Abstract
Various types of mesenchymal stromal cells (MSCs) have been used in urological tissue engineering but to date the existence of MSCs has not been reported in the human bladder. The present study provided evidence that a small number of MSC-like cells exist in the human bladder and designated this class of cells ‘human bladder-derived MSC-like cells’ (hBSCs). It was demonstrated that hBSCs can be cultured to yield a large population. These hBSCs expressed the surface markers of MSCs and exhibited the capacity for osteogenic, adipogenic and chondrogenic differentiation. On induction with appropriate media in vitro, hBSCs could differentiate into bladder-associated cell types, including urothelial, endothelial and smooth muscle cell-like lineages. In addition, the average telomerase activity of adult hBSCs was higher compared with adult human bone marrow-derived MSCs, but lower than that of human umbilical cord Wharton's jelly-derived MSCs. These findings may inspire future studies on the role of hBSCs in urological tissue engineering applications and in other fields.
Keywords: mesenchymal stromal cells, bladder, differentiation
Introduction
Multipotent mesenchymal stromal cells (MSCs) are found in mammalian stromal tissue compartments (1). The umbilical cord, bone marrow and adipose tissue are most commonly used as a source of MSCs (2). The International Society for Cellular Therapy has proposed several criteria (3) to define MSCs based on their plastic adherent growth, subsequent expansion and in vitro and in vivo differentiation multipotency (osteoblasts, chondrocytes and adipocytes). The phenotypic definition requires the expression of cell surface markers cluster of differentiation (CD)73, CD90 and CD105 in addition to the lack of expression of hematopoietic lineage markers, including CD11b, CD14, CD19, CD34 and CD45, and human leukocyte antigen (HLA)-DR.
The bladder consists of a urothelial layer, the lamina propria, a layer of stromal cells and submucosal, smooth muscle and serous layers (4,5). Basal cells, which are a type of stem cells capable of renewing and differentiating into intermediate and superficial cells, exist in the adult urothelium. CD44 is a basal cell surface marker (6) and is also a major surface receptor of hyaluronic acid, which is involved in various cellular functions including cell proliferation, differentiation, migration, presentation of cytokines and chemokines, and signaling for cell survival (7). Studies have demonstrated that MSCs also express CD44 (8–10). However, MSCs have not yet been described in the normal human bladder.
Tissue engineering offers a promising alternative technique for urethral reconstruction. This process involves biodegradable scaffolds that can be used to seed cells to promote bladder reconstruction (11). The present study provided evidence that there is a small number of MSC-like cells in the bladder, which the present study termed ‘human bladder-derived MSC-like cells’ (hBSCs). Cell culture experiments show that hBSCs can be cultured to a large number of cells. These cells possess the capacity to differentiate into osteogenic, adipogenic and chondrogenic cells. In addition, hBSCs expressed MSC markers. Following induction with appropriate media in vitro, hBSCs differentiated into bladder-related cell types. These findings reinforce the understanding of MSCs and may inspire future research on the role of hBSCs in urological tissue engineering applications.
Materials and methods
Primary isolation of hBSCs
Human BSCs were isolated from 10 bladder samples collected from seven individuals with urinary bladder cancer (five men and two women; tumor stage, T2-T3; two tissue samples were taken from three of the bladders) ranging between 38 and 65 years of age at Fuzhou General Hospital (between February 2014 and November 2014). These patients had undergone whole bladder removal surgery and the tumors had not spread to adjacent tissues from the site of origin. During bladder resection, the bladder was infused with epirubicin. Normal bladder tissue was cut >2 cm away from the tumor. Following organ removal, 0.5–0.8 cm3 of normal bladder tissue was pulled from the internal face of the bladder. The tissues were stored in antibiotics-containing phosphate-buffered saline (PBS) for ≤2 h prior to isolation. In order to facilitate single cell extraction and reduce epidermal cell contamination, the middle tissue of the bladder was taken. The inner dense mucosa tissue and the outer bladder wall were not included. The tissues were then cut into small pieces. The trimmed tissue was washed three times in PBS containing antibiotics (300 µg/ml penicillin and streptomycin; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) to remove red blood cells, and minced thoroughly using curved scissors. The fragments were reincubated in collagenase solution (1 mg/ml type IV collagen; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 1 h at 37°C. The supernatant containing single cells was transfer to a new centrifuge tube, then centrifuged at 300 × g for 5 min at room temperature. The pellet was resuspended in 10 ml of growth medium for MSCs (12), containing low-glucose Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences), 1% MEM non-essential amino acids (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100 µg/ml penicillin/streptomycin, and plated in a 75-cm2 culture flask. After 48 h, non-adherent cells were removed and maintained in a humidified atmosphere of 5% CO2 at 37°C. Once the cells achieved 70–80% confluence, they were propagated by trypsinization. All cell isolation steps were carried out following the receipt of informed consent from the patients. The isolation and use of human tissues were approved by the Fuzhou General Hospital IRB (Fuzhou, China) with written consent (no. 2014-012).
Fluorescence-activated cell sorting (FACS)
As is standard practice at our centers (Fujian Provincial Key Laboratory of Transplant Biology and the Organ Transplant Institute, Fuzhou General Hospital) (13,14), cell surface marker analysis was performed. Cultured bladder-derived cells at passages 3 were trypsinized and stained with specific human antibodies labeled for CD11b-phycoerythrin (PE) (cat. no. 557321), CD13-PE (cat. no. 347837), CD14-PE (cat. no. 555398), CD29-PE (cat. no. 555443), CD31-PE (cat. no. 340297), CD34-PE/CD45-fluorescein isothiocyanate (FITC) (cat. no. 341071), CD73-PE (cat. no. 550257), CD90-PE (cat. no. 555596), CD105-PE (cat. no. 560839), HLA-ABC-PE (cat. no. 555553), and HLA-DR-PE (cat. no. 347367) (all 1:100; BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA); CD19-PE-cy7 (cat. no. E10328-1633), CD44-PE (cat. no. E01238-1632) (both 1:100; eBioscience; Thermo Fisher Scientific, Inc.); PE-(cat. no. 555749) or PE/FITC-conjugated isotype control antibodies (cat. no. 349526) (1:100; BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) and PE-cy7-conjugated isotype control antibodies (1:100; cat. no. E10143-1633, eBioscience; Thermo Fisher Scientific, Inc.) were used to determine background fluorescence. Following staining, the cells were analyzed using a FACS analytical fluorescence-activated cell sorter (FACSAria II; BD Biosciences).
Differentiation potential of hBSCs
Osteogenic induction
hBSCs were seeded at a density of 4,000 cells/cm2 in a 6-well culture dish in MSC medium (low-glucose DMEM containing 10% FBS). After 24 h of culture, the media was replaced with StemPro osteogenic supplements (Gibco; Thermo Fisher Scientific, Inc.) for 14 additional days (15). The medium was replaced every two to three days. Osteogenic differentiation was assessed by 0.2% Alizarin red staining at room temperature (16). The cells were observed using an inverted microscope (X81; Olympus Corporation, Tokyo, Japan; magnification, ×100).
Adipogenic induction
hBSCs were plated at a density of 8,000 cells/cm2. After 24 h of culture, the medium was replaced with StemPro adipogenic supplements (Gibco; Thermo Fisher Scientific, Inc.) for 14 additional days (17). Cells were fixed with 4% paraformaldehyde for 15 min at room temperature and stained by Oil Red O (0.5% Oil red O in isopropyl alcohol) for 30 min at room temperature (18). The cells were observed using an inverted microscope (X81; Olympus Corporation; magnification, ×100).
Chondrogenic induction
Chondrogenic supplements were used to generate a cell solution of 1.6×107 viable cells/ml. To generate micromass cultures, 5-µl droplets of cell solution were seeded in a 6-well culture plate for 2 h. Then 2 ml of StemPro chondrogenic supplements (Gibco; Thermo Fisher Scientific, Inc.) was added (19). After 14 days, cells were fixed with 4% paraformaldehyde for 15 min at room temperature and stained by 1% Alcian blue for 30 min at room temperature (20). The cells were observed using an inverted microscope (X81; Olympus Corporation, Tokyo, Japan; magnification, ×100).
Urothelial induction
hBSCs were seeded in a plate at 3,000 cells/cm2. After 24 h, the medium was replaced for 14 days with a mixture of media containing 49% embryo fibroblast medium (EFM) (Lonza Group, Ltd., Basel, Switzerland) (19) and 49% keratinocyte serum-free medium (21) (Gibco; Thermo Fisher Scientific, Inc.) with 2% FBS and 30 ng/ml epidermal growth factor (Gibco; Thermo Fisher Scientific, Inc.), per the protocol reported by Bharadwaj et al (22).
Endothelial induction
hBSCs were plated at a density of 5,000 cells/cm2 and grown for 2 days. Endothelial basal medium (Lonza Group, Ltd.) containing 50 ng/ml vascular endothelial growth factor was used to culture hBSCs for 14 days for induction (22).
Smooth muscle cell induction
hBSCs were seeded in a 6-well culture plate at 2,000 cells/cm2. After 24 h, the media was replaced with smooth muscle differentiation medium containing 45% high-glucose DMEM and 45% EFM with 10% FBS, 2.5 ng/ml transforming growth factor β 1 and 5 ng/ml platelet-derived growth factor-BB (PeproTech, Inc., Rocky Hill, NJ, USA) (22). Cell morphology was evaluated for ≤14 days.
Cells that were continuously cultured in the growth medium were assayed together with the induced cells and used as a negative control for each of the differentiation experiments.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from each type of induced and non-induced control cell was extracted using TRIzol® (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The purity and concentration were detected by spectrophotometer (Nanodrop 2000c; Thermo Fisher Scientific, Inc., Wilmington, DE, USA). cDNA (3 µg) was synthesized by reverse-transcription using a First Strand cDNA Synthesis kit (Fermentas; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol (1 h at 42°C). qPCR was performed with the SYBR Green PCR Master Mix on an ABI 7900 Real-time PCR (Applied Biosystems; Thermo Fisher Scientific, Inc.) and was run for 40 cycles under the following conditions: 94°C for 15 sec, 58°C for 15 sec and 72°C for 30 sec. Specific primer sequences for human alkaline phosphatase, runt-related transcription factor 2 (RUNX2), peroxisome proliferator-activated receptor (PPAR)γ, CCAAT-enhancer-binding protein (C/EBP)α, SRY-Box (Sox)9, collagen II, uroplakin-Ia, cytokeratin (CK)-7, von Willebrand factor (vWF), CD31, desmin, smoothelin and actin are provided in Table I. Actin was used as an endogenous control. Relative fold-changes in mRNA expression were calculated using the 2−ΔΔCq formula (23). The assay was replicated six times for each sample.
Table I.
Details of primers used for gene expression analysis and their expected product size.
| Target gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Amplicon (bp) |
|---|---|---|---|
| hALP | CCACGTCTTCACATTTGGTG | AGACTGCGCCTGGTAGTTGT | 196 |
| hRunx2 | TCTGGCCTTCCACTCTCAGT | GACTGGCGGGGTGTAAGTAA | 161 |
| hPPARG | GAGCCCAAGTTTGAGTTTGC | CTGTGAGGACTCAGGGTGGT | 198 |
| hCEBPA | TGGACAAGAACAGCAACGAG | TTGTCACTGGTCAGCTCCAG | 130 |
| hSox9 | AGTACCCGCACTTGCACAAC | CGTTCTTCACCGACTTCCTC | 177 |
| hCol-2 | TCACGTACACTGCCCTGAAG | CTATGTCCATGGGTGCAATG | 126 |
| hUPK1A | GATCACCAAGCAGATGCTGA | CAGTCCATGGGACCAGATGT | 123 |
| hCK7 | GGCTGAGATCGACAACATCA | GCTTCACGCTCATGAGTTCC | 191 |
| hvWF | AGTGTGCCTGCAACTGTGTC | CCACAGGGTAGATGGTGCTT | 144 |
| hCD31 | GGTTCTGAGGGTGAAGGTGA | TTGCAGCACAATGTCCTCTC | 97 |
| hDesmin | CAGTGGCTACCAGGACAACA | CTCAGAACCCCTTTGCTCAG | 238 |
| hSMTN | CCTGGTGCACAACTTCTTCC | TACACGCACTTCCAGTCAGG | 174 |
| hActin | AGCGAGCATCCCCCAAAGTT | GGGCACGAAGGCTCATCATT | 285 |
RUNX2, runt-related transcription factor 2; PPAR, peroxisome proliferator-activated receptor; C/EBP, CCAAT-enhancer-binding protein; Sox, SRY-Box; ALP, alkaline phosphatase; vWF, von Willebrand factor; CK, cytokeratin; CD, cluster of differentiation; Col-1, collagen; SMTN, smoothelin; UPK1A, uroplakin 1A.
Immunofluorescence
Differentiated cells were fixed with 4% paraformaldehyde overnight at 4°C, washed with PBS and permeabilized with 1% Triton X-100 in PBS. Cells were blocked with PBS containing 5% bovine serum albumin (Sangon Biotech Co., Ltd., Shanghai, China) and 0.5% Triton X-100 for 30 min at 25°C. The urothelium-specific marker uroplakin-Ia (1:300; cat. no. orb186483; Biorbyt Ltd., Cambridge, UK) was assessed and vWF (1:500; cat. no. ab154193; Abcam, Cambridge, UK) was assessed for endothelial differentiation. The smooth muscle-like cell (SMC)-specific marker desmin (1:500; cat. no. ab32362; Abcam) was used. Antibodies were diluted in blocking buffer and applied to slides overnight at 4°C. The sections were then incubated with Dylight 594-conjugated IgG secondary antibodies (1:100; cat. no. 35560; Thermo Fisher Scientific, Inc.) for 30 min at 37°C and counterstained with DAPI (Sigma-Aldrich, Merck KGaA) for 5 min at room temperature. Slides were analyzed using an epifluorescence microscope (X81; Olympus Corporation; magnification, ×400). Surface marker assays were performed ≥3 times to ensure consistent results.
Determination of telomerase activity
Cell extracts from 1×105 hBSCs (passage 3) were assayed using a TRAPeze RT Telomerase Detection kit (Merck KGaA), according to the manufacturer's protocols. In brief, frozen samples were lysed on ice with the TRAPeze 1X CHAPS lysis buffer (EMD Millipore, Billerica, MA, USA) to release cellular proteins. Telomerase activity was measured with 1 µg of protein lysate using the TRAPeze RT telomerase detection kit (EMD Millipore). Briefly, PCR was performed with a volume of 12.5 µl, including 2.5 µl of 5X TRAPeze RT reaction mixture, 8.8 µl of nuclease-free water, 0.2 µl of Taq polymerase and 1 µl of protein sample. A series of diluted TSR8 control templates was used as a standard curve. The ABI 7900 PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.) was used for PCR amplification. Cycling conditions included one cycle at 30°C for 30 min and 95°C for 2 min, followed by 45 cycles at 94°C for 15 sec, 59°C for 1 min and 45°C for 10 sec. Analyses were performed in triplicate. The log10 for each well of each reaction was calculated. In Excel 2016 (MSO, 16.0.4549.1000; Microsoft Corporation, Redmond, WA, USA), different concentrations of TSR8 data points were fitted to a linear regression plot according to the curve fitting option. The linear equation obtained from the data curve fitting is used to infer the enzyme concentration of the experimental sample. Telomerase-positive samples were provided by the kit. CHAPS lysis buffer (EMD Millipore) was used as the negative control. Human bone marrow-derived MSCs (hBM-MSCs, passage 3) and umbilical cord Wharton's jelly-derived MSCs (hUC-MSCs, passage 4) were used as control cells for hBSCs. The growth media for hBM-MSCs and hUC-MSCs were similar to that for hBSCs.
Statistical analysis
SPSS software version 17.0 (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. Data were expressed as the mean ± standard deviation (SD). Data were analyzed using one- or two-way analysis of variance, followed by Bonferroni's multiple comparison tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Isolation and expansion of hBSCs
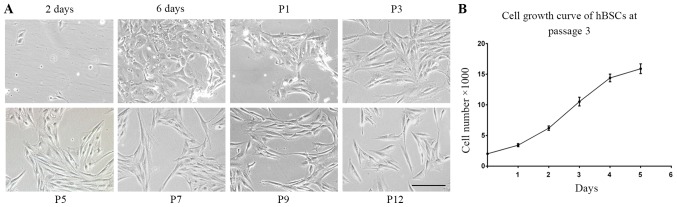
A total of 10 bladder samples were collected from 7 individuals. Following 2 days of culture, 99% of the cells did not attach to plates and non-adherent cells were removed when the medium was replaced with fresh. After 6 days of plating, certain single cells became a cluster of cells that appeared compact and uniform. hBSCs did not exhibit a homogeneous appearance in primary culture. However, these cells became symmetrical, spindle-shaped cells (MSC-like cells) following the first propagation and no epidermal-like cells appeared. This appearance was consistently observed at every subsequent passage (Fig. 1A). A high yield of cells was achieved from these cells. In ~2 weeks, the cells were expanded to ~5 million cells in 3, 75-cm2 plates at passage 1. Average population doubling time was 28 h in growth medium. These cells demonstrated normal exponential cell growth patterns with a steady increase in number during a 6-day culture period (Fig. 1B).
Figure 1.
Morphology of adherent cells. (A) Morphology of hBSCs with passage. A single line of hBSCs is followed through passage 0 (p0, 2 and 6 days) to p12 (magnification, ×100). (B) hBSC growth curve. The results are representative data from 3 individual experiments. hBSCs, human bladder-derived mesenchymal stromal cells.
Cell surface markers
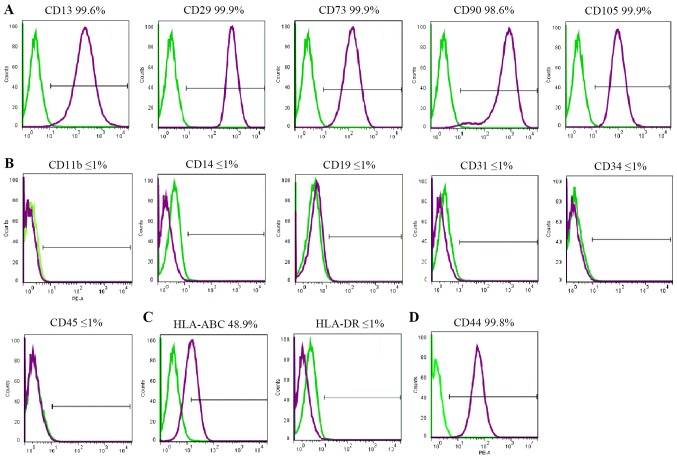
FACS was used to assess cell surface marker expression. All cell lines demonstrated similar results. hBSCs consistently expressed MSC-like cell surface markers (CD13, CD29, CD44, CD73, CD90 and CD105), but not hematopoietic markers (CD11b, CD14, CD19, CD34 and CD45), endothelial cell markers (CD31), or HLA-DR (Fig. 2). HLA-ABC was expressed at a lower level. CD44 was expressed in MSCs and considered a cell surface marker for urothelial basal cells (6). In the hBSC populations, CD44 was expressed and its mean expression was high.
Figure 2.
Immunophenotypes of hBSCs. hBSCs at passage 3 were labeled with antibodies against (A) mesenchymal stem cell markers (CD13, CD29, CD73, CD90 and CD105), (B) hematopoietic stem cell markers (CD14, CD19, CD31, CD34 and CD45), (C) HLA-ABC and HLA-DR, and (D) CD44, and then analyzed by fluorescence-activated cell sorting. Representative histograms are demonstrated (purple). The respective isotype control is indicated by a green line. hBSCs, human bladder-derived mesenchymal stromal cells; CD, cluster of differentiation; HLA, human leukocyte antigen.
Osteogenic, adipogenic and chondrogenic differentiation capacity of hBSCs
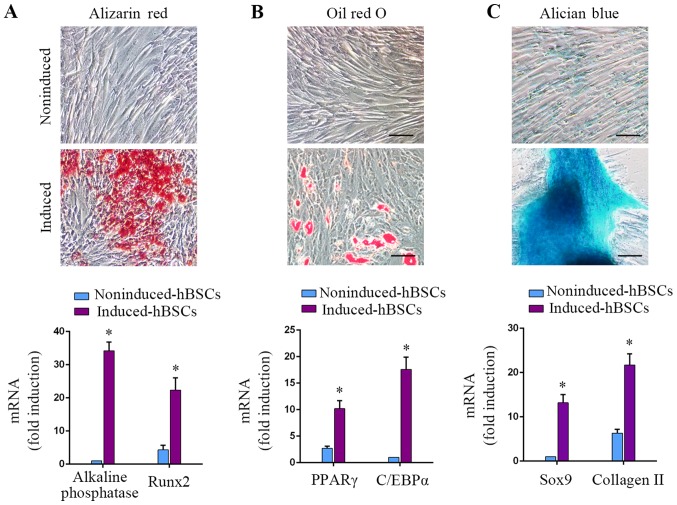
MSCs can be induced to differentiate into osteoblasts, adipocytes and chondrocytes under specific culture conditions (1). In the hBSC populations of the present study, osteogenic differentiation could be induced, as demonstrated by Alizarin red S staining for calcium deposition. Osteogenically-induced hBSCs expressed osteoblastic gene markers alkaline phosphatase and RUNX2 (P<0.05; Fig. 3A; 34.2±2.6-fold and 5.2±1.4-fold, respectively). Adipogenic-differentiated hBSCs were positive for Oil Red O staining. Induction of the adipogenic markers PPARγ and C/EBPα increased in adipogenically-induced cells (P<0.05; Fig. 3B; 3.8±1.1-fold and 17.6±2.3-fold, respectively). Chondrogenic-differentiated hBSCs were examined with Alcian blue staining. Differentiated hBSCs expressed the chondrogenic lineage markers Sox9 and collagen II (P<0.05; Fig. 3C, 13.2±1.8-fold and 3.4±1.6-fold, respectively). These results demonstrated that hBSCs could differentiate into the 3 above-mentioned cell lineages. This differentiation capacity of BSCs was similar to that of MSCs. Therefore, this class of bladder-derived cells were designated as MSC-like cells.
Figure 3.
hBSCs were induced to differentiate into osteogenic, adipogenic and chondrogenic lineages using specific media cocktails. hBSCs cultured in different induction media were assessed for differentiation into 3 lineages. (A) Osteogenic differentiation, day 14: Alizarin red staining for alkaline phosphatase activity. RT-qPCR analysis for lineage-specific transcripts alkaline phosphatase and RUNX2. Error bars indicate the SD of 6 biological replicates. (B) Adipogenic differentiation, day 14: Oil red O staining for lipid droplets. RT-qPCR analysis for lineage-specific transcripts PPARγ and C/EBPα. Error bars indicate the SD of 6 biological replicates. (C) Chondrogenic differentiation, day 14: Alcian blue staining for glycosaminoglycans. RT-qPCR analysis for lineage-specific transcripts Sox9 and collagen II. Error bars indicate the SD of 6 biological replicates. *P<0.05 vs. noninduced-hBSCs. hBSCs, human bladder-derived mesenchymal stromal cells; RUNX2, runt-related transcription factor 2; PPAR, peroxisome proliferator-activated receptor; C/EBP, CCAAT-enhancer-binding protein; Sox, SRY-Box; SD, standard deviation; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Differentiation of hBSCs into urothelial, endothelial and smooth muscle cells in vitro
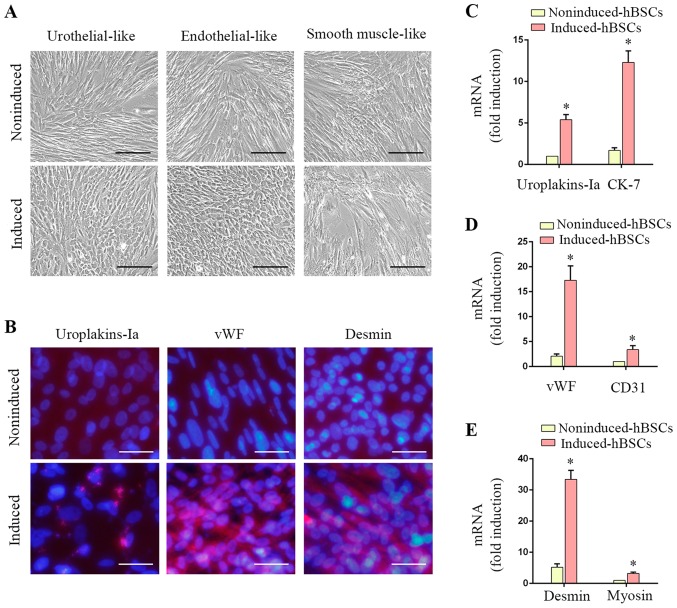
hBSCs demonstrated varying degrees of differentiation potential as presented by positive expression of urothelial, endothelial and SMC lineage-specific markers (Fig. 4). Urothelial-differentiated hBSCs became ‘rice-grain’ shaped (Fig. 4A). Immunofluorescence revealed that ~37.4±2.3% of the induced cells expressed the urothelial-specific protein uroplakin-Ia (Fig. 4B). Transcripts of uroplakin-Ia and CK-7 were significantly increased in induced hBSCs (P<0.05; Fig. 4C, 5.4±0.6-fold and 7.2±1.1-fold, respectively). Endothelial-differentiated hBSCs became ‘cobble-stone’ shaped (Fig. 4A). Induced hBSCs expressed the endothelial cell-specific protein marker vWF (Fig. 4B) and gene transcripts (vWF and CD31) were significantly increased (P<0.05; Fig. 4D; 8.2±1.5-fold and 3.5±0.8-fold, respectively). SMC-differentiated hBSCs became elongated (Fig. 4A) and ~84.1±5.2% of the induced cells expressed desmin (Fig. 4B). SMC-specific gene transcripts (desmin and myosin) were induced (P<0.05; Fig. 4E; 6.4±1.2-fold and 3.3±0.4-fold, respectively). These data suggested that hBSCs are a potential source for urological tissue reconstruction.
Figure 4.
hBSCs undergo urothelial, endothelial and smooth myogenic differentiation in vitro. (A) Morphology of hBSCs following induction. The results are representative data from 3 individual experiments. Scale bar, 200 µm. (B) Immunofluorescence staining using lineage-specific antibodies was performed to confirm the induction of hBSCs. The results are representative data from 3 individual experiments. Epithelial: Uroplakin-Ia; Endothelial: vWF; and SMC: Desmin. Specific staining is observed in red and nuclei in blue (DAPI). Scale bar, 50 µm. (C) RT-qPCR analysis was performed using urothelial-specific primers (uroplakins-Ia and CK-7). Error bars indicate the standard deviation (SD) of 6 biological replicates. (D) RT-qPCR analysis was performed using endothelial-specific primers (vWF and CD31). Error bars indicate the SD of 6 biological replicates. (E) RT-qPCR analysis was performed using SMC-specific primers (desmin and myosin). Error bars indicate the SD of 6 biological replicates. *P<0.05 vs. noninduced-hBSCs. hBSCs, human bladder-derived mesenchymal stromal cells; vWF, von Willebrand factor; SMC, smooth muscle-like cell; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; CK, cytokeratin; CD, cluster of differentiation.
Telomerase activity of hBSCs
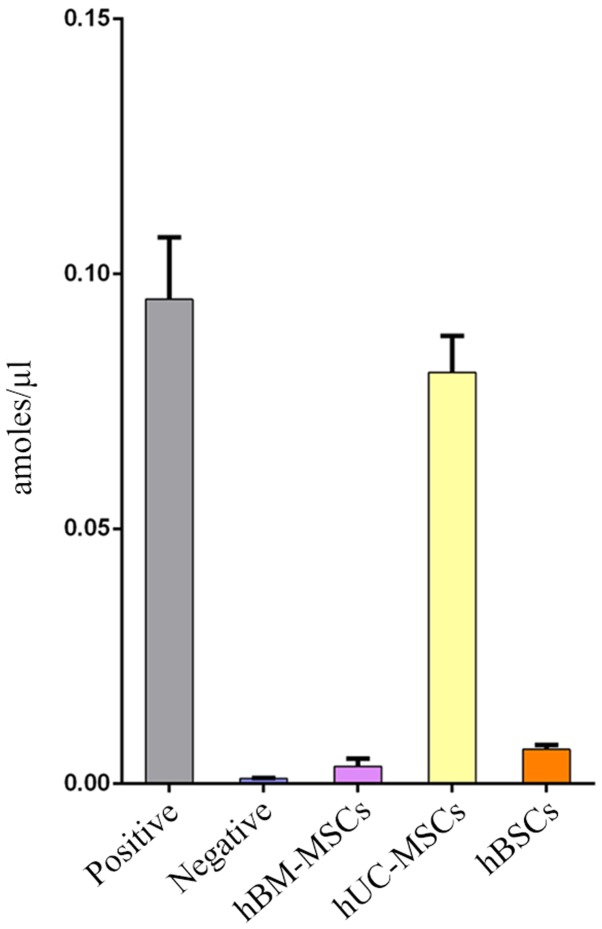
In the present study, telomerase was detected in 10 lines of hBSC samples at passage 4. hBM-MSCs and hUC-MSCs at the same passage were used as controls. Fig. 5 demonstrates that the average telomerase activity of adult hBSCs was lower compared with hUC-MSCs but higher compared with hBM-MSCs. Telomerase activity is correlated with lifespan and replicative capacity in cell lines (24). In the present study, hBSCs could proliferate for ≤20 generations (data not shown). This is far higher than the proliferative potential of hBM-MSCs, which usually stop growing prior to passage 10 (25). Although the cells can be passaged for ~20 generations, as the number of passages increases, they may still exhibit senescence and be unable to be immortalized.
Figure 5.
Telomerase activity in hBSCs. A total of 10 of the hBSCs samples were tested for telomerase activity. The quantitative data are presented as the mean ± standard deviation (n=3 per group). The average telomerase activity of adult hBSCs is indicated by the orange bar. hBM-MSCs: Purple bar; hUC-MSCs: Yellow bar; positive control: Dark gray bar; negative control: Blue bar. hBSCs, human bladder-derived mesenchymal stromal cells; human bone marrow-derived mesenchymal stromal cells; hUC-MSCs; umbilical cord Wharton's jelly-derived mesenchymal stromal cells.
Discussion
The latest discoveries in urological tissue engineering have demonstrated that bladder regeneration can be achieved using autologous bladder cells (11), embryonic stem cells (26) and several types of MSCs, including those derived from bone marrow (27), skeletal muscle (28) and adipose tissue (29–31). The present study identified that MSC-like cells exist in human bladder tissues. They express MSC surface markers, including CD13, CD29, CD73, CD90, CD105 and CD44, and exhibit telomerase activity. Importantly, these culture-expanded cells possess multipotent differentiation capacity. In addition to osteogenic, adipogenic and chondrogenic cells, hBSCs give rise to multiple lineages that express urothelium, endothelial and smooth muscle cell markers in vitro, suggesting that this type of progenitor cells can be planted in the cellular matrix to repair damaged tissues. Identification of the sources of hBSCs may provide a better understanding of their biological role. hBM-MSCs and urothelial basal cells could potentially be the origin of BSCs. The present study provided no evidence to suggest that hBSCs are actually hBM-MSCs. The maximum number of passages for hBM-MSCs is ~10 (24) while for hBSCs, it could reach ~20. The evidence from the current study suggested that hBSCs may originate from urothelial basal cells, as these cells expressed CD44. Since basal cells can self-renew, proliferate and differentiate into intermediate and superficial cells, including umbrella cells, basal cells are referred to as urothelial progenitor cells or stem cells. Perivascular cells are another possible origin of MSCs (32). Human perivascular cells can be sorted from diverse human tissues and long-term culture produces adherent, multilineage progenitor cells with MSC characteristics.
The urothelial, endothelial and SMC differentiation ability of hBSCs was demonstrated by the present study, suggesting that hBSCs can be an alternative to the urological tissue biopsies used in cell therapy. Notably, an advantage to using hBSCs is their expansion property. It was demonstrated that one bladder sample (~0.5 cm3) cultured for 4 weeks would yield a sufficient quantity of cells at below passage 5 for differentiation and tissue engineering. hBSC can be planted on biological materials in the same way as other MSC cells (33) and can be used for tissue damage repair following differentiation.
The adult bladder is composed of the urothelium, lamina propria, muscularis propria and perivesical soft tissues (11). Future studies may attempt to scrape the bladder tissues more precisely to identify the layer that is the main source of hBSCs. In addition, the hBSCs in the present study were obtained from non-carcinoma biopsy of urothelial bladder cancer patients. The interaction between hBSCs and urothelial cancer cells still requires study. The limitation of hBSC applications is mainly in cell acquisition. Autologous tissue cell separation is more traumatic. In view of the evidence that hBSC and MSC are similar and that the expression of HLA class 2 antigen is extremely low, allogeneic cells can be used. However, there is little chance of obtaining normal bladder tissue with informed consent. Donation after Cardiac Death Organ donation may be the way to obtain normal bladder tissue in the future.
In conclusion, the present study highlighted that the progenitor cells in human bladder tissues are MSC-like cells, which possess the capacity to expand and differentiate into multiple cells under directed treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by The National Natural Science Foundation of China (grant no. 81570748).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL and JMT designed the experiments. LFZ, HYD, LZ and YMC performed the experiments. LFZ provided the patient samples. JL and LZ analyzed the data. JL and YMC wrote the manuscript.
Ethics approval and consent to participate
The isolation and use of human tissues were approved by the Fuzhou General Hospital IRB, (Fuzhou, China), with written consent (2014–12).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 2.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. International Society for Cellular Therapy: Clarification of the nomenclature for MSC: The international society for cellular therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 4.Wong-You-Cheong JJ, Woodward PJ, Manning MA, Sesterhenn IA. From the archives of the AFIP: Neoplasms of the urinary bladder: Radiologic-pathologic correlation. Radiographics. 2006;26:553–580. doi: 10.1148/rg.266065126. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, Montironi R, Davidson DD, Lopez-Beltran A. Staging and reporting of urothelial carcinoma of the urinary bladder. Mod Pathol. 2009;22(Suppl 2):S70–S95. doi: 10.1038/modpathol.2009.1. [DOI] [PubMed] [Google Scholar]
- 6.Desai S, Lim SD, Jimenez RE, Chun T, Keane TE, McKenney JK, Zavala-Pompa A, Cohen C, Young RH, Amin MB. Relationship of cytokeratin 20 and CD44 protein expression with WHO/ISUP grade in pTa and pT1 papillary urothelial neoplasia. Mod Pathol. 2000;13:1315–1323. doi: 10.1038/modpathol.3880241. [DOI] [PubMed] [Google Scholar]
- 7.Zöller M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 8.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 9.Bian XH, Zhou GY, Wang LN, Ma JF, Fan QL, Liu N, Bai Y, Guo W, Wang YQ, Sun GP, et al. The role of CD44-hyaluronic acid interaction in exogenous mesenchymal stem cells homing to rat remnant kidney. Kidney Blood Press Res. 2013;38:11–20. doi: 10.1159/000355749. [DOI] [PubMed] [Google Scholar]
- 10.Ma F, Chen D, Chen F, Chi Y, Han Z, Feng X, Li X, Han Z. Human umbilical cord mesenchymal stem cells promote breast cancer metastasis by interleukin-8- and interleukin-6-dependent induction of CD44(+)/CD24(−) cells. Cell Transplant. 2015;24:2585–2599. doi: 10.3727/096368915X687462. [DOI] [PubMed] [Google Scholar]
- 11.Becker C, Jakse G. Stem cells for regeneration of urological structures. Eur Urol. 2007;51:1217–1228. doi: 10.1016/j.eururo.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Akram KM, Samad S, Spiteri MA, Forsyth NR. Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respir Res. 2013;14:9. doi: 10.1186/1465-9921-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, Wang Q, Huang L, Dong H, Lin L, Lin N, Zheng F, Tan J. Palmitate causes endoplasmic reticulum stress and apoptosis in human mesenchymal stem cells: Prevention by AMPK activator. Endocrinology. 2012;153:5275–5284. doi: 10.1210/en.2012-1418. [DOI] [PubMed] [Google Scholar]
- 14.Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 15.Oikonomopoulos A, van Deen WK, Manansala AR, Lacey PN, Tomakili TA, Ziman A, Hommes DW. Optimization of human mesenchymal stem cell manufacturing: The effects of animal/xeno-free media. Sci Rep. 2015;5:16570. doi: 10.1038/srep16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregory CA, Gunn WG, Peister A, Prockop DJ. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Swioklo S, Constantinescu A, Connon CJ. Alginate-encapsulation for the improved hypothermic preservation of human adipose-derived stem cells. Stem Cells Transl Med. 2016;5:339–349. doi: 10.5966/sctm.2015-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez-Zacarias JL, Castro-Muñozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Nyman JS, Ono K, Stevenson DA, Yang X, Elefteriou F. Mice lacking Nf1 in osteochondroprogenitor cells display skeletal dysplasia similar to patients with neurofibromatosis type I. Hum Mol Genet. 2011;20:3910–3924. doi: 10.1093/hmg/ddr310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa R, Mizuno H, Hyakusoku H, Watanabe A, Migita M, Shimada T. Chondrogenic and osteogenic differentiation of adipose-derived stem cells isolated from GFP transgenic mice. J Nippon Med Sch. 2004;71:240–241. doi: 10.1272/jnms.71.240. [DOI] [PubMed] [Google Scholar]
- 21.Itoh M, Kiuru M, Cairo MS, Christiano AM. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci USA. 2011;108:8797–8802. doi: 10.1073/pnas.1100332108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharadwaj S, Liu G, Shi Y, Wu R, Yang B, He T, Fan Y, Lu X, Zhou X, Liu H, et al. Multipotential differentiation of human urine-derived stem cells: Potential for therapeutic applications in urology. Stem Cells. 2013;31:1840–1856. doi: 10.1002/stem.1424. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 25.Vidal MA, Walker NJ, Napoli E, Borjesson DL. Evaluation of senescence in mesenchymal stem cells isolated from equine bone marrow, adipose tissue, and umbilical cord tissue. Stem Cells Dev. 2012;21:273–283. doi: 10.1089/scd.2010.0589. [DOI] [PubMed] [Google Scholar]
- 26.Bajada S, Mazakova I, Richardson JB, Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008;2:169–183. doi: 10.1002/term.83. [DOI] [PubMed] [Google Scholar]
- 27.Kim A, Yu HY, Heo J, Song M, Shin JH, Lim J, Yoon SJ, Kim Y, Lee S, Kim SW, et al. Mesenchymal stem cells protect against the tissue fibrosis of ketamine-induced cystitis in rat bladder. Sci Rep. 2016;6:30881. doi: 10.1038/srep30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corona BT, Ward CL, Baker HB, Walters TJ, Christ GJ. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng Part A. 2014;20:705–715. doi: 10.1089/ten.tea.2012.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, Yoshida T, Takahashi S, Mugishima H. Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol. 2009;182:355–365. doi: 10.1016/j.juro.2009.02.103. [DOI] [PubMed] [Google Scholar]
- 30.Hou X, Shi C, Chen W, Chen B, Jia W, Guo Y, Ma C, Ye G, Kang J, Dai J. Transplantation of human adipose-derived mesenchymal stem cells on a bladder acellular matrix for bladder regeneration in a canine model. Biomed Mater. 2016;11:031001. doi: 10.1088/1748-6041/11/3/031001. [DOI] [PubMed] [Google Scholar]
- 31.Qiu X, Zhang S, Zhao X, Fu K, Guo H. The therapeutic effect of adipose-derived mesenchymal stem cells for radiation-induced bladder injury. Stem Cells Int. 2016;2016:3679047. doi: 10.1155/2016/3679047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Liu Y, Bharadwaj S, Atala A, Zhang Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials. 2011;32:1317–1326. doi: 10.1016/j.biomaterials.2010.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.