Abstract
The present study aimed to identify potential novel biomarkers in synovial tissue obtained from patients with Rheumatoid Arthritis (RA) and Osteoarthritis (OA) for differential diagnosis. The genome-wide expression profiling datasets of synovial tissues from RA and OA cohorts, including GSE55235, GSE55457 and GSE55584 datasets, were retrieved and used to identify differentially expressed genes (DEGs; P<0.05; false discovery rate <0.05 and Fold Change >2) between RA and OA using R software. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses of DEGs were performed to determine molecular and biochemical pathways associated with the identified DEGs, and a protein-protein interaction (PPI) network of the DEGs was constructed using Cytoscape software. Significant modules in the PPI network and candidate driver genes were screened using the Molecular Complex Detection Algorithm. Potential biomarkers were evaluated by receiver operating characteristic and logistic regression analyses. Large numbers of DEGs were detected, including 273, 205 and 179 DEGs in the GSE55235, GSE55457 and GSE55584 datasets, respectively. Among them, 80 DEGs exhibited identical expression trends in all the three datasets, including 49 upregulated and 31 downregulated genes in patients with RA. DEGs in patients suffering from RA compared with patients suffering from OA were predominantly associated with the primary immunodeficiency pathway, including interleukin 7 receptor (IL7R) and signal transducer activator of transcription 1 (STAT1). The sensitivity of IL7R + STAT1 to differentiate RA from OA was 93.94% with a specificity of 80.77%. The results generated from analyses of the GSE36700 dataset were closely associated with results generated from analyses of GSE55235, GSE55457 and GSE55584 datasets, which further verified the reliability of the aforementioned results. The results of the present study suggested that increased expression of IL7R and STAT1 in synovial tissue as well as in the primary immunodeficiency may be associated with RA occurrence. These identified novel biomarkers may be used to predict disease occurrence and clinically differentiate RA from OA.
Keywords: rheumatoid arthritis, osteoarthritis, pathway, protein-protein interaction, sensitivity, specificity
Introduction
Rheumatoid arthritis (RA) and osteoarthritis (OA) are the two most frequent types of degenerative joint diseases and exhibit similar etiology (1,2). RA is a complex, chronic inflammatory and autoimmune arthritis that typically causes pain, swelling, stiffness and loss of function in the joints (1). It has been estimated that RA affects 0.5–1% of the adult population worldwide with 20–50 novel cases per 100,000 people occurring annually, which most frequently occurs in women aged >40 years old (3,4). RA has become one of the most common causes of reduced productivity and disability in affected patients and may additionally pose a substantial financial burden on the family of the patient as well as society (5). RA manifests as osteoporosis around the joint and joint space narrowing in the knees of patients (6). The bone anatomy degeneration and cystic degeneration of the bone joint surface may additionally occur with bone defects (7). During RA, the intercondylar fossa is enlarged and the tibial plateau sinks (8,9). Patients with late-stage RA may suffer from articular surface sclerosis, joint subluxation or joint stiffness (10). Furthermore, OA, the most prevalent form of arthritis worldwide, is a multi-gene and multi-factorial disease, and is characterized by cartilage degeneration and subchondral bone alterations, involving synovial tissue and articular cartilage (10–12). OA may reduce the quality of life for patients and eventually lead to disability due to pain. The joint most commonly affected by OA is the knee (8). Similar to RA, OA additionally has an increased occurrence rate in older adults, particularly in women.
In routine clinical practice, the diagnostic criteria for RA and OA are outlined by the American College of Rheumatology (Atlanta, USA). RA and OA exhibit overlapping symptoms, making differential diagnosis particularly challenging. In addition, differentiation between RA and OA is difficult in late-stage cases, primarily because disease progression frequently begins prior to the onset of symptoms. Therefore, accurate diagnosis of RA and OA may significantly improve the clinical outcomes and prognosis for affected patients. However, the mechanisms underlying the initiation and progression of RA and OA remain unclear. Previously, important genes and diagnostic markers that interact with each other and with environmental and stochastic factors have been identified in the two diseases (13). However, these markers may not entirely elucidate the complex pathogenesis of RA and OA.
Therefore, the present study aimed to investigate the developmental differences between RA and OA. An updated comprehensive analysis was performed to identify the potential novel biomarkers associated with synovial tissues obtained from patients with RA and OA. In the present study, three multicenter genome-wide transcriptomic datasets, including 33 patients with RA and 26 patients with OA were retrieved and analyzed. The present study aimed to investigate the different mechanisms underlying the differential pathogenesis of RA and OA, and thus improve the diagnosis and treatment strategies available for patients suffering from the two diseases in clinical practice.
Materials and methods
Microarray dataset source
A systematic search of microarray datasets was performed to examine differentially expressed genes (DEGs) between RA and OA. The National Center for Biotechnology Information's Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) was utilized to retrieve appropriate microarray datasets. The key words ‘Osteoarthritis’ and ‘Rheumatoid Arthritis’ were used for the screening. Datasets were included if they met the following inclusion criteria: i) were based on gene expression profiling of synovial membrane samples from the same platform. When the microarray datasets are obtained from the same platform, their homogeneity is usually good. Subsequent to screening OA-associated microarray datasets, the GPL96 platform was used at the highest frequency. Therefore, the OA-associated microarray datasets obtained from the GPL96 platform were included in the present study; ii) case (RA)-control (OA) studies; iii) patients with RA were diagnosed and classified based on the American College of Rheumatology criteria (14) and patients with OA were classified according to the criteria of Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association (15); and iv) the number of synovial tissue samples in each group of patients with RA and OA was ≥6. Three gene expression datasets, GSE55235 (nRA=10 and nOA=10), GSE55584 (nRA=10 and nOA=6) and GSE55457 (nRA=13 and nOA=10) met the inclusion criteria and were included in the present study (16).
A further dependent dataset, GSE36700 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE36700) (17), including microarray data from synovial biopsies of patients with RA (n=7) and OA (n=5), was used to validate the results obtained from the GSE55235, GSE55584 and GSE55457 datasets. The GSE36700 dataset was created based on the Affymetrix Human Genome U133 Plus 2.0 Array (Affymetrix UK Ltd., High Wycombe, UK) and was submitted by Nzeusseu Toukap et al (17) March 22, 2012 and updated on Aug 09, 2018. Patients with RA were diagnosed and classified based on the 1987 American College of Rheumatology criteria (14) and patients with OA were classified according to X-ray evidence of osteoarthritis (15).
Data preprocessing and differential expression analysis
The three databases were created based on the Affymetrix Human Genome U133A Array (Affymetrix UK Ltd.). A robust multi-array average algorithm using the Affy package (justMRA; http://ugrad.stat.ubc.ca/R/library/affy/html/00Index.html) was conducted for background adjustment, normalization and summarization of the three datasets to minimize data inconsistency and heterogeneity. The probe sets were converted into corresponding gene annotation using R/Bioconductor package (version 3.22.4; http://www.bioconductor.org/packages/release/BiocViews.html#___ChipManufacturer) and the Affymetrix Human Genome U133 Plus 2.0 Array. The probes with no gene annotation were excluded from the analysis. The expression values of all probes for a given gene were calculated from the average expression value. DEGs [P<0.05; false discovery rate (FDR) <0.05] between RA and OA from the three datasets were investigated using R software (v3.4.0; http://bioconductor.org/biocLite.R). FDR was applied based on the Benjamini & Hochberg method (18) and two independent sample Student's t-test was performed to select sets of DEGs. To reduce the false positive rate, DEGs of the three datasets were identified, and subsequently Venn diagrams (Venn 2.1; http://bioinfogp.cnb.csic.es/tools/venny/index.html) were used to screen the overlapping DEGs of the three datasets to improve the stability of the subsequent results.
Biological functions and pathway enrichment analyses
To further elucidate the biological functions of DEGs between RA and OA, the identified DEGs were subjected to Gene Ontology (GO; http://www.geneontology.org/) term enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.kegg.jp/) pathway analyses using Database for Annotation, Visualization and Integrated Discovery (DAVID; http://david.ncifcrf.gov/), respectively. Significantly enriched pathways and GO terms (P≤0.05; number of enrichment genes ≥2) were identified using Cytoscape 3.5.1 software (http://www.softpedia.com/get/Science-CAD/Cytoscape.shtml) and GeneClip 2.0 (http://gsds.cbi.pku.edu.cn/). The identified genes were classified into three functional categories, including the Biological Process (BP), Molecular Function (MF) and Cellular Component (CC). Fisher's exact tests (two-sided) or χ2 tests were performed to categorize the pathway and GO terms. The FDR (Benjamini & Hochberg method) (18) was applied to obtain the corrected P-values. Significantly enriched pathways and GO terms (P£0.05; number of enrichment genes ≥2) were identified using Cytoscape 3.5.1 software.
Analysis of pathway networks
The establishment of a pathway network of DEGs between RA and OA may help to identify important pathways associated with the development of RA and OA. Furthermore, pathway network analysis may reveal the possible interactions and crosstalk among these pathways. Therefore, pathway networks were constructed based on the identified DEGs using the ClueGO plugin on the Cytoscape platform (http://www.softpedia.com/get/Science-CAD/Cytoscape.shtml).
Protein-protein interaction (PPI) network analysis of DEGs
The Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (http://string-db.org/) was used to analyze the PPI of DEGs, and Cytoscape software was used to construct the PPI network. FDR<0.05 was considered as the cut-off criterion. A network was constructed consisting of nodes and lines in which each node represents a protein and the lines represent direct interactions between proteins. The PPI network was constructed based on human data alone. The number of nodes that may interact with a given node was expressed as the degree of the node. The greater the degree values of the included genes, the greater the degree in the whole network.
Identification of candidate genes between RA and OA
Identification of genes that may affect the development of RA and OA within the genome may provide a comprehensive understanding of the differences between the pathogenesis of RA and OA. Candidate genes that may affect the development of RA and OA based on the DEGs were predicted using Molecular Complex Detection Algorithm (MCODE) in Cytoscape (19). Furthermore, MCODE cluster analysis was performed using Cytocluster 3.5.1 software (20) (Degree cutoff=2; Node score cutoff=0.2; K-core=2; Max Depth=100) to identify the most significant MCODE clusters, according to clustering scores. The GeneMANIA Cytoscape plugin was used to identify and prioritize novel candidate genes involved in RA and OA. The establishment of entire PPI networks of DEGs between RA and OA were identified based on the biological network using the GeneMANIA plugin (21). The PPI networks were composed of genes included in the list of 80 DEGs and predicted candidate genes that may affect the development of RA and OA. Following the selection of Homo sapiens as the organism, common DEGs were entered into the GeneMANIA search bar, and the PPI network was constructed. Following this, the whole PPI network was filtered using a degree-filtering approach to include the most critical biomarkers in the occurrence of the two diseases using Cytoscape 3.5.1 software.
Statistical analysis
The raw expression data of patients with RA and OA were obtained from GEO datasets and logarithmically transformed. The means of two continuous, normally distributed variables were compared by independent sample Student's t-tests. Mann-Whitney U tests were used to compare the means of two groups of variables not normally distributed. Receiver Operating Characteristic (ROC) analysis was performed to identify a more accurate cut-off point in the gene expression level, which may aid the classification of RA and OA. ROC curves were generated by plotting the range of sensitivity (true positive fractions) and specificity (false positive fractions) pairs for each subject's error rate, with case status (RA vs. OA) representing the classifier variable. Youden's index was used for capturing the performance of a dichotomous diagnostic test. Youden's index=sensitivity + specificity −1 (22).
The area under the ROC curve (AUC), which provides an estimate of the accuracy of the diagnostic test for the discrimination between patients with RA and patients with OA, was used to assess the performance of the test. Binary logistic regression using backward stepwise selection mode was performed to screen out potential biomarkers that were positively correlated with RA diagnosis when identified biomarkers were detected together. Following this, ROC analysis was performed to determine the performance of the established logistic regression models. All statistical analyses in the present study (except for the screening of DEGs) were performed using SPSS version 24.0 for Windows (IBM Corp., Armonk, NY, USA) and GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Identification of DEGs between patients with RA and OA
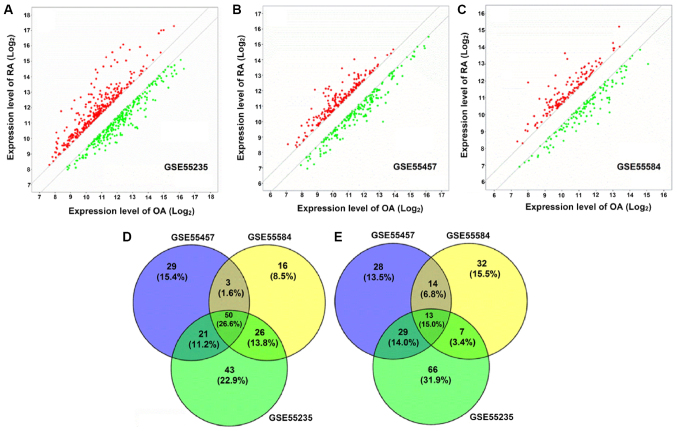
DEGs were identified by the t-test statistical algorithm. Based on the cutoff criteria, 140, 103 and 95 genes were identified in GSE55235, GSE55457 and GSE55584 datasets, respectively, which were upregulated in patients with RA (Fig. 1A-C). In addition, 133, 102 and 84 genes were identified in GSE55235, GSE55457 and GSE55584 datasets, respectively, which were downregulated in patients with RA (Fig. 1A-C). Notably, 50 upregulated (Fig. 1D) and 31 downregulated DEGs (Fig. 1E) in patients with RA were identified as being overlapped between the three datasets. One DEG without a symbol was excluded from the upregulated DEGs, therefore 80 DEGs in total, containing 49 upregulated and 31 downregulated, were included in the final analysis. The list of 80 DEG symbols is available upon request.
Figure 1.
Identification of DEGs between patients with RA and OA. (A) In total, 140 upregulated and 133 downregulated genes were identified in patients with RA from GSE55235 datasets. (B) In total, 103 upregulated and 102 downregulated genes were identified in patients with RA from GSE55457 datasets. (C) In total, 95 upregulated and 84 downregulated genes were identified in patients with RA from GSE55584 datasets. (D) In total, 50 upregulated DEGs in patients with RA were identified as being overlapped between the three datasets. (E) In total, 31 downregulated DEGs in patients with RA were identified as being overlapped between the three datasets. One DEG without a symbol was excluded from the upregulated DEGs, therefore 80 DEGs in total were included in the final analysis. RA, rheumatoid arthritis; OA, osteoarthritis; DEG, differentially expressed gene.
Biological functions and KEGG pathways
Cytoscape 3.5.1 and GeneClip 2.0 were used for biological function and pathway enrichment analyses. The results of these analyses identified 80 overlapped DEGs, which are presented in Table I. The results demonstrated that 80 overlapped DEGs were significantly enriched in immune, inflammation, apoptosis and antioxidant stress-associated functions and pathways.
Table I.
Top five biological functions and top ten KEGG pathways of the overlapped differentially expressed genes.
| A, Biological process | |||
|---|---|---|---|
| ID | GO term description | Count | FDR |
| GO:0007166 | Cell surface receptor signaling pathway | 38 | 1.10×10-13 |
| GO:0051239 | Regulation of multicellular organismal process | 40 | 1.10×10-13 |
| GO:0030155 | Regulation of cell adhesion | 23 | 1.23×10-13 |
| GO:2000026 | Regulation of multicellular organismal development | 32 | 1.41×10-12 |
| GO:0070887 | Cellular response to chemical stimulus | 37 | 6.78×10-12 |
| B, Molecular function | |||
| ID | GO term description | Count | FDR |
| GO:0005515 | Protein binding | 51 | 3.83×10-11 |
| GO:0005102 | Receptor binding | 25 | 1.05×10-09 |
| GO:0005003 | Ephrin receptor activity | 6 | 4.91×10-08 |
| GO:0008201 | Heparin binding | 10 | 5.03×10-07 |
| GO:0042802 | Identical protein binding | 20 | 5.17×10-07 |
| C, Cellular component | |||
| ID | GO term description | Count | FDR |
| GO:0005615 | Extracellular space | 28 | 4.35×10-11 |
| GO:0009986 | Cell surface | 19 | 2.35×10-08 |
| GO:0005576 | Extracellular region | 42 | 8.80×10-07 |
| GO:0009897 | External side of plasma membrane | 11 | 8.80×10-07 |
| GO:0098552 | Side of membrane | 13 | 2.42×10-06 |
| D, KEGG pathway | |||
| ID | GO term description | Count | FDR |
| 4,060 | Cytokine-cytokine receptor interaction | 16 | 3.66×10-12 |
| 4,360 | Axon guidance | 9 | 3.42×10-07 |
| 4,062 | Chemokine signaling pathway | 10 | 3.69×10-07 |
| 5,340 | Primary immunodeficiency | 6 | 4.88×10-07 |
| 4,151 | PI3K/AKT signaling pathway | 10 | 8.41×10-05 |
| 4,630 | JAK-STAT signaling pathway | 7 | 1.87×10-04 |
| 5,162 | Measles | 6 | 7.14×10-04 |
| 4,640 | Hematopoietic cell lineage | 5 | 1.05×10-03 |
| 4,064 | NF-κB signaling pathway | 5 | 1.11×10-03 |
| 4,660 | T cell receptor signaling pathway | 5 | 1.52×10-03 |
| 5,142 | American trypanosomiasis | 5 | 1.52×10-03 |
ID, identification; FDR, False Discovery Rate; GO, Gene Ontology; KEGG, Kyoto Encyclopedia Genes and Genomes; NF-κB, nuclear factor-κB; JAK, Janus kinase; STAT, signal transducer and activator of transcription; AKT, protein kinase B; PI3K, phosphatidylinositol-4,5-bisphosphate 3-kinase.
PPI network analysis
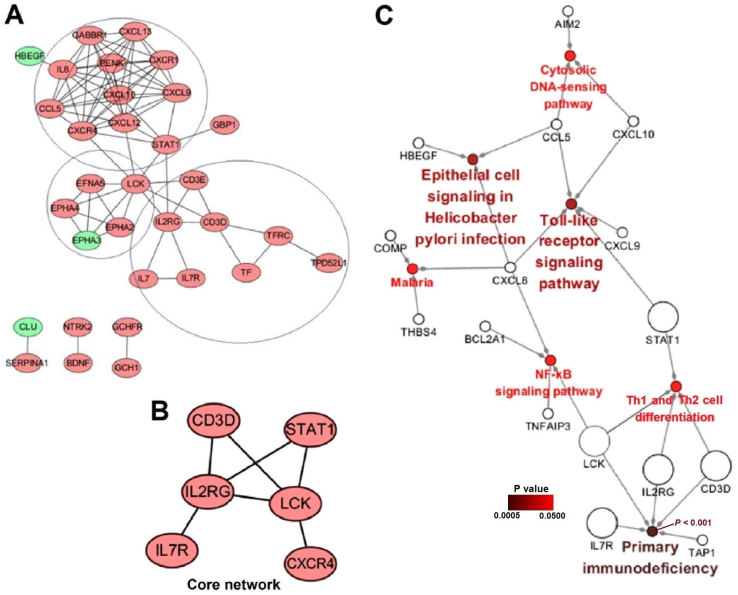
A PPI network was constructed based on the biological interactions of the 80 identified DEGs to further elucidate their associations at the protein level. As presented in Fig. 2A, 32 nodes were screened out, including 29 upregulated genes and three downregulated genes (heparin-binding epidermal growth factor-like growth factor, ephrin type-A receptor 3 and clusterin) in patients with RA. The PPI network included three primary sub-clusters: i) A sub-cluster including C-X-C motif chemokine receptor 4 (CXCR4) and signal transducer and activator of transcription 1 (STAT1), and at its core was predominantly associated with chemokines and immune functions (Fig. 2A; top circle); ii) a sub-cluster including LCK proto-oncogene, Src family tyrosine kinase (LCK), and at its core was predominantly correlated with the regulation of developmental events, notably in the nervous system (Fig. 2A; middle circle); iii) a sub-cluster including interleukin (IL)2 receptor, γ chain (IL2RG) and CD3d molecule (CD3D), and at its core was primarily associated with immunodeficiency (Fig. 2A; bottom circle).
Figure 2.
PPI network analysis, Core network and Gene-pathway network. (A) PPI network of differentially expressed genes (light red, upregulated; green, downregulated). (B) Core of the specific network affecting RA development. (C) Gene-pathway network associated with the development of RA. Larger circles represent genes in the core network. In Cytoscape 3.5.1 software, there are two visual styles, groups and significance. When ‘significance’ and ‘show only pathways with P-values <0.05’ were selected, the colors and the names of the enriched pathways in the figures are consistent with the P-values, therefore the colors of the pathway circles and their accompanying names represent P-values. RA, rheumatoid arthritis; PPI, protein-protein interaction.
Candidate genes and core network
To identify the potential pathological molecular network of the 80 identified DEGs, the specific network among them based on the human interactome network using MCODE algorithm was extracted using the default settings on GeneMANIA. This approach included maximal members of candidate genes with the minimal interaction associations. The network, capturing the 80 DEGs as its seeds, contained 323 nodes and 602 edges, including 30 genes of the 80 DEGs and 293 candidate genes that may affect RA and OA progression. To identify the most important core network, networks were filtered according to their degree using the degree-filtering approach. Finally, a core network including six genes (CD3D, CXCR4, IL2RG, IL7R, LCK and STAT1) was identified and presented in Fig. 2B, which suggested that these six genes may represent important biomarkers associated with RA and OA development and diagnosis.
Gene-pathway network
To further understand how important genes in the core PPI network affect RA development, a gene-pathway network was constructed using ClueGO (Fig. 2C). From the gene-pathway network, five genes (CD3D, IL2RG, IL7R, LCK and STAT1) were included in the PPI network. Notably, the results demonstrated that CD3D, IL2RG, IL7R, LCK and STAT1 interacted with the primary immunodeficiency pathway, either directly or indirectly (P<0.001), which suggested that upregulated genes may activate the primary immunodeficiency pathway and increase the risk of RA development.
Evaluation of the core network for RA identification
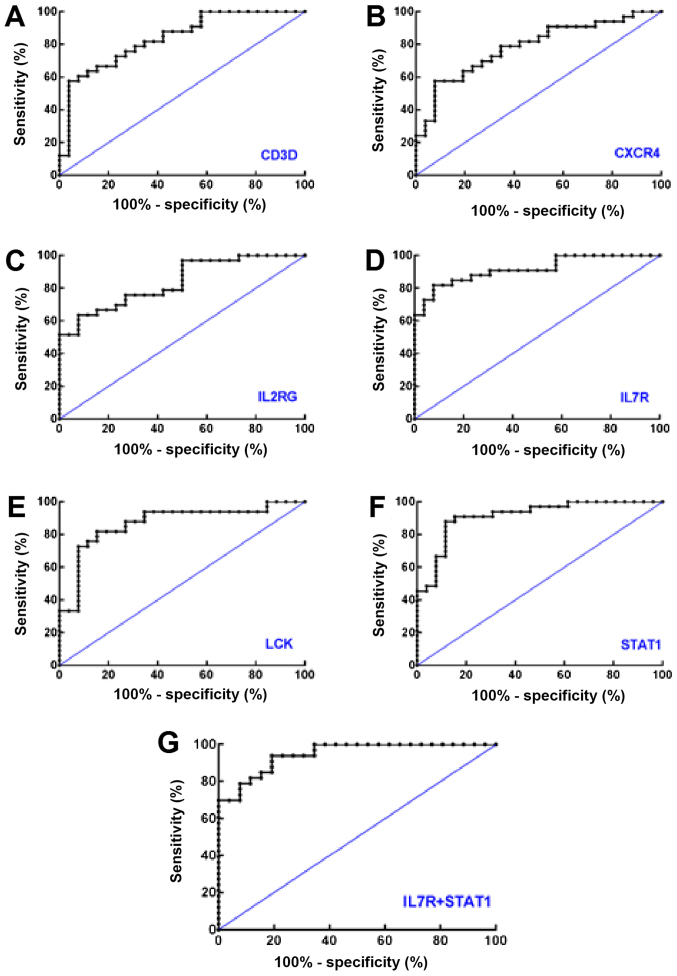
ROC curves were constructed to calculate the AUCs of CD3D (0.8357), CXCR4 (0.7855), IL2RG (0.8368), IL7R (0.9161), LCK (0.8683) and STAT1 (0.9138; all P<0.0001). Taking the maximum value of the Youden's index, the Log2 expression value of CD3D (8.65), CXCR4 (10.86), IL2RG (9.27), IL7R (8.11), LCK (6.58) and STAT1 (7.36) were determined (Fig. 3A-F and Table II). For RA identification, at the ROC-derived optimum cut-offs, the highest sensitivity exhibited by STAT1 was 90.91%. The specificities exhibited by CXCR4, IL2RG and IL7R were equal and reached a maximum value of 92.31%.
Figure 3.
Receiver operating characteristic curves of the six genes in the core network to distinguish rheumatoid arthritis from osteoarthritis using data from the GSE55235, GSE55457 and GSE55584 datasets. Receiver operating characteristic curves of (A) CD3D, (B) CXCR4, (C) IL2RG, (D) IL7R, (E) LCK, (F) STAT1 and (G) IL7R+STAT1 are presented. CD3D, T-cell surface glycoprotein CD3 δ chain; CXCR4, C-X-C motif chemokine receptor 4; IL2RG, interleukin 2 receptor γ; IL7R, interleukin 7 receptor; LCK, LCK proto-oncogene, Src family tyrosine kinase; STAT1, signal transducer and activator of transcription 1.
Table II.
Optimal cut-off points and associated diagnostic values of six genes in the core network as determined by receiver operator characteristic analysis.
| Genes | Cut-off value, Log2 | Sensitivity, % | Specificity, % | AUC | AUC 95% CI |
|---|---|---|---|---|---|
| CD3D | 8.65 | 66.67 | 84.62 | 0.8357 | 0.7329–0.9384 |
| CXCR4 | 10.86 | 57.58 | 92.31 | 0.7855 | 0.6696–0.9015 |
| IL2RG | 9.27 | 63.64 | 92.31 | 0.8368 | 0.7381–0.9356 |
| IL7R | 8.11 | 81.82 | 92.31 | 0.9161 | 0.8466–0.9856 |
| LCK | 6.58 | 81.82 | 84.62 | 0.8683 | 0.7718–0.9648 |
| STAT1 | 7.36 | 90.91 | 69.23 | 0.9138 | 0.8404–0.9871 |
Greater AUC values indicated a greater diagnostic value. The six AUCs all demonstrated statistical significances, P<0.0001. CD3D, T-cell surface glycoprotein CD3 δ chain; CXCR4, C-X-C motif chemokine receptor 4; IL2RG, interleukin 2 receptor γ; IL7R, interleukin 7 receptor; LCK, LCK proto-oncogene, Src family tyrosine kinase; STAT1, signal transducer and activator of transcription 1; AUC, area under the curve; CI, confidence interval.
Subsequent binary logistic regression analysis demonstrated that IL7R [odds ratio (OR) RA vs. OA=4.551; OR 95% confidence interval (CI): 1.517–13.657; P=0.007] and STAT1 (ORRA vs. OA=2.923; OR 95% CI: 1.091–7.829; P=0.033) were inputted into the regression, which suggested that IL7R and STAT1 may be detected together (Fig. 3G and Table III). Finally, ROC analyses suggested that the detection of IL7R + STAT1 together exhibited a higher diagnostic performance compared with the detection of either IL7R or STAT1 alone (AUC=0.9464; 95% CI: 0.8962–0.9966), with a sensitivity of 93.94% and a specificity of 80.77% (data not shown).
Table III.
Binary logistic regression results of the core network for rheumatoid arthritis diagnosis.
| Genes | β | S.E. | Wald | OR | OR 95% CI | P-value |
|---|---|---|---|---|---|---|
| IL7R | 1.515 | 0.561 | 7.307 | 4.551 | 1.517–13.657 | 0.007 |
| STAT1 | 1.073 | 0.503 | 4.551 | 2.923 | 1.091–7.829 | 0.033 |
IL7R, interleukin 7 receptor; STAT1, signal transducer and activator of transcription 1; β, coefficient of logistic regression; S.E., standard error of β value; Wald, Wald value of Wald tests; OR, Odds ratio; OR 95% CI, 95% confidence interval of OR value.
Validation using an additional, dependent dataset
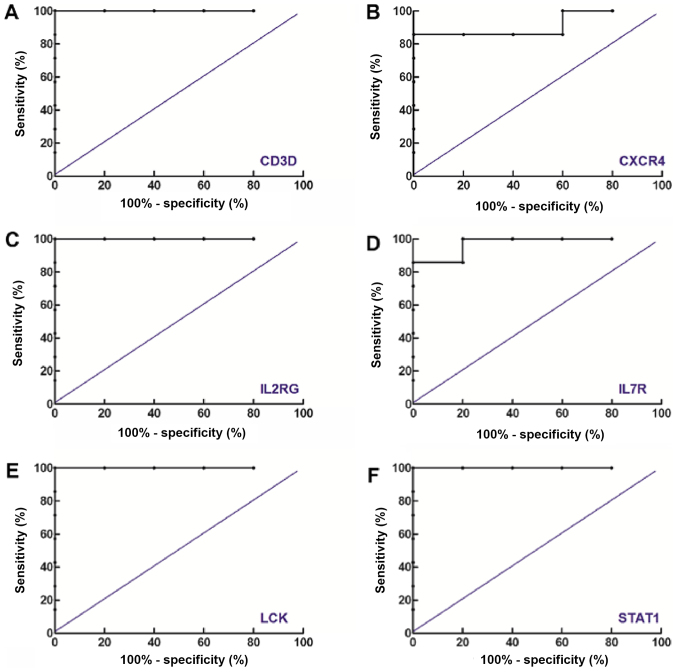
To investigate the reliability of the results of the ROC analyses obtained from all the three datasets and to identify if there was any possible overlapping between them, the same ROC analysis, including data from the GSE36700 dataset was performed, and the results are presented in Fig. 4. Notably, it was demonstrated that the six genes in the core network exhibited good performance in distinguishing RA from OA. In addition, the AUCs of genes identified in the GSE36700 dataset were increased compared with the results obtained from the aforementioned three datasets. In conclusion, the results suggested that the results obtained from the GSE36700 dataset were closely associated with those obtained from the GSE55235, GSE55584 and GSE55457 datasets, which further confirmed the reliability of the aforementioned results.
Figure 4.
Receiver operating characteristic curves of the six genes in the core network to investigate the differentiation between rheumatoid arthritis and osteoarthritis using data from the GSE36700 dataset. Receiver operating characteristic curves of (A) CD3D, (B) CXCR4, (C) IL2RG, (D) IL7R, (E) LCK and (F) STAT1 are presented. CD3D, T-cell surface glycoprotein CD3 δ chain; CXCR4, C-X-C motif chemokine receptor 4; IL2RG, interleukin 2 receptor γ; IL7R, interleukin 7 receptor; LCK, LCK proto-oncogene, Src family tyrosine kinase; STAT1, signal transducer and activator of transcription 1.
Discussion
RA and OA are the most common forms of degenerative joint diseases. They are the leading cause of chronic disability and may exhibit common clinical etiology (23,24). However, there remains a paucity of studies investigating the sensitivity and specificity of detection indicators for identification of the two diseases, particularly for patients with advanced-stage RA or OA. Recently, epigenetic dysregulation of cartilage genes has been demonstrated to have an important role in RA and OA development (24). Despite advances in the field, biomarkers associated with the pathogenesis and progression of RA and OA are not well characterized. Therefore, investigation of the gene signatures associated with disease development in RA and OA may elucidate the molecular mechanisms underlying pathogenesis and identify potential therapeutic strategies for the development of a biomarker of differential diagnosis.
In previous years, bioinformatics has had an increasingly important role in examining the pathogenesis of multifactorial disorders (25). In the present study, a comprehensive and systematic bioinformatics analysis of three gene expression profile datasets identified 80 significant DEGs, including 49 upregulated and 31 downregulated genes that may be associated with the development of RA and OA. These results suggested that alterations in gene expression profiles in synovial tissue may affect the development of RA and OA. Therefore, detailed analysis of the biological functions of the DEGs may be utilized to further understand the pathogenesis of the two diseases and may additionally reveal biomarkers for more accurate identification of RA and OA.
A previous study demonstrated that RA development may depend on a common alteration in the expression pattern of specific key genes (26), which was consistent with the results of the present study. Numerous previous studies have identified specific genes associated with RA development. For example, Ma et al (27) identified numerous genes (including adiponectin, C1Q and collagen domain containing, 3′-phosphoadenosine 5′-phosphosulfate synthase 1, DNA methyltransferase 1 and TIMP metallopeptidase inhibitor 1) involved in immune responses and inflammatory responses. Microarray analysis has additionally identified disease spectrum features in rheumatology and identified additional genes that may be associated with RA (28,29). Biswas et al (30) identified a number of different biomarkers, genes and pathways, the majority of which have not been revealed in other studies. Differential diagnoses of RA and OA remain clinically challenging due to substantial etiological similarities (16). Microarray experiments performed by Wang et al (31) identified an overview of differences in OA gene expression compared with healthy patients and identified 85 DEGs. In conclusion, these studies suggested that RA and OA have complex pathogenic mechanisms, and future studies should perform comprehensive and systematic analyses to further elucidate these mechanisms.
Biological function and KEGG pathway enrichment analyses identified that 80 overlapped DEGs were significantly enriched in immune, inflammation, apoptosis and antioxidant stress-associated functions and pathways, including ‘cytokine-cytokine receptor interaction’, ‘axon guidance’, ‘chemokine signaling pathway’ and ‘primary immunodeficiency’. A constructed PPI network additionally demonstrated that RA progression was associated with immunodeficiency. RA has been well established to represent a progressive, chronic, inflammatory and destructive joint disease (2). These results were based on three high throughput microarray datasets with multi-center design and containing large sample numbers of synovial tissue, which may provide further evidence for future research. In the present study, the PPI network studies demonstrated that CXCR4, LCK, IL2RG and CD3D may represent potential biomarkers associated with immunodeficiency in RA. To confirm this inference, a more complete and specific biological network based on GeneMANIA was determined, from which a core network of 293 candidate genes that may affect RA and OA development was obtained. Furthermore, the fact that the core network was closely aligned with the constructed PPI network further suggested that the six genes in the core network are involved in the occurrence and development of RA and OA.
To further investigate how these genes exhibit their biological function and affect the occurrence of RA, a gene-pathway interaction network was constructed. The results demonstrated that in the gene-pathway interaction network, five genes in the core network (CD3D, IL2RG, IL7R, LCK and STAT1) were included and notably, these genes were demonstrated to interact with the primary immunodeficiency pathway either directly (CD3D, IL2RG, IL7R and LCK) or indirectly (STAT1). Therefore, the results suggested that altered expression levels of CD3D, IL2RG, IL7R, LCK and STAT1 may activate the primary immunodeficiency pathway and subsequently lead to primary immune system dysfunction and the development of RA.
Primary immunodeficiencies are a heterogeneous group of disorders that cause increased susceptibility to infection, autoimmune disease and malignancy (32). From the primary immunodeficiency pathway, IL7R, LCK and Janus kinase (JAK)3/STAT1 primarily affect T-cell differentiation and antibody production (33), which may represent the basis of RA development. Investigation of the diagnostic capacity to distinguish RA from OA suggested that the genes in the core network may be detected alone to predict and diagnose RA occurrence with high sensitivity and specificity; however, the combined detection of important indicators may improve the effectiveness of this diagnostic strategy. Therefore, binary logistic regression analysis was used to screen for IL7R and STAT1 simultaneously to improve RA diagnosis.
A previous study identified that STAT1 is important in RA occurrence and is upregulated in patients with RA (34), which corroborates the results of the present study. STAT1 has been widely regarded to represent an important transcription factor involved in joint inflammation and destruction (33,35). STAT1 may be activated by numerous cytokines that are expressed in RA synovium, including interferon (IFN)γ, type I IFNs, IL6, IL10 and IL27, which induce inflammation via direct or indirect activation of mitogen-activated protein kinase, JAK-STAT and nuclear factor-κB signaling pathways (36). The functional defects in important proteins (including IL7R and IL7) associated with the IL7 signaling pathway may be involved in the pathogenesis of severe combined immunodeficiency (SCID) (36). IL7R was identified as a novel molecule with a potential role in RA in the present study. IL7R has been identified to have a critical role in V(D)J recombination during lymphocyte development, and thus mutations in this gene may increase the risk of SCID (37).
The identification of these two key biomarkers and a key pathway associated with immunodeficiency in the development of RA and reveals novel therapeutic targets for anti-immunotherapy for patients with RA.
In conclusion, the present study demonstrated that STAT1 and the primary immunodeficiency pathway may be precisely utilized to differentiate RA from OA. In addition, the present study additionally identified a previously unreported novel biomarker (IL7R), which may serve as potential candidate biomarker to differentiate RA from OA at the time of diagnosis. Therefore, the present study demonstrated potential implications for future clinical management of patients with RA and OA.
Acknowledgements
The authors would like to thank Dr Zhenzhong Li from Beijing Compass Biotechnology Co., Ltd. (Beijing, China) for his help with part of the data analysis.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81773372 and 81573104).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the National Center for Biotechnology Information's Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/).
Authors' contributions
YX and RZ designed the study. JW, LH, XY, AY, JZ, BL, DZ and ZL acquired the data. RZ, YX and JW analyzed and interpreted the data, and drafted the manuscript. All authors critically revised the manuscript, and read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.García-Bermúdez M, López-Mejías R, González-Juanatey C, Castañeda S, Miranda-Filloy JA, Blanco R, Fernández-Gutiérrez B, Balsa A, González-Alvaro I, Gómez-Vaquero C, et al. Lack of association between TLR4 rs4986790 polymorphism and risk of cardiovascular disease in patients with rheumatoid arthritis. DNA Cell Biol. 2012;31:1214–1220. doi: 10.1089/dna.2011.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song YJ, Li G, He JH, Guo Y, Yang L. Bioinformatics-based identification of MicroRNA-regulated and rheumatoid arthritis-associated genes. PLoS One. 2015;10:e0137551. doi: 10.1371/journal.pone.0137551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu G, Jiang Y, Chen X, Zhang R, Ma G, Feng R, Zhang L, Liao M, Miao Y, Chen Z, et al. Measles contributes to rheumatoid arthritis: Evidence from pathway and network analyses of genome-wide association studies. PLoS One. 2013;8:e75951. doi: 10.1371/journal.pone.0075951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballard DH, Aporntewan C, Lee JY, Lee JS, Wu Z, Zhao H. A pathway analysis applied to genetic analysis workshop 16 genome-wide rheumatoid arthritis data. BMC Proc. 2009;3(Suppl 7):S91. doi: 10.1186/1753-6561-3-s7-s91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Guo J, Jiang J, Wu W, Chang X, Zhou H, Li Z, Zhao J. New genes associated with rheumatoid arthritis identified by gene expression profiling. Int J Immunogenet. 2017;44:107–113. doi: 10.1111/iji.12313. [DOI] [PubMed] [Google Scholar]
- 6.Mc Ardle A, Flatley B, Pennington SR, FitzGerald O. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res Ther. 2015;17:141. doi: 10.1186/s13075-015-0652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson A, Bothner B, June R. Toward OA biomarkers: Metabolomic profiles of synovial fluid from OA, RA, and healthy patients. Osteoarthritis Cartilage. 2017;25(Suppl 1):S94–S95. doi: 10.1016/j.joca.2017.02.151. [DOI] [Google Scholar]
- 8.Atif U, Philip A, Aponte J, Woldu EM, Brady S, Kraus VB, Jordan JM, Doherty M, Wilson AG, Moskowitz RW, et al. Absence of association of asporin polymorphisms and osteoarthritis susceptibility in US Caucasians. Osteoarthritis Cartilage. 2008;16:1174–1177. doi: 10.1016/j.joca.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bijsterbosch J, Kloppenburg M, Reijnierse M, Rosendaal FR, Huizinga TW, Slagboom PE, Meulenbelt I. Association study of candidate genes for the progression of hand osteoarthritis. Osteoarthritis Cartilage. 2013;21:565–569. doi: 10.1016/j.joca.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Fang H, Zhang F, Li F, Shi H, Ma L, Du M, You Y, Qiu R, Nie H, Shen L, et al. Mitochondrial DNA haplogroups modify the risk of osteoarthritis by altering mitochondrial function and intracellular mitochondrial signals. Biochim Biophys Acta. 2015;1862:829–836. doi: 10.1016/j.bbadis.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Mu H, Lv H, Duan L, Shang Z, Li J, Jiang Y, Zhang R. Integrative analysis of genome-wide association studies and gene expression analysis identifies pathways associated with rheumatoid arthritis. Oncotarget. 2016;7:8580–8589. doi: 10.18632/oncotarget.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.González-Huerta NC, Borgonio-Cuadra VM, Zenteno JC, Cortés-González S, Duarte-Salazar C, Miranda-Duarte A. D14 repeat polymorphism of the asporin gene is associated with primary osteoarthritis of the knee in a Mexican Mestizo population. Int J Rheum Dis. 2017;20:1935–1941. doi: 10.1111/1756-185X.12797. [DOI] [PubMed] [Google Scholar]
- 13.Young SP, Kapoor SR, Viant MR, Byrne JJ, Filer A, Buckley CD, Kitas GD, Raza K. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum. 2013;65:2015–2023. doi: 10.1002/art.38021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 16.Woetzel D, Huber R, Kupfer P, Pohlers D, Pfaff M, Driesch D, Häupl T, Koczan D, Stiehl P, Guthke R, Kinne RW. Identification of rheumatoid arthritis and osteoarthritis patients by transcriptome-based rule set generation. Arthritis Res Ther. 2014;16:R84. doi: 10.1186/ar4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nzeusseu Toukap A, Galant C, Theate I, Maudoux AL, Lories RJ, Houssiau FA, Lauwerys BR. Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum. 2007;56:1579–1588. doi: 10.1002/art.22578. [DOI] [PubMed] [Google Scholar]
- 18.Green GH, Diggle PJ. On the operational characteristics of the Benjamini and Hochberg false discovery rate procedure. Stat Appl Genet Mol Biol. 2007;6:Article27. doi: 10.2202/1544-6115.1302. [DOI] [PubMed] [Google Scholar]
- 19.Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4:2. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Li D, Tang Y, Wu F, Wang J. CytoCluster: A cytoscape plugin for cluster analysis and visualization of biological networks. Int J Mol Sci. 2017;18(pii):E1880. doi: 10.3390/ijms18091880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montojo J, Zuberi K, Rodriguez H, Kazi F, Wright G, Donaldson SL, Morris Q, Bader GD. GeneMANIA Cytoscape plugin: Fast gene function predictions on the desktop. Bioinformatics. 2010;26:2927–2928. doi: 10.1093/bioinformatics/btq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Bay-Jensen AC, Bihlet A, Byrjalsen I, Andersen J, He Y, Siebuhr A, Thudium C, Guehring H, Michaelis M, Ladel C, et al. Elevated levels of CRPM, an inflammatory biomarker correlating with disease activity in RA, are prognostic of radiographic knee OA. Osteoarthritis Cartilage. 2017;25(Suppl 1):S32. doi: 10.1016/j.joca.2017.02.065. [DOI] [Google Scholar]
- 24.Castrejon I, Chua JR, Malfait AM, Block JA, Pincus T. Disease burden in rheumatoid arthritis (RA) patients who have secondary osteoarthritis (OA) is lower than in primary OA but higher than in RA with no secondary OA. Osteoarthritis Cartilage. 2017;25:S218–S219. doi: 10.1016/j.joca.2017.02.378. [DOI] [Google Scholar]
- 25.Can T. Introduction to bioinformatics. Methods Mol Biol. 2014;1107:51–71. doi: 10.1007/978-1-62703-748-8_4. [DOI] [PubMed] [Google Scholar]
- 26.Huber R, Hummert C, Gausmann U, Pohlers D, Koczan D, Guthke R, Kinne RW. Identification of intra-group, inter-individual, and gene-specific variances in mRNA expression profiles in the rheumatoid arthritis synovial membrane. Arthritis Res Ther. 2008;10:R98. doi: 10.1186/ar2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma C, Lv Q, Teng S, Yu Y, Niu K, Yi C. Identifying key genes in rheumatoid arthritis by weighted gene co-expression network analysis. Int J Rheum Dis. 2017;20:971–979. doi: 10.1111/1756-185X.13063. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Han N, Li Z, Lu Q. Identification of transcription regulatory relationships in rheumatoid arthritis and osteoarthritis. Clin Rheumatol. 2013;32:609–615. doi: 10.1007/s10067-012-2143-9. [DOI] [PubMed] [Google Scholar]
- 29.Yi CQ, Ma CH, Xie ZP, Cao Y, Zhang GQ, Zhou XK, Liu ZQ. Comparative genome-wide gene expression analysis of rheumatoid arthritis and osteoarthritis. Genet Mol Res. 2013;12:3136–3145. doi: 10.4238/2013.March.11.3. [DOI] [PubMed] [Google Scholar]
- 30.Biswas S, Manikandan J, Pushparaj PN. Decoding the differential biomarkers of Rheumatoid arthritis and Osteoarthritis: A functional genomics paradigm to design disease specific therapeutics. Bioinformation. 2011;6:153–157. doi: 10.6026/97320630006153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Ning Y, Guo X. Integrative meta-analysis of differentially expressed genes in osteoarthritis using microarray technology. Mol Med Rep. 2015;12:3439–3445. doi: 10.3892/mmr.2015.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nayan S, Alizadehfar R, Desrosiers M. Humoral primary immunodeficiencies in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2015;15:46. doi: 10.1007/s11882-015-0547-8. [DOI] [PubMed] [Google Scholar]
- 33.Paciolla M, Pescatore A, Conte MI, Esposito E, Incoronato M, Lioi MB, Fusco F, Ursini MV. Rare mendelian primary immunodeficiency diseases associated with impaired NF-κB signaling. Genes Immun. 2015;16:239–246. doi: 10.1038/gene.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang LJ, Zhang NN, Ding F, Li XY, Chen L, Zhang HX, Zhang W, Chen SJ, Wang ZG, Li JM, et al. RA-inducible gene-I induction augments STAT1 activation to inhibit leukemia cell proliferation. Proc Natl Acad Sci USA. 2011;108:1897–1902. doi: 10.1073/pnas.1019059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lim JY, Im KI, Lee ES, Kim N, Nam YS, Jeon YW, Cho SG. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci Rep. 2016;6:26851. doi: 10.1038/srep26851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yokota A, Narazaki M, Shima Y, Murata N, Tanaka T, Suemura M, Yoshizaki K, Fujiwara H, Tsuyuguchi I, Kishimoto T. Preferential and persistent activation of the STAT1 pathway in rheumatoid synovial fluid cells. J Rheumatol. 2001;28:1952–1959. [PubMed] [Google Scholar]
- 37.Pongratz G, Anthofer JM, Melzer M, Anders S, Grässel S, Straub RH. IL-7 receptor α expressing B cells act proinflammatory in collagen-induced arthritis and are inhibited by sympathetic neurotransmitters. Ann Rheum Dis. 2014;73:306–312. doi: 10.1136/annrheumdis-2012-202944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the National Center for Biotechnology Information's Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/).