Abstract
Electrical stimulation of the baroreflex chronically suppresses sympathetic activity and arterial pressure and is currently being evaluated for the treatment of resistant hypertension. The antihypertensive effects of baroreflex activation are often attributed to renal sympathoinhibition. However, baroreflex activation also decreases heart rate, and robust blood pressure lowering occurs even after renal denervation. Because controlling renal sympathetic nerve activity (RSNA) and cardiac autonomic activity cannot be achieved experimentally, we used an established mathematical model of human physiology (HumMod) to provide mechanistic insights into their relative and combined contributions to the cardiovascular responses during baroreflex activation. Three-week responses to baroreflex activation closely mimicked experimental observations in dogs including decreases in blood pressure, heart rate, and plasma norepinephrine and increases in plasma atrial natriuretic peptide (ANP), providing validation of the model. Simulations showed that baroreflex-induced alterations in cardiac sympathetic and parasympathetic activity lead to a sustained depression of cardiac function and increased secretion of ANP. Increased ANP and suppression of RSNA both enhanced renal excretory function and accounted for most of the chronic blood pressure lowering during baroreflex activation. However, when suppression of RSNA was blocked, the blood pressure response to baroreflex activation was not appreciably impaired due to inordinate fluid accumulation and further increases in atrial pressure and ANP secretion. These simulations provide a mechanistic understanding of experimental and clinical observations showing that baroreflex activation effectively lowers blood pressure in subjects with previous renal denervation.
NEW & NOTEWORTHY Both experimental and clinical studies have shown that the presence of renal nerves is not an obligate requirement for sustained reductions in blood pressure during chronic electrical stimulation of the carotid baroreflex. Simulations using HumMod, a mathematical model of integrative human physiology, indicated that both increased secretion of atrial natriuretic peptide and suppressed renal sympathetic nerve activity play key roles in mediating long-term reductions in blood pressure during chronic baroreflex activation.
Keywords: atrial natriuretic peptide, baroreflex, blood pressure, modeling, sympathetic nervous system
INTRODUCTION
Clinical trials evaluating the efficacy of chronic electrical stimulation of the carotid baroreflex in the treatment of resistant hypertension and heart failure are ongoing. Although it is well established that baroreflex activation chronically suppresses sympathetic activity (21, 22), the mechanisms that account for its sustained cardiovascular effects are still unclear. This lack of knowledge adds to the uncertainty as to which patients are most likely to benefit from this therapy. In addition to the clinical importance of baroreflex activation as a treatment option for hypertension, this device provides a unique tool for elucidating the mechanisms that account for blood pressure lowering during chronic suppression of central sympathetic outflow.
Because chronic activation of the arterial baroreflex suppresses renal sympathetic nerve activity (RSNA) (21), it is logical to hypothesize that the natriuretic effects of renal sympathoinhibition play a critical role in long-term blood pressure lowering during baroreflex activation. However, one of the more surprising experimental observations is that the presence of renal nerves is not an obligate requirement for chronically reducing blood pressure during baroreflex activation (20). Consistent with this finding, chronic baroreflex activation reduced blood pressure in patients in whom catheter-based renal nerve ablation was unsuccessful in treating their hypertension (13, 35).
In addition to suppressing RSNA, baroreflex activation has sustained autonomic effects on the heart, actions typically overlooked when accounting for the antihypertensive effects of this device-based therapy. Most noticeably, reciprocal effects of baroreflex activation on cardiac sympathetic and parasympathetic nerve activity leading to sustained bradycardia were observed in both experimental (15, 17, 19, 20, 22, 23) and clinical studies (1, 5, 13, 36). Less conspicuously, neurally mediated suppression of cardiac function during baroreflex activation may increase cardiac pressures and the concomitant secretion of atrial natriuretic peptide (ANP), a hypothesis consistent with our experimental findings during baroreflex activation in normotensive canines (20). Accordingly, by promoting Na+ excretion, increased plasma levels of ANP may contribute to chronic blood pressure lowering during baroreflex activation. Thus, the major goal of the present study was to provide mechanistic insights into the relative and combined contributions of changes in RSNA and cardiac autonomic activity to the cardiovascular responses to baroreflex activation. Because controlling RSNA and cardiac autonomic activity during baroreflex activation cannot be achieved experimentally, we used an established, integrative mathematical model of human physiology, HumMod, to achieve this goal (4, 11). This model is based on physiological principles and well-established physiological relationships. HumMod serves as a framework for understanding complex adaptive responses to physiological and pathophysiological conditions and can demonstrate interactions among variables that may not be intuitively obvious otherwise.
We hypothesized that baroreflex-mediated alterations in central autonomic outflow to the kidneys and heart both contribute significantly to chronic reductions in blood pressure during baroreflex activation through neurohormonal mechanisms that increase renal excretory function. We further hypothesize that when reductions in prevailing levels of RSNA are not possible during baroreflex activation, excess fluid retention leads to exaggerated increases in ANP secretion, which enhances the role of this natriuretic hormone in blood pressure lowering.
METHODS
Model Description
The simulations presented in the present study were performed using HumMod, a large model of human physiology composed of mathematical relationships that describe the detailed integration of organ, tissue, and cellular systems. With considerable content added to the backbone of the Guyton-Coleman model published over 45 yr ago, HumMod comprises over 8,000 independent variables and ~2,000 parameters and equations (4, 8–11). It should be emphasized that before this study was conducted, there were no significant changes in the model since the previous version of HumMod used in a recent study to simulate blood pressure salt sensitivity (4).
The model integrates multiple physiological systems, including those most relevant to this study, i.e., the fluid compartments and renal, autonomic, endocrine, and cardiovascular systems. Detailed analysis of the entire model is beyond the scope of this study; however, the graphical user interface and the version of the model with added content have been previously described in recent publications (4, 11). The latest version of HumMod used in this study can be downloaded at no cost as a single ZIP file at http://hummod.org/hummod-baro.zip. This link also provides a detailed listing of the mathematical relationships within HumMod, general instructions on how to use the model (General Instructions), and specific information for replicating the current simulations (Specific Experimental Protocols). Currently, HumMod can only be run on computers with or emulating a Windows operating system.
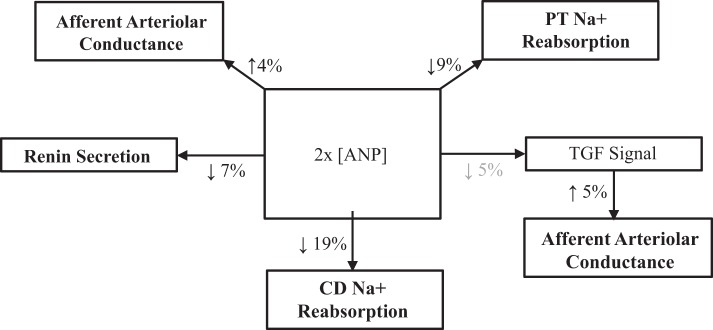
The most pertinent aspects of the model relevant to the present simulations are briefly discussed below. A critical component of HumMod is the renal fluid volume mechanism for long-term control of blood pressure (8–10). This feedback control mechanism is based on the interrelationships of body fluid volume and blood pressure through changes in pressures, flows, and the renal excretion of Na+. In HumMod, the key determinants that account for long-term blood pressure lowering during baroreflex activation through alterations in renal excretory function are provided in the appendix (Figs. A1–A3). Additionally, an integrated overview is shown in Fig. A4 in the appendix.
In the present simulations, alterations in central sympathetic outflow and cardiac vagal activity were primarily determined by afferent input from arterial baroreceptors. Afferent input from cardiopulmonary receptors was fixed at control levels for the following reasons: 1) the time dependency for resetting of these mechanoreceptors is unknown, and there is no evidence that the reflex responses emanating from these receptors are sustained chronically; and 2) based on our previous observations showing that the reduced levels of blood pressure, whole body norepinephrine (NE) spillover, and plasma NE concentration are constant throughout week 1 and from week 1 to week 3 of baroreflex activation (21, 22), we reasoned that cardiopulmonary and aortic baroreflex reflexes do not have a significant long-term influence on central sympathetic outflow during activation of the carotid baroreflex. In addition to global changes in sympathetic activity, there is differential control of efferent neural outflow to the renal, hepatic, cardiac, adrenal, and splanchnic territories in response to some stimuli. However, although not measured in experimental studies, in the model there was equal suppression of neural outflow to these districts during baroreflex activation. The quantitative relationships between changes in RSNA and Na+ excretion in HumMod are based on the studies by Miki et al. (26, 27) conducted in conscious dogs.
Plasma levels of hormones were calculated from rates of secretion/production, clearance rates, and volume of distribution. A similar calculation determined plasma NE concentration based on spillover from nerve terminals and secretion from the adrenal glands. The net secretion of ANP was calculated from the sum of the secretion rates from the left and right atria, which were based on their individual pressures. The quantitative relationship between changes in atrial pressure and plasma ANP concentration in HumMod are based on data generated in chronically instrumented dogs during the control of atrial pressure (24, 25, 33). Physiological responses to ANP in the model were derived from studies conducted in both dogs and rats (3, 12, 14, 18, 29–31, 34). Quantification of the multiple responses to ANP during baroreflex activation is provided in Fig. A5 in the appendix. In the simulations, the key determinants of renin secretion are β-adrenergic stimulation of juxtaglomerular cells and NaCl delivery to the macula densa, with the latter influenced by both RSNA and ANP. The primary determinants of aldosterone secretion are plasma angiotensin II (ANG II) and K+ concentration.
The organs and tissues that make up the circulation in HumMod include the kidneys, heart, skeletal muscle, gastrointestinal tract, liver, bone, brain, fat, skin, and lungs. The flow through these tissues is affected by sympathetic activity and plasma ANG II concentration as well as local tissue factors such as oxygen levels, which together regulate vascular tone and blood flow. Total peripheral resistance was calculated as the difference between mean arterial pressure (MAP) and right atrial pressure divided by cardiac output.
Protocols
To mimic experimental conditions in our canine study (22), Na+ intake in the model was fixed at normal levels (180 mmol/day), which is equivalent on a weight basis to the fixed normal Na+ intake of dogs (60 mmol/day). Water intake was ad libitum, and food intake was fixed to be consistent with the protocol in the canine study. The simulation was run for 4 wk to allow model variables to reach a steady state. After 1 wk of baseline values were recorded, responses to 3 wk of baroreflex activation were determined for each of the following four simulations:
-
1.
Normal: baroreflex activation alone.
-
2.
Renal clamp: baroreflex activation with RSNA maintained at control levels; thus, in this protocol, the effect of baroreflex activation on blood pressure was independent of suppression of RSNA.
-
3.
Cardiac clamp: baroreflex activation with cardiac sympathetic and vagal activity maintained at control levels; thus, in this protocol, the effect of baroreflex activation on blood pressure was independent of changes in cardiac autonomic outflow.
-
4.
Renal + cardiac clamp: baroreflex activation with both RSNA and cardiac autonomic activity maintained at control levels.
A 1-wk recovery period followed baroreflex activation in each protocol. The clamps were maintained during the control, baroreflex activation, and recovery periods.
Baroreflex activation was achieved by increasing carotid baroreceptor input into the central nervous system to 70% above normal to mimic constant electrical stimulation of the carotid sinus. This level was fixed for 3 wk in all four simulations. This level of baroreceptor afferent input was chosen to produce ~a 20-mmHg fall in blood pressure and ~a 40% decrease in plasma NE concentration, to match experimental studies (22).
RESULTS
Model Performance: Response to 3 wk of Baroreflex Activation With Comparisons With Experimental Data
Although HumMod accurately predicts many physiological and pathological processes, it was important to first determine whether this model realistically reproduces experimental baroreflex activation responses.
The following are congruent model and experimental responses to 3 wk of baroreflex activation (22).
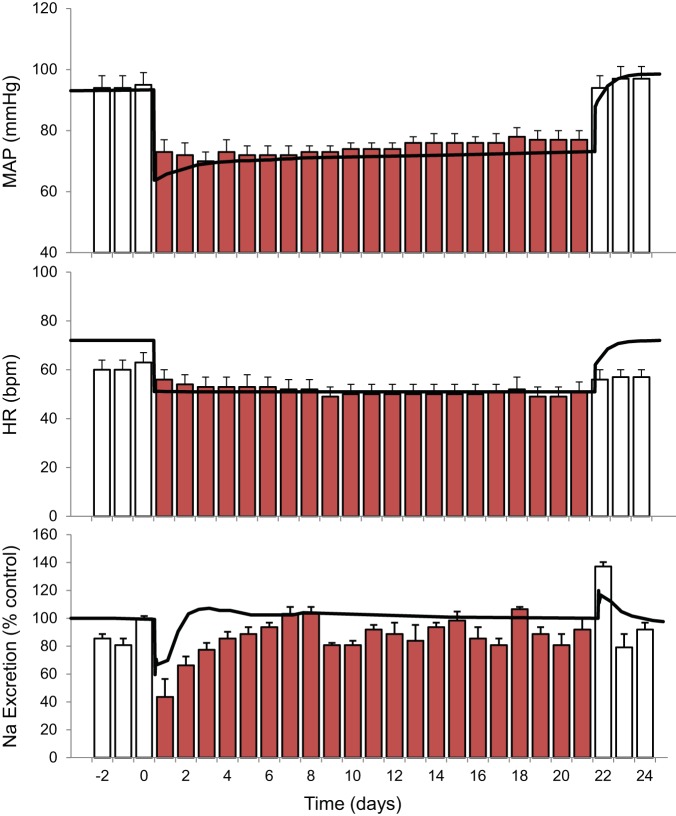
With an intensity of activation designed to produce a decrease in MAP of ~20 mmHg on day 1, this fall in MAP was sustained for the entire 3 wk of baroreflex activation. After cessation of baroreflex activation, full recovery of MAP to control levels was achieved within 2 days (Fig. 1).
Fig. 1.
Changes in mean arterial pressure (MAP), heart rate [HR; in beats/min (bpm)], and Na+ excretion during 3 wk of baroreflex activation. Red bars indicate experimental data. Values are means ± SE; n = 6 dogs. Black lines show the results from the simulation.
Heart rate decreased ~15 beats/min during chronic baroreflex activation (Fig. 1).
During the initial days of baroreflex activation, Na+ excretion decreased along with the acute fall in MAP. Subsequently, Na+ excretion actually exceeded intake on some days before finally returning to control levels. Conversely, with cessation of baroreflex activation, there was a transient increase in Na+ excretion above control levels along with the rise in MAP before Na+ balance was achieved once again (Fig. 1). Despite these transient changes in Na+ excretion, there was no sustained increase in extracellular fluid volume either in the simulation (see below) or when measured experimentally (15).
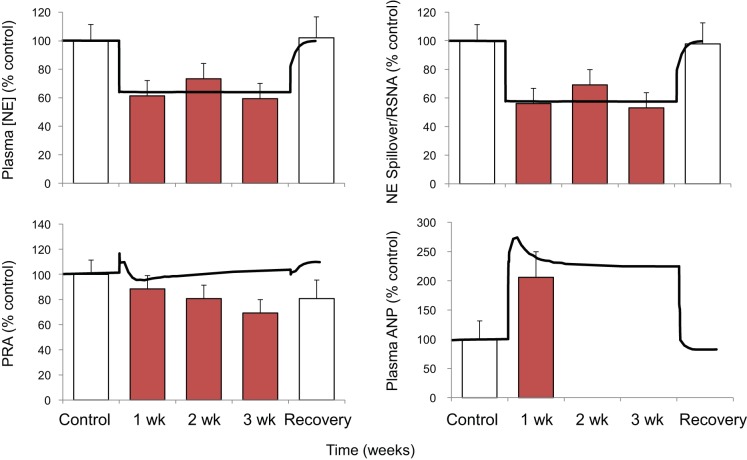
Plasma NE concentration decreased ~40% concomitant with similar reductions in whole body NE spillover (22) and RSNA (simulation), indicating sustained suppression of central sympathetic outflow (Fig. 2).
Fig. 2.
Changes in plasma concentrations of norepinephrine (NE) and atrial natriuretic peptide (ANP), plasma renin activity (PRA), and either NE spillover (red bars) or renal sympathetic nerve activity (black line) during 3 wk of baroreflex activation. Red bars indicate experimental data. Values are means ± SE; n = 6 dogs. Black lines show the results from the simulation.
Despite the pronounced fall in MAP during baroreflex activation, there was little or no change in plasma renin activity (Fig. 2). Similarly, there was virtually no change in plasma aldosterone concentration during baroreflex activation.
In the simulation, plasma ANP concentration increased two- to threefold throughout the 3 wk of baroreflex activation (Fig. 2). Although 3 wk experimental responses were not reported, the simulated increase in ANP compares favorably to experimental measurements made after 1 wk of baroreflex activation (20).
Despite appreciable reductions in MAP, daily Na+ balance was achieved even with a substantial decrease in the glomerular filtration rate (GFR) (see below). In the simulation, GFR was reduced ~20% along with the decrease in MAP of 20 mmHg. A smaller reduction in GFR (~10%) occurred experimentally at a level of baroreflex activation producing a somewhat smaller fall in MAP (17 mmHg) (15).
Relevant Model Predictions to 3 wk of Baroreflex Activation Including Variables Not Measured in Experimental Studies
Blood pressure, heart rate, and systemic hemodynamics.
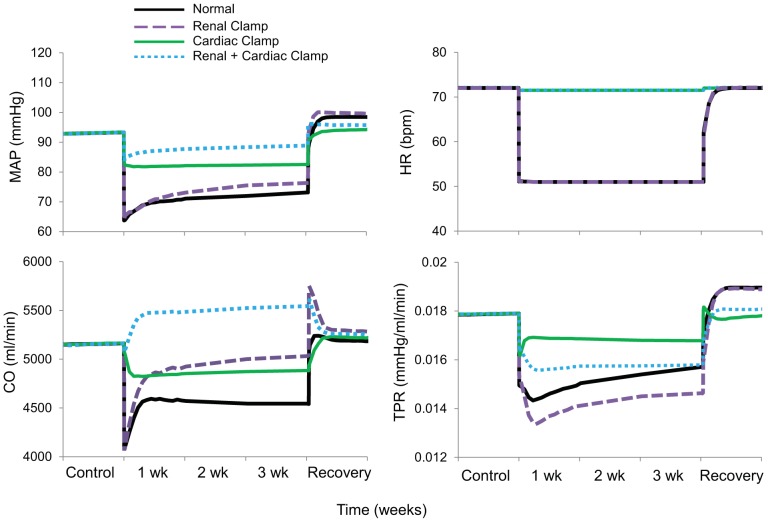
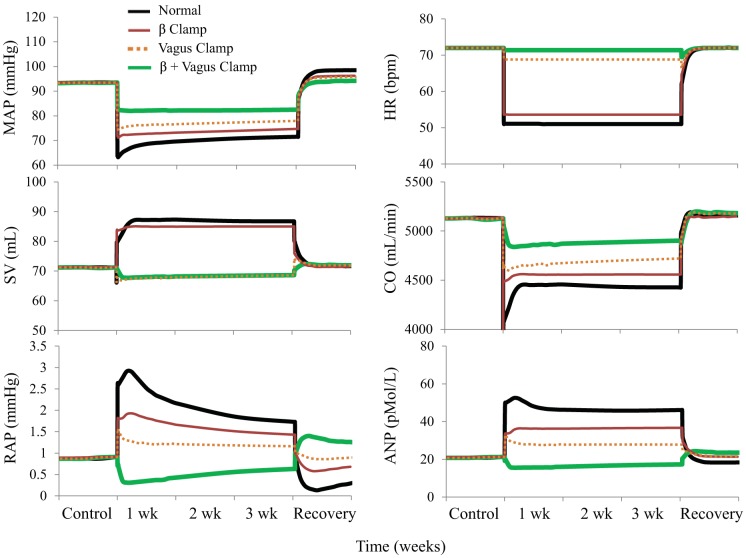
Fig. 3 shows simulated blood pressure and heart rate responses to baroreflex activation along with hemodynamic variables not measured experimentally. In addition to the findings in the normal simulation, Fig. 3 shows comparative hemodynamic responses to the same level of baroreflex activation but 1) without the 40% suppression of renal sympathetic outflow by fixing RSNA at control levels (renal clamp simulation), 2) without alterations in cardiac autonomic activity by fixing cardiac sympathetic and parasympathetic activity at control levels (cardiac clamp simulation), and 3) with a combination of these perturbations (renal + cardiac clamp simulation). Most significantly, while there was a sustained decrease in MAP of 20 mmHg in the normal simulation, clamping autonomic activity simultaneously to the heart and kidneys reduced MAP only 4 mmHg during baroreflex activation. When the clamps were applied separately, the fall in MAP during baroreflex activation was also diminished, but less severely. More specifically, chronic blood pressure lowering during baroreflex activation was 11 mmHg with the cardiac clamp simulation and 17 mmHg with the renal clamp simulation. That is, when compared with the normal simulation, the fall in MAP was reduced 45% by the cardiac clamp simulation but only 15% by the renal clamp simulation. In the simulations without clamping cardiac autonomic nerve activity (both normal and renal clamp simulations), heart rate decreased 15–20 beats/min in response to baroreflex activation, whereas, as expected, heart rate remained unchanged from baseline in both simulations with the cardiac clamp. Activation of the parasympathetic nervous system played a greater role than inhibition of cardiac sympathetic activity in mediating reductions in heart rate and cardiac output during baroreflex activation (Fig. A6 in the appendix). While cardiac output did not fall in the renal + cardiac clamp simulation, it was depressed in all other simulations throughout the 3 wk of baroreflex activation, especially in the normal simulation. During baroreflex activation, stroke volume increased in the normal simulation by 24% (Fig. A6 in the appendix) and by 38% in the renal clamp simulation (not shown), whereas increases in stroke volume were minimal in the renal + cardiac clamp simulation (9%, not shown) or absent in the cardiac clamp simulation (Fig. A6 in the appendix). There was also an immediate and sustained fall in total peripheral resistance in all simulations, being most pronounced during the renal clamp simulation.
Fig. 3.
Simulations showing changes in mean arterial pressure (MAP), heart rate [HR; in beats/min (bpm)], cardiac output (CO), and total peripheral resistance (TPR) during 3 wk of baroreflex activation. Simulations are 1) for normal conditions (normal), 2) without suppression of renal sympathetic outflow (renal clamp), 3) without changes in cardiac autonomic activity (cardiac clamp), and 4) without suppression of renal sympathetic outflow and without changes in cardiac autonomic activity combined (renal + cardiac clamp).
Na+ balance, body fluid volumes, and atrial pressures.
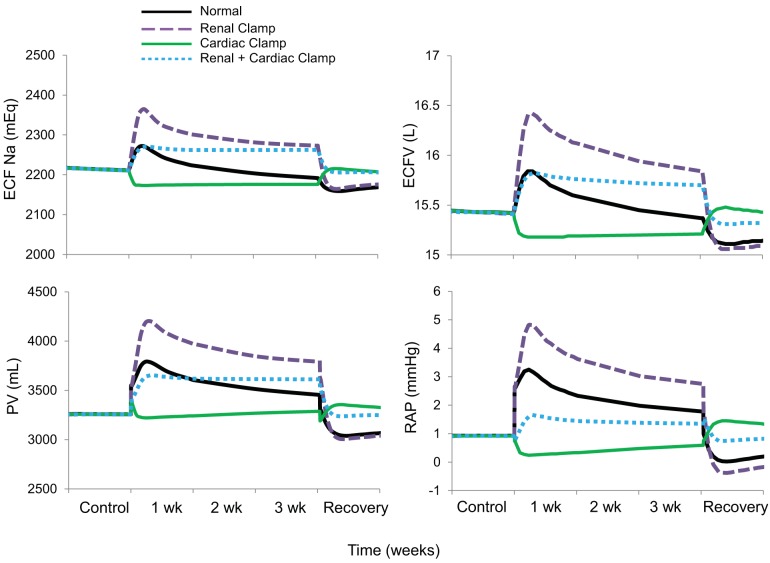
Along with the initial inhibition of sympathetic activity and fall in MAP, there was an increase in extracellular fluid Na+, extracellular fluid volume, plasma volume, and right atrial pressure during the early days of baroreflex activation in the normal simulation (Fig. 4), concomitant with the initial decrease in Na+ excretion (Fig. 1). However, only plasma volume and right atrial pressure remained elevated during the subsequent 2–3 wk of baroreflex activation, due to fluid redistribution from the interstitial space, as interstitial fluid volume decreased in all simulations in proportion to the fall in MAP (not shown). When there was no suppression of RSNA during baroreflex activation (renal clamp simulation), the fall in cardiac output was less pronounced (Fig. 3) as increases in extracellular fluid volume and plasma volume were exacerbated. Increases in body fluid volumes were either diminished (renal + cardiac clamp simulation) or nonexistent (cardiac clamp simulation) in the absence of autonomic changes to the heart during baroreflex activation. Increases in right atrial pressure were correlated with expansions in plasma volume during baroreflex activation. Thus, the greatest increases in right atrial pressure occurred during the renal clamp simulation, exceeding those in the normal simulation, whereas there was no increase in plasma volume or right atrial pressure in the cardiac clamp simulation. In parallel with expansion of body fluid volumes, blood viscosity decreased modestly during baroreflex activation (not shown), but initial reductions waned as body fluid volumes returned toward baseline levels.
Fig. 4.
The four simulations described in Fig. 3 showing changes in extracellular fluid (ECF) Na+, extracellular fluid volume (ECFV), plasma volume (PV), and right atrial pressure (RAP) during 3 wk of baroreflex activation. Simulations are 1) for normal conditions (normal), 2) without suppression of renal sympathetic outflow (renal clamp), 3) without changes in cardiac autonomic activity (cardiac clamp), and 4) without suppression of renal sympathetic outflow and without changes in cardiac autonomic activity combined (renal + cardiac clamp).
Neurohormonal responses.
In the normal simulation, there was a modest increase in renin secretion and plasma levels of ANG II and aldosterone at the onset of baroreflex activation, corresponding to the nadir of MAP and Na+ delivery to the macula densa (Figs. 5–7). However, despite the sustained decrease in MAP and sodium delivery to the macula densa, there was little or no chronic activation of the renin-angiotensin-aldosterone system throughout the remainder of baroreflex activation (Figs. 5 and 6). In this regard, it is relevant that the temporal renin response in the normal simulation was associated with suppression of RSNA and plasma NE concentration as well as increased ANP secretion, all of which would be expected to inhibit renin secretion (Figs. 2, 5, and 6). Similarly, there was little or no activation of the renin-angiotensin-aldosterone system during baroreflex activation in any of the other simulations. In contrast, plasma levels of ANP increased under all conditions except the cardiac clamp simulation, where there were no increases in atrial pressure. Increases in plasma ANP concentration and right atrial pressure occurred in parallel in all simulations (Figs. 4 and 5).
Fig. 5.
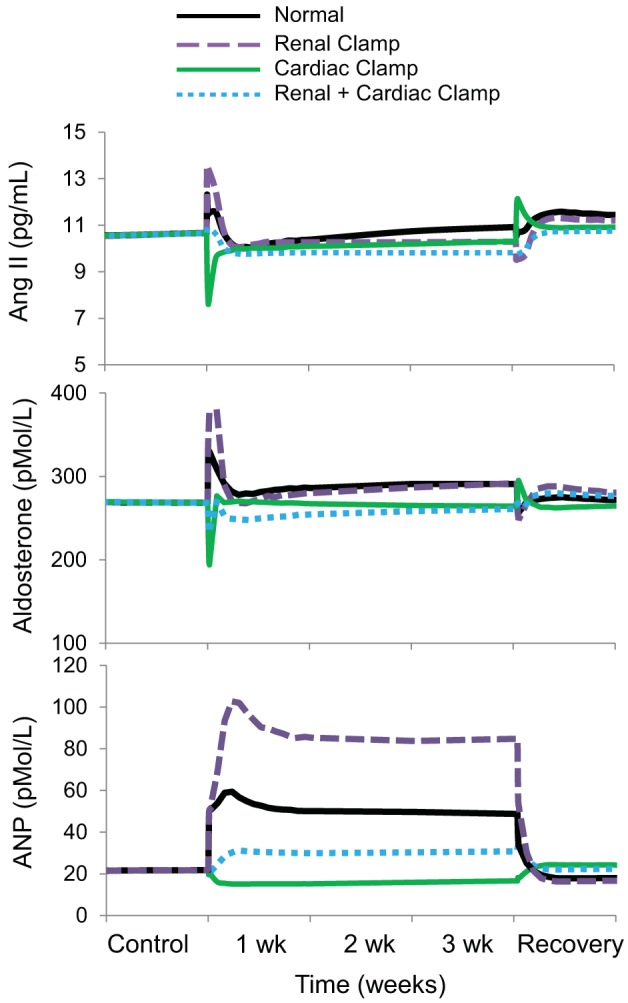
The four simulations described in Fig. 3 showing changes in plasma levels of angiotensin II, aldosterone, and atrial natriuretic peptide (ANP) during 3 wk of baroreflex activation. Simulations are 1) for normal conditions (normal), 2) without suppression of renal sympathetic outflow (renal clamp), 3) without changes in cardiac autonomic activity (cardiac clamp), and 4) without suppression of renal sympathetic outflow and without changes in cardiac autonomic activity combined (renal + cardiac clamp).
Fig. 7.
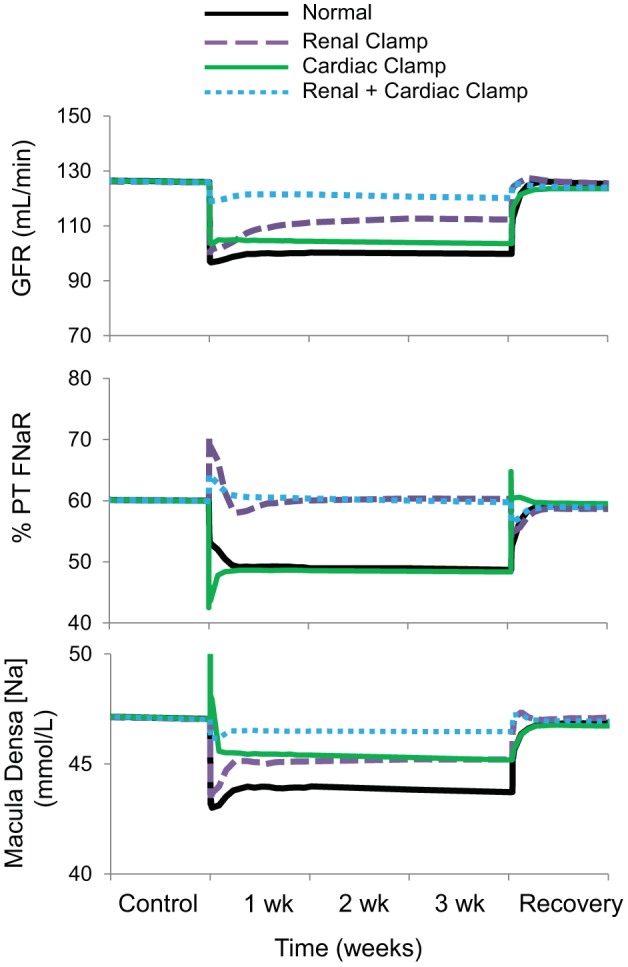
The four simulations described in Fig. 3 showing changes in glomerular filtration rate (GFR), fractional Na+ reabsorption in the proximal tubule (PT FNaR), and concentration of Na+ in the macula densa during 3 wk of baroreflex activation. Simulations are 1) for normal conditions (normal), 2) without suppression of renal sympathetic outflow (renal clamp), 3) without changes in cardiac autonomic activity (cardiac clamp), and 4) without suppression of renal sympathetic outflow and without changes in cardiac autonomic activity combined (renal + cardiac clamp).
Fig. 6.
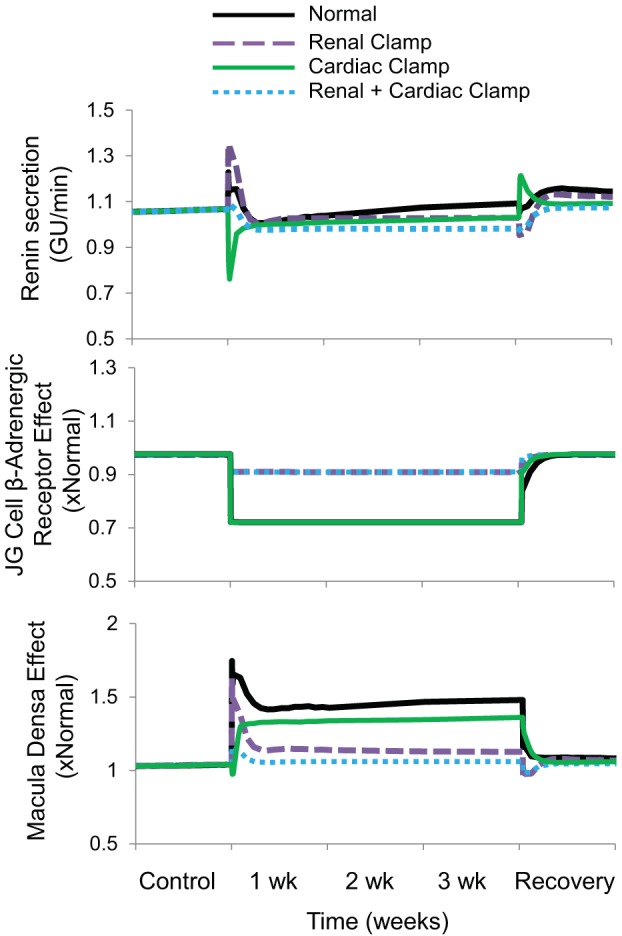
The four simulations described in Fig. 3 showing changes in renin secretion and its primary determinants during 3 wk of baroreflex activation. The direct effect of sympathetic activity on renin secretion is illustrated by β-adrenergic stimulation of juxtaglomerular (JG) cells. The indirect influence of renal sympathetic nerve activity and atrial natriuretic peptide on renin secretion, mediated through changes in NaCl delivery to the macula densa, is included in the overall macula densa effect. Simulations are 1) for normal conditions (normal), 2) without suppression of renal sympathetic outflow (renal clamp), 3) without changes in cardiac autonomic activity (cardiac clamp), and 4) without suppression of renal sympathetic outflow and without changes in cardiac autonomic activity combined (renal + cardiac clamp).
Renal responses.
normal simulation.
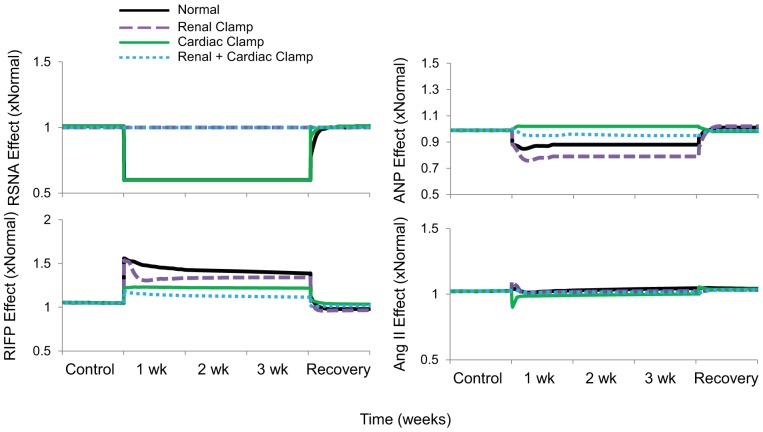
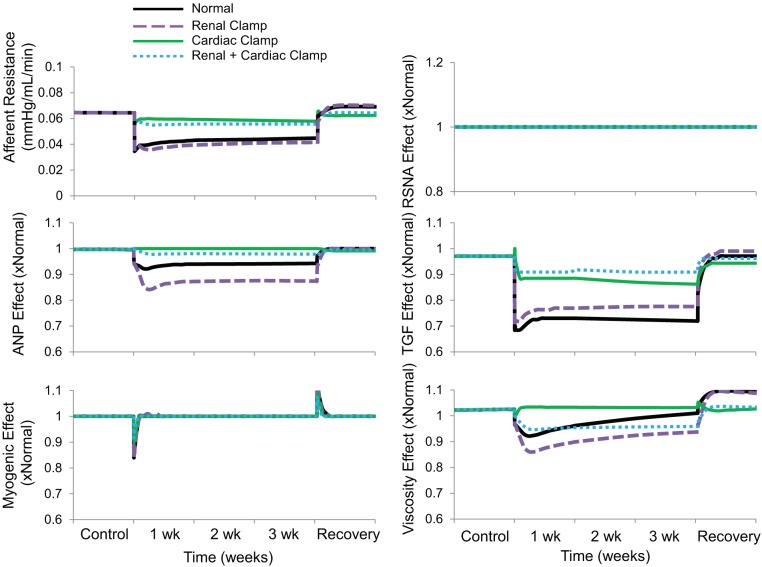
Proximal tubule fractional Na+ reabsorption decreased by ~10% during baroreflex activation, primarily due to suppression of RSNA and increased plasma levels of ANP (Figs. 7 and 8 and Table 1) There was also a 5% decrease in collecting duct fractional Na+ reabsorption concomitant with increased ANP secretion (Table 1). Despite vasodilation of the afferent arteriole during baroreflex activation (Fig. 9), GFR still decreased ~20% in association with the 20-mmHg reduction in MAP (Fig. 7). In this and in all simulations, the decrease in afferent arteriolar resistance was proportional to the combined influence of 1) reduced Na+ delivery to the macula densa and attendant suppression of tubuloglomerular feedback, 2) the direct vasodilatory effects of increased plasma levels of ANP on the afferent arteriole, and 3) decreases in blood viscosity (Fig. 9).
Fig. 8.
The four simulations described in Fig. 3 showing the influence of the major determinants of Na+ reabsorption in the proximal tubule during 3 wk of baroreflex activation. The determinants are renal sympathetic nerve activity (RSNA), renal interstitial fluid pressure (RIFP), and plasma concentrations of angiotensin II and atrial natriuretic peptide (ANP). Simulations are 1) for normal conditions (normal), 2) without suppression of renal sympathetic outflow (renal clamp), 3) without changes in cardiac autonomic activity (cardiac clamp), and 4) without suppression of renal sympathetic outflow and without changes in cardiac autonomic activity combined (renal + cardiac clamp).
Table 1.
Na+ handling in nephron segments (in mmol/min)
| Segment | Inflow | Reabsorption | Outflow | Fractional Na+ Reabsorption |
|---|---|---|---|---|
| Baseline | ||||
| PT | 9.63 | 5.79 | 3.83 | 0.60 |
| Loop | 3.83 | 2.84 | 0.99 | 0.74 |
| DT | 0.99 | 0.72 | 0.27 | 0.73 |
| CD | 0.27 | 0.20 | 0.07 | 0.74 |
| BA | ||||
| PT | 7.58 | 3.70 | 3.88 | 0.49 |
| Loop | 3.88 | 2.95 | 0.93 | 0.76 |
| DT | 0.93 | 0.71 | 0.22 | 0.76 |
| CD | 0.22 | 0.15 | 0.07 | 0.69 |
| BA + renal clamp | ||||
| PT | 8.60 | 5.21 | 3.40 | 0.61 |
| Loop | 3.40 | 2.54 | 0.86 | 0.75 |
| DT | 0.86 | 0.67 | 0.18 | 0.79 |
| CD | 0.18 | 0.12 | 0.07 | 0.63 |
| BA + cardiac clamp | ||||
| PT | 7.91 | 3.84 | 4.07 | 0.49 |
| Loop | 4.07 | 3.07 | 0.99 | 0.75 |
| DT | 1.00 | 0.72 | 0.28 | 0.72 |
| CD | 0.28 | 0.21 | 0.07 | 0.75 |
| BA + renal + cardiac clamp | ||||
| PT | 9.19 | 5.51 | 3.69 | 0.60 |
| Loop | 3.69 | 2.74 | 0.94 | 0.74 |
| DT | 0.94 | 0.69 | 0.25 | 0.74 |
| CD | 0.25 | 0.18 | 0.07 | 0.72 |
BA, baroreflex activation; PT, proximal tubule; Loop, loop of Henle; DT, distal tubule; CD, collecting duct.
Fig. 9.
The four simulations described in Fig. 3 showing changes in afferent arteriolar resistance and its determinants during 3 wk of baroreflex activation. The determinants are renal sympathetic nerve activity (RSNA), plasma atrial natriuretic peptide (ANP) concentration, tubuloglomerular feedback (TGF), the myogenic mechanism, and blood viscosity.
renal clamp simulation.
In comparison, despite even higher plasma levels of ANP than in the normal simulation, there was no decrease in proximal tubule fractional Na+ reabsorption during baroreflex activation in the absence of suppressed RSNA (Figs. 7 and 8 and Table 1). Nonetheless, the fall in MAP during baroreflex activation in the renal clamp simulation was still substantial (17 mmHg). With these higher levels of ANP, there was a greater decrease in collecting duct fractional Na+ reabsorption (14% vs. 7%; Table 1), greater vasodilation of the afferent arteriole (42% vs. 30%; Fig. 9), and only a small decrease in GFR (11% vs. 21%; Fig. 7) compared with the normal simulation.
cardiac clamp simulation.
As in the normal simulation, proximal tubule fractional Na+ reabsorption decreased substantially along with suppression of RSNA during baroreflex activation (Figs. 7 and 8 and Table 1). However, without increased ANP secretion during baroreflex activation, fractional Na+ reabsorption in the collecting duct did not fall (Table 1), vasodilation of the afferent arteriolar did not occur to any appreciable degree (Fig. 9), and blood pressure lowering was reduced by ~50% compared with the normal simulation (Fig. 3). Under these conditions, there was an appreciable decrease in GFR comparable to that in the normal simulation (Fig. 7).
renal + cardiac clamp simulation.
Without suppression of RSNA and with only small increases in plasma levels of ANP in this simulation, reductions in MAP (Fig. 3), proximal tubule fractional Na+ reabsorption (Figs. 7 and 8 and Table 1), collecting duct fractional Na+ reabsorption (Table 1), afferent arteriolar resistance (Fig. 9), and GFR (Fig. 7) were substantially attenuated during baroreflex activation compared with these responses in the normal simulation.
DISCUSSION
HumMod is an established, comprehensive, integrative mathematical model of human physiology that has evolved considerably from its precursor published by Guyton and associates more than over 45 yr ago (4, 8–11). With added content, HumMod accurately reproduces the acute and chronic responses to many physiological perturbations and the findings in many pathophysiological states (4, 11, 16). Therefore, this model has credibility in elucidating physiological mechanisms that are not obvious intuitively and not readily testable in experimental studies. Once again, it should be emphasized that the mathematical relationships in the model have not been calibrated to match experimental data shown in Figs. 1 and 2. The updated version of HumMod used in this study differed in only minor respects from the model used to generate the simulations presented in a recent publication focusing on blood pressure salt sensitivity (4) and had little or no impact on simulation outcomes.
An essential initial finding in the present study was that HumMod closely reproduced empirical data during 3 wk of baroreflex activation, thus suggesting validation of the following model predictions: 1) suppression of RSNA and increases in plasma levels of ANP are critical determinants of blood pressure lowering during chronic baroreflex activation, 2) autonomic-induced depression of cardiac function increases atrial pressure during baroreflex activation and contributes importantly to blood pressure lowering by increasing ANP secretion, and 3) when suppression of RSNA is not possible, there is little long-term impairment in blood pressure lowering during baroreflex activation because exaggerated fluid retention and attendant increases in atrial pressure stimulate ANP secretion even further.
The importance of the baroreflex in the acute regulation of blood pressure is well established and the hemodynamic responses engaged during its activation are clearly evident in the present simulations. These include reductions in heart rate, cardiac output, and total peripheral resistance, responses mediated by suppression of sympathetic activity and activation of parasympathetic outflow to the heart. These changes in neural activity are sustained during chronic baroreflex activation (17, 22). However, because long-term regulation of blood pressure is critically linked to the protracted regulation of body fluid volumes by the kidneys, the mechanisms that mediate blood pressure lowering during chronic baroreflex activation cannot be ascertained from acute observations, and they remain incompletely defined. In this regard, HumMod provides unique insights into these incompletely defined mechanisms by predicting fundamental chronic responses to baroreflex activation that have not been measured experimentally. Knowledge of these responses is critical in understanding the mechanisms mediating blood pressure lowering during chronic baroreflex activation.
Because the natural activation of the baroreflex in hypertension chronically suppresses RSNA and increases renal excretory function (21), a most unexpected experimental finding in dogs with renal denervation, now confirmed in the present simulations (renal clamp simulation), is that inhibition of sympathetic outflow to the kidneys is not an obligate requirement for chronically achieving Na+ balance at substantially lower blood pressure during baroreflex activation (20). Consistent with these findings, recent clinical studies have reported impressive reductions in blood pressure during baroreflex activation in patients with hypertension with prior renal nerve ablation that was unsuccessful in controlling their hypertension (13, 35). However, it should be acknowledged that interpretation of these clinical observations is confounded by the uncertainty to which renal denervation was achieved. Regardless, the mechanisms apart from renal sympathoinhibition that account for chronic blood pressure lowering during baroreflex activation have remained an enigma. In this regard, little consideration has been given to the impact of changes in sympathetic and parasympathetic activity to the heart and whether in the absence of suppression of RSNA compensatory mechanisms might gain a more prominent role in increasing renal excretory function during baroreflex activation. Based on our findings showing a two- to threefold increase in plasma levels of ANP during baroreflex activation in normotensive canines (20), one possibility is that ANP may be a critical link between the heart and kidneys that contributes importantly to blood pressure lowering by increasing renal excretory function. The simulations discussed below support this hypothesis and provide novel mechanistic insights into this cardiorenal interaction.
Responses to Baroreflex Activation With and Without Suppression of RSNA
The normal + renal clamp simulations, which include previously unmeasured changes in body fluid volumes, cardiac output, and right atrial pressure, are especially insightful in accounting for several unexplained observations and for discerning the mechanisms that account for chronic lowering of blood pressure during baroreflex activation in the absence of suppression of RSNA. First, the normal simulation shows that in the absence of pathophysiological conditions baroreflex activation has a sustained effect to depress cardiac output primarily by decreasing heart rate through parasympathetic activation and suppression of cardiac sympathetic outflow, with the former having the more prominent effect on cardiac function. Because the model predicts that baroreflex activation depresses cardiac output through these autonomic effects on the heart, this indicates that attendant increases in right atrial pressure account for the increase in plasma ANP concentration reported experimentally (20). Accordingly, the bradycardia associated with β-adrenergic blockade has been reported to increase plasma levels of ANP in humans with hypertension, a response that may contribute to the antihypertensive effects of this therapy (2, 28). Second, the initial Na+ retention and concomitant expansion of body fluid volumes that occur with suppression of sympathetic activity during baroreflex activation can be attributed to the acute fall in blood pressure, due to reductions in total peripheral resistance and cardiac output, with an increase in venous capacity contributing to the latter by decreasing venous return. However, both experimental observations and the normal simulation show that there is no net retention of Na+ and no sustained increase in extracellular fluid volume during chronic baroreflex activation, presumably due to the protracted natriuretic forces, especially reduced RSNA and increased ANP. Nonetheless, although there is no chronic increase in extracellular fluid volume, the normal simulation does show an initial and sustained increase in plasma volume, indicating a redistribution of fluid from the interstitial space, consistent with the fall in interstitial fluid volume during baroreflex activation (not shown). By increasing right atrial pressure, this increase in plasma volume not only stimulates ANP secretion but also contributes to the early time-dependent recovery of cardiac output through the Frank-Starling mechanism. Third, the renal clamp simulation provides novel insight into the potential mechanisms that may account for chronic blood pressure lowering during baroreflex activation in the absence of the renal nerves. In contrast to the normal simulation, blocking suppression of RSNA during the renal clamp simulation led to distinctly positive sodium balance during the entire 3 wk of baroreflex activation, resulting in more substantial increases in extracellular fluid volume, plasma volume, right atrial pressure, and ANP secretion. With the normal increase in heart rate in response to volume expansion abolished by baroreflex activation, the exaggerated volume retention and stroke volume in the renal clamp simulation indicates an important role for the Frank-Starling mechanism in augmenting cardiac output and restoring cardiac output to higher levels than achieved in the normal simulation. More to the point of blood pressure control, the above two simulations suggest that without the usual suppression of RSNA and with the impaired capacity of the heart to respond to volume expansion during baroreflex activation, excess fluid retention leads to exaggerated ANP secretion and attendant increases in renal excretory function, thus enhancing the role of this hormone in blood pressure lowering. Indeed, when increases in plasma levels of ANP were prevented from rising above baseline during the renal clamp simulation (not shown), protracted Na+ retention and massive increases in total body sodium, body fluid volumes and right atrial pressure ensued during the 3 wk of baroreflex activation. That is, with both RSNA and ANP clamped at control levels during baroreflex activation, Na+ balance could not be achieved at reduced blood pressure. Taken together, these simulations show that in the absence of renal sympathoinhibition, there is a compensatory increase in renal excretory function that can be attributed to an exaggerated increase in ANP secretion. It is likely that this accounts for the experimental and clinical observations showing that renal denervation does not appreciably impair blood pressure lowering during baroreflex activation.
Renin-angiotensin system.
Studies in chronically instrumented conscious dogs show that reductions in renal perfusion pressure of ≥15 mmHg lead to substantial increases in renin secretion (7, 32). However, in both normotensive and hypertensive dogs, increases in plasma renin activity do not occur when blood pressure decreases as much as 20–25 mmHg during chronic baroreflex activation (15, 19, 20, 22, 23) or when controlled reductions in renal perfusion pressure of this magnitude are achieved acutely by constriction of the aorta while simultaneously infusing physiological levels of ANP (32). Similarly, in the present simulations, there was little or no increase in renin secretion during baroreflex activation in the normal simulation despite appreciably reduced blood pressure. Therefore, in the presence of low blood pressure and attendant reductions in GFR during baroreflex activation, inhibitory signals must counteract the stimulation of renin in response to reduced NaCl delivery to the macula densa. In the model, both suppressed sympathetic activity and increased ANP inhibit renin secretion during baroreflex activation.
Neurally mediated stimulation of renin secretion is achieved by stimulating β-adrenergic receptors on juxtaglomerular cells and indirectly by decreasing NaCl delivery to the macula densa by promoting Na+ reabsorption in the proximal tubule through activation of α-adrenergic receptors. The inhibitory effects of baroreflex activation on renin secretion are achieved, in part, by attenuating these actions of the sympathetic nervous system. Furthermore, the simulations show that both suppression of RSNA and reductions in circulating NE contribute to the inhibition of renin secretion during baroreflex activation (Fig. 6). However, the renal clamp simulation indicates that even without the normal suppression of RSNA, there is still no increase in renin secretion during chronic baroreflex activation, a prediction consistent with experimental findings in dogs with denervated kidneys (20). The simulations do show that in the absence of suppression of RSNA, reductions in plasma NE concentration contribute to the inhibition of renin secretion during baroreflex activation by suppressing activation of juxtaglomerular β-adrenergic receptors (Fig. 6). But, the more prominent inhibitory effect on renin secretion during baroreflex activation in the renal clamp simulation can be attributed primarily to the exaggerated increases in ANP secretion that promote increased NaCl delivery to the macula densa through the mechanisms discussed below. The importance of these circulating neurohormonal responses in counteracting pressure-dependent renin release cannot be overemphasized as even small increases in plasma ANG II levels greatly diminish the magnitude of blood pressure lowering during baroreflex activation (19).
Renal responses.
There is a paucity of information from clinical studies relating to the effects of baroreflex activation on renal function. The scarce data comprises a few measurements of serum/plasma creatinine concentration in a small numbers of patients with resistant hypertension maintained on multiple drug combinations that were varied somewhat during the baroreflex activation trials. However, one study stands out as it was conducted in a relatively large group of patients (n = 236) with resistant hypertension. In this clinical trial, Alnima et al. (1) reported a 10% decrease in estimated GFR in their cohort when measured after 12 mo of baroreflex activation. Reductions in systolic blood pressure, diastolic blood pressure, and heart rate were 27 mmHg, 13 mmHg, and 8 beats/min, respectively. Alnima et al. (1) attributed the fall in GFR to the reduced renal perfusion pressure associated with the antihypertensive effects of baroreflex activation. In normotensive canines, we (15) previously reported a 10% decrease in GFR after 7 days of baroreflex activation that lowered MAP by 17 mmHg. This compares to the somewhat larger fall in GFR (20%) and MAP (20 mmHg) during baroreflex activation in the present simulations.
Because Na+ balance was achieved in the normal simulation in the presence of chronic reductions in blood pressure and GFR, this indicates that baroreflex activation had persistent effects to increase renal excretory function by inhibiting tubular sodium reabsorption. This is consistent with the model predictions indicating sustained reductions in fractional Na+ reabsorption in both the proximal tubule and collecting duct during baroreflex activation (Fig. 7 and Table 1). Increased plasma levels of ANP contribute modestly to the sustained inhibition of Na+ reabsorption in the proximal tubule, whereas the predominant inhibitory effect of this hormone on Na+ transport occurs in the collecting duct. Most of the inhibition of Na+ reabsorption in the proximal tubule can be attributed to suppression of RSNA (Fig. 8). Furthermore, as blood pressure falls in response to these natriuretic forces, this leads to a decrease in glomerular pressure, which accounts for the decrease in GFR. It is relevant to point out that the model shows that the pressure-dependent fall in GFR occurs despite a decrease in afferent arteriolar resistance (Fig. 9), which is mediated primarily by the vasoactive effects of ANP and the diminished tubuloglomerular feedback response associated with reduced NaCl delivery to the macula densa.
Compared with the Normal simulation, the most expected response to blocking reductions in RSNA during baroreflex activation (renal clamp simulation) was the marked increase in fractional proximal tubule Na+ reabsorption (Figs. 7 and 8 and Table 1), illustrating the dominant effect of renal sympathoinhibition, when present, in inhibiting Na+ reabsorption in this nephron segment. Nonetheless, as discussed above, preventing suppression of RSNA did not appreciably attenuate blood pressure lowering during baroreflex activation because attendant fluid retention stimulated ANP secretion further. Greater increases in ANP in the renal clamp simulation enhanced its actions to suppress Na+ reabsorption in the proximal tubule and collecting duct (Fig. 8 and Table 1). Moreover, by dilating the afferent arteriole directly, ANP increased GFR compared with the normal simulation (Fig. 7). Similarly, albeit in a small group of 28 patients with hypertension with prior renal denervation, Wallbach et al. (35) reported that when measured after 6 mo of therapy, GFR did not fall concomitantly with blood pressure during baroreflex activation. Thus, we propose that suppression of RSNA does not appear to be an obligate requirement for appreciable blood pressure lowering during baroreflex activation because of compensatory increases in ANP that increase renal excretory function through both renal vascular effects that increase GFR and tubular actions that depress Na+ reabsorption.
Responses to Baroreflex Activation Without Changes in Cardiac Autonomic Activity
Along with the above simulations, the predictions from the cardiac clamp simulation provide novel insights into the relative importance of changes in cardiac autonomic activity and suppression of renal sympathetic outflow to blood pressure lowering during baroreflex activation. In contrast to the excessive Na+ retention in the above renal clamp simulation, there was no net Na+ retention and only a trivial increase in plasma volume during baroreflex activation when suppression of RSNA was permitted while abolishing changes in cardiac autonomic activity in the cardiac clamp simulation. Accordingly, rather than increases in right atrial pressure and ANP secretion, both actually decreased. Taken together, these responses suggest that in the absence of autonomic depression of cardiac function and with sustained suppression of RSNA, the modest reduction in cardiac output can be attributed to reduced venous return. More specifically, inhibition of Na+ reabsorption during renal sympathoinhibition presumably prevented sufficient fluid retention to sustain a normal level of cardiac filling in the presence of the increased vascular capacitance concomitant with global suppression of sympathetic activity. Regardless, an especially novel prediction from the simulations was that the degree of blood pressure lowering during baroreflex activation is influenced more by the induced changes in cardiac autonomic activity than by suppression of sympathetic outflow to the kidneys.
Responses to Baroreflex Activation Without Suppression of RSNA and Changes in Cardiac Autonomic Activity
As presented in the results, most of the hemodynamic, renal, body fluid, and hormonal responses to global suppression of sympathetic activity by baroreflex activation were greatly attenuated in the renal + cardiac clamp simulation with the exception that total peripheral resistance remained depressed. Of greatest relevance to the present study, the renal + cardiac clamp simulation shows that changes in neural activity to the heart and kidneys play the dominant role in chronically lowering blood pressure during baroreflex activation. More specifically, a conspicuous difference in outcomes between the normal and renal + cardiac clamp simulations was the degree of blood pressure lowering during baroreflex activation, being 20 mmHg in the former and only 5 mmHg in the latter. Taken together, the simulations in this study indicate that increases in renal excretory function, mediated by suppression of RSNA and increased ANP secretion, account for essentially all of the chronic blood pressure lowering during baroreflex activation, even in the presence of a sustained decrease in total peripheral resistance, which was a common finding associated with global suppression of sympathetic activity in all simulations. These findings further support the concept that alterations in renal excretory function and not (nonrenal) peripheral resistance are the primary determinants of long-term blood pressure control (4, 8, 9).
Limitations
HumMod closely reproduced the empirical findings reported in dogs in response to chronic baroreflex activation. However, it should be emphasized that model predictions for previously unmeasured variables and outcomes must be considered hypotheses until formally tested in future experimental studies. Additionally, potential determinants of renal excretory function that currently are not incorporated in the model (such as nitric oxide, endothelin, or cytokines) may need to be included in future models to better understand the cardiovascular responses to baroreflex activation in pathophysiological states including heterogeneous forms of hypertension and heart failure. This will require additional cycles of experimentation and model refinement. Accordingly, with regard to the present study, the integration of multiple empirical findings in HumMod suggests that increased ANP secretion plays a significant role in contributing to blood pressure lowering during baroreflex activation. Thus, based on the conceptual framework provided by the current simulations, measurement of plasma ANP levels in patients with baroreflex activation therapy would be a critical test of this hypothesis.
Perspectives
A critical initial determination in this study was the simulation showing that HumMod accurately predicted the empirical data previously reported during baroreflex activation in normotensive dogs. This demonstration suggested credibility for the subsequent simulations providing novel insight into responses and mechanisms that have not been measured or determined previously during baroreflex activation. Nevertheless, further validation of the model will require experimental and clinical studies that include key measurements not commonly made including cardiac output, renal function, and plasma ANP concentration. In addition, experimental and clinical studies will be required to validate the relevance of the present model predictions under conditions associated with high basal levels of sympathetic activity, including heart failure and some forms of hypertension. In this regard, the results of the present simulations are also consistent with the experimental findings in a clinically relevant, sympathetically driven model of hypertension. More specifically, chronic baroreflex activation abolished the sympathetic activation, hypertension, tachycardia, cardiac autonomic imbalance, glomerular hyperfiltration, and increased plasma renin activity associated with obesity in dogs (17, 23). Additionally, after renal denervation, which also abolished the hypertension in these animals, baroreflex activation lowered blood pressure even further (unpublished observations), showing once again that the blood pressure response is not critically dependent on suppression of RSNA. Furthermore, even with the encouraging antihypertensive responses to baroreflex activation in hypertensive patients (5, 13), a potentially more important and unique application for baroreflex activation therapy may in patients with heart failure. In heart failure, increased sympathetic and decreased parasympathetic activity play a major role in disease progression, and a particularly favorable effect of baroreflex activation is that it has unique central actions to substantially correct this imbalance (6, 17, 22). However, despite having similar long-term effects on central autonomic outflow, not all responses to baroreflex activation in patients with heart failure are equivalent to those reported in normotensive and hypertensive subjects. More specifically, in patients with heart failure, blood pressure and GFR do not decrease and plasma levels of brain natriuretic peptide actually fall in association with improvement in cardiac function during chronic baroreflex activation (6, 37). Future simulations using HumMod may prove to be of value in accounting for these differential effects in hypertension and heart failure and may provide critical insight into the design of pivotal studies evaluating the efficacy of baroreflex activation in these conditions.
GRANTS
This work was supported by American Heart Association Grant 17POST33661071, National Institutes of Health Grants PO1-HL-51971 and P20-GM-104357, and National Science Foundation Grant EPS 0903787.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S.C. and T.E.L. conceived and designed research; J.S.C. and T.E.L. performed experiments; J.S.C. and T.E.L. analyzed data; J.S.C., W.A.P., R.L.H., R.I., and T.E.L. interpreted results of experiments; J.S.C. and W.A.P. prepared figures; J.S.C. and T.E.L. drafted manuscript; J.S.C., W.A.P., R.L.H., R.I., and T.E.L. edited and revised manuscript; J.S.C., W.A.P., R.L.H., R.I., and T.E.L. approved final version of manuscript.
APPENDIX
Described below are model relationships including details on determinants of Na+ reabsorption, renal vascular resistance, and GFR as well as relationships between systems in HumMod.
Figure A1 shows determinants of Na+ reabsorption in nephron segments.
Fig. A1.
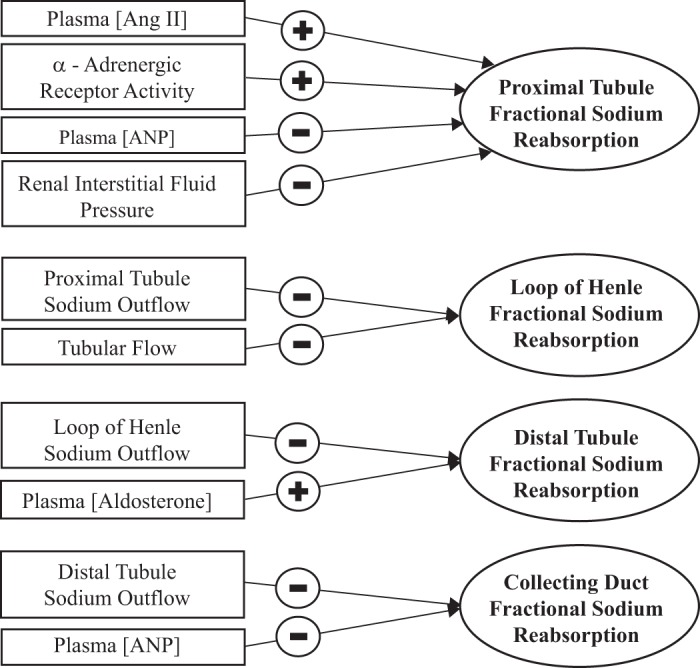
Determinants of Na+ reabsorption in nephron segments. ANP, atrial natriuretic peptide.
Figure A2 shows determinants of afferent and efferent arteriolar conductance.
Fig. A2.
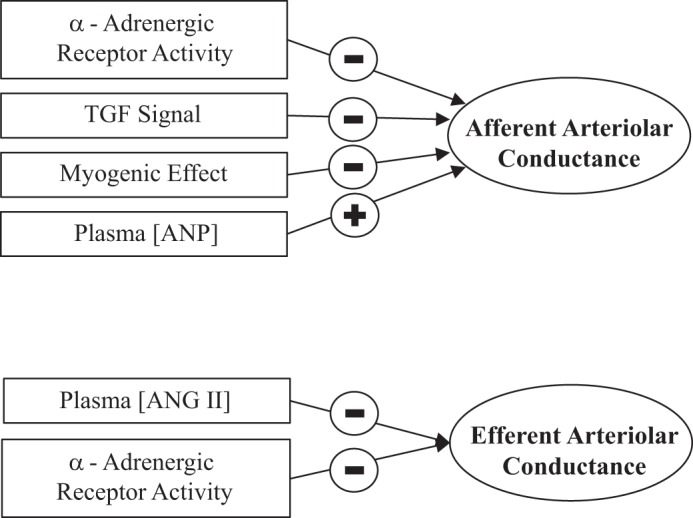
Determinants of afferent and efferent arteriolar conductance. TGF, tubuloglomerular feedback; ANP, atrial natriuretic peptide.
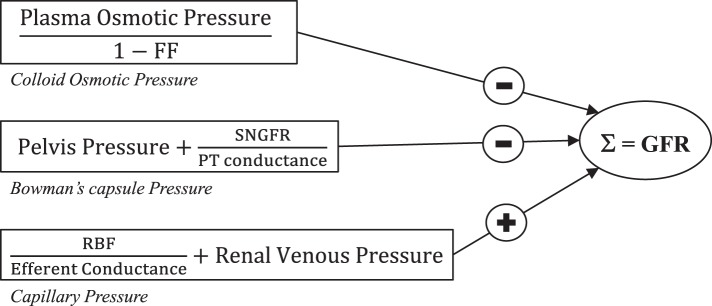
Figure A3 shows determinants of GFR.
Fig. A3.
Determinants of glomerular filtration rate (GFR). FF, filtration fraction; SNGFR, single nephron GFR; PT, proximal tubule; RBF, renal blood flow.
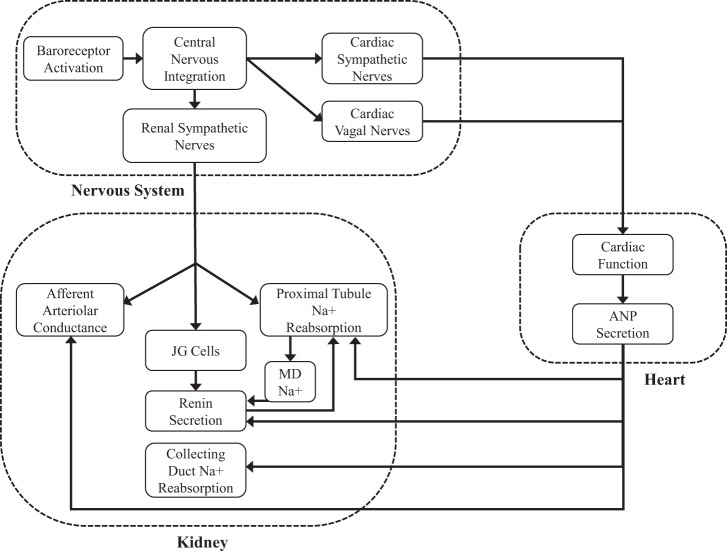
Figure A4 shows a diagram of the relationships between the nervous, cardiac, and renal systems in HumMod.
Fig. A4.
Diagram of the relationships between the nervous, cardiac, and renal systems in HumMod. JG, juxtaglomerular cells; MD, macula densa; ANP, atrial natriuretic peptide.
Figure A5 shows physiological effects of ANP during baroreflex activation.
Fig. A5.
Physiological effects of atrial natriuretic peptide (ANP) during baroreflex activation. PT, proximal tubule; CD, collecting duct; MD, macula densa; TGF, tubuloglomerular feedback.
Figure A6 shows simulations showing changes in MAP, heart rate, stroke volume, cardiac output, right atrial pressure, and the concentration of plasma ANP during 3 wk of baroreflex activation. The simulations shown are 1) for the normal condition, 2) without changes in cardiac β-adrenergic receptor activity (β-clamp), 3) without changes in cardiac vagal activity (vagus clamp), and 4) without changes in cardiac β-adrenergic receptor activity and without changes in cardiac vagal activity combined (β + vagus glamp).
Fig. A6.
Simulations showing changes in mean arterial pressure (MAP), heart rate [HR; in beats/min (bpm)], stroke volume (SV), cardiac output (CO), right atrial pressure (RAP), and concentration of plasma atrial natriuretic peptide (ANP) during 3 wk of baroreflex activation. Simulations are 1) for normal conditions, 2) without changes in cardiac β-adrenergic receptor activity (β clamp), 3) without changes in cardiac vagal activity (vagus clamp), and 4) without changes in cardiac β-adrenergic receptor activity and without changes in cardiac vagal activity combined (β + vagus clamp).
REFERENCES
- 1.Alnima T, de Leeuw PW, Tan FE, Kroon AA; Rheos Pivotal Trial Investigators . Renal responses to long-term carotid baroreflex activation therapy in patients with drug-resistant hypertension. Hypertension 61: 1334–1339, 2013. doi: 10.1161/HYPERTENSIONAHA.113.01159. [DOI] [PubMed] [Google Scholar]
- 2.Baláti B, Phung H, Pousset F, Isnard R, Boisvieux A, Carayon A, Komajda M, Lechat P. Relationships between the antihypertensive effects of bisoprolol and levels of plasma atrial natriuretic peptide in hypertensive patients. Fundam Clin Pharmacol 16: 361–368, 2002. doi: 10.1046/j.1472-8206.2002.00072.x. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev 70: 665–699, 1990. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- 4.Clemmer JS, Pruett WA, Coleman TG, Hall JE, Hester RL. Mechanisms of blood pressure salt sensitivity: new insights from mathematical modeling. Am J Physiol Regul Integr Comp Physiol 312: R451–R466, 2017. doi: 10.1152/ajpregu.00353.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Leeuw PW, Bisognano JD, Bakris GL, Nadim MK, Haller H, Kroon AA; DEBuT-HT and Rheos Trial Investigators . Sustained reduction of blood pressure with baroreceptor activation therapy: results of the 6-year open follow-up. Hypertension 69: 836–843, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09086. [DOI] [PubMed] [Google Scholar]
- 6.Dell’Oro R, Gronda E, Seravalle G, Costantino G, Alberti L, Baronio B, Staine T, Vanoli E, Mancia G, Grassi G. Restoration of normal sympathetic neural function in heart failure following baroreflex activation therapy: final 43-month study report. J Hypertens 35: 2532–2536, 2017. doi: 10.1097/HJH.0000000000001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finke R, Gross R, Hackenthal E, Huber J, Kirchheim HR. Threshold pressure for the pressure-dependent renin release in the autoregulating kidney of conscious dogs. Pflugers Arch 399: 102–110, 1983. doi: 10.1007/BF00663904. [DOI] [PubMed] [Google Scholar]
- 8.Guyton AC. Arterial Pressure and Hypertension. Philadelphia, PA: Saunders, 1980. [Google Scholar]
- 9.Guyton AC. Long-term arterial pressure control: an analysis from animal experiments and computer and graphic models. Am J Physiol Regul Integr Comp Physiol 259: R865–R877, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Annu Rev Physiol 34: 13–44, 1972. doi: 10.1146/annurev.ph.34.030172.000305. [DOI] [PubMed] [Google Scholar]
- 11.Hester RL, Brown AJ, Husband L, Iliescu R, Pruett D, Summers R, Coleman TG. HumMod: a modeling environment for the simulation of integrative human physiology. Front Physiol 2: 12, 2011. doi: 10.3389/fphys.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildebrandt DA, Mizelle HL, Brands MW, Gaillard CA, Smith MJ Jr, Hall JE. Intrarenal atrial natriuretic peptide infusion lowers arterial pressure chronically. Am J Physiol Regul Integr Comp Physiol 259: R585–R592, 1990. doi: 10.1152/ajpregu.1990.259.3.R585. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe UC, Brandt MC, Wachter R, Beige J, Rump LC, Kroon AA, Cates AW, Lovett EG, Haller H. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens 6: 270–276, 2012. doi: 10.1016/j.jash.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Huang CL, Cogan MG. Atrial natriuretic factor inhibits maximal tubuloglomerular feedback response. Am J Physiol Renal Fluid Electrolyte Physiol 252: F825–F828, 1987. doi: 10.1152/ajprenal.1987.252.5.F825. [DOI] [PubMed] [Google Scholar]
- 15.Iliescu R, Irwin ED, Georgakopoulos D, Lohmeier TE. Renal responses to chronic suppression of central sympathetic outflow. Hypertension 60: 749–756, 2012. doi: 10.1161/HYPERTENSIONAHA.112.193607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iliescu R, Lohmeier TE. Lowering of blood pressure during chronic suppression of central sympathetic outflow: insight from computer simulations. Clin Exp Pharmacol Physiol 37: e24–e33, 2010. doi: 10.1111/j.1440-1681.2009.05291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iliescu R, Tudorancea I, Irwin ED, Lohmeier TE. Chronic baroreflex activation restores spontaneous baroreflex control and variability of heart rate in obesity-induced hypertension. Am J Physiol Heart Circ Physiol 305: H1080–H1088, 2013. doi: 10.1152/ajpheart.00464.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtz A, Della Bruna R, Pfeilschifter J, Taugner R, Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc Natl Acad Sci USA 83: 4769–4773, 1986. doi: 10.1073/pnas.83.13.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lohmeier TE, Dwyer TM, Hildebrandt DA, Irwin ED, Rossing MA, Serdar DJ, Kieval RS. Influence of prolonged baroreflex activation on arterial pressure in angiotensin hypertension. Hypertension 46: 1194–1200, 2005. doi: 10.1161/01.HYP.0000187011.44201.2e. [DOI] [PubMed] [Google Scholar]
- 20.Lohmeier TE, Hildebrandt DA, Dwyer TM, Barrett AM, Irwin ED, Rossing MA, Kieval RS. Renal denervation does not abolish sustained baroreflex-mediated reductions in arterial pressure. Hypertension 49: 373–379, 2007. doi: 10.1161/01.HYP.0000253507.56499.bb. [DOI] [PubMed] [Google Scholar]
- 21.Lohmeier TE, Iliescu R. The baroreflex as a long-term controller of arterial pressure. Physiology (Bethesda) 30: 148–158, 2015. doi: 10.1152/physiol.00035.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol 299: H402–H409, 2010. doi: 10.1152/ajpheart.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 59: 331–338, 2012. doi: 10.1161/HYPERTENSIONAHA.111.185074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohmeier TE, Mizelle HL, Reinhart GA. Role of atrial natriuretic peptide in long-term volume homeostasis. Clin Exp Pharmacol Physiol 22: 55–62, 1995. doi: 10.1111/j.1440-1681.1995.tb01919.x. [DOI] [PubMed] [Google Scholar]
- 25.Metzler CH, Lee ME, Thrasher TN, Ramsay DJ. Increased right or left atrial pressure stimulates release of atrial natriuretic peptides in conscious dogs. Endocrinology 119: 2396–2398, 1986. doi: 10.1210/endo-119-5-2396. [DOI] [PubMed] [Google Scholar]
- 26.Miki K, Hayashida Y, Sagawa S, Shiraki K. Renal sympathetic nerve activity and natriuresis during water immersion in conscious dogs. Am J Physiol Regul Integr Comp Physiol 256: R299–R305, 1989. doi: 10.1152/ajpregu.1989.256.2.R299. [DOI] [PubMed] [Google Scholar]
- 27.Miki K, Hayashida Y, Tajima F, Iwamoto J, Shiraki K. Renal sympathetic nerve activity and renal responses during head-up tilt in conscious dogs. Am J Physiol Regul Integr Comp Physiol 257: R337–R343, 1989. doi: 10.1152/ajpregu.1989.257.2.R337. [DOI] [PubMed] [Google Scholar]
- 28.Nakaoka H, Kitahara Y, Amano M, Imataka K, Fujii J, Ishibashi M, Yamaji T. Effect of beta-adrenergic receptor blockade on atrial natriuretic peptide in essential hypertension. Hypertension 10: 221–225, 1987. doi: 10.1161/01.HYP.10.2.221. [DOI] [PubMed] [Google Scholar]
- 29.Opgenorth TJ, Burnett JC Jr, Granger JP, Scriven TA. Effects of atrial natriuretic peptide on renin secretion in nonfiltering kidney. Am J Physiol Renal Fluid Electrolyte Physiol 250: F798–F801, 1986. doi: 10.1152/ajprenal.1986.250.5.F798. [DOI] [PubMed] [Google Scholar]
- 30.Pollock DM, Arendshorst WJ. Native tubular fluid attenuates ANF-induced inhibition of tubuloglomerular feedback. Am J Physiol Renal Fluid Electrolyte Physiol 258: F189–F198, 1990. doi: 10.1152/ajprenal.1990.258.1.F189. [DOI] [PubMed] [Google Scholar]
- 31.Salazar FJ, Fiksen-Olsen MJ, Opgenorth TJ, Granger JP, Burnett JC Jr, Romero JC. Renal effects of ANP without changes in glomerular filtration rate and blood pressure. Am J Physiol Renal Fluid Electrolyte Physiol 251: F532–F536, 1986. doi: 10.1152/ajprenal.1986.251.3.F532. [DOI] [PubMed] [Google Scholar]
- 32.Scheuer DA, Thrasher TN, Quillen EW Jr, Metzler CH, Ramsay DJ. Atrial natriuretic peptide blocks renin response to renal hypotension. Am J Physiol Regul Integr Comp Physiol 252: R423–R427, 1987. doi: 10.1152/ajpregu.1987.252.2.R423. [DOI] [PubMed] [Google Scholar]
- 33.Shin Y, Lohmeier TE, Hester RL, Kivlighn SD, Smith MJ Jr. Hormonal and circulatory responses to chronically controlled increments in right atrial pressure. Am J Physiol Regul Integr Comp Physiol 261: R1176–R1187, 1991. doi: 10.1152/ajpregu.1991.261.5.R1176. [DOI] [PubMed] [Google Scholar]
- 34.Villarreal D, Freeman RH, Davis JO, Verburg KM, Vari RC. Renal mechanisms for suppression of renin secretion by atrial natriuretic factor. Hypertension 8: II28–II35, 1986. doi: 10.1161/01.HYP.8.6_Pt_2.II28. [DOI] [PubMed] [Google Scholar]
- 35.Wallbach M, Halbach M, Reuter H, Passauer J, Lüders S, Böhning E, Zenker D, Müller GA, Wachter R, Koziolek MJ. Baroreflex activation therapy in patients with prior renal denervation. J Hypertens 34: 1630–1638, 2016. doi: 10.1097/HJH.0000000000000949. [DOI] [PubMed] [Google Scholar]
- 36.Wustmann K, Kucera JP, Scheffers I, Mohaupt M, Kroon AA, de Leeuw PW, Schmidli J, Allemann Y, Delacrétaz E. Effects of chronic baroreceptor stimulation on the autonomic cardiovascular regulation in patients with drug-resistant arterial hypertension. Hypertension 54: 530–536, 2009. doi: 10.1161/HYPERTENSIONAHA.109.134023. [DOI] [PubMed] [Google Scholar]
- 37.Zile MR, Abraham WT, Weaver FA, Butter C, Ducharme A, Halbach M, Klug D, Lovett EG, Müller-Ehmsen J, Schafer JE, Senni M, Swarup V, Wachter R, Little WC. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction: safety and efficacy in patients with and without cardiac resynchronization therapy. Eur J Heart Fail 17: 1066–1074, 2015. doi: 10.1002/ejhf.299. [DOI] [PubMed] [Google Scholar]