Abstract
Skeletal muscle is the largest and most important site of capillary-tissue exchange, especially during high-energy demand tasks such as exercise; however, information regarding the role of the microcirculation in maintaining skeletal muscle health is limited. Changes in microcirculatory function, as observed with aging, chronic and cardiovascular diseases, and exercise, likely precede any alterations that arise in larger vessels, although further investigation into these changes is required. One of the main barriers to addressing this knowledge gap is the lack of methodologies for quantifying microvascular function in vivo; the utilization of valid and noninvasive quantification methods would allow the dynamic evaluation of microvascular flow during periods of clinical relevance such as during increased demand for flow (exercise) or decreased demand for flow (disuse). Contrast-enhanced ultrasound (CEUS) is a promising noninvasive technique that has been used for diagnostic medicine and more recently as a complementary research modality to investigate the response of the microcirculation in insulin resistance, diabetes, and aging. To improve the reproducibility of these measurements, our laboratory has optimized the quantification protocol associated with a bolus injection of the contrast agent for research purposes. This brief report outlines the assessment of microvascular flow using the raw time-intensity curve incorporated into gamma variate response modeling. CEUS could be used to compliment any macrovascular assessments to capture a more complete picture of the aging vasculature, and the modified methods presented here provide a template for the general analysis of CEUS within a research setting.
Keywords: aging, contrast-enhanced ultrasound, microcirculation, skeletal muscle
INTRODUCTION
Noninvasive measurements of large feed artery flow and function are commonly used as being indicative of overall vasculature health. The prognostic value of conduit artery stiffness as a proxy for vascular health and endothelial function is supported by large observational studies (32, 36, 38). Nonetheless, there is significant evidence indicating that the microvasculature, the intricate network of arterioles and venules connected by a capillary bed, is affected both earlier and more distinctly than larger conduit arteries. Thus, changes in microvascular function likely precede and are possibly more closely linked to problems that arise later in larger vessels. Within skeletal muscle, the microvasculature is the interface by which circulating nutrients, hormones, gases, and electrolytes must pass when moving to and from the systemic circulation. Furthermore, skeletal muscle is the largest and most important site of capillary-tissue exchange, especially during high-energy demand tasks such as exercise. Skeletal muscle blood flow and oxygen delivery are predictors of aerobic capacity, all of which exhibit an inevitable continual decline with age during exercise (16, 26, 28, 29), resulting in the age-related decline in overall exercise capacity. Given the multifaceted role of the microcirculation in maintaining skeletal muscle health, more attention should be placed on its role in affecting functional declines with aging and chronic and cardiovascular diseases as well as evaluating how targeted therapeutic options, such as exercise, can prevent or attenuate age-related declines in microcirculatory function.
The size of the microvascular network can expand with tissue growth, whereas with aging, the branching patterns of arterioles and venules undergo limited alterations (2). The subtle age-associated changes in the microvascular network are generally linked, with remodeling of the terminal capillaries manifesting as reductions in capillary density and capillary surface area (10, 12, 22). It is possible that these structural microvascular changes are impacted through chronic disease in addition to the independent effect of aging. Aging is also associated with reduced microvascular function, such as a reduced vasodilation in response to insulin and exercise (8, 24), which may be the result of impairments in vasodilation (transmission or mediation) in response to elevations in shear stress (3, 7). It is now apparent that the skeletal muscle microvasculature plays a critical role in determining insulin sensitivity by actively regulating both delivery and access of insulin and glucose to the musculature (18, 27); however, the relationship between the skeletal muscle fiber and the associated microvasculature and how this regulation is affected by chronic disease or dysfunction needs to be further studied. One of the main barriers to addressing this knowledge gap is the lack of methodologies for quantifying microvascular function in vivo; the utilization of valid and noninvasive quantification methods would allow the dynamic evaluation of microvascular flow during periods of clinical relevance such as during increased demand for flow (exercise) or decreased demand for flow (disuse).
Contrast-enhanced ultrasound (CEUS) is a promising, noninvasive imaging technique with diagnostic, therapeutic, and research applications (13, 14, 17, 23). CEUS involves the injection of gas-filled phospholipid microbubbles (~1.0–3.0 µm) into the systemic circulation to act as acoustic reflectors to allow improved contrast in ultrasound images. These bubbles have thin permeable shells containing high-molecular-weight gases that do not readily diffuse or dissolve within the bloodstream. These microbubbles act as nearly perfect intravascular reverberators of the acoustic ultrasound energy without local disruption, ultimately allowing enhanced contrast fidelity of the captured ultrasound images (25). The microbubbles themselves are smaller than red blood cells, which allows them passage through the microcirculation but prevents them from diffusing through vessel walls. Their ability to conform to fluid-filled intravascular spaces and their echogenic properties are advantages of their use in perfusion studies and can be quantified as the level of contrast in a tissue over time to indicate the perfusion capacity. Presently, CEUS is gaining traction for its use in diagnostic medicine because of its use in measuring neovascularization, tissue perfusion, echocardiography, and tumor vascularity (11, 14, 23, 37), primarily because of the relatively noninvasive methods for rapid image acquisition. Recently, CEUS has been used as a complementary research modality to demonstrate that the loss of microvascular insulin action (vasodilation) is manifest in both insulin resistance and aging but occurs before the development of tissue-based (muscle) insulin resistance (1, 5, 15, 24, 33). CEUS has also been instrumental in establishing that resistance exercise training improves the microvascular response in type 2 diabetes, ultimately improving overall glycemic status (31) and the effects of aging on skeletal muscle blood flow distribution (35). Although CEUS has some methodological barriers such as brief transit time and muscle movement artifacts, on balance this methodology offers an accessible, noninvasive, and reproducible assessment of microvasculature function. Presently, there are no standardized methodologies available for quantification of skeletal muscle perfusion. To improve the reproducibility of these measurements, our laboratory has optimized the quantification protocol associated with a bolus injection of the contrast agent for research purposes. Our methodology combines systemic perfusion of microbubbles with unilateral limb interventions, ultimately using the participant as the participant’s own internal control as the participant has an affected and a control limb (21). Here, we detail how assessment of microvascular flow can be attained using the raw time-intensity curve data incorporated into gamma variate response modeling, with unilateral exercise as a functional stimulus.
METHODS
The two representative subjects used for this methodological description were analyzed from a previous study (6). In brief, older subjects (age: 65 and 69 yr, body mass index: 27.6 and 29.7 kg/m2, respectively) completed a 2-wk protocol that required an abrupt reduction in activity (via step reduction: <1,500 steps/day for 2 wk), during which the experimental leg performed knee extension exercises 3 times/wk at 30% one-repetition maximum and the control leg remained sedentary. Participants were asked to attend experimental visits after an overnight fast (no caffeine or alcohol) and to take all prescribed medications and vitamins as usual except for any vasoactive medications (i.e., nitroglycerin). Contrast imaging was used to record the contrast-enhanced acoustic time-intensity curve after microbubble bolus injection using curve-fitting techniques. Perflutren-containing lipid microspheres (Definity, Lantheus) were activated by a vial mixer (Vialmix, Lantheus) at 75 Hz for 45 s, after which the activated microbubbles were prepared for bolus injection. Before microbubble administration, two ultrasound transducers were placed in custom-made stereotactic clamp stands that were attached to the examination bed and positioned to permit visualization of the vastus lateralis muscle tissue of each leg and to reduce movement artifacts. Participants were instructed to remain as still as possible and then received an intravenous bolus injection of 10 µl/kg of activated Definity via an antecubital vein followed immediately by a 10-ml saline flush. Data acquisition was initiated simultaneously in both ultrasound machines and coincided with the bolus agent injection. Microbubble appearance was assessed in the vastus lateralis muscle of both legs simultaneously. Contrast ultrasound measurements were recorded using two identical Vivid q BT10 ultrasound machines (GE Medical Systems, Horten, Norway) equipped with 1.5 to 3.6-MHz M4S-RS matrix phased-array transducers in left ventricular contrast mode (Fig. 1). The ultrasound depth was set at 5.0 cm, and the acquisition rate was 28 Hz. These settings were identical between ultrasound machines and for all time points throughout the protocol. A maximum ultrasound buffer of a 180-s limited recording length captured the full time-intensity curves in all subjects. Data were stored using the EchoPAC software package and analyzed using the Q-Analysis package (EchoPAC version 110.0.2, GE Medical Systems). Acoustic intensity (AI) was determined using a commercially available software package (EchoPAC) by manually selecting five independent circular regions of interest of 3 cm in diameter within the image, which were judged to be representative of the changes in AI upon bolus arrival. All AI traces were further evaluated for motion artifacts and unstable baseline, and a frame-by-frame average of suitable regions of interest was calculated. Average traces were imported to MATLAB (The MathWorks, Natick, MA) and digitally filtered (second-order, dual-pass Butterworth, cutoff frequency: 0.06 Hz) to identify the washin time from bolus injection (first continuous positive derivative) and to remove the AI bias from the signal by setting the AI at bolus arrival to a 0-dB reference. From these adjusted curves, the slope of the bolus perfusion into the muscle could be calculated (25–75% of bolus arrival to peak intensity; see Fig. 2). Raw data were also fit to a gamma variate function using a nonlinear least-squares method in the MATLAB Curve Fit Toolbox (Fig. 2), as follows:
where t is time, Ymax is the peak intensity, t′ is t/tmax (where tmax is time at the peak intensity), α is a curve shape constant, and e is Euler’s number. Initial curve estimates of Ymax and tmax were determined from the filtered data, and α was initially set to 1. From the gamma variate fit, we report the washin time, maximum AI, and integral of the peak intensity. The main limitation of a bolus CEUS approach is the variable washin time, which causes slight variability in response identification. However, the interrater and intrarater reliability were excellent for bolus arrival {intraclass correlation coefficient [ICC(2,1)]: 0.98 and 0.97, respectively}, maximum AI [ICC(2,1): 0.99 and 0.98, respectively], and arrival slope [ICC(2,1): 0.91 and 0.93, respectively].
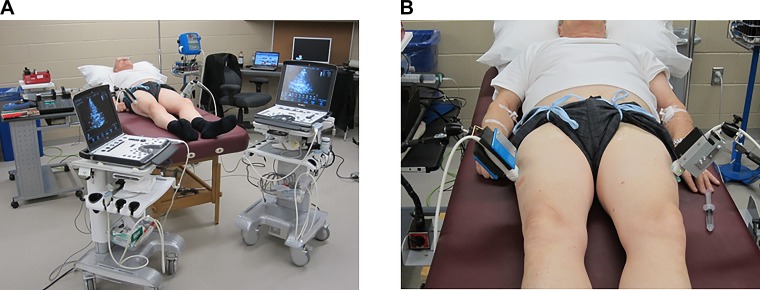
Fig. 1.
A and B: images of the within-subject unilateral experimental setup.
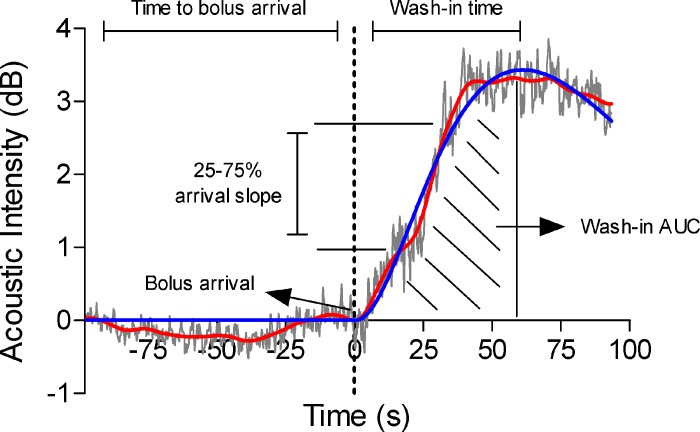
Fig. 2.
Representative example of contrast-enhanced ultrasound analysis methods from the vastus lateralis of a 69-yr-old man. Raw data (gray), filtered data (red), and gamma variate fit (blue) are plotted as acoustic intensity against time, relative to the point of bolus arrival. The filtered data were used to identify the time to bolus arrival and slope of the bolus arrival (from 25% to 75% of peak filtered acoustic intensity). The gamma variate fit was used to identify the peak intensity, washin time, and washin time area under the curve (AUC).
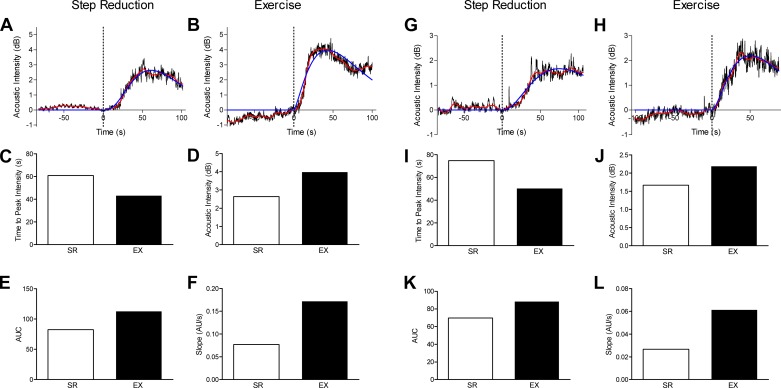
As shown in Fig. 3, from the gamma variate fit, we assessed several aspects of perfusion of the skeletal muscle: the time to peak intensity of the contrast agent, the maximal AI of the contrast agent, the area under the curve generated by the time-intensity trace, and the slope of the arrival of the contrast agent. This information gives us an accurate quantification of the perfusion capacity of the muscle at rest in an affected limb (inactive) and after short-term exercise. With the bolus method, there is some interindividual variability (i.e., bolus arrival time, absolute AI, and imaging parameters), which is why we have used the within-subject unilateral design. The data shown in Fig. 3 demonstrate that the specific gamma outcomes extracted from the bolus arrival trace are largely consistent within the same individual (e.g., bolus arrival, shape of curve, and root mean square error of the gamma variate fit) regardless of the leg being examined.
Fig. 3.
Representative examples of the application of contrast-enhanced ultrasound (CEUS) to assess the effect of resistance exercise on the vastus lateralis in two healthy older men (65 and 69 yr old). Participants underwent a 14-day reduced-step count intervention combined with a unilateral leg resistance exercise protocol to assess whether resistance training performed during step reduction would prevent impairments to skeletal muscle microvascular perfusion. Unilateral leg resistance exercise, at 30% of one-repetition maximum, was performed every second day during the period of step reduction. After the 2-wk intervention, simultaneous assessment of resting vastus lateralis microvascular function was completed using CEUS in both the step-reduced (SR) and step-reduced plus exercise (EX) legs. Time-intensity curves were generated for each leg (A, B, G, and H), and measurements of time to peak intensity (C and I), acoustic intensity (D and J), area under the curve (AUC; E and K), and slope (F and L) were extracted from the gamma variate fit. AU, arbitrary units.
CONCLUSIONS
CEUS is a relatively noninvasive, comprehensive, and promising tool that can, we propose, advance investigations into the skeletal muscle microvasculature, particularly in the context of aging, exercise, and disuse. While there are no standardized methodologies associated with the assessment of skeletal muscle perfusion, the present available assessments (Table 1) are limited to surrogate measures, such as conduit artery structure and function, that do not differentiate between the skin, skeletal muscle, and microvasculature (30) or expensive but innovative techniques such as blood oxygen level-dependent magnetic resonance imaging (19, 34). Future studies examining the effectiveness of interventions to enhance microvascular function in aging and populations with reduced microvascular function, such as those with diabetes or peripheral artery disease, are needed. Further work to identify the stability of the gamma variate fit discussed here in different populations with varying microvascular health is also needed. As we have detailed here, CEUS could be used to compliment any macrovascular assessments to capture a more complete picture of the aging vasculature, and the modified methods presented here provide a template for the general analysis of CEUS within a research setting.
Table 1.
Techniques to measure skeletal muscle microcirculation in humans
| Technique | Method | Strengths | Weaknesses |
|---|---|---|---|
| Laser-Doppler flowmetry | Uses a small probe touching the skin, measuring blood flow over a small volume (0.5- to 1.5-mm skin depth or smaller); quantifies the Doppler shift induced by the laser light scattered by moving blood cells | Noninvasive, able to measure fast alterations in blood flow, can use unilateral limb study design | Quantification based on average red blood cell concentration and velocity; not an exact measure (flux; has a linear relationship to the actual flow); measures cutaneous flux, not skeletal muscle; cannot compare perfusion between individuals |
| Near-infrared spectroscopy | Measures regional skeletal muscle hemoglobin oxygenation/deoxygenation | Noninvasive, can be measured during exercise, can detect hemoglobin in vessels of <2 mm, portable, multichannel measures for spatial differences, can use unilateral limb study design | Difficult to predict the hemoglobin distribution ratio between artery, capillary, and vein |
| Venous occlusion plethysmography | Uses pneumatic cuffs to induce venous occlusion but allow arterial inflow; blood flow is then measured as linear increases in volume over time and is thought to be proportional to the rate of arterial inflow | Noninvasive, portable, no radiation or contrast agent | Global indicator of perfusion, not able to differentiate microvasculature |
| Contrast-enhanced ultrasound | Quantifies the concentration of an injected contrast agent (lipid microbubbles); the microbubbles are smaller than red blood cells, which allows them to travel throughout the muscle microcirculation | Noninvasive; portable; can use unilateral limb study design; can use exercise, diet, and cuff occlusion to induce changes in microcirculation activation; useful for vascular pathologies; can use either bolus or burst replenishment method | Can be influenced by limb movement, brief transit time, requires catheter for contrast introduction, bolus arrival time can be limiting |
| Blood oxygen level-dependent magnetic resonance imaging | Quantifies the oxygenation of hemoglobin within the skeletal muscle through the measurement of changes in the local ratio of oxyhemoglobin and deoxyhemoglobin | Useful for vascular pathologies, noninvasive, can use exercise and cuff occlusion to induce changes in hemoglobin oxygenation, high spatial resolution, no radiation dose, no contrast agent | Expensive; can be influenced by hydration status, vessel orientation, and limb movement |
| Quantitative dynamic contrast-enhanced magnetic resonance imaging | Quantifies the temporal enhancement pattern of a paramagnetic contrast agent introduced into the vasculature; magnetic resonance images are acquired before, during, and after the intravenous injection of a contrast agent | Measures blood flow and tissue perfusion, can be used in a clinical setting | Uses gadolinium contrast agent (risks include headache, nausea, dizziness, possible allergic reaction, gadolinium retention, or nephrogenic systemic fibrosis in renal-insufficient patients), indirect measure of contrast agent using water protons, expensive, complex data acquisition and interpretation |
| Positron emission tomography | Measures skeletal muscle blood flow and glucose metabolism through the quantification of injected radioactive molecules labeled with positron-emitting nuclides with subsequent tomographic detection of the radioactive nuclide within an organ of interest | Noninvasive, can use unilateral limb study design, can compare blood flow to glucose utilization, provides three-dimensional insights into capillary-level blood flow | Expensive, can be influenced by limb movement, uses ionizing radiation |
GRANTS
This work was supported by a Natural Sciences and Engineering Research Council of Canada (Conseil de Recherches en Sciences Naturelles et en Génie du Canada) grant (to S. M. Phillips).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.D., S.M.P., and M.J.M. conceived and designed research; E.C.D., M.C.D., and M.J.M. performed experiments; E.C.D. and J.S.A. analyzed data; E.C.D. and J.S.A. interpreted results of experiments; E.C.D. and J.S.A. prepared figures; E.C.D. drafted manuscript; E.C.D., J.S.A., S.M.P., and M.J.M. edited and revised manuscript; S.M.P. and M.J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. James Lacefield for insights into and comments on the adoption of the gamma variate fit for our skeletal muscle CEUS model.
REFERENCES
- 1.Amarteifio E, Wormsbecher S, Demirel S, Krix M, Braun S, Rehnitz C, Delorme S, Kauczor HU, Weber MA. Assessment of skeletal muscle microcirculation in type 2 diabetes mellitus using dynamic contrast-enhanced ultrasound: a pilot study. Diab Vasc Dis Res 10: 468–470, 2013. doi: 10.1177/1479164113484165. [DOI] [PubMed] [Google Scholar]
- 2.Bearden SE. Effect of aging on the structure and function of skeletal muscle microvascular networks. Microcirculation 13: 279–288, 2006. doi: 10.1080/10739680600618892. [DOI] [PubMed] [Google Scholar]
- 3.Behringer EJ, Segal SS. Spreading the signal for vasodilatation: implications for skeletal muscle blood flow control and the effects of ageing. J Physiol 590: 6277–6284, 2012. doi: 10.1113/jphysiol.2012.239673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey DP, Curry TB, Joyner MJ. Measuring muscle blood flow: a key link between systemic and regional metabolism. Curr Opin Clin Nutr Metab Care 11: 580–586, 2008. doi: 10.1097/MCO.0b013e32830b5b34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan A, Barrett EJ, Anderson SM, Kovatchev BP, Breton MD. Muscle microvascular recruitment predicts insulin sensitivity in middle-aged patients with type 1 diabetes mellitus. Diabetologia 55: 729–736, 2012. doi: 10.1007/s00125-011-2402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devries MC, Breen L, Von Allmen M, MacDonald MJ, Moore DR, Offord EA, Horcajada MN, Breuillé D, Phillips SM. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol Rep 3: e12493, 2015. doi: 10.14814/phy2.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dinenno FA, Joyner MJ. α-Adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13: 329–341, 2006. doi: 10.1080/10739680600618843. [DOI] [PubMed] [Google Scholar]
- 8.Durham WJ, Casperson SL, Dillon EL, Keske MA, Paddon-Jones D, Sanford AP, Hickner RC, Grady JJ, Sheffield-Moore M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J 24: 4117–4127, 2010. doi: 10.1096/fj.09-150177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gliemann L, Mortensen SP, Hellsten Y. Methods for the determination of skeletal muscle blood flow: development, strengths and limitations. Eur J Appl Physiol 118: 1081–1094, 2018. doi: 10.1007/s00421-018-3880-5. [DOI] [PubMed] [Google Scholar]
- 10.Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, van Loon LJ. Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol 116: 998–1005, 2014. doi: 10.1152/japplphysiol.00919.2013. [DOI] [PubMed] [Google Scholar]
- 11.He MN, Lv K, Jiang YX, Jiang TA. Application of superb microvascular imaging in focal liver lesions. World J Gastroenterol 23: 7765–7775, 2017. doi: 10.3748/wjg.v23.i43.7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrandt W, Schwarzbach H, Pardun A, Hannemann L, Bogs B, König AM, Mahnken AH, Hildebrandt O, Koehler U, Kinscherf R. Age-related differences in skeletal muscle microvascular response to exercise as detected by contrast-enhanced ultrasound (CEUS). PLoS One 12: e0172771, 2017. doi: 10.1371/journal.pone.0172771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotfiel T, Heiss R, Swoboda B, Kellermann M, Gelse K, Grim C, Strobel D, Wildner D. Contrast-enhanced ultrasound as a new investigative tool in diagnostic imaging of muscle injuries: a pilot study evaluating conventional ultrasound, CEUS, and findings in MRI. Clin J Sport Med 28: 332–338, 2017. doi: 10.1097/JSM.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 14.Kaspar M, Partovi S, Aschwanden M, Imfeld S, Baldi T, Uthoff H, Staub D. Assessment of microcirculation by contrast-enhanced ultrasound: a new approach in vascular medicine. Swiss Med Wkly 145: w14047, 2015. doi: 10.4414/smw.2015.14047. [DOI] [PubMed] [Google Scholar]
- 15.Keske MA, Premilovac D, Bradley EA, Dwyer RM, Richards SM, Rattigan S. Muscle microvascular blood flow responses in insulin resistance and ageing. J Physiol 594: 2223–2231, 2016. doi: 10.1113/jphysiol.2014.283549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111: 220–230, 2012. doi: 10.1161/CIRCRESAHA.112.269571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krix M, Weber MA, Krakowski-Roosen H, Huttner HB, Delorme S, Kauczor HU, Hildebrandt W. Assessment of skeletal muscle perfusion using contrast-enhanced ultrasonography. J Ultrasound Med 24: 431–441, 2005. doi: 10.7863/jum.2005.24.4.431. [DOI] [PubMed] [Google Scholar]
- 18.Kusters YH, Barrett EJ. Muscle microvasculature’s structural and functional specializations facilitate muscle metabolism. Am J Physiol Endocrinol Metab 310: E379–E387, 2016. doi: 10.1152/ajpendo.00443.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledermann HP, Schulte AC, Heidecker HG, Aschwanden M, Jäger KA, Scheffler K, Steinbrich W, Bilecen D. Blood oxygenation level-dependent magnetic resonance imaging of the skeletal muscle in patients with peripheral arterial occlusive disease. Circulation 113: 2929–2935, 2006. doi: 10.1161/CIRCULATIONAHA.105.605717. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Ko SH, Chai W, Cao W. Regulation of muscle microcirculation in health and diabetes. Diabetes Metab J 36: 83–89, 2012. doi: 10.4093/dmj.2012.36.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacInnis MJ, McGlory C, Gibala MJ, Phillips SM. Investigating human skeletal muscle physiology with unilateral exercise models, when one limb is more powerful than two. Appl Physiol Nutr Metab 42: 563–570, 2017. doi: 10.1139/apnm-2016-0645. [DOI] [PubMed] [Google Scholar]
- 22.Martin WH III, Ogawa T, Kohrt WM, Malley MT, Korte E, Kieffer PS, Schechtman KB. Effects of aging, gender, and physical training on peripheral vascular function. Circulation 84: 654–664, 1991. doi: 10.1161/01.CIR.84.2.654. [DOI] [PubMed] [Google Scholar]
- 23.Mehta KS, Lee JJ, Taha AG, Avgerinos E, Chaer RA. Vascular applications of contrast-enhanced ultrasound imaging. J Vasc Surg 66: 266–274, 2017. [Erratum in J Vasc Surg 66: 1311, 2017. 10.1016/j.jvs.2017.08.022.] doi: 10.1016/j.jvs.2016.12.133. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell WK, Phillips BE, Williams JP, Rankin D, Smith K, Lund JN, Atherton PJ. Development of a new Sonovue™ contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol Rep 1: e00119, 2013. doi: 10.1002/phy2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulvagh SL, Rakowski H, Vannan MA, Abdelmoneim SS, Becher H, Bierig SM, Burns PN, Castello R, Coon PD, Hagen ME, Jollis JG, Kimball TR, Kitzman DW, Kronzon I, Labovitz AJ, Lang RM, Mathew J, Moir WS, Nagueh SF, Pearlman AS, Perez JE, Porter TR, Rosenbloom J, Strachan GM, Thanigaraj S, Wei K, Woo A, Yu EH, Zoghbi WA; American Society of Echocardiography . American Society of Echocardiography consensus statement on the clinical applications of ultrasonic contrast agents in echocardiography. J Am Soc Echocardiogr 21: 1179–1201, 2008. doi: 10.1016/j.echo.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Nyberg M, Blackwell JR, Damsgaard R, Jones AM, Hellsten Y, Mortensen SP. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J Physiol 590: 5361–5370, 2012. doi: 10.1113/jphysiol.2012.239053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole DC, Copp SW, Ferguson SK, Musch TI. Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98: 1645–1658, 2013. doi: 10.1113/expphysiol.2013.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- 29.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- 30.Roustit M, Cracowski JL. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation 19: 47–64, 2012. doi: 10.1111/j.1549-8719.2011.00129.x. [DOI] [PubMed] [Google Scholar]
- 31.Russell RD, Hu D, Greenaway T, Blackwood SJ, Dwyer RM, Sharman JE, Jones G, Squibb KA, Brown AA, Otahal P, Boman M, Al-Aubaidy H, Premilovac D, Roberts CK, Hitchins S, Richards SM, Rattigan S, Keske MA. Skeletal muscle microvascular-linked improvements in glycemic control from resistance training in individuals with type 2 diabetes. Diabetes Care 40: 1256–1263, 2017. doi: 10.2337/dc16-2750. [DOI] [PubMed] [Google Scholar]
- 32.Shechter M, Issachar A, Marai I, Koren-Morag N, Freinark D, Shahar Y, Shechter A, Feinberg MS. Long-term association of brachial artery flow-mediated vasodilation and cardiovascular events in middle-aged subjects with no apparent heart disease. Int J Cardiol 134: 52–58, 2009. doi: 10.1016/j.ijcard.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Song Y, Li Y, Wang PJ, Gao Y. Contrast-enhanced ultrasonography of skeletal muscles for type 2 diabetes mellitus patients with microvascular complications. Int J Clin Exp Med 7: 573–579, 2014. [PMC free article] [PubMed] [Google Scholar]
- 34.Stacy MR, Caracciolo CM, Qiu M, Pal P, Varga T, Constable RT, Sinusas AJ. Comparison of regional skeletal muscle tissue oxygenation in college athletes and sedentary control subjects using quantitative BOLD MR imaging. Physiol Rep 4: e12903, 2016. doi: 10.14814/phy2.12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas KN, Cotter JD, Lucas SJ, Hill BG, van Rij AM. Reliability of contrast-enhanced ultrasound for the assessment of muscle perfusion in health and peripheral arterial disease. Ultrasound Med Biol 41: 26–34, 2015. doi: 10.1016/j.ultrasmedbio.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Verwoert GC, Elias-Smale SE, Rizopoulos D, Koller MT, Steyerberg EW, Hofman A, Kavousi M, Sijbrands EJ, Hoeks AP, Reneman RS, Mattace-Raso FU, Witteman JC. Does aortic stiffness improve the prediction of coronary heart disease in elderly? The Rotterdam Study. J Hum Hypertens 26: 28–34, 2012. doi: 10.1038/jhh.2010.124. [DOI] [PubMed] [Google Scholar]
- 37.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Basis for detection of stenosis using venous administration of microbubbles during myocardial contrast echocardiography: bolus or continuous infusion? J Am Coll Cardiol 32: 252–260, 1998. doi: 10.1016/S0735-1097(98)00212-5. [DOI] [PubMed] [Google Scholar]
- 38.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the Multi-ethnic Study of Atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]