Abstract
Pulmonary arterial hypertension (PAH) is characterized by remodeling of the extracellular matrix (ECM) of the pulmonary arteries with increased collagen deposition, cross-linkage of collagen, and breakdown of elastic laminae. Extracellular matrix remodeling occurs due to an imbalance in the proteolytic enzymes, such as matrix metalloproteinases, elastases, and lysyl oxidases, and tissue inhibitor of matrix metalloproteinases, which, in turn, results from endothelial cell dysfunction, endothelial-to-mesenchymal transition, and inflammation. ECM remodeling and pulmonary vascular stiffness occur early in the disease process, before the onset of the increase in the intimal and medial thickness and pulmonary artery pressure, suggesting that the ECM is a cause rather than a consequence of distal pulmonary vascular remodeling. ECM remodeling and increased pulmonary arterial stiffness promote proliferation of pulmonary vascular cells (endothelial cells, smooth muscle cells, and adventitial fibroblasts) through mechanoactivation of various signaling pathways, including transcriptional cofactors YAP/TAZ, transforming growth factor-β, transient receptor potential channels, Toll-like receptor, and NF-κB. Inhibition of ECM remodeling and mechanotransduction prevents and reverses experimental pulmonary hypertension. These data support a central role for ECM remodeling in the pathogenesis of the PAH, making it an attractive novel therapeutic target.
Keywords: collagen, compliance, mechanotransduction, right ventricle, stiffness
INTRODUCTION
Pulmonary arterial hypertension (PAH) is characterized by decreased compliance of the pulmonary arterial system and progressive narrowing of the distal resistance pulmonary arteries, leading to higher right ventricular (RV) afterload, RV failure, and eventually death (85). It is a debilitating disease, associated with significant morbidity and a 1-yr mortality rate of 15–20% (5, 79). Median survival is poor at ~7 yr (5, 79). Moreover, the economic burden of PAH is high; estimated PAH-related health care costs in the United States are approximately $29 billion dollars per year (1). Current PAH-targeted therapies (prostaglandins, phosphodiesterase-5 inhibitors, endothelin receptor antagonists, and soluble guanylate cyclase stimulators) are predominantly vasodilators, and when used alone or in combination, they improve functional capacity and hemodynamics and reduce hospitalization (18). However, these vasodilators do not address key features of PAH pathogenesis and have not been shown to reduce mortality in randomized, controlled clinical trials (18). Thus, there is a need for novel therapies in PAH to better improve long-term outcomes.
The healthy pulmonary circulation is a low-pressure, high-compliance system. In PAH, there is increased vascular stiffness or reduced pulmonary arterial compliance (PAC) due to remodeling of the extracellular matrix (ECM) of the pulmonary vascular system. In addition to ECM remodeling, increased sympathetic activity and autonomic modulation may contribute to reduced PAC in PAH (87). Decreased PAC causes premature reflection of waves from the distal pulmonary vasculature, leading to increased pulsatile RV afterload and eventually RV failure. Growing evidence suggests that the decrease in PAC and increased pulsatility due to remodeling of the ECM of the pulmonary arteries plays a critical role in the pathogenesis of PAH and is a cause rather than a consequence of distal small vessel proliferative vasculopathy (76). The remodeling of the ECM and loss of PAC occur early in the disease process, even when pulmonary artery pressure and pulmonary vascular resistance are normal (64, 82). More importantly, decreased PAC has consistently been shown to be a predictor of increased mortality in patients with PAH, even better than increased pulmonary vascular resistance (10, 20, 43, 51, 74). ECM remodeling and stiffness also plays an essential role in other pulmonary disease processes, including pulmonary arterial endothelial cell permeability in acute lung injury (32) and fibroblast activation in idiopathic pulmonary fibrosis (52).
With the recognition of the prognostic importance of PAC, its impact on RV function, and its contributory role in the development and progression of distal small vessel proliferative vasculopathy, PAC and the ECM are attractive targets for developing novel therapies for PAH. Here, we review the evidence supporting the importance of ECM remodeling of the pulmonary vasculature in the pathogenesis of PAH and potential novel therapeutic targets for treatment of PAH.
REMODELING OF THE ECM OF THE PULMONARY ARTERIES IN PAH
Pulmonary vascular remodeling in PAH is characterized by increased thickness of all three layers of the distal pulmonary arteries, including the intima, media, and adventitia. There is endothelial cell and smooth muscle cell proliferation, leading to neointimal formation and plexiform lesions. Pulmonary artery smooth muscle hypertrophy and hyperplasia lead to increased medial thickness in small pulmonary arteries and muscularization of nonmuscularized precapillary arterioles. There is expansion of the ECM in all three layers of the pulmonary vascular wall leading to vascular fibrosis (84). Furthermore, there is an accumulation of perivascular inflammatory cells and in situ thrombosis (84). While the vascular fibrosis related to ECM expansion causes stiffening and reduced compliance of the pulmonary arteries, the endothelial and smooth muscle proliferative lesions reduce vascular luminal area and increase pulmonary vascular resistance.
The ECM of a normal human pulmonary artery consists of collagens, elastins, laminins, fibronectin, tenascin C, and proteoglycan (11). In PAH, several changes occur in the ECM. First, there is increased deposition and cross-linkage of collagen (converting soluble collagen to insoluble collagen) in the perivascular and intravascular compartments (9, 14, 26, 55, 83). Increased collagen accumulation occurs in both the proximal and distal pulmonary arteries (23, 26, 55, 89). The highest deposition of collagens occurs in the intima followed by the media and adventitia (26). As evidenced by compartment-specific gene expression analysis, patients with PAH display increased expression of genes encoding fibril-associated collagen (COL14A1), network-forming collagen (COL4A5), and the endostatin-producing collagen (COL18A1) in the intima and media of the pulmonary arteries compared with healthy donor pulmonary arteries (26). Second, there is increased breakdown of elastin in the pulmonary arteries in PAH, as evidenced by fragmentation of the internal elastic lamina in the pulmonary arteries from experimental models of pulmonary hypertension and children with PAH associated with congenital heart disease (57, 59, 83). Third, there is increased accumulation of tenascin and fibronectin in the pulmonary arteries in PAH (31, 56). Finally, diseased pulmonary arteries from patients with PAH and experimental models of pulmonary hypertension have increased expression of osteopontin (2, 62) and calcification (61).
MECHANISM OF PULMONARY VASCULAR ECM REMODELING IN PAH
The composition of ECM is regulated by the balance between proteolytic enzymes, such as matrix metalloproteinases (MMPs), metalloproteases [a disintegrin and metalloproteinases (ADAMs)], serine elastases, lysyl oxidases (LOXs), and their endogenous inhibitors tissue inhibitors of metalloproteinase (TIMPs). In PAH, an imbalance in the proteolytic enzymes and their endogenous tissue inhibitors leads to increased collagen deposition, cross-linkage of collagens, and elastin breakdown in the intravascular and perivascular compartments of the pulmonary arteries (11). The pulmonary arteries from animals with monocrotaline and hypoxia-induced pulmonary hypertension show increased expression of MMPs, ADAMs, serine elastases, LOXs, and TIMPs (8, 11). Likewise, diseased pulmonary arteries from patients with PAH show altered expression of several MMPs, ADAMs, elastases, LOXs, and TIMPs in the intima and media compared with healthy controls (8, 26, 39).
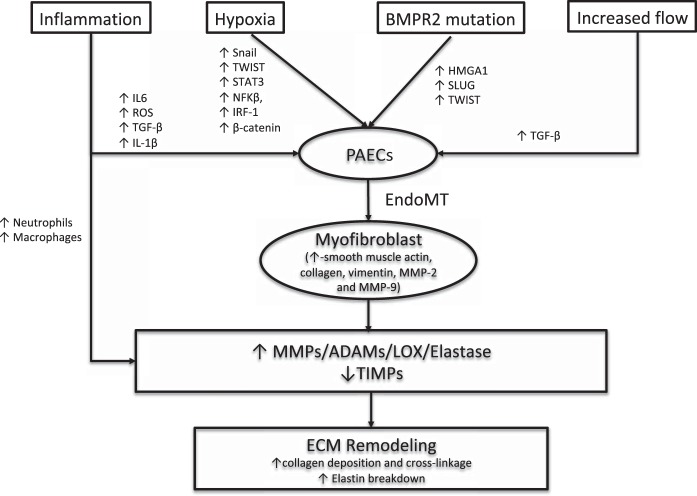
The exact mechanism that drives the imbalance in the secretion of the proteolytic enzymes in the pulmonary arteries in PAH is unclear. Several mechanisms have been suggested to play a role (Fig. 1). Alteration in the structure and function of pulmonary arterial endothelial cells has been proposed as the initiating event. Increase in flow, shear stress, pulsatility, and inflammation cause pulmonary arterial endothelial cell damage, loss of barrier function, and increased permeability. This allows an unidentified circulating serum factor or factors to enter the vessel wall and stimulate pulmonary artery smooth muscle cells to secrete serine elastases (35). In support of this hypothesis, pulmonary artery smooth muscles cells from patients with idiopathic PAH show increased expression of serine elastases (35). Serine elastases then cause degradation of the ECM and activate growth factors, such as fibroblast growth factor and transforming growth factor (TGF)-β, which normally are stored in an inactive form in the ECM (80). These growth factors subsequently cause increased deposition of collagen, elastin, fibronectin, and tenascin by stimulating the smooth muscle cells and fibroblasts. The degradation products of the ECM and growth factors also induce increased secretion of MMPs.
Fig. 1.
Pathogenesis of pulmonary vascular extracellular matrix (ECM) remodeling in pulmonary arterial hypertension (PAH). Increased shear stress from increased flow, hypoxia, inflammation, and altered bone morphogenic protein receptor 2 (BMPR2) signaling causes the endothelial-to-mesenchymal transition (EndoMT), in which endothelial cells acquire a mesenchymal phenotype with increased expression of a gene profile similar to smooth muscle cells. These myofibroblast-like cells cause remodeling of the ECM by increased deposition and cross-linkage of collagen through increased secretion of matrix metalloproteinases (MMPs), metalloproteases [a disintegrin and metalloproteinases (ADAMs)], serine elastases, and lysyl oxidases (LOXs), and decreased secretion of their endogenous inhibitors, like tissue inhibitors of metalloproteinase (TIMPs). HMGA1, high-mobility group AT-hook 1; IRF-1, interferon regulatory factor-1; PAECs, pulmonary artery endothelial cells; ROS, reactive oxygen species; TGF, transforming growth factor.
Inflammation has also been proposed to initiate the imbalance in the proteolytic enzymes and their inhibitors and cause remodeling of the pulmonary vascular ECM (29). Reactive oxygen species resulting from inflammation increase secretion of MMPs and decrease secretion of TIMPs by smooth muscle cells, endothelial cells, and fibroblasts (19). Also, inflammatory cytokines activate and recruit macrophages and neutrophils, which, in turn, secrete MMPs and serine elastases, respectively. The breakdown products of collagen and elastin resulting from the increased proteolytic enzymes are proinflammatory, which further activate inflammation, leading to a positive feedback loop. There is ample evidence suggesting inflammation is an important driver for pulmonary vascular remodeling in PAH (24, 37). There is perivascular accumulation of inflammatory cells in the pulmonary arteries in human and animal models of PAH (47, 65). PAH can be induced experimentally in animals with exposure to various immune stimuli, including human immunodeficiency virus, schistosomiasis, and IL-6 overexpression (22, 36, 72). Clinically, PAH is associated with autoimmune diseases, including scleroderma and systemic lupus erythematosis, and infectious diseases, such as human immunodeficiency virus and schistosomiasis infection (67). Depletion of inflammatory macrophages in chronic hypoxic calves and rats prevents remodeling of the pulmonary vascular ECM and pulmonary hypertension (17). Finally, increased serum cytokine levels in patients with PAH have been associated with increased mortality (69). In aggregate, these data suggest an important role for inflammation in initiating pulmonary vascular ECM remodeling and pulmonary vascular stiffness. However, unlike in coronary artery disease (13, 75, 92), the hypothesis that inflammation is therapeutically targetable in PAH has not yet been demonstrated clinically, but it is being investigated (90).
More recently, ECM remodeling in PAH has been attributed to endothelial-to-mesenchymal transition (EndoMT), in which the endothelial cells acquire a mesenchymal phenotype with increased expression of a gene profile similar to smooth muscle cells (73). In this process, endothelial cells separate from the intimal monolayer due to loss of cell-to-cell interactions, resulting from downregulation of endothelial gene markers, such as vascular-endothelial cadherin, platelet endothelial adhesion molecule, desmoplakin, and cytokeratin (73). Subsequently, endothelial cells migrate into the media and dedifferentiate into myofibroblast-like mesenchymal cells with increased expression of α-smooth muscle actin, collagen, vimentin, MMP-2, and MMP-9 (73). These myofibroblast-like cells cause remodeling of the ECM by increased deposition and cross-linkage of collagen. EndoMT has been demonstrated in experimental models of pulmonary hypertension and in human PAH (27, 58).
Various stimuli commonly implicated in the pathogenesis of PAH initiate EndoMT. First, loss of bone morphogenic protein receptor 2 (BMPR2) function has been demonstrated to initiate EndoMT in experimental pulmonary hypertension and human PAH by two different groups (27, 58). Hopper et al. (27) showed that loss of BMPR2 function in pulmonary artery endothelial cells causes an elevation in high-mobility group AT-hook 1 (HMGA1), a chromatin remodeling and scaffolding protein. Elevated HMGA1 induces increased expression of a transcription factor called Slug that upregulates expression of smooth muscle genes, such as smooth muscle actin, and downregulates expression of endothelial cell genes, including cell junctional proteins, platelet endothelial cell adhesion molecule, and vascular-endothelial cadherin, resulting in a mesenchymal phenotype (27). Similarly, Ranchoux et al. (58) showed EndoMT in a genetically modified rat model of pulmonary hypertension (with loss of BMPR2 function) through increased expression of a transcription factor called Twist. Second, mechanical cues from increased pulsatility and shear stress lead to endothelial dysfunction and EndoMT through TGF-β signaling. Third, chronic hypoxia initiates EndoMT through increased expression of transcription factors Snail, Twist, STAT3, NF-KB, interferon regulatory factor-1, and β-catenin through interactions with hypoxia-inducible-factor and TGF-β signaling (44, 73). Finally, inflammatory cytokines IL-1β, IL-6, TNF-α and reactive oxygen species also cause EndoMT through activation of TGF-β signaling (73).
Pulmonary arterial endothelial cells in PAH also contribute to vascular fibrosis, in the absence of EndoMT phenotype switch, through upregulation of neural precursor cell-expressed developmentally downregulated 9 (NEDD9) genes by oxidative stress (63). In PAH, there is elevation of aldosterone, which is an important regulator of reactive oxygen species generation (45, 49). Oxidative modification of NEDD9 by aldosterone reduces its degradation. Increased NEDD9, in turn, promotes collagen formation through upregulation of the collagen type III-forming gene (COL3A1). In vivo inhibition of NEDD9 prevents fibrotic vascular remodeling and pulmonary hypertension in animal models of PAH (63).
Pulmonary arterial calcification also occurs in PAH. Recent work by Ruffenach et al. (61) has shed light on the mechanism of pulmonary artery calcification in PAH. Downregulation of miRNA-204 activates Runt-related transcription factor 2 (RUNX2), which, in turn, through activation of hypoxia-inducible factor-1α, drives pulmonary artery smooth muscle cell proliferation, resistance to apoptosis, and their differentiation into osteoblast-like cells that secrete calcium. Patients with PAH have increased expression of RUNX2 in their lungs, and inhibition of RUNX2 in Sugen/hypoxia rat model of PA prevents pulmonary arterial calcification, reduces pulmonary arterial remodeling, and improves pulmonary hypertension and RV function (61). Interestingly, Runx2 is a downstream target of bone morphogenic protein signaling, which is frequently altered in PAH (53).
Osteopontin, an ECM protein, may play a role in the remodeling of the pulmonary vascular ECM in PAH. Anwar et al. (2) reported that osteopontin is an essential contributor to pulmonary vascular fibroblast proliferation and migration in brisket disease in cattle. Work from Saker et al. (62) has demonstrated that osteopontin is a key mediator in the proliferation of pulmonary artery smooth muscle and is increased in idiopathic PAH lungs. It is possible that the increase in osteopontin expression in PAH is triggered by pulsatile flow, as osteopontin production increases in mesenchymal stem cells in response to pulsatile flow (33). In addition, increased MMP activity has been shown to cleave osteopontin to create a number of peptides with a variety of biological activities in left ventricular remodeling after myocardial infarction (41).
INHIBITION OF ECM REMODELING PREVENTS AND REVERSES EXPERIMENTAL PAH
Manipulation of ECM remodeling prevents and reverses pulmonary vascular remodeling and pulmonary hypertension in several experimental models. In rats with monocrotaline- and chronic hypoxia-induced pulmonary hypertension, oral and intravenous serine elastase inhibitors decrease elastolytic activity, reduce muscularization of nonmuscular distal pulmonary arteries, and lower pulmonary artery pressure (12, 28, 48, 91). These effects were observed when serine elastase was inhibited either before or after the onset of pulmonary vascular changes (91). Similarly, transgenic mice with overexpression of elafin, a natural serine elastase inhibitor, when exposed to hypoxia, have no increase in serine elastase and MMPs and an attenuated increase in pulmonary artery pressure and pulmonary vascular remodeling compared with nontransgenic mice (93). Conversely, transgenic mice with overexpression of MMP-9 have exaggerated pulmonary vascular remodeling and pulmonary hypertension after monocrotaline treatment compared with wild-type mice (21). In chronic hypoxic mice, inhibition of LOXs attenuates collagen deposition and cross-linkage, decreases muscularization of pulmonary arterioles, and reduces pulmonary artery pressure (50). Finally, administration of proline analog cis-4-hydroxy-l-proline, a specific inhibitor of collagen synthesis, reduces collagen and elastin deposition and pulmonary arterial remodeling in rats exposed to chronic hypoxia (34). These experimental data support the hypothesis that ECM remodeling, due to an imbalance between the proteolytic enzymes and their tissue inhibitors, plays a critical role in initiating pulmonary vascular remodeling in PAH.
REMODELING OF THE ECM PRECEDES OTHER VASCULAR CHANGES IN PAH
Experimental studies have suggested that remodeling of the ECM of the pulmonary arteries occurs early in the course of PAH pathogenesis, before the onset of an increase in medial thickness and an increase in pulmonary artery pressure. In rats with monocrotaline-induced PAH, there is increased collagen deposition and cross-linkage of the collagen on day 3 after exposure to monocrotaline (8). This is followed by a decrease in elastin content in the media on day 8 and an increase in insoluble elastin in the media on day 16 (83). These changes are accompanied by an increase in the activity of serine elastases and LOXs on day 4 (8, 91). However, medial hypertrophy of the pulmonary arteries, increase in pulmonary artery pressure, and RV hypertrophy were noted only 3–4 wk after monocrotaline injection (8, 30, 83). Likewise, in the chronic hypoxic mouse model of pulmonary hypertension, there is increased arterial collagen deposition and breakdown of elastin on day 3 after exposure to hypoxia, before the increase in medial thickness and RV systolic pressure (8, 93). This is associated with an increase in the activity of several proteolytic enzymes involved in matrix remodeling, including serine elastases, MMP, and LOXs, and also with an increased pulmonary vascular stiffness on atomic microscopy (8, 93). The early increase in pulmonary arteriolar collagen has also been noted in other experimental models of pulmonary hypertension, including Schistosoma mansoni-infected mice, transgenic mice with increased expression of IL-6, the surgical lamb model of congenital heart disease, and von Hippel-Lindau gene-null mice (8).
Although there is no similar serial histological analysis of pulmonary arteries from patients with various stages of PAH, several clinical observations in patients with PAH complement the experimental data, thus suggesting the early occurrence of pulmonary vascular ECM remodeling in the pathogenesis of PAH. Clinically, in patients with PAH, this is evident from inspection of the resistance/compliance curve where compliance can be seen to fall before the resistance increases outside the normal range. Consistent with this, pulmonary arterial compliance maintains an inverse hyperbolic relationship with pulmonary vascular resistance (38, 78). Furthermore, patients with exercise-induced pulmonary hypertension, who have normal resting pulmonary artery pressure and pulmonary vascular resistance, have reduced pulmonary artery compliance compared with normal healthy controls (64).
These experimental and human data suggest that pulmonary vascular fibrosis and decrease in PAC precede the increase in pulmonary artery medial and intimal thickness, as well as the increase in pulmonary artery pressure.
ECM STIFFNESS PROMOTES PULMONARY VASCULAR REMODELING
Emerging evidence suggests that, in PAH, increased stiffness of the proximal and distal pulmonary arteries, due to ECM remodeling, triggers proliferation of pulmonary arterial smooth muscle and endothelial cells in the distal pulmonary arteries through several potential mechanisms (Fig. 2), similar to what has also been described in other disease states, such as cancer and pulmonary fibrosis (4, 81). Mechanotransduction, a process in which extracellular mechanical cues alter intracellular signaling, is known to have substantial regulatory effects on cell behavior, shape, size, and differentiation state in multiple tissue types, including the vasculature. Although much less is known about mechanotransduction in the pulmonary vasculature, integrin-mediated signaling plays a major role functionally connecting ECM proteins to alterations of the intracellular cytoskeleton. In the peripheral vasculature and cardiac valve tissue, transmission of forces from the stiffened matrix can promote proliferation and calcification via osteochondrogenic differentiation of myofibroblasts (13, 75, 92), whereas reduction of the substrate modulus can reverse this differentiation process (88). TGF-β superfamily members may mediate one such relevant process, whereby when released from stiff but not the soft matrix, these factors specifically embed between cellular integrins and other matrix proteins when stretched open by matrix traction (90). In pulmonary hypertension, direct integrin expression in the pulmonary vasculature is also altered, consistent with the importance of integrins in pathogenic mechanotransduction in the pulmonary vessel as well (86). In acute lung injury, emerging data also indicate an important role for intracellular RhoA GTPase signaling in stiffness-dependent alterations of lung endothelial inflammation and permeability; it remains to be seen how such mechanosensitive signaling molecules behave in PH.
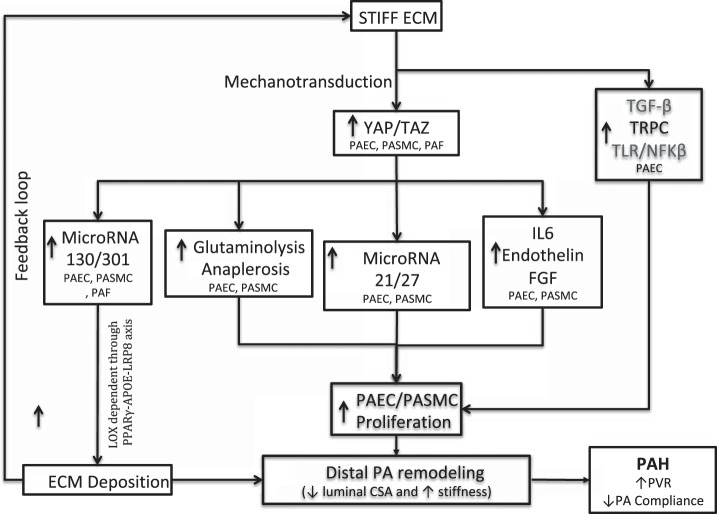
Fig. 2.
Pulmonary vascular extracellular matrix (ECM) stiffness induces pulmonary vascular remodeling. Stiff ECM activates yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) signaling in pulmonary artery endothelial cells (PAECs), pulmonary artery smooth muscle cells (PASMCs), and pulmonary adventitial fibroblasts (PAFs) through mechanotransduction. Activation of YAP/TAZ signaling causes increased proliferation of PAECs and PASMCs through three different mechanisms: increased glutaminolysis and anaplerosis, upregulation of microRNA-21/27, and increased secretion of IL-6, endothelin, and fibroblast growth factor (FGF). In addition, stiff ECM increases PAEC proliferation by activating transforming growth factor (TGF)-β signaling, transient receptor potential channels (TRPCs), and Toll-like receptors (TLRs) as well as the NF-κB signaling axis through mechanotransduction. Finally, activation of YAP/TAZ signaling, in turn, leads to increased deposition of ECM by PAECs, PASMCs, and PAFs by upregulation of microRNA-130/301, leading to a feedback loop of increased ECM stiffness and mechanotransduction. ApoE, apolipoprotein E; CSA, cross-sectional area; LOX, lysyl oxidase; LRP8, LDL receptor-related protein 8; PAH, pulmonary arterial hypertension; PPARγ, peroxisome proliferator-activated receptor-γ; PVR, pulmonary vascular resistance.
More recently, more effort has been devoted to defining the ways in which mechanical cues sensed by distal endothelial cells are transduced into several intracellular signals that ultimately promote pulmonary arterial endothelial and smooth muscle cell proliferation. For instance, Bertero et al. (7) showed that ECM remodeling and pulmonary vascular stiffness activate the transcriptional coactivators yes-associated protein 1 (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ), which, in turn, induce expression of the miR-130/301 family in pulmonary artery adventitial fibroblasts, pulmonary artery endothelial cells, and pulmonary artery smooth muscle cells. miR-130/301 members promote increased deposition of collagen and cross-linking of collagen in a LOX-dependent fashion through an peroxisome proliferator-activated receptor (PPAR)-γ-apolipoprotein E (apoE)-LDL receptor-related protein 8 (LRP8) axis in all three pulmonary vascular cell types (7). The miR-130/301 family activates YAP and TAZ through a feedback mechanotransduction loop resulting in further ECM remodeling (7). Furthermore, the miR-130/301 family promotes proliferation of pulmonary artery smooth muscle and endothelial cell proliferation through pulmonary vascular cell cross-talk via increased expression of miR-21/27. In support of this, in vivo pharmacological inhibition of miR-130/301, apoE, or LOX activity in various experimental models of pulmonary hypertension ameliorated ECM remodeling and pulmonary hypertension (7). On the basis of these findings, beyond PAH, miR-130/301 family members have been predicted computationally to promote fibrotic mechanisms in a variety of other human conditions in health and disease, including liver fibrosis and lung fibrosis (6).
YAP/TAZ activation also increases proliferation of pulmonary artery endothelial and smooth muscle cells through metabolic modulation (8). In PAH, pulmonary artery smooth muscle cells, endothelial cells, and adventitial fibroblasts undergo metabolic reprogramming characterized by increased aerobic glycolysis, a shift in energy production from oxidative phosphorylation to glycolysis despite normoxia (3, 15, 46, 94). This metabolic reprogramming drives increased proliferation of all three pulmonary vascular cell types (3, 15, 46, 94), but emerging data have implicated an additional process called anaplerosis in PAH, crucial to providing additional tricarboxylic acid cycle carbon intermediates for cellular biomass important to proliferation. Specifically, two important anaplerotic pathways, include glutaminolysis (conversion of glutamine to glutamate by glutaminase) and conversion of pyruvate to oxaloacetate by pyruvate carboxylase (54). Bertero et al. (8) found that mechanoactivation of YAP/TAZ causes increased proliferation of pulmonary artery endothelial cells and smooth muscle cells by inducing the key metabolic enzymes glutaminase, pyruvate carboxylase, and lactate dehydrogenase A to promote glutaminolysis and glycolysis in several experimental models of pulmonary hypertension and human PAH, thus maintaining the metabolic needs of hyperproliferative pulmonary vascular cells and thereby fostering pulmonary vascular remodeling and hemodynamic manifestations of pulmonary hypertension. As a testament for this, in vivo pharmacological inhibition of YAP/TAZ activation with verteporfin and pharmacological inhibition of glutaminase by CB-839 and C968 prevents and reverses monocrotaline-induced pulmonary hypertension in rats (8). Additionally, in patients with human immunodeficiency virus-associated PAH, pulmonary arterial compliance, a marker of vascular fibrosis and stiffness, inversely correlates with a decreased serum glutamine-to-glutamate ratio and an increased serum lactate-to-pyruvate ratio, markers of glutaminolysis and glycolysis, respectively (8).
In addition to driving pulmonary arterial endothelial and smooth muscle cell proliferation, it is possible that YAP/TAZ activation may play a role upstream in initiating pulmonary vascular ECM remodeling by activating pulmonary adventitial fibroblast proliferation in response to increased pulsatility and shear stress. However, it is unclear whether mechanical cues from increased pulsatility and shear stress alone, in the absence of a stiff matrix, can activate YAP/TAZ in the adventitial fibroblasts.
ECM remodeling and increased stiffness of the proximal pulmonary arteries also induce a proinflammatory response in the endothelial cells of the distal pulmonary arteries characterized by increased expression of Toll-like receptor-2 expression and NF-κB signaling (40, 77). Furthermore, high pulsatile flow decreases expression of endothelial nitric oxide synthase, which generates nitric oxide (a potent vasodilator) and increases expression of endothelin and angiotensin (potent vasoconstrictors and smooth muscle mitogens) and of TGF-β in pulmonary artery endothelial cells (66). Together, the inflammatory response, vasoactive cytokines, and growth factors from the pulmonary artery endothelial cells cause pulmonary artery smooth muscle hypertrophy with increased expression of contractile proteins, smooth muscle α-actin, and smooth muscle myosin heavy chain (66).
Suppression of cyclooxygenase (COX)-2 expression has also been implicated in the pathogenesis of pulmonary vascular remodeling induced by increased pulmonary vascular stiffness. Absence of COX-2 enhances stiffness-induced proliferation of human pulmonary artery smooth muscle cells, production of ECM proteins collagen and fibronectin, and increased pulmonary hypertension in mice exposed to chronic hypoxia. Conversely, overexpression of COX-2 reduces stiffness-induced increases in ECM deposition (16). Finally, highly pulsatile flow has also been shown to increase expression of mechanosensitive transient receptor potential channels in pulmonary artery smooth muscle cells, which leads to more intracellular calcium signaling and smooth muscle proliferation (68).
Taken together, these data suggest that pulmonary vascular stiffness, resulting from ECM remodeling causes proliferation of pulmonary artery smooth muscle and endothelial cells in the distal pulmonary arteries (Fig. 2).
THERAPEUTIC IMPLICATIONS
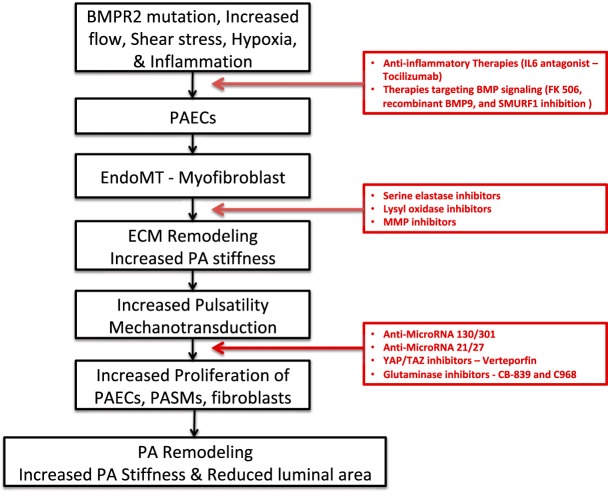
Figure 3 highlights the main steps in the development from pulmonary arterial collagen deposition to pulmonary arterial narrowing. Given the central role of ECM remodeling in the pathogenesis of PAH, it provides several potential novel therapeutic targets that can be translated from bench to bedside for treating PAH (Fig. 3). These include 1) inhibition of serine elastases, lysyl oxidases, or MMPs (there are several ongoing clinical trials in oncology using a similar approach; importantly, a phase 2 clinical trial evaluating the safety and efficacy of elafin, a serine elastase inhibitor, in the treatment of PAH was recently initiated and is ongoing); 2) inhibition of miR-130/301 and miR-21/27; 3) inhibition of YAP/TAZ activation with verteporfin, which is already used clinically in photodynamic therapy to treat neovascularization in age-related macular degeneration (25); and 4) inhibition of glutaminolysis with glutaminase inhibitors, such as CB-839 and C968, which are currently in clinical trials for the treatment of renal cell carcinoma (NCT03428217), melanoma (NCT02771626), and colorectal carcinoma (NCT02861300). Moreover, therapeutic approaches that modulate BMPR2 pathways, including recombinant BMP9 (42), FK506 (70, 71), and SMURF1 inhibition (60), may also target ECM remodeling as BMP signaling is involved in the process of EndoMT and pulmonary artery calcification.
Fig. 3.
Schematic illustration of the major steps in the development from pulmonary arterial collagen deposition to pulmonary arterial narrowing and potential therapeutic targets for treatment of pulmonary arterial hypertension (PAH). Increased shear stress, increased pulsatility, hypoxia, inflammation, and altered bone morphogenic protein receptor 2 (BMPR2) signaling cause the endothelial-to-mesenchymal transition (EndoMT), which, in turn, leads to remodeling of the extracellular matrix (ECM) and increased vascular stiffness through increased deposition and cross-linkage of collagen and breakdown of elastin. Increased stiffness promotes pulmonary endothelial cell, smooth muscle cell, and fibroblast cell proliferation. This causes pulmonary arterial remodeling with decreased compliance and cross-sectional luminal area leading to elevated pulmonary artery pressure and pulmonary vascular resistance. Potential novel therapeutic targets to treat PAH are presented in red boxes.
CONCLUSIONS
In summary, there is growing evidence that suggests that ECM remodeling may occur early in the course of PAH and play a critical role in the pathogenesis of distal pulmonary vascular remodeling. This makes vascular ECM remodeling an increasingly attractive and novel potential therapeutic target for both the prevention and treatment of PAH.
GRANTS
This work was supported by American Heart Association Scientific Development Grant 15SDG25560048 (to T. Thenappan), National Institutes of Health Grants R01-HL-124021, HL-122596, HL-138437, and UH2-TR-002073, and an American Heart Association Established Investigator award (to S. Y. Chan).
DISCLOSURES
T. Thenappan has served as a consultant for Actelion and Gilead (modest). S. Y. Chan has served as a consultant for Actelion (Significant), Gilead, Pfizer, and Vivus (Modest).
AUTHOR CONTRIBUTIONS
T.T., S.Y.C., and E.K.W. drafted manuscript; T.T. prepared figures; S.Y.C and E.K.W reviewed and edited manuscript; T.T., S.Y.C., and E.K.W. approved final version of manuscript.
REFERENCES
- 1.Anand V, Roy SS, Archer SL, Weir EK, Garg SK, Duval S, Thenappan T. Trends and outcomes of pulmonary arterial hypertension-related hospitalizations in the United States: analysis of the nationwide inpatient sample database from 2001 through 2012. JAMA Cardiol 1: 1021–1029, 2016. doi: 10.1001/jamacardio.2016.3591. [DOI] [PubMed] [Google Scholar]
- 2.Anwar A, Li M, Frid MG, Kumar B, Gerasimovskaya EV, Riddle SR, McKeon BA, Thukaram R, Meyrick BO, Fini MA, Stenmark KR. Osteopontin is an endogenous modulator of the constitutively activated phenotype of pulmonary adventitial fibroblasts in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 303: L1–L11, 2012. doi: 10.1152/ajplung.00050.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer SL. Pyruvate kinase and Warburg metabolism in pulmonary arterial hypertension: uncoupled glycolysis and the cancer-like phenotype of pulmonary arterial hypertension. Circulation 136: 2486–2490, 2017. doi: 10.1161/CIRCULATIONAHA.117.031655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellaye PS, Shimbori C, Upagupta C, Sato S, Shi W, Gauldie J, Ask K, Kolb M. Lysyl oxidase-like 1 protein deficiency protects mice from AdTGF-β1-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 58: 461–470, 2018. doi: 10.1165/rcmb.2017-0252OC. [DOI] [PubMed] [Google Scholar]
- 5.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, Liou TG, McGoon MD. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 122: 164–172, 2010. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 6.Bertero T, Cottrill KA, Annis S, Bhat B, Gochuico BR, Osorio JC, Rosas I, Haley KJ, Corey KE, Chung RT, Nelson Chau B, Chan SY. A YAP/TAZ-miR-130/301 molecular circuit exerts systems-level control of fibrosis in a network of human diseases and physiologic conditions. Sci Rep 5: 18277, 2015. doi: 10.1038/srep18277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, Hale A, Bhat B, Kaimal V, Zhang YY, Graham BB, Kumar R, Saggar R, Saggar R, Wallace WD, Ross DJ, Black SM, Fratz S, Fineman JR, Vargas SO, Haley KJ, Waxman AB, Chau BN, Fredenburgh LE, Chan SY. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Reports 13: 1016–1032, 2015. doi: 10.1016/j.celrep.2015.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J III, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botney MD, Liptay MJ, Kaiser LR, Cooper JD, Parks WC, Mecham RP. Active collagen synthesis by pulmonary arteries in human primary pulmonary hypertension. Am J Pathol 143: 121–129, 1993. [PMC free article] [PubMed] [Google Scholar]
- 10.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Champion HC, Lechtzin N, Wigley FM, Girgis RE, Hassoun PM. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. Am J Respir Crit Care Med 182: 252–260, 2010. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chelladurai P, Seeger W, Pullamsetti SS. Matrix metalloproteinases and their inhibitors in pulmonary hypertension. Eur Respir J 40: 766–782, 2012. doi: 10.1183/09031936.00209911. [DOI] [PubMed] [Google Scholar]
- 12.Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 6: 698–702, 2000. doi: 10.1038/76282. [DOI] [PubMed] [Google Scholar]
- 13.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 14.Estrada KD, Chesler NC. Collagen-related gene and protein expression changes in the lung in response to chronic hypoxia. Biomech Model Mechanobiol 8: 263–272, 2009. doi: 10.1007/s10237-008-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Federti E, Matté A, Ghigo A, Andolfo I, James C, Siciliano A, Leboeuf C, Janin A, Manna F, Choi SY, Iolascon A, Beneduce E, Melisi D, Kim DW, Levi S, De Franceschi L. Peroxiredoxin-2 plays a pivotal role as multimodal cytoprotector in the early phase of pulmonary hypertension. Free Radic Biol Med 112: 376–386, 2017. doi: 10.1016/j.freeradbiomed.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Fredenburgh LE, Liang OD, Macias AA, Polte TR, Liu X, Riascos DF, Chung SW, Schissel SL, Ingber DE, Mitsialis SA, Kourembanas S, Perrella MA. Absence of cyclooxygenase-2 exacerbates hypoxia-induced pulmonary hypertension and enhances contractility of vascular smooth muscle cells. Circulation 117: 2114–2122, 2008. doi: 10.1161/CIRCULATIONAHA.107.716241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N, Stenmark KR. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 168: 659–669, 2006. doi: 10.2353/ajpath.2006.050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37: 67–119, 2016. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 19.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res 90: 251–262, 2002. doi: 10.1161/res.90.3.251. [DOI] [PubMed] [Google Scholar]
- 20.Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 132: 1906–1912, 2007. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 21.George J, D’Armiento J. Transgenic expression of human matrix metalloproteinase-9 augments monocrotaline-induced pulmonary arterial hypertension in mice. J Hypertens 29: 299–308, 2011. doi: 10.1097/HJH.0b013e328340a0e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George MP, Champion HC, Simon M, Guyach S, Tarantelli R, Kling HM, Brower A, Janssen C, Murphy J, Carney JP, Morris A, Gladwin MT, Norris KA. Physiologic changes in a nonhuman primate model of HIV-associated pulmonary arterial hypertension. Am J Respir Cell Mol Biol 48: 374–381, 2013. doi: 10.1165/rcmb.2011-0434OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golob MJ, Wang Z, Prostrollo AJ, Hacker TA, Chesler NC. Limiting collagen turnover via collagenase-resistance attenuates right ventricular dysfunction and fibrosis in pulmonary arterial hypertension. Physiol Rep 4: e12815, 2016. doi: 10.14814/phy2.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54, Suppl: S10–S19, 2009. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Hatz K, Schneider U, Henrich PB, Braun B, Sacu S, Prunte C. Ranibizumab plus verteporfin photodynamic therapy in neovascular age-related macular degeneration: 12 months of retreatment and vision outcomes from a randomized study. Ophthalmologica 233: 66–73, 2015. doi: 10.1159/000367603. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann J, Marsh LM, Pieper M, Stacher E, Ghanim B, Kovacs G, König P, Wilkens H, Haitchi HM, Hoefler G, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G. Compartment-specific expression of collagens and their processing enzymes in intrapulmonary arteries of IPAH patients. Am J Physiol Lung Cell Mol Physiol 308: L1002–L1013, 2015. doi: 10.1152/ajplung.00383.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, Hennigs JK, Gu M, Wang L, Rabinovitch M. In pulmonary arterial hypertension, reduced BMPR2 promotes endothelial-to-mesenchymal transition via HMGA1 and its target slug. Circulation 133: 1783–1794, 2016. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilkiw R, Todorovich-Hunter L, Maruyama K, Shin J, Rabinovitch M. SC-39026, a serine elastase inhibitor, prevents muscularization of peripheral arteries, suggesting a mechanism of monocrotaline-induced pulmonary hypertension in rats. Circ Res 64: 814–825, 1989. doi: 10.1161/01.RES.64.4.814. [DOI] [PubMed] [Google Scholar]
- 29.Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA. “Inflammation and arterial stiffness in humans”. Atherosclerosis 237: 381–390, 2014. doi: 10.1016/j.atherosclerosis.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Jones JE, Mendes L, Rudd MA, Russo G, Loscalzo J, Zhang YY. Serial noninvasive assessment of progressive pulmonary hypertension in a rat model. Am J Physiol Heart Circ Physiol 283: H364–H371, 2002. doi: 10.1152/ajpheart.00979.2001. [DOI] [PubMed] [Google Scholar]
- 31.Jones PL, Rabinovitch M. Tenascin-C is induced with progressive pulmonary vascular disease in rats and is functionally related to increased smooth muscle cell proliferation. Circ Res 79: 1131–1142, 1996. doi: 10.1161/01.RES.79.6.1131. [DOI] [PubMed] [Google Scholar]
- 32.Karki P, Birukova AA. Substrate stiffness-dependent exacerbation of endothelial permeability and inflammation: mechanisms and potential implications in ALI and PH (2017 Grover Conference Series). Pulm Circ 8: 2045894018773044, 2018. doi: 10.1177/2045894018773044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavlock KD, Goldstein AS. Effect of pulse frequency on the osteogenic differentiation of mesenchymal stem cells in a pulsatile perfusion bioreactor. J Biomech Eng 133: 091005, 2011. doi: 10.1115/1.4004919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerr JS, Ruppert CL, Tozzi CA, Neubauer JA, Frankel HM, Yu SY, Riley DJ. Reduction of chronic hypoxic pulmonary hypertension in the rat by an inhibitor of collagen production. Am Rev Respir Dis 135: 300–306, 1987. doi: 10.1164/arrd.1987.135.2.300. [DOI] [PubMed] [Google Scholar]
- 35.Kim YM, Haghighat L, Spiekerkoetter E, Sawada H, Alvira CM, Wang L, Acharya S, Rodriguez-Colon G, Orton A, Zhao M, Rabinovitch M. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am J Pathol 179: 1560–1572, 2011. doi: 10.1016/j.ajpath.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolosionek E, King J, Rollinson D, Schermuly RT, Grimminger F, Graham BB, Morrell N, Butrous G. Schistosomiasis causes remodeling of pulmonary vessels in the lung in a heterogeneous localized manner: detailed study. Pulm Circ 3: 356–362, 2013. doi: 10.4103/2045-8932.114764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar R, Graham B. How does inflammation contribute to pulmonary hypertension? Eur Respir J 51: 1702403, 2018. doi: 10.1183/13993003.02403-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lankhaar JW, Westerhof N, Faes TJ, Gan CT, Marques KM, Boonstra A, van den Berg FG, Postmus PE, Vonk-Noordegraaf A. Pulmonary vascular resistance and compliance stay inversely related during treatment of pulmonary hypertension. Eur Heart J 29: 1688–1695, 2008. doi: 10.1093/eurheartj/ehn103. [DOI] [PubMed] [Google Scholar]
- 39.Lepetit H, Eddahibi S, Fadel E, Frisdal E, Munaut C, Noel A, Humbert M, Adnot S, D’Ortho MP, Lafuma C. Smooth muscle cell matrix metalloproteinases in idiopathic pulmonary arterial hypertension. Eur Respir J 25: 834–842, 2005. doi: 10.1183/09031936.05.00072504. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Tan Y, Stenmark KR, Tan W. High pulsatility flow induces acute endothelial inflammation through overpolarizing cells to activate NF-κB. Cardiovasc Eng Technol 4: 26–38, 2013. doi: 10.1007/s13239-012-0115-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsey ML, Zouein FA, Tian Y, Padmanabhan Iyer R, de Castro Brás LE. Osteopontin is proteolytically processed by matrix metalloproteinase 9. Can J Physiol Pharmacol 93: 879–886, 2015. doi: 10.1139/cjpp-2015-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, Moore SD, Drake KM, Aldred MA, Yu PB, Upton PD, Morrell NW. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 21: 777–785, 2015. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 47: 799–803, 2006. doi: 10.1016/j.jacc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 44.Mammoto T, Muyleart M, Konduri GG, Mammoto A. Twist1 in hypoxia-induced pulmonary hypertension through transforming growth factor-β-Smad signaling. Am J Respir Cell Mol Biol 58: 194–207, 2018. doi: 10.1165/rcmb.2016-0323OC. [DOI] [PubMed] [Google Scholar]
- 45.Maron BA, Opotowsky AR, Landzberg MJ, Loscalzo J, Waxman AB, Leopold JA. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: a pilot study. Eur J Heart Fail 15: 277–283, 2013. doi: 10.1093/eurjhf/hfs173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, Chen CT, Archer SL. Lung 18F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med 185: 670–679, 2012. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh LM, Jandl K, Grünig G, Foris V, Bashir M, Ghanim B, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G. The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur Respir J 51: 1701214, 2018. doi: 10.1183/13993003.01214-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maruyama K, Ye CL, Woo M, Venkatacharya H, Lines LD, Silver MM, Rabinovitch M. Chronic hypoxic pulmonary hypertension in rats and increased elastolytic activity. Am J Physiol Heart Circ Physiol 261: H1716–H1726, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A. Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol 16: 2906–2912, 2005. doi: 10.1681/ASN.2005040390. [DOI] [PubMed] [Google Scholar]
- 50.Nave AH, Mižíková I, Niess G, Steenbock H, Reichenberger F, Talavera ML, Veit F, Herold S, Mayer K, Vadász I, Weissmann N, Seeger W, Brinckmann J, Morty RE. Lysyl oxidases play a causal role in vascular remodeling in clinical and experimental pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 34: 1446–1458, 2014. doi: 10.1161/ATVBAHA.114.303534. [DOI] [PubMed] [Google Scholar]
- 51.Pellegrini P, Rossi A, Pasotti M, Raineri C, Cicoira M, Bonapace S, Dini FL, Temporelli PL, Vassanelli C, Vanderpool R, Naeije R, Ghio S. Prognostic relevance of pulmonary arterial compliance in patients with chronic heart failure. Chest 145: 1064–1070, 2014. doi: 10.1378/chest.13-1510. [DOI] [PubMed] [Google Scholar]
- 52.Philp CJ, Siebeke I, Clements D, Miller S, Habgood A, John AE, Navaratnam V, Hubbard RB, Jenkins G, Johnson SR. Extracellular matrix cross-linking enhances fibroblast growth and protects against matrix proteolysis in lung fibrosis. Am J Respir Cell Mol Biol 58: 594–603, 2018. doi: 10.1165/rcmb.2016-0379OC. [DOI] [PubMed] [Google Scholar]
- 53.Phimphilai M, Zhao Z, Boules H, Roca H, Franceschi RT. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res 21: 637–646, 2006. doi: 10.1359/jbmr.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piao L, Fang YH, Parikh K, Ryan JJ, Toth PT, Archer SL. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl) 91: 1185–1197, 2013. doi: 10.1007/s00109-013-1064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poiani GJ, Tozzi CA, Yohn SE, Pierce RA, Belsky SA, Berg RA, Yu SY, Deak SB, Riley DJ. Collagen and elastin metabolism in hypertensive pulmonary arteries of rats. Circ Res 66: 968–978, 1990. doi: 10.1161/01.RES.66.4.968. [DOI] [PubMed] [Google Scholar]
- 56.Rabinovitch M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin Chest Med 22: 433–449, 2001. doi: 10.1016/S0272-5231(05)70282-3. [DOI] [PubMed] [Google Scholar]
- 57.Rabinovitch M, Bothwell T, Hayakawa BN, Williams WG, Trusler GA, Rowe RD, Olley PM, Cutz E. Pulmonary artery endothelial abnormalities in patients with congenital heart defects and pulmonary hypertension. A correlation of light with scanning electron microscopy and transmission electron microscopy. Lab Invest 55: 632–653, 1986. [PubMed] [Google Scholar]
- 58.Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F, Planté S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, Perros F. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 131: 1006–1018, 2015. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg HG, Williams WG, Trusler GA, Higa T, Rabinovitch M. Structural composition of central pulmonary arteries. Growth potential after surgical shunts. J Thorac Cardiovasc Surg 94: 498–503, 1987. [PubMed] [Google Scholar]
- 60.Rothman AM, Arnold ND, Pickworth JA, Iremonger J, Ciuclan L, Allen RM, Guth-Gundel S, Southwood M, Morrell NW, Thomas M, Francis SE, Rowlands DJ, Lawrie A. MicroRNA-140-5p and SMURF1 regulate pulmonary arterial hypertension. J Clin Invest 126: 2495–2508, 2016. doi: 10.1172/JCI83361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruffenach G, Chabot S, Tanguay VF, Courboulin A, Boucherat O, Potus F, Meloche J, Pflieger A, Breuils-Bonnet S, Nadeau V, Paradis R, Tremblay E, Girerd B, Hautefort A, Montani D, Fadel E, Dorfmuller P, Humbert M, Perros F, Paulin R, Provencher S, Bonnet S. Role for runt-related transcription factor 2 in proliferative and calcified vascular lesions in pulmonary arterial hypertension. Am J Respir Crit Care Med 194: 1273–1285, 2016. doi: 10.1164/rccm.201512-2380OC. [DOI] [PubMed] [Google Scholar]
- 62.Saker M, Lipskaia L, Marcos E, Abid S, Parpaleix A, Houssaini A, Validire P, Girard P, Noureddine H, Boyer L, Vienney N, Amsellem V, Marguerit L, Maitre B, Derumeaux G, Dubois-Rande JL, Jourdan-Lesaux C, Delcroix M, Quarck R, Adnot S. Osteopontin, a key mediator expressed by senescent pulmonary vascular cells in pulmonary hypertension. Arterioscler Thromb Vasc Biol 36: 1879–1890, 2016. doi: 10.1161/ATVBAHA.116.307839. [DOI] [PubMed] [Google Scholar]
- 63.Samokhin AO, Stephens T, Wertheim BM, Wang RS, Vargas SO, Yung LM, Cao M, Brown M, Arons E, Dieffenbach PB, Fewell JG, Matar M, Bowman FP, Haley KJ, Alba GA, Marino SM, Kumar R, Rosas IO, Waxman AB, Oldham WM, Khanna D, Graham BB, Seo S, Gladyshev VN, Yu PB, Fredenburgh LE, Loscalzo J, Leopold JA, Maron BA. NEDD9 targets COL3A1 to promote endothelial fibrosis and pulmonary arterial hypertension. Sci Transl Med 10: eaap7294, 2018. doi: 10.1126/scitranslmed.aap7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanz J, Kariisa M, Dellegrottaglie S, Prat-González S, Garcia MJ, Fuster V, Rajagopalan S. Evaluation of pulmonary artery stiffness in pulmonary hypertension with cardiac magnetic resonance. JACC Cardiovasc Imaging 2: 286–295, 2009. doi: 10.1016/j.jcmg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 65.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 897–908, 2012. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 66.Scott D, Tan Y, Shandas R, Stenmark KR, Tan W. High-pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. Am J Physiol Lung Cell Mol Physiol 304: L70–L81, 2013. doi: 10.1152/ajplung.00342.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D34–D41, 2013. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 68.Song S, Yamamura A, Yamamura H, Ayon RJ, Smith KA, Tang H, Makino A, Yuan JX. Flow shear stress enhances intracellular Ca2+ signaling in pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension. Am J Physiol Cell Physiol 307: C373–C383, 2014. doi: 10.1152/ajpcell.00115.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122: 920–927, 2010. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 70.Spiekerkoetter E, Sung YK, Sudheendra D, Scott V, Del Rosario P, Bill M, Haddad F, Long-Boyle J, Hedlin H, Zamanian RT. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J 50: 1602449, 2017. doi: 10.1183/13993003.02449-2016. [DOI] [PubMed] [Google Scholar]
- 71.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L, de Jesus Perez V, Wang L, Reddy S, Zhao M, Bernstein D, Solow-Cordero DE, Beachy PA, Wandless TJ, Ten Dijke P, Rabinovitch M. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 123: 3600–3613, 2013. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104: 236–244, 2009. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stenmark KR, Frid M, Perros F. Endothelial-to-mesenchymal transition: an evolving paradigm and a promising therapeutic target in PAH. Circulation 133: 1734–1737, 2016. doi: 10.1161/CIRCULATIONAHA.116.022479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swift AJ, Capener D, Johns C, Hamilton N, Rothman A, Elliot C, Condliffe R, Charalampopoulos A, Rajaram S, Lawrie A, Campbell MJ, Wild JM, Kiely DG. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 196: 228–239, 2017. doi: 10.1164/rccm.201611-2365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science 341: 1240104, 2013. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan W, Madhavan K, Hunter KS, Park D, Stenmark KR. Vascular stiffening in pulmonary hypertension: cause or consequence? (2013 Grover Conference series). Pulm Circ 4: 560–580, 2014. doi: 10.1086/677370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tan Y, Tseng PO, Wang D, Zhang H, Hunter K, Hertzberg J, Stenmark KR, Tan W. Stiffening-induced high pulsatility flow activates endothelial inflammation via a TLR2/NF-κB pathway. PLoS One 9: e102195, 2014. doi: 10.1371/journal.pone.0102195. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Tedford RJ, Hassoun PM, Mathai SC, Girgis RE, Russell SD, Thiemann DR, Cingolani OH, Mudd JO, Borlaug BA, Redfield MM, Lederer DJ, Kass DA. Pulmonary capillary wedge pressure augments right ventricular pulsatile loading. Circulation 125: 289–297, 2012. doi: 10.1161/CIRCULATIONAHA.111.051540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thenappan T, Shah SJ, Rich S, Tian L, Archer SL, Gomberg-Maitland M. Contemporary survival in patients with pulmonary arterial hypertension: a Reappraisal of the National Institutes of Health Risk Stratification Equation. Eur Respir J 35:1079–1087, 2010. doi: 10.1183/09031936.00072709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thompson K, Rabinovitch M. Exogenous leukocyte and endogenous elastases can mediate mitogenic activity in pulmonary artery smooth muscle cells by release of extracellular-matrix bound basic fibroblast growth factor. J Cell Physiol 166: 495–505, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 81.Tilghman RW, Blais EM, Cowan CR, Sherman NE, Grigera PR, Jeffery ED, Fox JW, Blackman BR, Tschumperlin DJ, Papin JA, Parsons JT. Matrix rigidity regulates cancer cell growth by modulating cellular metabolism and protein synthesis. PLoS One 7: e37231, 2012. doi: 10.1371/journal.pone.0037231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Todorovich-Hunter L, Dodo H, Ye C, McCready L, Keeley FW, Rabinovitch M. Increased pulmonary artery elastolytic activity in adult rats with monocrotaline-induced progressive hypertensive pulmonary vascular disease compared with infant rats with nonprogressive disease. Am Rev Respir Dis 146: 213–223, 1992. doi: 10.1164/ajrccm/146.1.213. [DOI] [PubMed] [Google Scholar]
- 83.Todorovich-Hunter L, Johnson DJ, Ranger P, Keeley FW, Rabinovitch M. Altered elastin and collagen synthesis associated with progressive pulmonary hypertension induced by monocrotaline. A biochemical and ultrastructural study. Lab Invest 58: 184–195, 1988. [PubMed] [Google Scholar]
- 84.Tuder RM. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res 367: 643–649, 2017. doi: 10.1007/s00441-016-2539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tuder RM, Archer SL, Dorfmüller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D4–D12, 2013. doi: 10.1016/j.jacc.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Umesh A, Paudel O, Cao YN, Myers AC, Sham JS. Alteration of pulmonary artery integrin levels in chronic hypoxia and monocrotaline-induced pulmonary hypertension. J Vasc Res 48: 525–537, 2011. doi: 10.1159/000329593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vaillancourt M, Chia P, Sarji S, Nguyen J, Hoftman N, Ruffenach G, Eghbali M, Mahajan A, Umar S. Autonomic nervous system involvement in pulmonary arterial hypertension. Respir Res 18: 201, 2017. doi: 10.1186/s12931-017-0679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One 7: e39969, 2012. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Z, Schreier DA, Abid H, Hacker TA, Chesler NC. Pulmonary vascular collagen content, not cross-linking, contributes to right ventricular pulsatile afterload and overload in early pulmonary hypertension. J Appl Physiol 122: 253–263, 2017. doi: 10.1152/japplphysiol.00325.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor β1—an intimate relationship. Eur J Cell Biol 87: 601–615, 2008. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Ye CL, Rabinovitch M. Inhibition of elastolysis by SC-37698 reduces development and progression of monocrotaline pulmonary hypertension. Am J Physiol Heart Circ Physiol 261: H1255–H1267, 1991. doi: 10.1152/ajpheart.1991.261.4.H1255. [DOI] [PubMed] [Google Scholar]
- 92.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol 29: 936–942, 2009. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 93.Zaidi SH, You XM, Ciura S, Husain M, Rabinovitch M. Overexpression of the serine elastase inhibitor elafin protects transgenic mice from hypoxic pulmonary hypertension. Circulation 105: 516–521, 2002. doi: 10.1161/hc0402.102866. [DOI] [PubMed] [Google Scholar]
- 94.Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, Riddle S, Kumar S, Flockton A, McKeon BA, Frid MG, Reisz JA, Caruso P, El Kasmi KC, Ježek P, Morrell NW, Hu CJ, Stenmark KR. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a MicroRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation 136: 2468–2485, 2017. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]