Abstract
The endothelial-to-mesenchymal transition (EndoMT) is a cellular process featuring decreased expression of endothelial marker genes but increased expression of mesenchymal marker genes. The EndoMT is involved in endothelial dysfunction and the pathogenesis of atherosclerosis. To investigate the dynamic expression of EndoMT genes in vascular endothelial cells under atheroprotective pulsatile shear stress (PS) and atheroprone oscillatory shear stress (OS), we analyzed RNA sequencing data from multitimepoint shear-stress experiments. This unbiased analysis involving next-generation sequencing confirmed that PS and OS had an opposite effect in regulating EndoMT genes. Further experimental validations with H2O2 and gain- and loss-of-function approaches indicated that reactive oxygen species are involved in OS-induced EndoMT, whereas AMP-activated protein kinase and sirtuin-1 could inhibit OS-induced EndoMT. Furthermore, compared with PS, OS increased the DNA methylation of the promoter regions of von Willebrand factor, CD31, and cadherin 5 genes but decreased that of cadherin 2, fibroblast-specific protein 1, and vimentin. The translational implication of the present study builds on the ability of the antidiabetic drug metformin and cholesterol-lowering drug atorvastatin to suppress the EndoMT in cultured endothelial cells and in mouse aortas.
NEW & NOTEWORTHY Our RNA sequencing data provided a genome-wide and unbiased view of the shear stress regulation of the endothelial-to-mesenchymal transition (EndoMT) in the endothelium. Furthermore, epigenetic regulation (e.g., DNA methylation) is a key mechanism involved in shear stress-regulated EndoMT. The translational implication of this study is that cardiovascular medications such as statins and metformin have similar beneficial effects as that of atheroprotective flow by mitigating EndoMT.
Keywords: AMP-activated protein kinase, endothelial-to-mesenchymal transition, endothelium, shear stress, sirtuin 1
INTRODUCTION
Defined as having a gain-of-mesenchymal but loss-of-endothelial phenotype, the endothelial-to-mesenchymal transition (EndoMT) is a cellular process involved in the pathogenesis of vascular diseases such as atherosclerosis (6, 17, 52). At the molecular level, the EndoMT is associated with decreased levels of endothelial cell (EC) markers [e.g., von Willebrand factor (vWF), CD31, and vascular-endothelial cadherin (i.e., CDH5)] but increased levels of mesenchymal cell markers [e.g., α-smooth muscle actin (α-SMA), neural cadherin (i.e., CDH2), fibroblast-specific protein 1 (FSP1), and vimentin] (12). Functionally, ECs lose their cell polarity and specific barrier functions but gain migratory and profibrotic properties resembling myofibroblast-like cells (28). The EndoMT was initially observed in cardiac valve formation during embryonic development (20). Thereafter, it was found to be involved in many adult cardiovascular diseases such as vascular remodeling, inflammatory vasculitis, and cardiac fibrosis (3, 24, 51, 52).
Atherosclerosis is prevalent in the bifurcations and curvatures of the arterial tree, where the blood flow pattern is disturbed. Such focal distribution of atherosclerosis is seen in human patients and also in various animal models, which indicates that the local blood flow pattern predisposes other atherogenesis risk factors (e.g., hyperlipidemia, hypertension, smoking, etc.) (10). We and others have extensively studied mechanotransduction and gene expression in ECs in response to different flow patterns and the consequent phenotypic modulations (5, 11, 55). In brief, atheroprotective flow [i.e., pulsatile shear stress (PS)] exerts anti-inflammatory, antioxidative, and antiproliferative effects in ECs, thereby maintaining EC homeostasis. In contrast, atheroprone flow [i.e., oscillatory shear stress (OS)] causes an inflammatory response and increases oxidative stress and EC migration (55). Studies by Moonen et al. (36) and Mahmoud et al. (31) have shown that the EndoMT could be induced by atheroprone flow in vitro and in vivo. Although these authors suggested that fibroblast growth factor receptor 1, Snail1, and MAPKs (MEK5/ERK5) were involved in the EndoMT (6, 31, 36), a detailed mechanism by which flow regulates the EndoMT remains unclear.
Among the mechanosensitive molecules in ECs that are activated/induced by atheroprotective flow, AMP-activated protein kinase (AMPK) is a master regulator of energy homeostasis and sirtuin-1 (SIRT1) is a NAD+-dependent class III histone deacetylase (33, 54). Activation of AMPK or SIRT1 has antioxidative and anti-inflammatory effects in ECs (8, 40, 44). Importantly, apolipoprotein E-null mice crossed with EC-specific AMPK-α2 or SIRT1 knockout mice show enhanced atherosclerosis, including in atheroprotective areas (8, 44). AMPK synergistically regulates gene expression with SIRT1, in part via epigenetic regulation (4, 32). Evidence supporting this postulation includes our recent finding that flow-activated AMPK can phosphorylate DNA methyltransferase (DNMT)1 to inhibit its activity and thus desuppress the expression of several mitochondrial genes (32). Although we and others have reported phenotypic changes of the EndoMT under diverse flow patterns (1, 30, 31, 34, 36), details of the mechanism of how shear stress induces mechanosensitive molecules contributing to the EndoMT remain to be studied.
In the present study, we used RNA sequencing (RNA-seq) data to further explore the effect of different flow patterns on the EndoMT in ECs. Such genome-wide and unbiased analysis of mRNA expression profiles revealed that atheroprotective and atheroprone flow had opposite effects in regulating the EndoMT. Further conventional experiments showed that metformin and atorvastatin had similar effects as atheroprotective flow in suppressing the EndoMT via AMPK and SIRT1 signaling.
MATERIALS AND METHODS
Cell culture, shear stress, and reagents.
Human umbilical vein ECs (HUVECs) were cultured in medium 199 supplemented with 20% FBS, acidic fibroblast growth factor (10 ng/ml), l-glutamine (2 mmol/l), 100 μg/ml heparin, penicillin (100 U/ml), and streptomycin (100 U/ml). Bovine aortic ECs were maintained in DMEM with 10% FBS, penicillin (100 U/ml), and streptomycin (100 U/ml). ECs were seeded on collagen-coated glass slides and subjected to PS (12 ± 4 dyn/cm2) or OS (1 ± 4 dyn/cm2) as previously described (29). Slides not exposed to flow were used as static controls. The flow system was enclosed in a chamber and kept at a constant temperature at 37°C with pH maintained at 7.4 by continuous gassing with 95% humidified air plus 5% (vol/vol) CO2. Atorvastatin, metformin, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), compound C, sirtinol, and 5-aza-2′-deoxycytidine (5-Aza) were from Sigma-Aldrich. Antibodies against vimentin, CD31, and fluorescent secondary antibodies (goat anti-mouse, goat anti-rat, and goat anti-rabbit) were from Cell Signal Technology. Anti-β-actin and horseradish peroxidase-labeled secondary antibodies (goat anti-mouse, goat anti-rabbit, and donkey anti-goat) were from Santa Cruz Biotechnology. Anti-α-SMA was from Abcam and anti-vWF was from R&D.
Quantitative real-time PCR.
Total RNA was isolated from cultured ECs and mouse aortas with the use of TRIzol reagent (Invitrogen) and reverse transcribed with Superscript reverse transcriptase (ThermoFisher Scientific) and oligo (DT) primers. Real-time quantitative PCR involved the use of SYBR Green PCR Supermix (Promega) in the ABI 7500 real-time detection system. Primer sequences for CD31, vWF, CDH5, α-SMA, CDH2, FSP1, vimentin, and GAPDH are shown in Table 1. GAPDH was used as an internal control. The PCR conditions were 95°C for 10 min and 45 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 30 s, concluded by the melting curve analysis process. The fold change in mRNA level was calculated by the method (where Ct is threshold cycle).
Table 1.
Primers used for quantitative PCR
| Gene | Species | Sequence |
|---|---|---|
| vWF | Homo sapiens | Forward: 5′-CCGATGCAGCCTTTTCGGA-3′ |
| Reverse: 5′-TCCCCAAGATACACGGAGAGG-3′ | ||
| Mus musculus | Forward: 5′-TCCCAAGAGGAAGTGGACAT-3′ | |
| Reverse: 5′-GGTACACGACAGAGCCATTG-3′ | ||
| CD31 | Homo sapiens | Forward: 5′-GAGTCCAGCCGCATATCC-3′ |
| Reverse: 5′-TGACACAATCGTATCTTCCTTC-3′ | ||
| Mus musculus | Forward: 5′-CTGCTCCACTTCTGAACTCC-3′ | |
| Reverse: 5′-TACATCCATGTTCTGGGGGT-3′ | ||
| VE-cadherin | Homo sapiens | Forward: 5′-CACCTTCTGCGAGGATATGG-3′ |
| Reverse: 5′-AGGAAGATGAGCAGGGTGAT-3′ | ||
| Mus musculus | Forward: 5′-CTTCTGTGAGGAGATGGCAG-3′ | |
| Reverse: 5′-CATCATAGCTGGTGGTGTCC-3′ | ||
| α-SMA | Homo sapiens | Forward: 5′-CAGGGCTGTTTTCCCATCCAT-3′ |
| Reverse: 5′-GCCATGTTCTATCGGGTACTTC-3′ | ||
| Mus musculus | Forward: 5′-CTGACAGGATGCAGAAGGAG-3′ | |
| Reverse: 5′-CTGGAAGGTAGACAGCGAAG-3′ | ||
| N-cadherin | Homo sapiens | Forward: 5′-AGCCAACCTTAACTGAGGAGT-3′ |
| Reverse: 5′-GGCAAGTTGATTGGAGGGATG-3′ | ||
| Mus musculus | Forward: 5′-TGAAACGGCGGGATAAAGAG-3′ | |
| Reverse: 5′-GGCTCCACAGTATCTGGTTG-3′ | ||
| FSP1 | Homo sapiens | Forward: 5′-GTCCACCTTCCACAAGTAC-3′ |
| Reverse: 5′-TGTCCAAGTTGCTCATCAG-3′ | ||
| Mus musculus | Forward: 5′-TCCTGGGGAAAAGGACAGAT-3′ | |
| Reverse: 5′-AATGCAGGACAGGAAGACAC-3′ | ||
| Vimentin | Homo sapiens | Forward: 5′-GCCCTAGACGAACTGGGTC-3′ |
| Reverse: 5′-GGCTGCAACTGCCTAATGAG-3′ | ||
| Mus musculus | Forward: 5′-GCCAACCTTTTCTTCCCTGA-3′ | |
| Reverse: 5′-TCAAGGTCATCGTGATGCTG-3′ | ||
| GAPDH | Homo sapiens and Mus musculus | Forward: 5′-ACCACAGTCCATGCCATCAC-3′ |
| Reverse: 5′-TCCACCACCCTGTTGCTGTA-3′ |
vWF, von Willebrand factor; VE-cadherin, vascular-endothelial cadherin; α-SMA, α-smooth muscle actin; N-cadherin, neural cadherin; FSP1, fibroblast-specific protein 1.
Western blot analysis.
Protein extracts were obtained using a protein extraction kit (QIAGEN). After the concentration was determined, protein samples were mixed with reducing buffer, boiled for 10 min, separated on SDS-PAGE, transferred to polyvinylidene difluoride membranes, blocked with 5% nonfat milk in Tris-buffered saline with Tween 20, and then incubated with primary antibodies at 4°C overnight. Protein bands were detected with horseradish peroxidase-conjugated secondary antibodies and visualized by using the ECL chemiluminescence system (Millipore).
RNA isolation from the mouse aorta.
RNA was isolated from intima of mouse aortas as previously described (37). Briefly, mice were euthanized by CO2 inhalation and pressure perfused with PBS via the left ventricle. The aortic arch (AA) and thoracic aorta (TA) were then isolated, and periadventitial fat was removed. The aorta lumen was quickly flushed with 250 μl TRIzol reagent (Invitrogen) using a 271/2-gauge syringe in a 35-mm petri dish. The intimal eluate was then collected for intimal RNA isolation.
Animal experiments.
Animal experiments were approved by and complied with the guidelines of the Institutional Animal Care and Use Committee of Xi’an Jiaotong University. Mice were fed ad libitum. Both sexes of C57BL/6 mice (6 wk old) were intraperitoneally injected with atorvastatin (50 mg/kg body wt), metformin (200 mg/kg body wt), 5-Aza (0.2 mg/kg body wt), or the same amount of saline as a control. At 24 h after injection, the intima was isolated from the AA or TA areas of C57BL/6 mice. Total RNA was isolated, and mRNA levels of vWF, CD31, CDH5, α-SMA, CDH2, FSP1, and vimentin were analyzed by quantitative PCR as previously described (29).
DNA methylation-specific quantitative PCR.
Genomic DNA was isolated from ECs by using the QIAamp DNA Mini Kit (Qiagen). Bisulfite conversion involved using the EpiTect Bisulfite kit (Qiagen), and the DNA methylation status of EndoMT-related genes was analyzed by quantitative PCR with primers that specifically recognize the methylated cytosines (Table 2).
Table 2.
Primers used for DNA methylation-specific quantitative PCR
| Gene | Species | Sequence |
|---|---|---|
| vWF | Homo sapiens | Forward: 5′-TTTCGAGTAGTTGGGATTATAGGC-3′ |
| Reverse: 5′-TAAAAAAAACCATTAAAAAACCGAA-3′ | ||
| Mus musculus | Forward: 5′-ATTGTTTTTTGTTATTGTTTTTCGT-3′ | |
| Reverse: 5′-AAATCTACTCAAACTCTAAACCGAC-3′ | ||
| CD31 | Homo sapiens | Forward: 5′-GGATTATAGGCGTGAGTTATTGC-3′ |
| Reverse: 5′-AAATTATAAAATTCCCGAATTTCGT-3′ | ||
| Mus musculus | Forward: 5′-TTATAGAAAGAAGTTGTTTGTTTCGG-3′ | |
| Reverse: 5′-TCTACAATCTTTACGACACATCGAT-3′ | ||
| VE-cadherin | Homo sapiens | Forward: 5′-GGTACGTAGATTATGAGGATAGGC-3′ |
| Reverse: 5′-AAAATAAACAAACAACAACTCAACG-3′ | ||
| Mus musculus | Forward: 5′-TGGTTTGAAGGTTATTTTAGAGACG-3′ | |
| Reverse: 5′-CTACCAAAAAAACAAAAAACACGAT-3′ | ||
| α-SMA | Homo sapiens | Forward: 5′-TTTACGTTTATTTTAACGTGGAGC-3′ |
| Reverse: 5′-ACCTAAAAACGACGAACTACTACGA-3′ | ||
| Mus musculus | Forward: 5′-TTTTTTAAAGGAATTGTTAGTTCGT-3′ | |
| Reverse: 5′-CAATAAATCCTTATCATAAACATCGTA-3′ | ||
| N-cadherin | Homo sapiens | Forward: 5′-GTGCGAGTTTTAGAGAGGAGTC-3′ |
| Reverse: 5′-TATCTCCTTAAAAACCTAACGTACG-3′ | ||
| Mus musculus | Forward: 5′-GGATTTAATATAGGGTTAGGGTCGT-3′ | |
| Reverse: 5′-ATAAAAAACCACCGAACTACGAA-3′ | ||
| FSP1 | Homo sapiens | Forward: 5′-GTTTTTGAGATGTGGGTTTGTATAC-3′ |
| Reverse: 5′-CCTTCTCGAACTAAACTACTACGTA-3′ | ||
| Mus musculus | Forward: 5′-GTTTCGGGATTTTAGAGATTTTAGC-3′ | |
| Reverse: 5′-CAAACCTAATAAACATAACTTCGAA-3′ | ||
| Vimentin | Homo sapiens | Forward: 5′-GTATGAATTGGTGGATTTTGTAGAC-3′ |
| Reverse: 5′-TTATACACTCTAAAACCCTAAACGAA-3′ | ||
| Mus musculus | Forward: 5′-GAGTTTTTGGAACGTTAGATGC-3′ | |
| Reverse: 5′-TTCTAAATCTCATCCTACAAACGAC-3′ | ||
| UBB | Homo sapiens | Forward: 5′-ATAGTGGGTTTTGTTGATTTGA-3′ |
| Reverse: 5′-CCTTTCTCACACTAAAATTCCA-3′ | ||
| Mus musculus | Forward: 5′-GATTTAGTAGGTTTTTAAATTTTTATT-3′ | |
| Reverse: 5′-ATACCCTCTTTATCCTAAATCTTAA-3′ |
vWF, von Willebrand factor; VE-cadherin, vascular-endothelial cadherin; α-SMA, α-smooth muscle actin; N-cadherin, neural cadherin; FSP1, fibroblast-specific protein 1; UBB, ubiquitin B.
Statistical analysis.
All data are presented as means ± SE. Student’s t-test or ANOVA followed by the Bonferroni post hoc correction involved using GraphPad Prism (GraphPad Software) to analyze differences among groups. P < 0.05 was considered statistically significant.
RESULTS
RNA-seq data reveal EndoMT induction by OS.
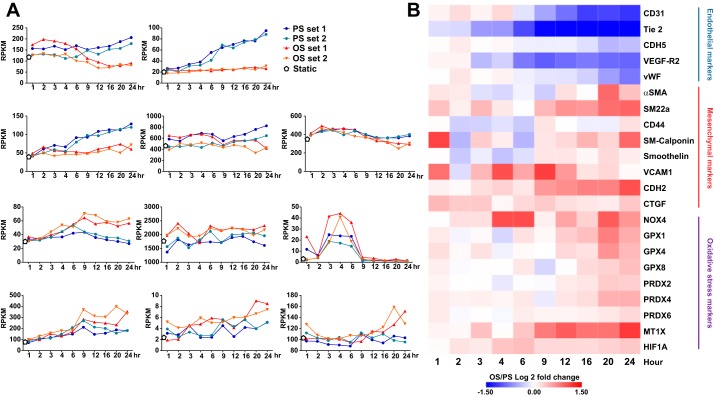
Using our established 10-timepoint RNA-seq data (1), we compared the effect of atheroprone OS and atheroprotective PS on the EndoMT in ECs at the genome-wide scale. With PS, mRNA levels of genes related to the EC phenotype (e.g., CD31, Tie2, VE growth factor receptor 2, vWF, and CDH5) were increased (Fig. 1A), whereas atheroprone OS had little effect on their expression. At 24 h after exposure to flow, the expression of all of these genes was increased under PS. In contrast, in general, OS induced mRNA levels of mesenchymal markers such as CDH2, connective tissue growth factor, VCAM-1, and smooth muscle protein-22α compared with PS. Some mesenchymal genes, such as vimentin and FSP1, were not greatly induced by OS. Additionally, genes involved in ROS induction, such as NADPH oxidase 4 and peroxiredoxin 4, were upregulated by OS. The heat map shown in Fig. 1B provides a comprehensive view of the opposite trend of EndoMT gene expression under OS and PS and the same direction of mesenchymal and oxidative gene expression induced by OS.
Fig. 1.
RNA-sequencing analysis of oscillatory shear stress (OS) induction of the endothelial-to-mesenchymal transition. Human umbilical vein endothelial cells were exposed to OS or pulsatile shear stress (PS) for the 10 timepoints as indicated. RNA samples collected at each timepoint and static controls underwent RNA-sequencing analysis. A: mRNA levels of selected endothelial cell, mesenchymal, and oxidative stress markers represented by reads per kilobase million mapped reads (RPKM). B: heat map representing the average of log2 fold changes of OS/PS at the 10 times for the indicated genes. Each time under OS or PS involved two biological repeats. VEGFR-2, VEGF receptor 2; vWF, von Willebrand factor; CDH, cadherin; CTGF, connective tissue growth factor; SM22a, smooth muscle-22α; NOX, NADPH oxidase 4; PRDX4, peroxiredoxin 4; α-SMA, α-smooth muscle actin; GPX, glutathione peroxidase; MT1X, metallothionein 1X; HIF1A, hypoxia-inducible factor-1α.
Atheroprone flow induces the EndoMT in vitro and in vivo.
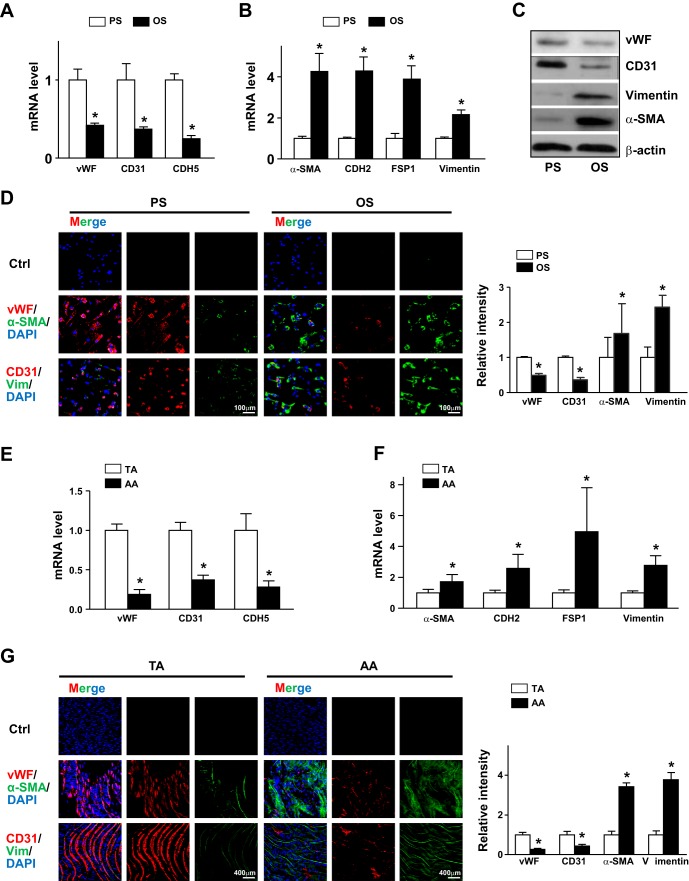
To confirm the results from RNA-seq data, we assessed the differential expression of EndoMT-related genes in cultured ECs responding to PS or OS. Compared with PS, OS reduced mRNA expression of vWF, CD31, and CDH5 but induced that of α-SMA, CDH2, FSP1, and vimentin (Fig. 2, A and B). Consistently, under OS, the protein levels of vWF and CD31 were lower and those of α-SMA and vimentin were higher (Fig. 2C). We also performed immunostaining to confirm OS-induced EndoMT in ECs further. Levels of vWF and CD31 as well as α-SMA and vimentin showed an opposite trend under PS and OS (Fig. 2D). EC markers were expressed higher under PS, whereas mesenchymal markers were expressed higher under OS.
Fig. 2.
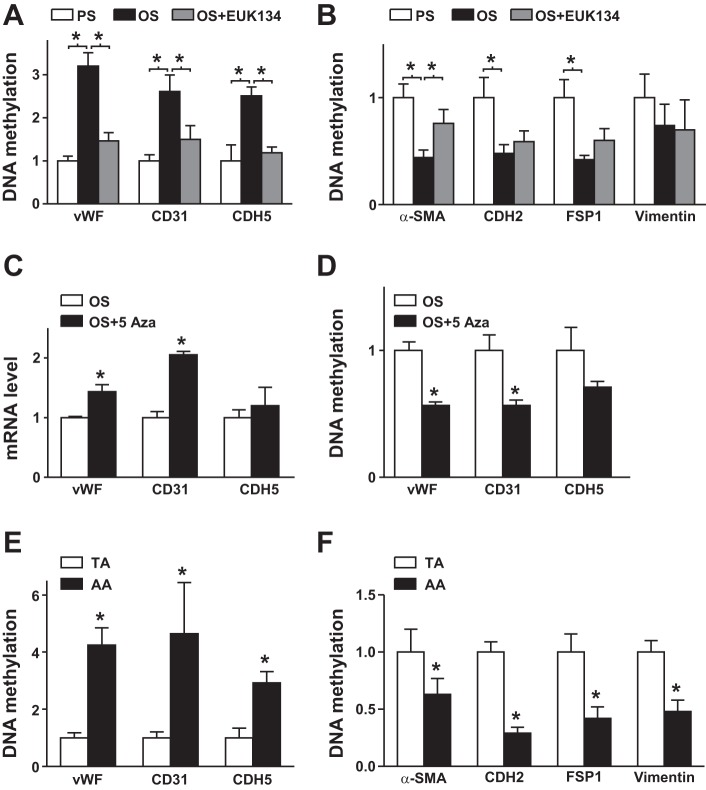
Pulsatile shear stress (PS) and oscillatory shear stress (OS) distinctly regulate the endothelial-to-mesenchymal transition in endothelial cells (ECs). A–D: human umbilical vein ECs were subjected to PS (12 ± 4 dyn/cm2) or OS (1 ± 4 dyn/cm2) for 24 h. mRNA levels of von Willebrand factor (vWF), CD31, caherin 5 (CDH5), α-smooth muscle actin (α-SMA), cadherin 2 (CDH2), fibroblast-specific protein 1 (FSP1), and vimentin (Vim) were measured by real-time quantitative PCR. C: protein levels of CD31, vWF, vimentin, and α-SMA were measured by Western blot analysis. The β-actin level was used as a loading control. D: immunofluorescence staining with anti-vWF (red), anti-CD31 (red), anti-α-SMA (green), and antivimentin (green) antibodies. E and F: intima were collected from aortic arch (AA) or thoracic aorta (TA) areas of C57BL/6 mice (n = 18). mRNA levels of the indicated genes were quantified by real-time quantitative PCR. G: en face immunofluorescence staining with anti-vWF (red), anti-CD31 (red), anti-α-SMA (green), and anti-vimentin (green) antibodies revealed the expression of EC and mesenchymal genes in TA or AA regions. In D and G, images were acquired and the fluorescent signal was analyzed by Nikon C2 confocal laser scanning microscopy. The fluorescence intensity of 100 randomly chosen cells was determined using ImageJ. Data are means ± SE from three independent experiments. *P < 0.05 between the indicated groups.
To validate the atheroprone flow induction of the EndoMT in vivo, we isolated the intima from atheroprotective areas (i.e., TA) and atheroprone areas (i.e., AA) and compared the expression of EndoMT genes. Levels of vWF, CD31, and CDH5 were higher in TA than AA areas, but those of α-SMA, CDH2, FSP1, and vimentin were lower (Fig. 2, E and F). Furthermore, we used en face immunostaining for vWF, CD31, α-SMA and vimentin in TA and AA areas of C57BL/6 mice. Consistently, levels of vWF and CD31 were higher in TA areas (Fig. 2G) but levels of α-SMA and vimentin were higher in AA areas (Fig. 2G). Thus, PS induced EC lineage-dependent markers and OS induced mesenchymal-related genes in ECs in vitro and in vivo.
OS induction of the EndoMT via elevated oxidative stress.
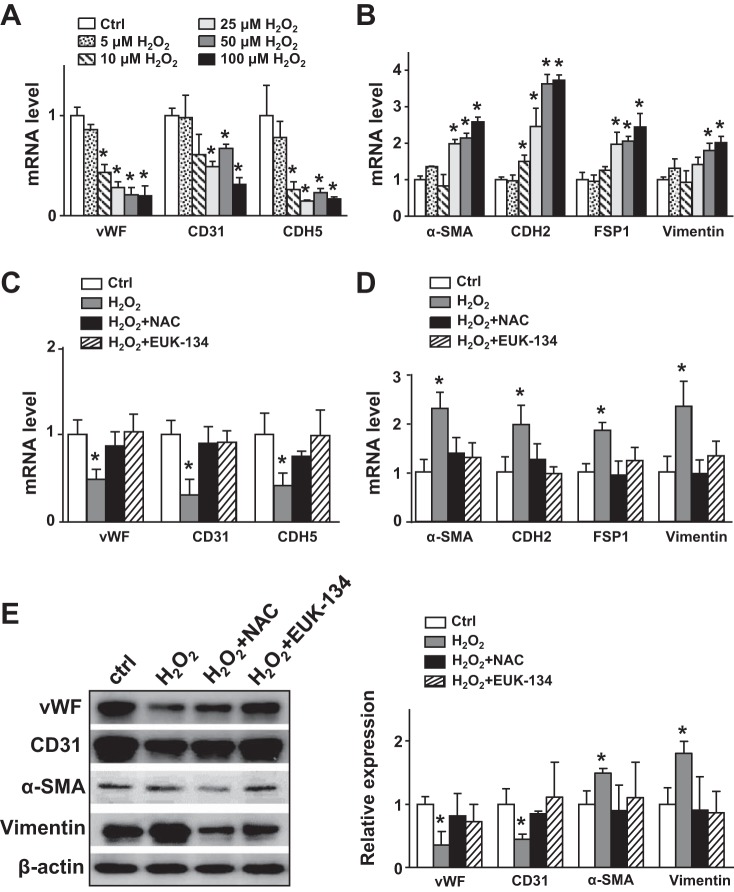
OS elevates levels of ROS in the endothelium in vitro and in vivo (10), and a dysregulated redox state can induce the EndoMT (17, 35). Therefore, we investigated whether the OS-induced EndoMT was mediated at least in part by increased ROS levels. We treated ECs with various concentrations of H2O2 (i.e., 5, 10, 25, 50, and 100 μmol/l) for 24 h. H2O2 dose dependently decreased EC marker expression but increased that of mesynchamal markers (Fig. 3, A and B). Pretreatment with N-acetylcysteine (a free radical scavenger) or EUK-134 (a catalase/superoxide dismutase mimetic) reversed the H2O2-induced EndoMT at both mRNA and protein levels (Fig. 3, C–E). These results strongly suggest that OS-mediated oxidative stress induces the EndoMT.
Fig. 3.
Oxidative stress in endothelial cells (ECs) promotes the endothelial-to-mesenchymal transition. A and B: human umbilical vein ECs were treated with H2O2 (5, 10, 25, 50, and 100 μmol/l) for 24 h. C–E: human umbilical vein ECs were pretreated with N-acetylcysteine (NAC; 5 mmol/l) or EUK-134 (1 μmol/l) for 5 h before incubation with H2O2 (100 μmol/l) for 18 h. mRNA levels of von Willebrand factor (vWF), CD31, cadherin 5 (CDH5, α-smooth muscle actin (α-SMA), cadherin 2 (CDH2), fibroblast-specific protein 1 (FSP1), and vimentin were measured by real-time quantitative PCR. E: protein levels of vWF, CD31, α-SMA, and vimentin were measured by Western blot analysis. Ctrl, control. Data are means ± SE from at least three independent experiments. *P < 0.05 between the indicated groups.
AMPK and SIRT1 suppress the EndoMT.
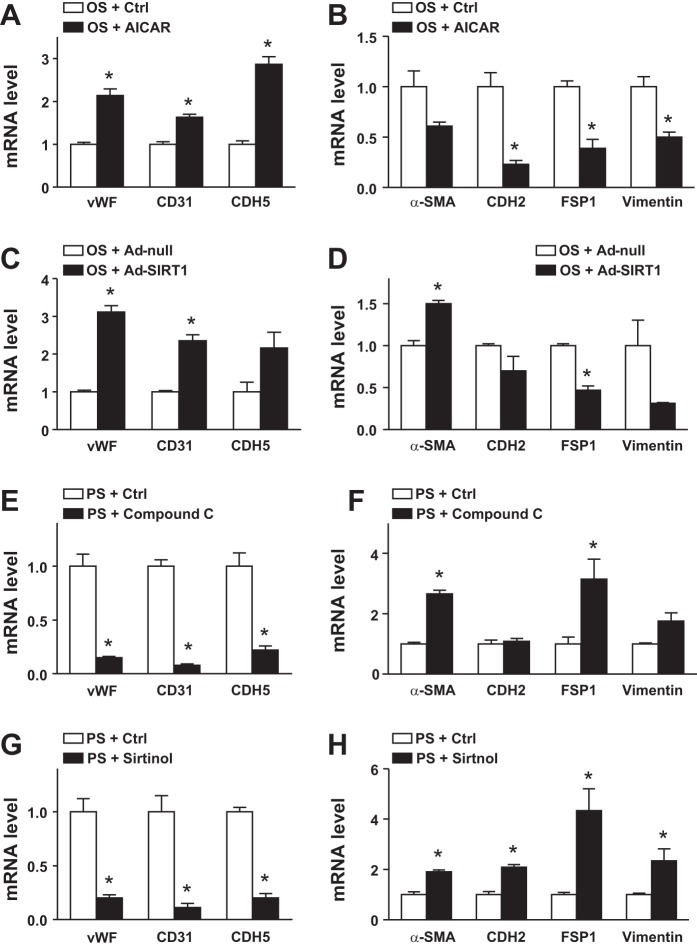
Atheroprotective flow activates AMPK and SIRT1 in the endothelium, which is crucial for shear stress-regulated EC homeostasis (8, 40, 44). We used a gain-of-function approach to examine whether AMPK and/or SIRT1 could suppress OS-induced EndoMT. ECs were treated with AICAR to activate AMPK or infected with adenovirus-SIRT to overexpress SIRT1 before the application of OS. AICAR increased the expression of vWF, CD31, and CDH5 and decreased that of CDH2, FSP1, and vimentin in ECs (Fig. 4, A and B). Overexpression of SIRT1 caused similar changes in levels of genes associated with the EndoMT (Fig. 4, C and D). Thus, AMPK or SIRT1 activation in the endotheium might decrease the EndoMT in response to OS.
Fig. 4.
AMP-activated protein kinase (AMPK) and sirtuin (SIRT1) are involved in pulsatile shear stress (PS)-suppressed endothelial-to-mesenchymal transition. Human umbilical vein endothelial cells were treated with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; 1 mmol/l), adenovirus-SIRT1 (multiplicity of infection: 20) and OS and with compound C (5 μmol/l) or sirtinol (20 μmol/l) and PS for 24 h. mRNA levels of von Willebrand factor (vWF), CD31, cadherin 5 (CDH5), α-smooth muscle actin (α-SMA), cadherin 2 (CDH2), fibroblast-specific protein 1 (FSP1), and vimentin were measured by real-time quantitative PCR. Ctrl, control. Data are means ± SE from three independent experiments. *P < 0.05 between the indicated groups.
We used a complementary loss-of-function experiment to elucidate the role of AMPK and SIRT1 in regulating the EndoMT in the context of flow-mediated gene expression in the endothelium furhter. ECs were treated with compound C or sirtinol to inhibit AMPK or SIRT1, respectively, before PS stimulation. PS-suppressed EndoMT was reversed with compound C or sirtinol treatment, as evidenced by the decreased expression of vWF, CD31, and CDH5 but increased expression of α-SMA, CDH2, FSP1, and vimentin (Fig. 4, E–H). Thus, PS-activated AMPK and SIRT1 may be beneficial for suppressing the EndoMT in ECs responding to PS.
Flow regulation of DNA methylation.
Epigenetic regulation constitutes an integral part of transcriptional regulation in ECs in response to shear stress, in part via DNMT-regulated DNA methylation (7, 15, 27). Thus, in addition to deciphering the upstream signaling molecules in the EndoMT (i.e., AMPK and SIRT1), we studied the role of DNA methylation in the EndoMT in response to shear stress. Compared with PS, OS increased DNA methylation in the promoter regions of vWF, CD31, and CDH5 (Fig. 5A) but reduced it in α-SMA, CDH2, FSP1, and vimentin (Fig. 5B). However, including EUK-134 in the shearing media alleviated OS-induced EndoMT (Fig. 5, A and B). To further demonstrate that OS-mediated EndoMT involved differential regulation of DNA methylation, ECs were treated with or without 5-Aza (an inhibitor of DNA methylation) and then stimulated with OS. 5-Aza treatment increased mRNA levels of vWF, CD31, and CDH5 in ECs under OS (Fig. 5C). Consistently, 5-Aza treatment significantly decreased DNA methylation of vWF, CD31, and CDH5 (Fig. 5D). The distinct regulation of DNA methylation by OS versus PS was also seen in mouse aortas in vivo. Specifically, DNA methylation of EC markers was significantly lower in the intima of TA than AA areas (Fig. 5E), but DNA methylation of mesenchymal markers was lower in the intima of AA than TA areas (Fig. 5F). Thus, DNA methylation may have a role in the differential expression of both EC and mesenchymal markers under atheroprotective versus atheroprone flow conditions.
Fig. 5.
Pulsatile shear stress (PS) and oscillatory shear stress (OS) regulation of DNA methylation of genes involved in the endothelial-to-mesenchymal transition. A and B: human umbilical vein endothelial cells were pretreated with or without EUK-134 (1 μmol/l) for 5 h before stimulation with PS or OS for 24 h. Genomic DNA was converted to nonmethylated uracil, and DNA methylation levels of promoter regions of von Willebrand factor (vWF), CD31, cadherin 5 (CDH5), α-smooth muscle actin (α-SMA), cadherin 2 (CDH2), fibroblast-specific protein 1 (FSP1), and vimentin genes were measured by methylation-specific quantitative PCR. C and D: human umbilical vein endothelial cells were treated with or without 5-aza-2′-deoxycytidine (5-Aza; 5 μmol/l) for 12 h and then stimulated with OS for an additional 12 h. C: mRNA levels of vWF, CD31, and CDH5 were quantified by real-time quantitative PCR. D: modifications of DNA methylation on the promoter region of the vWF, CD31, and CDH5 genes were measured by methylation-specific quantitative PCR. E and F: the intima was collected from thoracic aorta (TA) or aortic arch (AA) areas of C57BL/6 mice (n = 18). Genomic DNA was converted to nonmethylated uracil, and the DNA methylation of the indicated genes was measured accordingly. Ctrl, control. Data are means ± SE from three independent experiments. *P < 0.05 between the indicated groups.
Atheroprotective drugs suppress the EndoMT in vitro and in vivo.
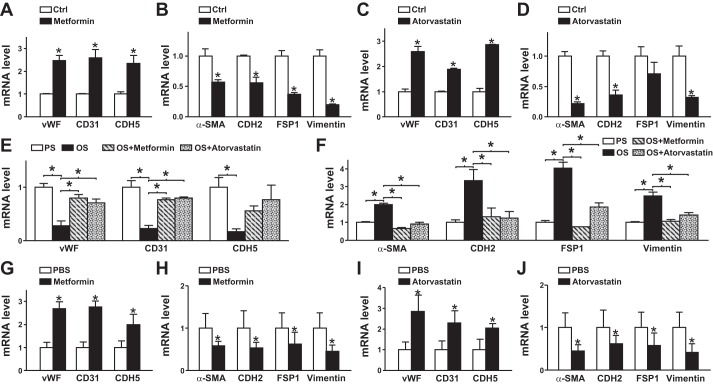
Because of the similar beneficial effects of atheroprotective flow and cardiovascular drugs (e.g., metformin and statins) on the vascular endothelium (41, 54), we next examined whether metformin and statins can decrease the EndoMT. Cultured ECs were treated with or without metformin or atorvastatin. Both metformin and atorvastatin increased the levels of EC markers but decreased those of mesenchymal markers (Fig. 6, A–D). Consistently, metformin and atorvastatin alleviated OS-induced EndoMT (Fig. 6, E and F). We also administered metformin or atorvastatin to C57BL/6 mice. As expected, both metformin and atorvastatin increased the expression of EC markers but suppressed that of mesenchymal markers in mouse aortas compared with PBS treatment (Fig. 6, G–J).
Fig. 6.
Metformin and atorvastatin suppress the endothelial-to-mesenchymal transition in vitro and in vivo. A–D: human umbilical vein endothelial cells were treated with or without metformin (1 mmol/l) or atorvastatin (0.1 μmol/l). E and F: human umbilical vein endothelial cells pretreated with or without metformin (1 mmol/l) or atorvastatin (0.1 μmol/l) for 12 h were exposed to pulsatile shear stress (PS) or oscillatory shear stress (OS) for 24 h. G–J: C57BL/6 mice (6 wk old) were intraperitoneally injected with or without metformin (200 mg/kg body wt) or atorvastatin (50 mg/kg body wt). At 24 h, the intima was isolated from the aortic arch area (n = 9, 3 independent experiments with samples pooled from 3 animals in each of the experiments). mRNA levels of vWF, von Willebrand factor (vWF), CD31, cadherin 5 (CDH5), α-smooth muscle actin (α-SMA), cadherin 2 (CDH2), fibroblast-specific protein 1 (FSP1), and vimentin were quantified by real-time quantitative PCR. Ctrl, control. Data are means ± SE from three independent experiments. *P < 0.05 between the indicated groups.
DISCUSSION
Increasing evidence indicates that ECs can undergo the EndoMT in the arterial wall, which leads to vascular fibrosis associated with many cardiovascular diseases, including atherosclerosis (6, 17). Among the reported EndoMT stimuli, an atheroprone flow pattern is a principal risk factor of atherosclerosis (10). In the present study, using RNA-seq data, we found that atheroprone flow could induce the EndoMT, which is consistent with previous reports (1, 30, 31, 34, 36). Our systemic analysis of RNA-seq data provided a genome-wide and unbiased view of the shear stress regulation of the EndoMT. However, the caveat of data generated from next-generation sequencing is that methodological issues associated with sequencing technology may restrict the experimental authentication. For example, quantitative PCR but not 10-timepoint data showed the induction of vimentin by OS (Figs. 1B and 2B). Other new findings include 1) PS and OS differentially regulating the EndoMT, as shown by the time-dependent RNA-seq data; 2) epigenetic regulation (i.e., DNA methylation) involved in the shear stress regulation of EndoMT; and 3) cardiovascular medicine such as statins and metformin acting similar to atheroprotective flow to regulate the EndoMT.
A number of inflammatory cytokines, including transforming growth factor (TGF)-β, TNF-α, and IL-1β, can induce the EndoMT (21). A maladaptive effect that is common to these inflammatory stimuli and atheroprone flow is the increased redox burden in the endothelium, in part by induction of NADPH oxidase (26). An increase in oxidative stress is an important factor for the epithelial-to-mesenchymal transition (38). Several studies have reported the involvement of hypoxia-inducible factor (HIF)-1α in the epithelial-to-mesenchymal transition (25, 53). ΗΙF-1α regulates TWIST and Snail, two transcription factors that transactivate mesenchymal genes (49). The increased oxidative burden in ECs under OS is supported by our RNA-seq data (Fig. 1). Critically, treatment of ECs with H2O2 induced the EndoMT (Fig. 3). The mechanism underlying oxidative stress-induced EndoMT remains elusive. However, aurora-like kinase 5 and NF-κB may be involved (3).
TGF-β-induced EndoMT can be suppressed in ECs costimulated with laminar shear stress (36). Similarly, TNF-α-induced inflammation can be suppressed in ECs by laminar flow (42). In terms of upregulation of endothelial markers by atheroprotective flow, Krüppel-like factor (KLF)2 and KLF4 seem to play a crucial role (16). Functioning as master transcription factors to regulate genes important for EC function, both KLF2 and KLF4 have been implicated in endothelial homeostasis (39). Egorova et al. (16) showed that ECs lacking primary cilia undergo shear-induced EndoMT, accompanied by downregulation of KLF4. Importantly, such EndoMT can be rescued by KLF4 overexpression or TGF-β signaling blockade (16). Our data also show that pharmacological inhibition of AMPK or SIRT1 enhanced the EndoMT under PS (Fig. 4). In contrast, activation of AMPK or overexpression of SIRT1 attenuated OS-induced EndoMT. Thus, AMPK and/or SIRT1 may negatively regulate the EndoMT in ECs. Atheroprotective flow is known to activate AMPK, leading to KLF2 induction in ECs, which is mediated by ERK5 and MEF2 (50). Moreover, resveratrol-activated SIRT1 increases the expression of KLF2 (22). Thus, the AMPK/SIRT1-KLF2/KLF4 axis may antagonize the EndoMT. The beneficial role of AMPK and SIRT1 in alleviating redox burden has been well documented. For example, Wang et al. (43) showed that AMPK-α2 functions as a physiological suppressor of NAD(P)H oxidase and ROS production in ECs. SIRT1 was found to mitigate oxidative stress by upregulating FOXO (2). Furthermore, resveratrol can protect ECs against oxidized low-density lipoprotein-induced oxidative damage via the AMPK/SIRT1 synergism (23).
We and others have shown that atheroprone flow induces microRNA-92a, which, in turn, targets KLF2 and KLF4 (9, 18, 46). Thus, an additional mechanism by which atheroprone flow induces the EndoMT would include microRNA-92a suppression of KLF2/KLF4, thereby decreasing the expression of EC marker genes. A recent study by Xiong et al. (48) showed that the endothelial metabolic status is critical in the regulation of the EndoMT. Intriguingly, shear stress regulates endothelial metabolism. For example, laminar flow inhibits glucose uptake and glycolysis via KLF2 (14). In contrast, disturbed flow induces glycolysis and reduces mitochondrial respiratory capacity in a HIF-1α-dependent manner (19, 45). Thus, flow-modulated EndoMT may also be associated with the metabolic changes tightly regulated by atheroprone versus atheroprotective flow patterns.
In response to atheroprotective flow, AMPK regulates KLF2 at the transcriptional level and also at the epigenetic level. Under PS, AMPK-mediated phosphorylation of DNMT1 results in inhibition of DNMT1 (32). The consequent nucleosome remodeling and decreased DNA methylation causes increased expression of genes encoding proteins involved in mitochondrial biogenesis (32). Consistent with AMPK activation, DNMT1 phosphorylation, and decreased DNMT1 activity, the promoter regions of EC marker genes (i.e., vWF, CD31, and CDH5) were hypomethylated (Fig. 5), which would account in part for the increased expression of these genes under PS. Paradoxically, the promoter regions of mesenchymal genes (i.e., α-SMA, CDH2, FSP1, and vimentin) were hypermethylated, which decreased the expression of these genes. The molecular insights underlying the hypermethylated upstream region of mesenchymal genes in connection with the decreased DNMT1 activity in ECs under PS deserve further investigation.
Antihyperglycemic drugs (e.g., metformin) and cholesterol-lowering drugs (e.g., atorvastatin) have important beneficial effects on the endothelium. Like atheroprotective flow, metformin and atorvastatin can activate AMPK, induce SIRT1 and KLF2/KLF4, and increase endothelial nitric oxide synthase-derived nitric oxide bioavailability in ECs (41, 50, 54). Furthermore, these drugs may attenuate TGF-β expression and profibrotic pathways (38a, 47). Given that metformin and statins have therapeutic effects in atherosclerosis, this beneficial effect may be mediated at least in part by suppressing the EndoMT in the vasculature.
GRANTS
This work was supported in part by funds from the National Natural Science Foundation of China Grants 81670452 and 91739112 (to J. Shyy) and National Heart, Lung, and Blood Institute Grant HL-125643 (to J. Shyy).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.L., Z.L., and J.Y.-J.S. conceived and designed research; B.L., Z.L., L.C., and Y.Y. performed experiments; B.L. and Z.L. analyzed data; B.L., Z.L., M.H., J.Z., and J.Y.-J.S. interpreted results of experiments; B.L., Z.L., M.H., and J.Z. prepared figures; B.L., Z.L., M.H., and J.Y.-J.S. drafted manuscript; B.L., Z.L., M.H., and J.Y.-J.S. edited and revised manuscript; B.L., Z.L., M.H., Y.W., L.C., J.Z., Y.Y., and J.Y.-J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Tse-Shun Huang, Jacobs School of Engineering and Dr. Brendan Gongol (School of Medicine, University of California-San Diego) for the help in data analysis, and Jin Zhang (School of Basic Medical Sciences, Xi’an Jiaotong University Health Science Center) for assistance in animal experiments.
REFERENCES
- 1.Ajami NE, Gupta S, Maurya MR, Nguyen P, Li JYS, Shyy JYJ, Chen Z, Chien S, Subramaniam S. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc Natl Acad Sci USA 114: 10990–10995, 2017. doi: 10.1073/pnas.1707517114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512–1521, 2007. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 3.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 293: L1–L8, 2007. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 4.Ayissi VB, Ebrahimi A, Schluesenner H. Epigenetic effects of natural polyphenols: a focus on SIRT1-mediated mechanisms. Mol Nutr Food Res 58: 22–32, 2014. doi: 10.1002/mnfr.201300195. [DOI] [PubMed] [Google Scholar]
- 5.Baeyens N, Bandyopadhyay C, Coon BG, Yun S, Schwartz MA. Endothelial fluid shear stress sensing in vascular health and disease. J Clin Invest 126: 821–828, 2016. doi: 10.1172/JCI83083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen PY, Qin L, Baeyens N, Li G, Afolabi T, Budatha M, Tellides G, Schwartz MA, Simons M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J Clin Invest 125: 4514–4528, 2015. doi: 10.1172/JCI82719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Z, Li S, Subramaniam S, Shyy JY, Chien S. Epigenetic regulation: a new frontier for biomedical engineers. Annu Rev Biomed Eng 19: 195–219, 2017. doi: 10.1146/annurev-bioeng-071516-044720. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Peng IC, Cui X, Li YS, Chien S, Shyy JY. Shear stress, SIRT1, and vascular homeostasis. Proc Natl Acad Sci USA 107: 10268–10273, 2010. doi: 10.1073/pnas.1003833107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, Wen L, Martin M, Hsu CY, Fang L, Lin FM, Lin TY, Geary MJ, Geary GG, Zhao Y, Johnson DA, Chen JW, Lin SJ, Chien S, Huang HD, Miller YI, Huang PH, Shyy JYJ. Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA-92a. Circulation 131: 805–814, 2015. doi: 10.1161/CIRCULATIONAHA.114.013675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91: 327–387, 2011. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies PF, Civelek M, Fang Y, Fleming I. The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 99: 315–327, 2013. doi: 10.1093/cvr/cvt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun 8: 14361, 2017. doi: 10.1038/ncomms14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doddaballapur A, Michalik KM, Manavski Y, Lucas T, Houtkooper RH, You X, Chen W, Zeiher AM, Potente M, Dimmeler S, Boon RA. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler Thromb Vasc Biol 35: 137–145, 2015. doi: 10.1161/ATVBAHA.114.304277. [DOI] [PubMed] [Google Scholar]
- 15.Dunn J, Qiu H, Kim S, Jjingo D, Hoffman R, Kim CW, Jang I, Son DJ, Kim D, Pan C, Fan Y, Jordan IK, Jo H. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest 124: 3187–3199, 2014. doi: 10.1172/JCI74792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egorova AD, Khedoe PP, Goumans MJ, Yoder BK, Nauli SM, ten Dijke P, Poelmann RE, Hierck BP. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res 108: 1093–1101, 2011. doi: 10.1161/CIRCRESAHA.110.231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evrard SM, Lecce L, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman KR, d’Escamard V, Li JR, Hadri L, Fujitani K, Moreno PR, Benard L, Rimmele P, Cohain A, Mecham B, Randolph GJ, Nabel EG, Hajjar R, Fuster V, Boehm M, Kovacic JC. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun 7: 11853, 2016. doi: 10.1038/ncomms11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol 32: 979–987, 2012. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng S, Bowden N, Fragiadaki M, Souilhol C, Hsiao S, Mahmoud M, Allen S, Pirri D, Ayllon BT, Akhtar S, Thompson AAR, Jo H, Weber C, Ridger V, Schober A, Evans PC. Mechanical activation of hypoxia-inducible factor 1α drives endothelial dysfunction at atheroprone sites. Arterioscler Thromb Vasc Biol 37: 2087–2101, 2017. doi: 10.1161/ATVBAHA.117.309249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvin KM, Donovan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MA Jr, Falb D, Huszar D. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet 24: 171–174, 2000. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 21.Good RB, Gilbane AJ, Trinder SL, Denton CP, Coghlan G, Abraham DJ, Holmes AM. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol 185: 1850–1858, 2015. doi: 10.1016/j.ajpath.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Gracia-Sancho J, Villarreal G Jr, Zhang Y, García-Cardeña G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc Res 85: 514–519, 2010. doi: 10.1093/cvr/cvp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H, Chen Y, Liao L, Wu W. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy upregulation via the AMPK/SIRT1 pathway. Cardiovasc Drugs Ther 27: 189–198, 2013. doi: 10.1007/s10557-013-6442-4. [DOI] [PubMed] [Google Scholar]
- 24.He M, Chen Z, Martin M, Zhang J, Sangwung P, Woo B, Tremoulet AH, Shimizu C, Jain MK, Burns JC, Shyy JY. miR-483 targeting of CTGF suppresses endothelial-to-mesenchymal transition: therapeutic implications in Kawasaki Disease. Circ Res 120: 354–365, 2017. doi: 10.1161/CIRCRESAHA.116.310233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang J, Ing MH, Salazar A, Lassègue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res 93: 1225–1232, 2003. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang YZ, Jiménez JM, Ou K, McCormick ME, Zhang LD, Davies PF. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-like factor 4 promoter in vitro and in vivo. Circ Res 115: 32–43, 2014. doi: 10.1161/CIRCRESAHA.115.303883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacic JC, Mercader N, Torres M, Boehm M, Fuster V. Epithelial-to-mesenchymal and endothelial-to-mesenchymal transition: from cardiovascular development to disease. Circulation 125: 1795–1808, 2012. doi: 10.1161/CIRCULATIONAHA.111.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin TY, Wei TW, Li S, Wang SC, He M, Martin M, Zhang J, Shentu TP, Xiao H, Kang J, Wang KC, Chen Z, Chien S, Tsai MD, Shyy JY. TIFA as a crucial mediator for NLRP3 inflammasome. Proc Natl Acad Sci USA 113: 15078–15083, 2016. doi: 10.1073/pnas.1618773114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahler GJ, Frendl CM, Cao Q, Butcher JT. Effects of shear stress pattern and magnitude on mesenchymal transformation and invasion of aortic valve endothelial cells. Biotechnol Bioeng 111: 2326–2337, 2014. doi: 10.1002/bit.25291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahmoud MM, Serbanovic-Canic J, Feng S, Souilhol C, Xing R, Hsiao S, Mammoto A, Chen J, Ariaans M, Francis SE, Van der Heiden K, Ridger V, Evans PC. Shear stress induces endothelial-to-mesenchymal transition via the transcription factor Snail. Sci Rep 7: 3375, 2017. doi: 10.1038/s41598-017-03532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marin TL, Gongol B, Zhang F, Martin M, Johnson DA, Xiao H, Wang Y, Subramaniam S, Chien S, Shyy JYJ. AMPK promotes mitochondrial biogenesis and function by phosphorylating the epigenetic factors DNMT1, RBBP7, and HAT1. Sci Signal 10: eaaf7478, 2017. doi: 10.1126/scisignal.aaf7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mina SG, Huang P, Murray BT, Mahler GJ. The role of shear stress and altered tissue properties on endothelial to mesenchymal transformation and tumor-endothelial cell interaction. Biomicrofluidics 11: 044104, 2017. doi: 10.1063/1.4991738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montorfano I, Becerra A, Cerro R, Echeverría C, Sáez E, Morales MG, Fernández R, Cabello-Verrugio C, Simon F. Oxidative stress mediates the conversion of endothelial cells into myofibroblasts via a TGF-β1 and TGF-β2-dependent pathway. Lab Invest 94: 1068–1082, 2014. doi: 10.1038/labinvest.2014.100. [DOI] [PubMed] [Google Scholar]
- 36.Moonen JRAJ, Lee ES, Schmidt M, Maleszewska M, Koerts JA, Brouwer LA, van Kooten TG, van Luyn MJA, Zeebregts CJ, Krenning G, Harmsen MC. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res 108: 377–386, 2015. doi: 10.1093/cvr/cvv175. [DOI] [PubMed] [Google Scholar]
- 37.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol 297: H1535–H1543, 2009. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436: 123–127, 2005. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.Rodrigues Díez R, Rodrigues-Díez R, Lavoz C, Rayego-Mateos S, Civantos E, Rodríguez-Vita J, Mezzano S, Ortiz A, Egido J, Ruiz-Ortega M. Statins inhibit angiotensin II/Smad pathway and related vascular fibrosis, by a TGF-β-independent process. PLoS One 5: e14145, 2010. doi: 10.1371/journal.pone.0014145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sangwung P, Zhou G, Nayak L, Chan ER, Kumar S, Kang DW, Zhang R, Liao X, Lu Y, Sugi K, Fujioka H, Shi H, Lapping SD, Ghosh CC, Higgins SJ, Parikh SM, Jo H, Jain MK. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight 2: e91700, 2017. doi: 10.1172/jci.insight.91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shentu TP, He M, Sun X, Zhang J, Zhang F, Gongol B, Marin TL, Zhang J, Wen L, Wang Y, Geary GG, Zhu Y, Johnson DA, Shyy JYJ. AMP-activated protein kinase and Sirtuin 1 coregulation of cortactin contributes to endothelial function. Arterioscler Thromb Vasc Biol 36: 2358–2368, 2016. doi: 10.1161/ATVBAHA.116.307871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun W, Lee TS, Zhu M, Gu C, Wang Y, Zhu Y, Shyy JY. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation 114: 2655–2662, 2006. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 42.Surapisitchat J, Hoefen RJ, Pi X, Yoshizumi M, Yan C, Berk BC. Fluid shear stress inhibits TNF-alpha activation of JNK but not ERK1/2 or p38 in human umbilical vein endothelial cells: Inhibitory crosstalk among MAPK family members. Proc Natl Acad Sci USA 98: 6476–6481, 2001. doi: 10.1073/pnas.101134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res 106: 1117–1128, 2010. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen L, Chen Z, Zhang F, Cui X, Sun W, Geary GG, Wang Y, Johnson DA, Zhu Y, Chien S, Shyy JY. Ca2+/calmodulin-dependent protein kinase kinase β phosphorylation of Sirtuin 1 in endothelium is atheroprotective. Proc Natl Acad Sci USA 110: E2420–E2427, 2013. doi: 10.1073/pnas.1309354110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu D, Huang RT, Hamanaka RB, Krause M, Oh MJ, Kuo CH, Nigdelioglu R, Meliton AY, Witt L, Dai G, Civelek M, Prabhakar NR, Fang Y, Mutlu GM. HIF-1α is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. eLife 6: e25217, 2017. doi: 10.7554/eLife.25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G Jr, Wang KC, Geary GG, Zhang Y, Wang WC, Huang HD, Zhou J, Li YS, Chien S, Garcia-Cardena G, Shyy JY. Flow-dependent regulation of Kruppel-like factor 2 is mediated by microRNA-92a. Circulation 124: 633–641, 2011. doi: 10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M, Shen Q, Zhu Y, Zhang Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res 87: 504–513, 2010. doi: 10.1093/cvr/cvq066. [DOI] [PubMed] [Google Scholar]
- 48.Xiong J, Kawagishi H, Yan Y, Liu J, Wells QS, Edmunds LR, Fergusson MM, Yu ZX, Rovira II, Brittain EL, Wolfgang MJ, Jurczak MJ, Fessel JP, Finkel T. A metabolic basis for endothelial-to-mesenchymal transition. Mol Cell 69: 689–698.e7, 2018. doi: 10.1016/j.molcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol 10: 295–305, 2008. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 50.Young A, Wu W, Sun W, Larman HB, Wang N, Li YS, Shyy JY, Chien S, García-Cardeña G. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Krüppel-like factor 2 expression. Arterioscler Thromb Vasc Biol 29: 1902–1908, 2009. [Erratum in Arterioscler Thromb Vasc Biol 30: e325, 2010]. doi: 10.1161/ATVBAHA.109.193540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19: 2282–2287, 2008. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Shi X, Peng Y, Wu M, Zhang P, Xie R, Wu Y, Yan Q, Liu S, Wang J. HIF-1α promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer. PLoS One 10: e0129603, 2015. doi: 10.1371/journal.pone.0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T JR, Shyy JY. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol 26: 1281–1287, 2006. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 55.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler Thromb Vasc Biol 34: 2191–2198, 2014. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]