present study demonstrated that IL-6 inhibited the expression of visfatin in BeWo cells, which was associated with PPAR-γ.
Figure 1.
IL-6 regulates the expression levels of PPAR-γ and visfatin in BeWo cells. BeWo cells were incubated with IL-6 (0–10 ng/ml) for 48 h, and in the presence or absence of IL-6 (5 ng/ml) for 12, 24, 48 and 72 h. (A and B) IL-6 reduced the mRNA levels of PPAR-γ and visfatin. (C and D) Time course experiments indicated the inhibitory effects of IL-6 (5 ng/ml) on visfatin and PPAR-γ mRNA, which peaked at 48 h. (E and F) Expression levels of visfatin were correlated with the expression of PPAR-γ. *P<0.05 vs. CON. CON, control; IL-6, interleukin 6; PPAR-γ, peroxisome proliferator-activated receptor-γ.
PPAR-γ agonist pioglitazone induces the expression of visfatin gene of BeWo cells
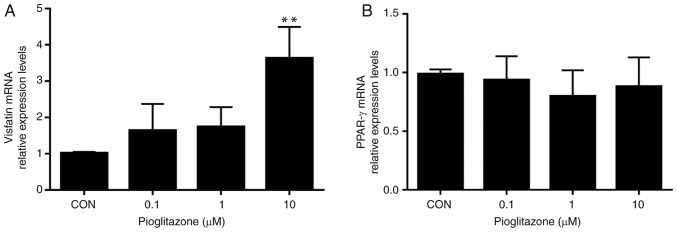
Treatment with increasing concentrations of pioglitazone (0.1, 1 and 10 µM) upregulated the mRNA expression levels of visfatin in a dose-dependent manner, whereas, no significant differences were observed in the mRNA levels of PPAR-γ among the treatment groups (Fig. 2). Furthermore, activation of PPAR-γ by exposure to pioglitazone (10 µM) for 48 h resulted in significantly increased expression of visfatin compared with the control, which abrogated the inhibitory effects of IL-6 on visfatin in BeWo cells (Fig. 3A). In summary, the results of the present study demonstrated that visfatin may be a target gene of PPAR-γ in BeWo cells and that the PPAR-γ pathway may be involved in the regulation of visfatin by IL-6.
Figure 2.
Effects of PPAR-γ agonist pioglitazone on the expression levels of visfatin and PPAR-γ in BeWo cells. The mRNA levels of visfatin and PPAR-γ were analyzed by reverse transcription-quantitative polymerase chain reaction. (A) PPAR-γ agonist pioglitazone induced the expression of visfatin in BeWo cells in a dose-dependent manner. (B) No significant differences were observed in the mRNA expression levels of PPAR-γ among the treatment groups. The results were representative of at least three independent experiments. Data were presented as the mean ± standard deviation. **P<0.01 vs. CON, and 0.1 and 1 µM pioglitazone. CON, control; PPAR-γ, peroxisome proliferator-activated receptor-γ.
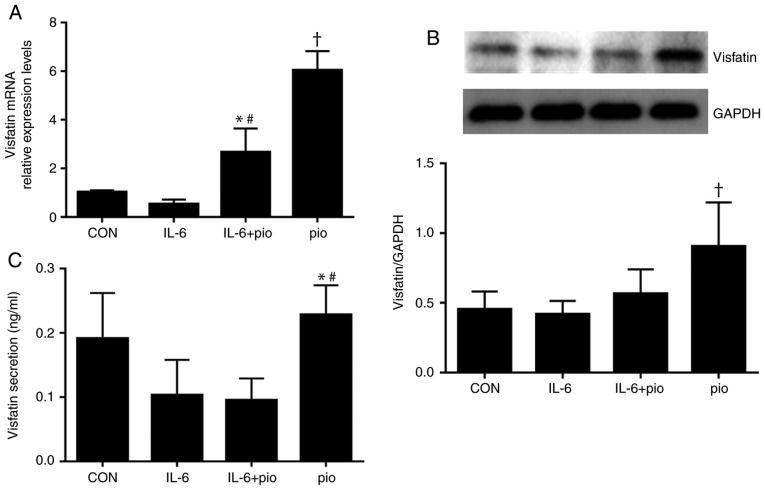
Figure 3.
Effects of pio and IL-6 on the expression of visfatin in BeWo cells. BeWo cells were treated with IL-6 (5 ng/ml) and/or pio (10 µM) for 48 h. (A and B) Pio significantly upregulated the expression levels of visfatin, which abrogated the inhibitory effects of IL-6 on visfatin in BeWo cells. *P<0.05 vs. IL-6; #P<0.05 vs. CON; †P<0.05 vs. CON, IL-6 and IL-6 + pio. (C) Treatment with pio significantly increased the secretion levels of visfatin in BeWo cell compared with IL-6 alone or in combination with pio. *P<0.05 vs. IL-6; #P<0.05 vs. IL-6 + pio. Data were presented as the mean ± standard deviation. CON, control; IL-6, interleukin 6; pio, pioglitazone.
PPAR-γ activation regulates the expression and secretion of visfatin protein of BeWo cells
To investigate whether pioglitazone-induced expression of visfatin resulted in increased protein levels, BeWo cells treated with IL-6 (5 ng/ml) and/or pioglitazone (10 µM) for 48 h were subjected to western blot analysis. IL-6 exhibited no significant effect on the protein expression of visfatin, while activation of PPAR-γ resulted in significantly increased protein expression levels of visfatin compared with the control, IL-6 treatment alone or in combination with pioglitazone (Fig. 3B); however, the expression levels of PPAR-γ protein were not affected by IL-6 or pioglitazone (data not shown). To examine whether induction by pioglitazone promoted the secretion of visfatin secretion, the ability of PPAR-γ to stimulate the release of visfatin was evaluated. As presented in Fig. 3C, treatment with pioglitazone (10 µM) significantly increased the levels of visfatin in BeWo cell compared with IL-6 alone (5 ng/ml) or in combination with pioglitazone. The findings suggested that pioglitazone upregulated the expression level of visfatin by activating the PPAR-γ pathway and the PPAR-γ pathway may be involved in the regulation of visfatin by IL-6.
Discussion
In the present study, the expression of visfatin in BeWo cells was evaluated in the presence of IL-6. Downregulation of visfatin was observed in BeWo cells treated with IL-6, which was consistent with the findings in 3T3-L1 adipocytes by Kralisch et al (19). The present study additionally identified that IL-6 reduced the mRNA expression level of PPAR-γ in BeWo cells and was associated with the expression of visfatin. The PPAR-γ agonist pioglitazone induced the expression of visfatin in BeWo cells, possibly in a PPAR-γ-dependent manner, which was consistent with the findings by Mayi et al (12) in resting primary human macrophages and adipose tissue macrophages. To the best of our knowledge, the present study is the first to demonstrate that visfatin may be a target gene of PPAR-γ in BeWo cells. In addition, the PPAR-γ pathway could be involved in the regulation of visfatin by IL-6; however, Lv et al (15) reported that pioglitazone ameliorates insulin resistance by downregulating visfatin expression in visceral adipose tissue and reducing circulating visfatin in high-fat-fed rats, whereas, no effect of pioglitazone was observed during the differentiation of 3T3-L1 adipocytes. This discrepancy may be due to the different sources of cells and tissues, which indicates that the effects of pioglitazone on visfatin expression could be cell- or tissue-specific.
PPARs are a member of the nuclear hormone receptor superfamily consisting of three isotypes: PPAR-α, PPAR-δ and PPAR-γ. All three PPAR isotypes are expressed in the placenta (30). The expression levels of PPAR-γ were downregulated in the placenta of females with GDM; however, that of PPAR-δ remained unchanged. A previous study demonstrated that PPAR-α agonist fenofibrate stimulated the expression of PPAR-α and induced the mRNA expression of visfatin in visceral fat deposits of Otsuka Long-Evans Tokushima fatty rats (31). The protein expression levels of PPAR-α in placenta were reduced, whereas, the mRNA levels were unchanged in females with GDM (24). Therefore, PPAR-γ was selected as the potential target in the present study. A chemical agonist of PPARs was easier to obtain compared with overexpression vectors and/or PPAR small interfering RNAs, thus, pioglitazone was used in the present study.
The results of the present study demonstrated that IL-6 downregulated PPAR-γ and visfatin at the transcriptional level; the expression levels of visfatin and PPAR-γ were strongly correlated in BeWo cells. In addition, visfatin and PPAR-γ expression was reduced as the concentration of IL-6 increased between 0 and 5 ng/ml in BeWo cells, but their expression levels increased as the concentration of IL-6 reached 10 ng/ml; however, the expression levels of visfatin and PPAR-γ were significantly decreased compared with control group. The results of the present study suggested that the inhibitory effect of IL-6 on visfatin and PPAR-γ did not occur in a dose-dependent manner. The underlying mechanisms require further investigation.
Following treatment with pioglitazone, the mRNA and protein expression levels of visfatin were significantly increased in a dose-dependent manner in the present study. Therefore, visfatin may serve within trophoblastic cells as a protective factor, and may be involved in the regulation of energy metabolism and inflammatory reactions. It is well-established that visfatin/Nampt promotes intracellular NAD+ synthesis and induces the activation of sirtuin1 (12,32–34). Furthermore, placental visfatin/Nampt may additionally serve as the Nampt enzyme within trophoblasts (34). The overexpression of visfatin/Nampt may increase intracellular NAD+ and stimulate sirtuin1, consequently affecting DNA repair, protecting telomeres and reducing inflammation (34). NAD+ is an important coenzyme in numerous oxidation-reduction reactions (34). NAD+ accepts and donates electrons in reactions, thus leading to the production of ATP, which is required by cells for most energy-consuming processes (34). Sirtuin1, additionally termed NAD+-dependent deacetylase, is a negative regulator of pro-inflammatory cytokines (35–37). In summary, visfatin/Nampt may be the regulatory factor underlying the survival of trophoblastic cells and maintenance of placental function in a hostile environment, including the inflammation response or hyperglycemia. Upon inflammation, reduced expression of visfatin may lead to disrupted placental function and increased risk of abortion, preterm birth, and/or fetal distress (33).
However, treatment with the PPAR-γ agonist pioglitazone induced the mRNA and protein expression of visfatin by increasing the secretion of visfatin and offsetting the inhibitory effect of IL-6 at the mRNA level. The findings of the present study suggested that visfatin was a potential target gene of PPAR-γ in BeWo cells, and pioglitazone upregulated the expression level of visfatin by activating the PPAR-γ pathway. Additionally, the mRNA expression levels of PPAR-γ remained unchanged following the treatment of pioglitazone. Subsequent to being activated by its ligands, PPAR-γ forms a heterodimer with the retinoic X receptor, which transactivates the PPAR-response elements of target genes involved in insulin sensitivity, glucose metabolism and immune responses (12,38,39). Therefore, upon activation, PPAR-γ upregulates its target genes, including visfatin, rather than promoting its own transcription. Furthermore, treatment with pioglitazone alone for 48 h resulted in increased secretion of visfatin compared with IL-6 alone or in combination with pioglitazone; however, the expression levels of visfatin in all groups remained low, indicating that visfatin may serve as an autocrine/paracrine factor rather than a typical endocrine agent in trophoblastic cells. Therefore, the present study hypothesized that visfatin was able to exert local effects in the placenta. Additionally, the activation of PPAR-γ suppressed the activity of nuclear factor-κB, consequently regulating the balance between cytokine production and lipid metabolism (40); however, the present study indicated that treatment with visfatin for 48 h did not alter the secretion levels of inflammatory cytokines, including IL-6 and TNF-α (data not shown). In summary, the results of the present study provide novel insight into the roles of visfatin in trophoblastic cells. Placental visfatin may serve as the Nampt enzyme by increasing NAD+ biosynthesis and activating sirtuin1, consequently functioning as an anti-inflammatory factor rather than a pro-inflammatory factor, and serving an important role in maintaining the energy metabolism of trophoblastic cells and the function of the placenta.
In conclusion, the findings of the present study suggested that IL-6 downregulated visfatin and PPAR-γ at the transcriptional level; in addition, activation of PPAR-γ induced the expression of visfatin in BeWo cells. The thiazolidinedione pioglitazone may promote the energy metabolism of trophoblastic cells, maintain the function of the placenta and improve the outcome of pregnancy; however, further study is required to confirm whether visfatin may be a target gene of PPAR-γ in BeWo cells. The roles of visfatin as a Nampt enzyme, as well as its downstream signaling pathway and underlying mechanisms in trophoblastic cells or placenta, require further investigation.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- GDM
gestational diabetes mellitus
- Nampt
nicotinamide phosphoribosyltransferase
- PPAR-γ
peroxisome proliferator-activated receptor γ
- IL-6
interleukin 6
- TNF-α
tumor necrosis factor-α
Funding
The present study was supported by the Hebei Natural Science Foundation (grant no. H2016307035).
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Authors' contributions
YZ and SL conducted the experiments, were involved in data collection and drafted the manuscript. YH and WH performed the statistical analyses and contributed to the study design. HL and LL helped with the literature review and collected the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shi KL, Qian JY, Qi L, Mao DB, Chen Y, Zhu Y, Guo XG. Atorvastatin antagonizes the visfatin-induced expression of inflammatory mediators via the upregulation of NF-κB activation in HCAECs. Oncol Lett. 2016;12:1438–1444. doi: 10.3892/ol.2016.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kocełak P, Olszanecka-Glinianowicz M, Owczarek AJ, Krupa W, Obirek P, Bożentowicz-Wikarek M, Brzozowska A, Mossakowska M, Zdrojewski T, Skalska A, et al. Plasma visfatin/nicotinamide phosphoribosyltransferase (visfatin/NAMPT) concentration in elderly subjects with metabolic syndrome. Pol Arch Med Wewn. 2015;125:402–413. doi: 10.20452/pamw.2878. [DOI] [PubMed] [Google Scholar]
- 3.Owczarek AJ, Olszanecka-Glinianowicz M, Kocełak P, Bożentowicz-Wikarek M, Brzozowska A, Mossakowska M, Puzianowska-Kuźnicka M, Grodzicki T, Więcek A, Chudek J. The relationship between circulating visfatin/nicotinamide phosphoribosyltransferase, obesity, inflammation and lipids profile in elderly population, determined by structural equation modeling. Scand J Clin Lab Invest. 2016;76:632–640. doi: 10.1080/00365513.2016.1230884. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y, Cheng Y, Wang J, Cheng H, Zhou S, Li X. The changes of visfatin in serum and its expression in fat and placental tissue in pregnant women with gestational diabetes. Diabetes Res Clin Pract. 2010;90:60–65. doi: 10.1016/j.diabres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Porter B, Babbar S, Ye SQ, Maulik D. The role of nicotinamide phosphoribosyltransferase in pregnancy: A review. Am J Perinatol. 2016;33:1327–1336. doi: 10.1055/s-0036-1582448. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Chen S, Gao H, Ren L, Song G. Visfatin induces the apoptosis of endothelial progenitor cells via the induction of pro-inflammatory mediators through the NF-κB pathway. Int J Mol Med. 2017;40:637–646. doi: 10.3892/ijmm.2017.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vejrazkova D, Lischkova O, Vankova M, Stanicka S, Vrbikova J, Lukasova P, Vcelak J, Vacinova G, Bendlova B. Distinct response of fat and gastrointestinal tissue to glucose in gestational diabetes mellitus and polycystic ovary syndrome. Physiol Res. 2017;66:283–292. doi: 10.33549/physiolres.933366. [DOI] [PubMed] [Google Scholar]
- 8.Liang Z, Wu Y, Xu J, Fang Q, Chen D. Correlations of serum visfatin and metabolisms of glucose and lipid in women with gestational diabetes mellitus. J Diabetes Investig. 2016;7:247–252. doi: 10.1111/jdi.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosseinzadeh-Attar MJ, Golpaie A, Foroughi M, Hosseinpanah F, Zahediasl S, Azizi F. The relationship between visfatin and serum concentrations of c-reactive protein, interleukin 6 in patients with metabolic syndrome. J Endocrinol Invest. 2016;39:917–922. doi: 10.1007/s40618-016-0457-1. [DOI] [PubMed] [Google Scholar]
- 10.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 11.Kendal CE, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor (Pbef/Visfatin) gene expression is modulated by NF-kappaB and AP-1 in human amniotic epithelial cells. Placenta. 2007;28:305–314. doi: 10.1016/j.placenta.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Mayi TH, Duhem C, Copin C, Bouhlel MA, Rigamonti E, Pattou F, Staels B, Chinetti-Gbaguidi G. Visfatin is induced by peroxisome proliferator-activated receptor gamma in human macrophages. FEBS J. 2010;277:3308–3320. doi: 10.1111/j.1742-4658.2010.07729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stromsdorfer KL, Yamaguchi S, Yoon MJ, Moseley AC, Franczyk MP, Kelly SC, Qi N, Imai S, Yoshino J. NAMPT-mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Rep. 2016;16:1851–1860. doi: 10.1016/j.celrep.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HS, Han SY, Sung HY, Park SH, Kang MK, Han SJ, Kang YH. Blockade of visfatin induction by oleanolic acid via disturbing Il-6-TRAF6-NF-κB signaling of adipocytes. Exp Biol Med (Maywood) 2014;239:284–292. doi: 10.1177/1535370213514511. [DOI] [PubMed] [Google Scholar]
- 15.Lv Q, Wang Y, Wang W, Wang L, Zhou X. Effect of pioglitazone on visfatin expression in 3T3-L1 adipocytes and SD rats. Endocr Res. 2009;34:130–141. doi: 10.3109/07435800903287061. [DOI] [PubMed] [Google Scholar]
- 16.Mrizak I, Grissa O, Henault B, Fekih M, Bouslema A, Boumaiza I, Zaouali M, Tabka Z, Khan NA. Placental infiltration of inflammatory markers in gestational diabetic women. Gen Physiol Biophys. 2014;33:169–176. doi: 10.4149/gpb_2013075. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Chi H, Xiao H, Tian X, Wang Y, Yun X, Xu Y. Interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) single nucleotide polymorphisms (SNPs), inflammation and metabolism in gestational diabetes mellitus in inner Mongolia. Med Sci Monit. 2017;23:4149–4157. doi: 10.12659/MSM.903565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telejko B, Kuzmicki M, Zonenberg A, Szamatowicz J, Wawrusiewicz-Kurylonek N, Nikolajuk A, Kretowski A, Gorska M. Visfatin in gestational diabetes: Serum level and mRNA expression in fat and placental tissue. Diabetes Res Clin Pract. 2009;84:68–75. doi: 10.1016/j.diabres.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Kralisch S, Klein J, Lossner U, Bluher M, Paschke R, Stumvoll M, Fasshauer M. Interlenkin-6 is a negative regulator of visfatin gene expression in 3T3-L1 adipocyts. Am J Physicol Endocrinol Metab. 2005;289:E586–E590. doi: 10.1152/ajpendo.00090.2005. [DOI] [PubMed] [Google Scholar]
- 20.McGee KC, Harte AL, da Silva NF, Al-Daghri N, Creely SJ, Kusminski CM, Tripathi G, Levick PL, Khanolkar M, Evans M, et al. Visfatin is regulated by rosiglitazone in Type 2 diabetes mellitus and influenced by NFκB and JNK in human abdominal subcutaneous adipocytes. PLoS One. 2011;6:e20287. doi: 10.1371/journal.pone.0020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 22.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: The good, the bad and the future. Nat Med. 2013;19:557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charrier A, Wang L, Stephenson EJ, Ghanta SV, Ko CW, Croniger CM, Bridges D, Buchner DA. Zinc finger protein 407 overexpression upregulates PPAR target gene expression and improves glucose homeostasis in mice. Am J Physiol Endocrinol Metab. 2016;311:E869–E880. doi: 10.1152/ajpendo.00234.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holdsworth-Carson SJ, Lim R, Mitton A, Whitehead C, Rice GE, Permezel M, Lappas M. Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: Gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta. 2010;31:222–229. doi: 10.1016/j.placenta.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Suwaki N, Masuyama H, Masumoto A, Takamoto N, Hiramatsu Y. Expression and potential role of peroxisome proliferator-activated receptor gamma in the placenta of diabetic pregnancy. Placenta. 2007;28:315–323. doi: 10.1016/j.placenta.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Capobianco E, Jawerbaum A, Romanini MC, White V, Pustovrh C, Higa R, Martinez N, Mugnaini MT, Soñez C, Gonzalez E. 15-Deoxy-∆12,14-prostaglandin J2 and peroxisome proliferator-activated receptor γ (PPARγ) levels in term placental tissues from control and diabetic rats: Modulatory effects of a PPARγ agonist on nitridergic and lipid placental metabolism. Reprod Fertil Dev. 2005;17:423–433. doi: 10.1071/RD04067. [DOI] [PubMed] [Google Scholar]
- 27.Szklanna PB, Wynne K, Nolan M, Egan K, Áinle FN, Maguire PB. Comparative proteomic analysis of trophoblast cell models reveals their differential phenotypes, potential uses and limitations. Proteomics. Mar 20; doi: 10.1002/pmic.201700037. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Orendi K, Gauster M, Moser G, Meiri H, Huppertz B. The choriocarcinoma cell line BeWo: Syncytial fusion and expression of syncytium-specific proteins. Reproduction. 2010;140:759–766. doi: 10.1530/REP-10-0221. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schimittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Barak Y, Sadovsky Y, Shalom-Barak T. PPAR signaling in placental development and function. PPAR Res. 2008;2008:142082. doi: 10.1155/2008/142082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi KC, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, Kim NH, Choi DS, Baik SH, Choi KM. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem Biophys Res Commun. 2005;336:747–753. doi: 10.1016/j.bbrc.2005.08.203. [DOI] [PubMed] [Google Scholar]
- 32.Bermudez B, Dahl TB, Medina I, Groeneweg M, Holm S, Montserrat-de la Paz S, Rousch M, Otten J, Herias V, Varela LM, et al. Leukocyte overexpression of intracellular NAMPT attenuates atherosclerosis by regulating PPARγ-dependent monocyte differentiation and function. Arterioscler Thromb Vasc Biol. 2017;37:1157–1167. doi: 10.1161/ATVBAHA.116.308187. [DOI] [PubMed] [Google Scholar]
- 33.Yang SJ, Choi JM, Kim L, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW, Park CY. Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes. J Nutr Biochem. 2014;25:66–72. doi: 10.1016/j.jnutbio.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Tsai PJ, Davis J, Thompson K, Bryant-Greenwood G. Visfatin/Nampt and SIRT1: Roles in postterm delivery in pregnancies associated with obesity. Reprod Sci. 2015;22:1028–1036. doi: 10.1177/1933719115570908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin QQ, Geng YW, Jiang ZW, Tian ZJ. SIRT1 regulates lipopolysaccharide-induced CD40 expression in renal medullary collecting duct cells by suppressing the TLR4-NF-κB signaling pathway. Life Sci. 2017;170:100–107. doi: 10.1016/j.lfs.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Li L, Sun Q, Li Y, Yang Y, Yang Y, Chang T, Man M, Zheng L. Overexpression of sirt1 induced by resveratrol and inhibitor of miR-204 suppresses activation and proliferation of microglia. J Mol Neurosci. 2015;56:858–867. doi: 10.1007/s12031-015-0526-5. [DOI] [PubMed] [Google Scholar]
- 37.Tian Y, Ma J, Wang W, Zhang L, Xu J, Wang K, Li D. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol Cell Biochem. 2016;422:75–84. doi: 10.1007/s11010-016-2807-x. [DOI] [PubMed] [Google Scholar]
- 38.Chigurupati S, Dhanaraj SA, Balakumar P. A step ahead of PPARγ full agonists to PPARγ partial agonists: Therapeutic perspectives in the management of diabetic insulin resistance. Eur J Pharmacol. 2015;755:50–57. doi: 10.1016/j.ejphar.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 39.Mayi TH, Rigamonti E, Pattou F, Staels B, Chinetti-Gbaguidi G. Liver X receptor (LXR) activation negatively regulates visfatin expression in macrophages. Biochem Biophys Res Commun. 2011;404:458–462. doi: 10.1016/j.bbrc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Kirwan AM, Lenighan YM, O'Reilly ME, McGillicuddy FC, Roche HM. Nutritional modulation of metabolic inflammation. Biochem Soc Trans. 2017;45:979–985. doi: 10.1042/BST20160465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.