Abstract
The hypothalamic paraventricular nucleus (PVN) is a unique and important brain region involved in the control of cardiovascular, neuroendocrine, and other physiological functions pertinent to homeostasis. The PVN is a major source of excitatory drive to the spinal sympathetic outflow via both direct and indirect projections. In this review, we discuss the role of the PVN in the regulation of sympathetic output in normal physiological conditions and in hypertension. In normal healthy animals, the PVN presympathetic neurons do not appear to have a major role in sustaining resting sympathetic vasomotor activity or in regulating sympathetic responses to short-term homeostatic challenges such as acute hypotension or hypoxia. Their role is, however, much more significant during longer-term challenges, such as sustained water deprivation, chronic intermittent hypoxia, and pregnancy. The PVN also appears to have a major role in generating the increased sympathetic vasomotor activity that is characteristic of multiple forms of hypertension. Recent studies in the spontaneously hypertensive rat model have shown that impaired inhibitory and enhanced excitatory synaptic inputs to PVN presympathetic neurons are the basis for the heightened sympathetic outflow in hypertension. We discuss the molecular mechanisms underlying the presynaptic and postsynaptic alterations in GABAergic and glutamatergic inputs to PVN presympathetic neurons in hypertension. In addition, we discuss the ability of exercise training to correct sympathetic hyperactivity by restoring blood-brain barrier integrity, reducing angiotensin II availability, and decreasing oxidative stress and inflammation in the PVN.
Keywords: autonomic nervous system, hypothalamus, paraventricular nucleus, sympathetic nervous system, synaptic plasticity, synaptic transmission
INTRODUCTION
Up until the 1970s, the hypothalamic paraventricular nucleus (PVN) was regarded mainly as the location of neurons that synthesized the hormones vasopressin and oxytocin, releasing them from the terminals in the pituitary gland. With the advent of the method of tracing neuronal connections using anterograde and retrograde transport, however, Saper et al. (149) discovered that there are neurons within the PVN that project directly to sympathetic preganglionic nuclei in the spinal cord as well as to other key autonomic nuclei, including the nucleus of the solitary tract (NTS) and rostral ventrolateral medulla (RVLM; Fig. 1). This landmark study led to many subsequent studies of the role of the PVN in autonomic regulation. In this review, we will focus on the role of the PVN in regulating the sympathetic outflow to the heart and blood vessels. In the first section of this review, we will describe our current understanding of the afferent and efferent connections of PVN neurons that regulate the cardiovascular system followed by a discussion of the role of PVN neurons in cardiovascular regulation under normal nonpathological conditions. The subsequent sections of the review will describe the changes in the synaptic inputs to PVN presympathetic neurons and the beneficial effects of exercise training on the autonomic control in hypertension. For the involvement of the PVN in the autonomic control in heart failure, readers are referred to two relevant review articles on this topic (133, 138).
Fig. 1.
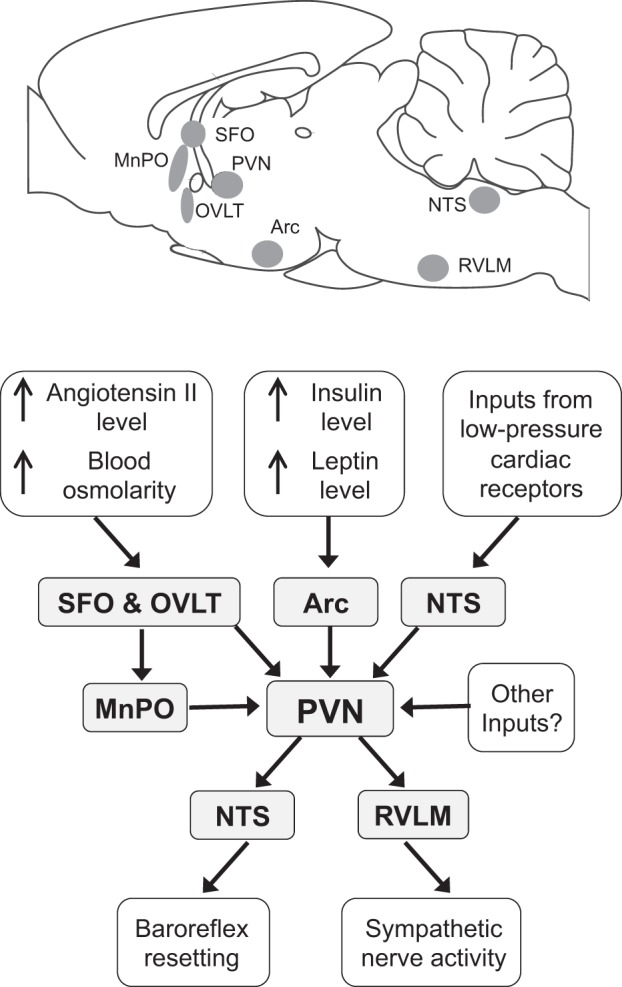
Top: sagittal section of the rat brain showing the location of the brain nuclei that have a major role in the regulation of the sympathetic vasomotor outflow by the hypothalamic paraventricular nucleus (PVN) under normal conditions. Bottom: flow diagram showing the inputs to, and outputs from, PVN presympathetic neurons that regulate the sympathetic vasomotor outflow under normal conditions. Note that under pathological conditions (e.g., hypertension, heart failure, or chronic intermittent hypoxia) or after sustained behavioral changes (e.g., exercise training) the activity of PVN presympathetic neurons may also be altered by other inputs. Arc, arcuate nucleus; MnPO, median preoptic nucleus; NTS, nucleus of the solitary tract; RVLM, rostral ventrolateral medulla; SFO, subfornical organ; OVLT, organum vasculosum lamina terminalis.
OUTPUT PATHWAYS FROM PVN PRESYMPATHETIC NEURONS REGULATING CARDIOVASCULAR FUNCTION
An important study by Strack et al. (165) using the method of transneuronal retrograde labeling demonstrated that the PVN is one of five brain regions that provide direct innervation of the entire sympathetic outflow. The same study also showed, however, that most presympathetic neurons within the PVN are organized topographically, such that PVN neurons that regulate the sympathetic outflow to different target organs have different locations within the nucleus. In agreement with this, physiological studies have indicated that PVN neurons that regulate the renal sympathetic outflow are a distinct group from those that regulate the cardiac sympathetic outflow (31). Furthermore, presympathetic neurons also differ in their neurochemical properties; different groups contain oxytocin, vasopressin, corticotrophin-releasing hormone, or various other peptides (8, 56). There is also evidence that some PVN presympathetic neurons may act as “command neurons,” with collateral projections to different sympathetic outflows, such as those to the heart and adrenal medulla (69). In addition, there are also PVN neurons with collateral projections to the RVLM and spinal cord (139). Thus, these observations imply that while many PVN presympathetic neurons specifically regulate particular targets, some may regulate the sympathetic outflow in a more global fashion.
Based on early studies of descending projections from the PVN, reviews of the anatomic properties of PVN presympathetic neurons have commonly emphasized that the main targets of these neurons are the spinal cord, NTS, dorsal motor nucleus of the vagus (DMV), and RVLM (8, 35, 159). A more recent study (52), however, using the anterograde tracer Phaseolus vulgaris leucoagglutinin found that there are also strong descending projections to the caudal pressor area in the medulla, a region that is known to contain sympathoexcitatory neurons but whose precise function remains unclear. In summary, there are multiple pathways by which PVN neurons can influence the sympathetic outflow to the cardiovascular system, although our knowledge of the specific functions of these pathways remains incomplete.
AFFERENT INPUTS TO PVN PRESYMPATHETIC NEURONS
PVN presympathetic neurons receive information from visceral receptors relayed by the NTS and A1 and A2 noradrenergic cell groups as well as information about temperature, osmolality, circulating angiotensin II, and other humoral factors from the median preoptic nucleus and two circumventricular organs: the subfornical organ (SFO) and organum vasculosum lamina terminalis (Fig. 1) (31, 119, 156, 159, 161). In addition, inputs to PVN presympathetic neurons from the arcuate nucleus relay signals related to leptin and insulin levels (Fig. 1) (20, 143). Thus, these neurons receive inputs signaling a wide range of physiological variables. Further details about the organization of inputs to PVN presympathetic neurons will be provided in the following sections, which discuss the role of these neurons in cardiovascular regulation.
ROLE OF THE PVN IN REGULATING RESTING MEAN ARTERIAL PRESSURE AND SYMPATHETIC NERVE ACTIVITY
In anesthetized rats, acute inhibition of the PVN with microinjection of muscimol, a GABAA receptor agonist, has been reported to result in decreases in the resting level of mean arterial blood pressure (MAP), heart rate, and sympathetic nerve activity (SNA) in some studies (2, 178, 181). In contrast, in other studies, it has been reported that PVN inhibition with muscimol or the ionotropic glutamate antagonist kynurenic acid has little effect on these variables (64, 161, 162, 178). Similarly, in conscious sheep, inhibition of the PVN with muscimol has little effect on resting MAP or SNA (144). A possible explanation for the fact that different investigators have reported different effects of muscimol inhibition of the PVN on resting MAP or SNA is that these studies were performed using different anesthetics (e.g., isoflurane, chloralose-urethane, pentobarbital sodium, or no anesthetic) and/or different species (rats or sheep). PVN presympathetic neurons receive both tonic excitatory and inhibitory inputs, the balance of which may vary under different anesthetic conditions or in different species.
There is general agreement, however, that PVN presympathetic neurons are tonically inhibited by GABAergic inputs, because in both anesthetized rats and conscious sheep bicuculline injection into the PVN increases MAP and SNA (66, 80, 144, 181). Furthermore, the fact that SNA is increased after blockade of GABA receptors in the PVN also implies that PVN presympathetic neurons receive tonic excitatory inputs. As we shall discuss below, the balance of tonic excitatory and inhibitory inputs to PVN presympathetic neurons is altered under conditions such as water deprivation or pregnancy or in pathological conditions such as hypertension.
ROLE OF PVN PRESYMPATHETIC NEURONS IN THE REFLEX REGULATION OF CARDIOVASCULAR FUNCTION
Blood Volume
It is well established that the PVN is a key component of the central mechanisms that regulate blood volume. Changes in blood volume are directly sensed by mechanoreceptors that are located at the venous-atrial junctions in the heart (55). Signals from these cardiac receptors are conveyed by vagal afferent fibers to the NTS. An increase in blood volume or direct stimulation of cardiac receptors triggers a reflex increase in heart rate accompanied by a reflex decrease in renal SNA, which, in turn, results in renal vasodilation, increased urine flow, and Na+ loss (79, 86, 140).
The PVN plays a critical role in this reflex, because lesions of the PVN in anesthetized rats or inhibition of the PVN by muscimol injection in conscious rabbits or sheep abolishes the reflex decrease in renal SNA or renal vasodilation induced by an acute increase in blood volume (58, 109, 144). Many of the PVN neurons that control renal sympathetic outflow via the spinal cord may use vasopressin as a neurotransmitter, based on the finding that increases in renal SNA evoked from the PVN can be blocked by intrathecal application of a vasopressin receptor antagonist (174). Consistent with this, a substantial proportion of PVN spinally projecting neurons contain vasopressin (22), and vasopressin receptors are located in the intermediolateral column of the spinal cord (155).
In electrophysiological studies in anesthetized rats, Lovick and Coote (107, 108) showed that some spinally projecting PVN neurons are inhibited by volume loading, leading to the suggestion that these neurons may project to renal sympathetic preganglionic neurons and be a component of the pathway by which volume expansion causes a reflex inhibition of renal SNA (31). According to this hypothesis, NTS neurons that receive inputs from cardiac receptors activated by volume loading project to the PVN and synapse with GABAergic interneurons that in turn inhibit presympathetic neurons that control the renal sympathetic outflow. Results from anatomic studies support this view: there are direct projections from NTS neurons to GABAergic neurons in the PVN (1) that in turn project to PVN presympathetic neurons (173).
In the conscious rabbit with denervated arterial baroreceptors, an increase in blood volume results in increased activation of PVN neurons (as indicated by c-Fos expression) (137). Very few of the activated PVN neurons contain vasopressin (137), consistent with the hypothesis (31) that the PVN neurons activated by volume loading are GABAergic interneurons that inhibit renal sympathoexcitatory responses. It has also been proposed that the reflex increase in heart rate evoked by volume loading is mediated by PVN presympathetic neurons (31). In contrast to the renal component of the reflex, however, lesions of the PVN do not significantly affect the cardiac component (109). Thus, the reflex effects on heart rate induced by volume loading are not dependent on the PVN.
Acute decreases in blood volume (e.g., as a result of hemorrhage) result in compensatory increases in SNA to blood vessels, the heart, and adrenal medulla, together with increases in the levels of circulating vasopressin and angiotensin II (150). Both arterial baroreceptors and cardiac receptors contribute to these reflex effects (110), but PVN presympathetic neurons appear to make only a minor contribution to the reflex increase in SNA. In the rat, a small proportion (~12%) of spinally projecting PVN neurons express c-Fos after nonhypotensive hemorrhage (6). A similar proportion of such neurons expresses c-Fos after hypotensive hemorrhage (6), suggesting that these neurons are activated by hypovolemia rather than hypotension. Consistent with this, Polson et al. (136) found that nitroprusside-induced hypotension activates very few PVN spinally projecting neurons. Thus, activation of PVN spinally projecting neurons by hypovolemia may be either due to inputs arising from cardiac receptors or due to an increase in the level of circulating angiotensin II, which activates PVN neurons via the circumventricular organs (118, 119).
Dehydration
In contrast to their minor role in generating responses to acute hypovolemia, PVN presympathetic neurons have a major role in generating increased SNA in response to sustained water deprivation. In anesthetized rats deprived of water for 48 h, inhibition of the PVN results in large decreases in arterial pressure and renal, splanchnic, and lumbar SNA (47, 64, 161, 162). There is strong evidence that resting blood pressure is maintained during water deprivation by the sustained activity of a glutamatergic pathway from the PVN to RVLM presympathetic neurons. First, blockade of excitatory amino acid receptors in the RVLM reduces blood pressure in water-deprived but not water-replete rats (14). Second, Stocker et al. (163) showed that the large majority (94%) of PVN neurons projecting to the RVLM that are activated by water deprivation in conscious rats are glutamatergic. This study also showed that a substantial proportion of RVLM-projecting neurons in the PVN (16–40%, depending on the PVN subregion) are activated by water deprivation.
Water deprivation results in increased levels of plasma osmolarity as well as a reduction in blood volume. Scrogin et al. (153, 154) found that restoration of normal levels of plasma osmolarity, but not restoration of blood volume, normalizes the elevated lumbar SNA in water-deprived rats. It thus appears that increased osmolarity is an adequate stimulus for triggering sympathetic responses to water deprivation via inputs to PVN presympathetic neurons. Increased osmolarity is sensed by osmoreceptors in neurons in the SFO and organum vasculosum lamina terminalis, which then project to the PVN either directly or via the median preoptic nucleus, as described above.
Na+ Balance
In normotensive animals and humans, a sustained increase in salt intake causes a decrease in renal SNA, which, together with inhibition of the renin-angiotensin-aldosterone system, increases salt excretion and thus maintains Na+ balance (106, 123). Studies by Kapusta et al. (77, 78) have indicated that the PVN plays an essential role in generating renal sympathoinhibition in response to the increased salt intake. In particular, they demonstrated that in normotensive rats a sustained increase in salt intake resulted in a decrease in circulating norepinephrine levels, indicative of a decreased SNA, which was associated with an increase in Gαi2 protein levels specifically within the PVN. Furthermore, they demonstrated that downregulation of brain Gαi2 proteins prevented this effect (77, 78).
An increase in salt intake leads to an increase in plasma volume (91), which, as discussed above, will lead to renal sympathoinhibition via a PVN-dependent mechanism. Thus, it seems likely that salt loading leads to renal sympathoinibition as a consequence of the increased plasma volume rather than an increase in osmolarity. Consistent with this, Kapusta et al. (78) found that downregulation of brain Gαi2 proteins also abolished the renal sympathoinhibitory response to isotonic volume expansion. Furthermore, as pointed out above, increased osmolarity results in sympathoexcitation rather than sympathoinhibition.
Blood Pressure
Signals from arterial baroreceptors do reach the PVN and can influence blood pressure via reflex effects on vasopressin release. In particular, hypotension-induced activation of vasopressin-synthesizing neurons in the PVN is dependent mainly on inputs from arterial baroreceptors (36). With regard to the role of the PVN in mediating baroreflex changes in SNA, electrophysiological studies in anesthetized rats have identified that PVN neurons projecting to the spinal cord or RVLM appear to be barosensitive, as they are spontaneously active and are inhibited by increases in blood pressure (7, 26, 27, 107, 108). Other studies have suggested, however, that PVN presympathetic neurons do not play a major role in the baroreflex regulation of sympathetic activity. In anesthetized rats, lesions of the PVN do not affect the baroreflex inhibition of SNA (58), and studies in conscious rabbits or rats using c-Fos as a marker of neuronal activation have shown that PVN neurons that project to the spinal cord or the RVLM are not activated by sustained hypotension (5, 6, 136).
Although the PVN may not be an essential component of the baroreceptor-sympathetic reflex pathways, there is evidence that PVN neurons that project to the NTS can modify the cardiac component of the baroreflex under certain conditions, such as exercise. Primary baroreceptor afferents terminate in the NTS, which is also a major target of PVN neurons that project to the brainstem (52). As summarized by Michelini (120), NTS-projecting PVN neurons that contain vasopressin or oxytocin modulate synaptic transmission within the NTS, with the result that the baroreflex control of heart rate is reset, so as to allow increases in heart rate and cardiac output during exercise without loss of baroreflex sensitivity.
Hypoxia
There has been considerable debate regarding the role of PVN presympathetic neurons in generating reflex changes in SNA evoked by peripheral chemoreceptor input. With the use of c-Fos as a marker of neuronal activation, sustained moderate hypoxia (10% O2) in conscious rats was found to activate NTS and A1 noradrenergic neurons that project to the PVN (82, 83) as well as PVN neurons immunoreactive for vasopressin and corticotrophin-releasing hormone (30, 160). This hypoxic stimulus, however, does not result in the activation of presympathetic PVN neurons that project to the RVLM or spinal cord (30). Similarly, in conscious rabbits, a hypoxic stimulus of the same magnitude does not activate PVN neurons projecting to the RVLM but does activate RVLM-projecting neurons in the NTS and Kölliker-Fuse nucleus (63).
Other studies, however, in which injection of KCN was used to activate peripheral chemoreceptors, indicated that the PVN does contribute to the sympathoexcitatory response evoked by that stimulus. In particular, in anesthetized rats, lesions or inhibition of the PVN reduce the sympathoexcitatory response to KCN injections (129, 145). Consistent with these findings, Cruz et al. (33) found that KCN injections in conscious rats activate RVLM-projecting PVN neurons. In contrast to the moderate hypoxia induced by breathing 10% O2, KCN injection may cause more intense stimulation of peripheral chemoreceptors or cause secondary effects that in turn evoke sympathoexcitation. In particular, KCN injection can cause a behavioral defense reaction (124) that is well known to be associated with sympathoexcitatory responses (34).
Thus, taking all the evidence together, acute systemic hypoxia does not normally lead to reflex activation of PVN presympathetic neurons except when the hypoxia is extreme or is associated with secondary behavioral reactions. At the same time, chronic intermittent hypoxia, such as occurs during sleep apnea, can lead to sustained increases in SNA. As reviewed by Mifflin et al. (122), studies in rats have indicated that chronic intermittent hypoxia increases the transmission of chemoreceptor inputs to the NTS and increases SNA via a circuit that includes the PVN and RVLM as essential components.
Psychological Stress
It is well known that psychological or emotional stress evokes cardiovascular responses, including increases in SNA, blood pressure, and heart rate (34). The PVN does not play a major role in generating sympathoexcitatory responses to acute psychological stress, because inhibition of the PVN does not significantly affect the increases in blood pressure and heart rate evoked by air jet stress, although it does block the neuroendocrine response to this stress (164). Nevertheless, PVN presympathetic neurons may make some contribution to the increase in SNA associated with some types of psychological stress, because Carrive and Gorissen (19) found that ~10% of spinally projecting PVN neurons are activated during conditioned fear. In addition, Furlong et al. (49) found that a small proportion (~7%) of PVN neurons projecting to the NTS are activated by air puff stress. Such NTS-projecting neurons may contribute to the resetting of the baroreflex that occurs during psychological stress (76).
It is well established that chronic stress, even when sustained over several weeks, is associated with sustained increases in sympathoadrenal activity, as indicated by increased levels of urinary norepinephrine excretion (48, 87). In addition, there is also a sustained increase in cortisol levels, indicating sustained activation of neuroendocrine neurons within the PVN (87). This has led to the suggestion that, in contrast to acute psychological stress, in chronic stress the PVN plays an important role in generating sustained increases in SNA (35, 113). There is, however, not yet direct evidence to support this hypothesis.
Pregnancy
Fluid balance and arterial pressure regulation are profoundly altered in pregnancy. Blood volume and cardiac output increase, but total resistance decreases, mainly as a consequence of hormonal changes (147). There is an overall increase in SNA in late pregnancy, which helps to sustain blood pressure in both humans (54, 70) and animals (15, 29, 89). The increases in SNA, however, appear to be nonuniform. In pregnant rats, lumbar, splanchnic, and cardiac SNA are markedly elevated (29, 157). In contrast, some (62, 128, 157) but not all (115) studies have reported that renal SNA is not increased in pregnancy. PVN presympathetic neurons contribute to the maintenance of this increased SNA in pregnancy because inhibition of the PVN in pregnant rats, but not nonpregnant rats, results in significant decreases in blood pressure, heart rate, and lumbar SNA (157).
A major factor responsible for the increased activity of PVN presympathetic neurons in pregnant rats is that, compared with nonpregnant rats, there is a reduction in tonic GABAergic inhibition of these neurons (90). The mechanisms responsible for the reduced tonic GABAergic inhibition have not been established, but several possibilities have been proposed. First, the level of circulating angiotensin II is increased in pregnancy (16, 57), and it has been shown that chronic intravenous infusion of angiotensin II results in decreased GABAergic inhibition of PVN neurons (92) and thus increased activation of these neurons (39). These observations led Kvochina et al. (90) to suggest that the increased levels of circulating angiotensin II in pregnancy may activate angiotensin II type 1 (AT1) receptors on neurons in the SFO that project to the PVN, ultimately causing a reduction in GABAergic inhibition of presympathetic neurons. In addition, pregnancy is also associated with decreased expression of nitric oxide synthase activity and expression (59). It has been shown that decreased levels of nitric oxide in the PVN results in decreased GABAergic inhibition of PVN presympathetic neurons (181). Thus, as occurs in heart failure (179), decreased nitric oxide synthase activity in the PVN may lead to disinhibition of PVN neurons and increased SNA. The molecular mechanisms by which inputs from the SFO or decreased levels of nitric oxide synthase activity cause decreased GABAergic inhibition in the PVN are unknown, but one factor that may be involved is a decrease in the expression of the GABAA receptor α5-subunit (32).
Inhibition of neurons in the arcuate nucleus decreases arterial pressure, heart rate, and lumbar SNA in pregnant but not nonpregnant rats (157). The arcuate nucleus is a major source of inputs to the PVN that release either neuropeptide Y (NPY) or α-melanocyte-stimulating hormone (MSH) (71, 127). Expression of NPY in the PVN is reduced in pregnant rats, and blockade of NPY receptors in the PVN increased arterial pressure, heart rate, and lumbar SNA in nonpregnant but not pregnant rats (157). Conversely, inhibition of melanocortin 3/4 receptors (normally activated by MSH) in the PVN decreased arterial pressure, heart rate, and lumbar SNA in pregnant but not nonpregnant rats (157). Taken together, these observations suggest that in pregnancy there is reduced NPY-mediated inhibition and increased MSH-mediated excitation of PVN presympathetic neurons from inputs arising from the arcuate nucleus.
In addition to effects on the resting levels of arterial pressure, heart rate, and SNA, pregnancy is associated with a marked reduction in the gain of the arterial baroreflex (for a review, see Ref. 13). There is evidence that the PVN plays a role in this effect during pregnancy. In particular, there is a high density of insulin receptors in the PVN (152), and activation of these receptors increases baroreflex gain (13). Therefore, during pregnancy, when the level of brain insulin is reduced (38), this may contribute to the reduction in baroreflex gain.
In summary, PVN presympathetic neurons do not appear to have a major role in regulating sympathetic responses to short-term homeostatic challenges. Their role is, however, much more significant during longer term challenges, such as sustained water deprivation, chronic intermittent hypoxia, and pregnancy. The following sections will consider the role of these neurons under the pathological state of hypertension.
SYNAPTIC PLASTICITY IN THE PVN IN HYPERTENSION
In contrast to normotensive animals, in the spontaneously hypertensive rat (SHR), the excitability of PVN presympathetic neurons is profoundly increased and is a major source of excitatory drive to maintain heightened sympathetic outflow (3, 94, 100, 176). Glutamate and GABA are the predominant excitatory and inhibitory neurotransmitters, respectively, in the brain, and the firing activity of PVN output neurons is finely tuned by these synaptic inputs (99, 100). The inhibitory actions of GABA are mediated primarily through ionotropic GABAA receptors and metabotropic GABAB receptors. Glutamate receptors are divided into ionotropic glutamate receptors, including N-methyl-d-aspartate (NMDA) receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), kainite receptors, and metabotropic glutamate receptors (mGluRs). In the following section, we review synaptic plasticity in the PVN from recent studies performed mainly using SHRs, a commonly used animal model of primary hypertension.
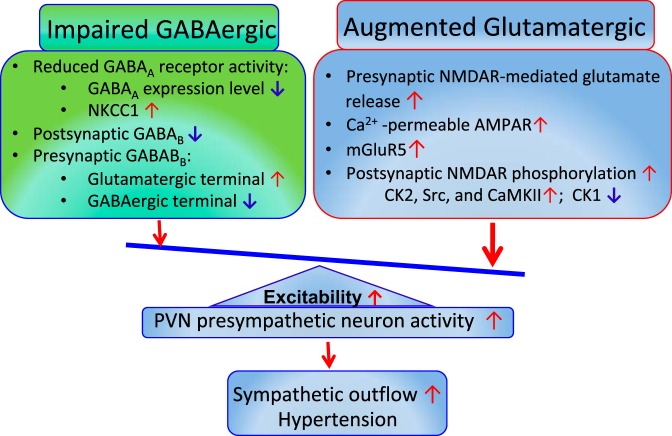
IMPAIRED GABAERGIC SYNAPTIC INPUTS TO THE PVN IN HYPERTENSION
GABAA Receptors
Normal GABAergic inhibition of PVN presympathetic neurons is impaired in hypertension (Fig. 2). In this regard, blockade of GABAA receptors with bicuculline or gabazine typically increases the excitability of presympathetic PVN neurons in normotensive Wistar-Kyoto (WKY) rats. However, bicuculline or gabazine either increases or has no effect on the firing activity of these neurons in adult SHRs (97, 175). The frequency of GABAergic inhibitory postsynaptic currents (IPSCs) of PVN presympathetic neurons is reduced in SHRs (97, 175). Also, GABAA receptor-binding sites are reduced in the PVN in SHRs (88). The diminished GABAergic inhibition of PVN presympathetic neurons may result from reduced presynaptic GABA release, decreased GABAA receptor number or function, or the loss of GABAergic neurons in hypertension. Notably, in rats with congestive heart failure, GABA-mediated neuronal inhibition in the PVN is also reduced and contributes to increased sympathetic outflow (18, 180).
Fig. 2.
Imbalance of excitatory and inhibitory synaptic inputs to paraventricular nucleus (PVN) presympathetic neurons in the spontaneously hypertensive rat model. Impaired inhibitory GABAergic synaptic inputs include a depolarizing shift of GABA reversal potential due to Na+-K+-Cl− cotransporter-1 (NKCC1) upregulation and reduced GABAB receptor activity. Enhanced excitatory glutamatergic synaptic inputs include increased N-methyl-d-aspartate receptors (NMDAR)-mediated presynaptic glutamate release, increased activity of postsynaptic NMDARs and metabotropic glutamate receptor 5 (mGluR5), and a switch to Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). The impaired GABAergic and enhanced glutamatergic inputs lead to hyperactivity of PVN presympathetic neurons and heightened sympathetic vasomotor tone in hypertension. CK, casein kinase; CaMKII, Ca2+/calmodulin-dependent protein kinase II.
GABAB Receptors
In contrast, GABAB receptor activity in the PVN is increased in SHRs, as evidenced by microinjection of the GABAB receptor agonist baclofen into the PVN inducing a greater inhibitory effect on sympathetic outflow in SHRs than in WKY rats (98). Also, blockade of GABAB receptors with CGP-55845 increases the firing activity of PVN presympathetic neurons in SHRs but has no effect in WKY rats (97). In addition, activation of GABAB receptors induces larger membrane hyperpolarization and larger outward currents in PVN presympathetic neurons in SHRs than in WKY rats (99). These findings suggest that the GABAB receptor is tonically activated and involved in the regulation of the excitability of PVN presympathetic neurons in SHRs (97). GABAB receptors are expressed presynaptically and can regulate both glutamate and GABA release. The GABAB receptor control of synaptic glutamate release to PVN presympathetic neurons is enhanced, whereas presynaptic GABAB receptor control of GABAergic synaptic inputs is attenuated, in SHRs compared with normotensive control rats (99).
Cation-Cl− Cotransporter
GABAA receptors are ligand-gated anion channels with a predominant permeability to Cl− and a limited permeability to (74, 75). When the intracellular Cl− concentration is high, GABAA receptor activation induces Cl− outflow to depolarize the cell membrane. On the other hand, GABAA receptor activation induces hyperpolarization when the intracellular Cl− concentration is low (74, 75). Both Na+-K+-Cl− cotransporter-1 (NKCC1) and K+-Cl− cotransporter-2 (KCC2) are involved in maintaining Cl− homeostasis and the GABA reversal potential (EGABA) (134, 146). mRNA and protein levels of NKCC1, but not KCC2, in the PVN are markedly increased in SHRs. The upregulation of NKCC1 induces an increase in the intracellular Cl− concentration, which leads to a depolarizing shift of EGABA in PVN presympathetic neurons and diminishes GABAergic inhibition in SHRs. This shift in EGABA corresponds to an elevation of intracellular Cl− concentration in PVN presympathetic neurons in SHRs (175). Furthermore, NKCC1 proteins on the plasma membrane in the PVN of SHRs are highly glycosylated, and inhibition of NKCC1 N-glycosylation normalizes EGABA and restores GABA inhibition of PVN presympathetic neurons in SHRs (175). In addition, intracerebroventricular administration of the NKCC1 inhibitor bumetanide decreases sympathetic vasomotor activity and restores sympathoinhibitory responses to the GABAA receptor agonist in the PVN in SHRs. Interestingly, a depolarizing shift of EGABA in PVN vasopressin neurons is also observed in deoxycorticosterone acetate-salt-treated hypertensive rats (81). This depolarizing shift of EGABA is associated with increased NKCC1 protein levels in the PVN. Intracerebroventricular injection of bumetanide delays the development of hypertension induced by deoxycorticosterone acetate-salt treatment (81). Together, these findings suggest that increased NKCC1 activity in the PVN may account for diminished GABAergic input to PVN presympathetic neurons in hypertension.
ENHANCED GLUTAMATERGIC SYNAPTIC INPUTS TO THE PVN IN HYPERTENSION
Synaptic NMDARs
Blockade of inotropic glutamate receptors in the PVN has little effect on vasomotor tone in normotensive rats, but it profoundly reduces sympathetic nerve discharges and MAP in SHRs (95). This augmented glutamatergic synaptic input in the PVN in hypertensive conditions has been reported in SHRs (142, 176), salt-sensitive hypertension (50), and angiotensin II-induced hypertension (53, 141). In brain slices, the basal frequency of miniature excitatory postsynaptic currents (mEPSCs; a measure of presynaptic quantal release of glutamate) of PVN presympathetic neurons is substantially increased in SHRs compared with normotensive controls (142, 176, 177). This increased presynaptic glutamate release is mediated by NMDARs because blockade of NMDARs abolishes the increased frequency of mEPSCs of PVN presympathetic neurons in SHRs (102, 142). However, blockade of NMDARs does not alter the frequency of mEPSCs of PVN neurons in normotensive rats. Thus, NMDAR-mediated presynaptic glutamate release in the PVN is latent under the normotensive condition but becomes tonically activated in SHRs (142, 176). GluN2A-containing NMDARs predominantly mediate enhanced synaptic glutamate release to PVN presympathetic neurons in hypertension (177).
Postsynaptic NMDAR activity in PVN presympathetic neurons is also enhanced in hypertension. For example, the NMDAR currents induced by puff NMDA application are much larger in spinally projecting PVN neurons in SHRs compared withWKY rats (100, 102, 142, 176). Blockade of NMDARs significantly decreases the firing activity of PVN presympathetic neurons in brain slices (100, 102, 176) and reduces sympathetic vasomotor activity in SHRs (94, 100, 103, 176). Also, angiotensin II-induced hypertension is associated with increased NMDAR activity of spinally projecting PVN neurons (170). Deletion of the NMDAR subunit GluN1 in the PVN attenuates systemic angiotensin II administration-induced increases in blood pressure in mice (53), demonstrating an important role of NMDARs in the PVN in hypertension caused by angiotensin II. In addition, upregulation of NMDARs and increased NMDAR activity in the PVN has been shown in rats with congestive heart failure (84, 85, 104).
NMDAR Regulation by Kinases/Phosphatases and α2δ-1
Many protein kinases are involved in the regulation of NMDAR activity through direct or indirect phosphorylation of NMDARs and/or NMDAR-interacting proteins. The kinases involved in NMDAR regulation of PVN presympathetic neurons in hypertension include casein kinase II (CK2) (176), casein kinase I (CK1) (101), Src kinases (142), and Ca2+/calmodulin-dependent protein kinase II (CaMKII) (102). CaMKII, a synapse-enriched serine/threonine protein kinase, binds to and modulates NMDAR activity in PVN presympathetic neurons in hypertension. CaMKII inhibition decreases both presynaptic and postsynaptic NMDAR activity in PVN presympathetic neurons in SHRs (102). Furthermore, the CaMKII-mediated phosphorylation level of GluN2B at Ser1303 in the PVN is much higher in SHRs than in WKY rats (102).
The CK2α protein level on the cell membrane in the PVN is substantially higher in SHRs compared with WKY rats (176). Inhibition of CK2 activity profoundly decreases NMDAR-mediated EPSCs, puff NMDAR currents, and NMDAR-mediated mEPSCs in spinally projecting PVN neurons in SHRs (176). In addition, intracerebroventricular administration of a CK2 inhibitor decreases SNA and MAP in SHRs but not in WKY rats (176). Src kinases are also crucially involved in the control of pre- and postsynaptic NMDAR activity of RVLM-projecting PVN neurons in SHRs (142). CK2 phosphorylates the Ser1480 residue of the GluN2B subunit (148), whereas Src kinase phosphorylates Tyr1325 of the GluN2A subunit (166). Thus, CK2 and Src kinases may increase the phosphorylation of different amino acid residues of NMDARs in SHRs. Also, Src kinases may interact with CK2 via phosphorylation of tyrosine residues in CK2 catalytic subunits to increase CK2 activity (41).
The net activity and phosphorylation level of NMDARs in the PVN in SHRs are tightly regulated by protein kinases and phosphatases as well as their reciprocal interactions (Fig. 2). In contrast to the increased NMDAR phosphorylation level by CK2, Src, and CaMKII kinases in the PVN, the CK1 (particularly CK1ε) protein level in the PVN is significantly decreased in SHRs (101). CK1 inhibition increases NMDAR-mediated EPSCs, puff NMDA-elicited currents, and the firing activity of spinally projecting PVN neurons in WKY rats but not in SHRs (101), suggesting that CK1 activity is attenuated in the PVN in SHRs. Because CK1 is a serine/threonine kinase that does not directly phosphorylate NMDARs (168), it may inhibit NMDAR activity indirectly by increasing the activity of protein phosphatases such as protein phosphatase (PP)1/2A (172) and/or PP2B (calcineurin) (105, 167). Interestingly, inhibition of PP1/2A or PP2B can mimic the effect of the CK1 inhibitor on NMDAR activity, and CK1 inhibitor does not further increase NMDAR activity of PVN presympathetic neurons after inhibition of PP1/2A and PP2B (101). Thus, decreased CK1 activity in the PVN may lead to reduced dephosphorylation of NMDARs by protein phosphatases in SHRs.
α2δ-1, previously considered a subunit of voltage-activated Ca2+ channels, can directly interact with NMDARs and potentiate presynaptic and postsynaptic NMDAR activity by promoting synaptic trafficking of NMDARs (25). A recent study has shown that α2δ-1-bound NMDARs in the PVN are essential for the angiotensin II-induced increase in NMDAR activity of PVN presympathetic neurons and augmented sympathetic outflow (111). Furthermore, the prevalence of synaptic α2δ-1-bound NMDARs in the PVN is increased and accounts for potentiated NMDAR activity of PVN presympathetic neurons sympathetic vasomotor activity in SHRs (112). The potential link between α2δ-1-bound NMDARs and various protein kinases in regulating NMDAR activity in hypertension is currently unknown. Because increased protein phosphorylation can strengthen protein-protein binding complexes (125), it is possible that certain protein kinases potentiate the phosphorylation of α2δ-1 and/or NMDAR proteins to promote their physical interactions by changing their physicochemical properties, stability, and dynamics.
Synaptic AMPARs
AMPARs containing the GluR2 subunit are impermeable to Ca2+ (65, 67), whereas AMPARs lacking the GluR2 subunit are permeable to Ca2+ and are blocked by intracellular polyamines in a voltage-dependent manner (11, 42, 67). AMPARs in the PVN presympathetic neurons in SHRs undergo a switch to Ca2+-permeable AMPARs. Specifically, AMPAR-mediated EPSCs display an inward rectification at positive holding potentials in spinally projecting PVN neurons in SHRs (93). Furthermore, a selective Ca2+-permeable AMPAR blocker, 1-naphthyl acetyl spermine, substantially reduces the amplitude of AMPAR-mediated EPSCs and excitability of spinally projecting PVN neurons in SHRs but not in WKY rats (93). These findings suggest that Ca2+-permeable AMPAR activity is increased and contributes to the hyperactivity of PVN presympathetic neurons in SHRs (93). Together with augmented NMDAR activity, a switch to Ca2+-permeable AMPARs can lead to an increased intracellular Ca2+ level of PVN presympathetic neurons in SHRs.
Group I mGluRs
mGluRs are typically activated by the excessive glutamate release in the PVN in SHRs and involved in regulating sympathetic outflow in hypertension (96). Group I mGluRs (mGluR1 and mGluR5) are coupled to Gq/11 proteins, and their activation triggers several signaling pathways, including PKC, to increase neuronal excitability and synaptic neurotransmitter release. Microinjection of a mGluR5 receptor antagonist into the PVN produces a greater inhibitory effect on SNA and MAP than that caused by a mGluR1 receptor antagonist in SHRs, suggesting that mGluR5 receptors play a dominant role in maintaining elevated sympathetic vasomotor activity in SHRs (96). In addition, mRNA and protein expression levels of mGluR5 in the PVN are markedly increased in SHRs compared with WKY rats (103). The NMDAR antagonist largely attenuates the sympathoexcitatory response to microinjection of a general group I mGluR agonist into the PVN (103). Hence, stimulation of group I mGluRs may excite PVN presympathetic neurons via NMDARs.
Together, recent findings from brain slice studies have revealed the signaling mechanisms of synaptic plasticity in the PVN in animal models of hypertension. The augmented glutamatergic input and diminished GABAergic input serve as the cellular and molecular basis of increased excitability of PVN presympathetic neurons, leading to heightened sympathetic outflow in hypertension. In addition to the synaptic plasticity described above, mineralocorticoid receptors and AT1 receptors in the PVN play a major role in the development of hypertension induced by aldosterone-salt or angiotensin II administration in rats (24, 51, 171). Also, brain-derived neurotrophic factor (BDNF) and its receptor TrkB in the PVN play a role in angiotensin II-induced hypertensions in rats (43, 151). However, it remains uncertain how BDNF-TrkB signaling and mineralocorticoid receptors contribute to synaptic plasticity of PVN presympathetic neurons in hypertension (111).
EFFECTS OF EXERCISE TRAINING ON THE PVN AND AUTONOMIC CONTROL IN HYPERTENSION
Apart from increased sympathetic vasomotor activity, hypertension is accompanied by increased expression of the vasoconstrictor, trophic, and proinflammatory axis of the renin-angiotensin system (RAS) and decreased expression of the contraregulatory vasodilator, antitrophic, and anti-inflammatory axis in the PVN and other brain regions that regulate the autonomic nervous system (4, 37, 46). Indeed, an imbalance between RAS axes, with the predominance of angiotensin II over angiotensin-(1-7)-mediated effects, occurs in hypertensive and old rodents (4, 126). In SHRs, augmented angiotensinogen and AT1 receptor expression levels are also accompanied by a high level of oxidative stress and increased synthesis of proinflammatory cytokines in the PVN, NTS, and RVLM (116, 158, 182). These changes are associated with impaired baroreceptor reflex control, increased blood pressure fluctuation, and reduced heart rate variability (73, 130), resulting in increased sympathetic outflow to the heart and blood vessels and reduced parasympathetic modulation of the heart in SHRs (10, 117). Increased blood-brain barrier (BBB) permeability may be another mechanism contributing to autonomic dysfunction in hypertension (9). BBB dysfunction allows circulating angiotensin II to enter the brain parenchyma to cause further increases in proinflammatory cytokines, oxidative stress, and sympathoexcitatory responses.
Low to moderate aerobic training can reduce or counterbalance the deleterious effects of hypertension on the autonomic control (45, 116, 117). Aerobic training in SHRs reduces angiotensinogen and angiotensin II levels within the PVN (Fig. 3A) and sympathetic vasomotor activity. It also normalizes sympathovagal balance to the heart and baroreceptor sensitivity (Fig. 4) (23, 116). In SHRs, exercise training also produces a transient, but not maintained, increase in Mas receptor expression and a reduction in AT1 receptor levels in the PVN in SHRs. Interestingly, the reduction in AT1 receptor expression is observed after downregulation of the brain RAS and improvement of the autonomic control in SHRs after exercise training (23). Similar effects of exercise training on the autonomic control by the PVN, including enhancing the inhibitory neuronal activity by nitric oxide and GABA and normalizing the excitatory glutamatergic and angiotensinergic input, have been shown in rats with heart failure (132, 133).
Fig. 3.
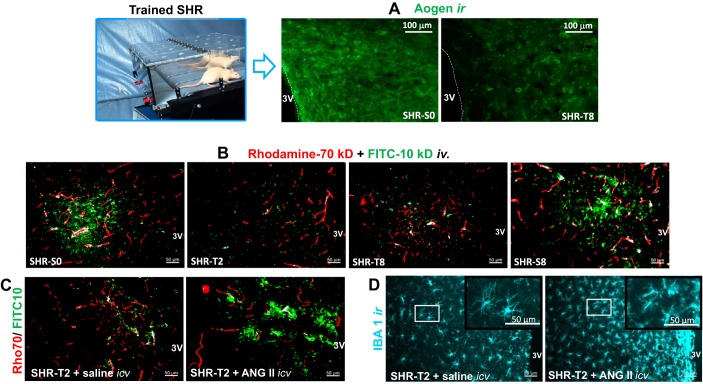
Effects of exercise training on the paraventricular nucleus (PVN) in spontaneously hypertensive rats (SHRs). Exercise training (T) decreases angiotensinogen immunoreactivity (Aogen ir; A) content and causes a prompt and maintained (from second up to eighth week of training) reduction on FITC-10 kDa (green) extravasation from capillaries (rhodamine-70 kDa, red) into the brain parenchyma (B). Simultaneous intracerebroventricular infusion of angiotensin II in trained SHRs blocks the beneficial effects of training observed in saline-infused SHRs (C) and augments microglia activation in the PVN (IBA1 immunoreactivity; D). [Modified from Chaar et al. (23) and Buttler et al. (17) with permission.]
Fig. 4.
Effects of a 2-wk exercise training (T) period on baroreceptor reflex control of heart rate (HR) in the spontaneously hypertensive rat (SHR) model. Right: sequential events induced by exercise training on local blood-brain barrier (BBB), the brain renin-angiotensin system, paraventricular nucleus intracellular signaling pathways, and neuronal activity-driven changes on baroreflex sensitivity (BrS). The baroreceptor function curve of sedentary normotensive rats (WKY-S) is shown for comparison. HR, heart rate [in beats/min (bpm)]; MAP, mean arterial pressure; Aogen ir, angiotensinogen immunoreactivity; PICs, proinflammatory cytokines; SNA, sympathetic nerve activity; PSNA, parasympathetic nerve activity; SAP, systolic arterial pressure; PI, pulse interval. *P < 0.05, vs. WKY-S; †P < 0.05, vs. SHR-S. [Modified from Masson et al. (116) with permission.]
A 2-wk period of aerobic training effectively restores BBB integrity within the PVN in SHRs (Fig. 3B). BBB dysfunction, characterized by the intense FITC (10 kDa) extravasation into the brain parenchyma present in sedentary SHRs, is markedly reduced by exercise training (17). Similar effects of exercise training on the attenuation of BBB leakage also occur in the NTS and RVLM in SHRs (17). A 2-wk exercise training period also reduces sympathetic vasomotor activity and the sympathovagal balance to the heart, and both changes are positively correlated with a decrease in BBB leakage within the PVN (17). Intracerebroventricular injection of a subpressor dose of angiotensin II blocks the beneficial effect of training on BBB leakage (Fig. 3C) (17). Angiotensin II infusion also abrogates the effect of training on reducing microglial activation in the PVN in SHRs (Fig. 3D) (17).
Exercise training also normalizes the increased expression of both the p47phox and gp91phox subunits of NADPH oxidase, an important downstream target of angiotensin II, and reduces the increased concentration of superoxide anion and hydrogen peroxide in the PVN (Fig. 4) (116). Exercise training does not change the expression level of p38 kinase, but it reduces ERK1/2 phosphorylation in the PVN in SHRs (Fig. 4). Furthermore, training reduces NF-κB translocation to the nucleus, therefore decreasing protein synthesis stimulated by angiotensin II and reactive oxygen species (116). In addition, both mRNA and protein levels of TNF-α and IL-6 in the PVN in trained SHRs are normalized to levels seen in age-matched normotensive rats (116). A 2-wk training period completely normalizes baroreceptor reflex sensitivity in SHRs (Fig. 4), an effect that precedes the reduction in resting arterial blood pressure (116).
Reactive oxygen species can alter ion channel properties and change neuronal excitability, and high concentrations of reactive oxygen radicals compromise neuronal excitability and the capability of generating action potentials (131, 135). Inflammatory cytokines such as TNF-α, IL-6, and IL-1β exhibit neuromodulatory properties on neuronal excitability (169). IL-10, an anti-inflammatory cytokine, acts on PVN neurons to offset the neuronal excitatory actions of angiotensin II (72). By reducing both oxidative stress and the proinflammatory profile in the PVN of SHRs, exercise training may change the redox balance toward an antioxidative/anti-inflammatory profile to improve neuronal function. These changes in the brain parenchyma may affect the neuronal circuitry involved in the control of the autonomic nervous system.
Oxytocinergic (OTergic) PVN neurons may also be involved in the beneficial effects produced by exercise training. Exercise training can increase mRNA and protein levels of oxytocin in both SHRs and normotensive control rats and increases the density of dorsal brain stem-projecting OTergic neurons in the PVN (21, 114, 121). Training augments the excitability of PVN OTergic neurons projecting to the brain stem (68), and exercise-induced OTergic drive to the NTS/DMV complex facilitates the appearance of resting bradycardia and the reduction in tachycardia associated with exercise (12, 60). Both exercise training and oxytocin administration into the NTS/DMV of sedentary rats equally improve the baroreceptor reflex control of heart rate (61). These effects are mediated by the oxytocin-induced increase in vagal outflow to the heart (61, 120). In addition, sinoaortic denervation abrogates the exercise-induced increase in the density of PVN OTergic neurons and simultaneously blocks the beneficial effects of training on the cardiovascular control (21).
These findings together indicate that hypertension impairs and exercise training improves the autonomic control of the circulation by affecting BBB function, the angiotensin II content, and the downstream signaling in the PVN. Exercise training can restore normal neuronal activity in the PVN, which is a critical region for the autonomic control of the circulation in hypertension. Although the mechanisms mediating the effect of excise training on BBB function are not clear, the similar temporal changes, such as the reduction in RAS, oxidative stress, proinflammatory mediators, and microglia activation, have been observed in the PVN. Thus, these factors may be involved in improving BBB function by exercise training.
Summary and Perspectives
As discussed in the first part of this review, the primary function of PVN presympathetic neurons in normal animals is to regulate sympathetic responses to longer-term challenges, such as sustained water deprivation, chronic intermittent hypoxia, and pregnancy. In normotensive animals, PVN presympathetic neurons do not make a significant contribution to the maintenance of resting SNA and blood pressure. In contrast, these neurons do make a major contribution to the increased sympathetic vasomotor activity that is responsible for hypertension.
Recent studies in the SHR have provided considerable evidence showing that the hyperactivity of PVN presympathetic neurons in hypertension is due to an imbalance of excitatory and inhibitory synaptic inputs to these neurons, resulting in heightened sympathetic vasomotor tone in hypertension. However, our understanding of the molecular or signaling mechanisms responsible for the increased NMDAR and Ca2+-permeable AMPAR activity in the PVN in hypertension remains incomplete. Also, the epigenetic mechanism responsible for the increased expression level of NKCC1 and mGluR5 in the PVN in hypertension warrants further studies. Because glutamate and GABA receptors are important for many physiological functions, directly targeting these receptors often produces intolerable adverse effects. NKCC1 and specific protein kinases or α2δ-1-bound NMDARs responsible for aberrant NMDAR activity in the PVN may be opportune targets for treating neurogenic hypertension. For example, gabapentinoids (gabapentin and pregabalin), commonly used for treating patients with neuropathic pain, act predominantly on α2δ-1-bound NMDARs (25). Because gabapentinoids do not affect basal α2δ-1-free NMDARs, targeting α2δ-1-bound NMDARs with these drugs could circumvent the adverse effects caused by blocking physiological NMDARs with nonselective NMDAR antagonists. It has been previously reported that gabapentinoids reduce tracheal intubation-elicited pressor response and brain injury caused paroxysmal sympathetic hyperactivity in patients (28, 40, 44). Thus, gabapentinoids may be considered for treating neurogenic hypertension.
Exercise training has emerged as an effective intervention for the management of hypertension. Regular physical activity is beneficial for treating hypertension without the side effects associated with pharmacological agents. Accumulating experimental evidence indicates that exercise training reverses several deficits induced by neurogenic hypertension. Exercise training can correct BBB dysfunction, downregulate the brain RAS, and reduce oxidative stress and the inflammatory profile in the PVN, thereby improving autonomic modulation and baroreflex control of the circulatory system. Further studies are needed to delineate precisely how exercise training differentially affects sympathetic and parasympathetic outflows in hypertension.
GRANTS
Work conducted in the laboratories of the authors was supported by the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia, São Paulo Research Foundation Grants 2011/51410-9 and 2015/24935-4 (Brazil), and National Heart, Lung, and Blood Institute Grants HL-131161 and HL-139523.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A.D., L.C.M., D.-P.L., and H.-L.P. drafted manuscript; R.A.D., L.C.M., D.-P.L., and H.-L.P. edited and revised manuscript; R.A.D., L.C.M., D.-P.L., and H.-L.P. approved final version of manuscript.
REFERENCES
- 1.Affleck VS, Coote JH, Pyner S. The projection and synaptic organisation of NTS afferent connections with presympathetic neurons, GABA and nNOS neurons in the paraventricular nucleus of the hypothalamus. Neuroscience 219: 48–61, 2012. doi: 10.1016/j.neuroscience.2012.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akine A, Montanaro M, Allen AM. Hypothalamic paraventricular nucleus inhibition decreases renal sympathetic nerve activity in hypertensive and normotensive rats. Auton Neurosci 108: 17–21, 2003. doi: 10.1016/j.autneu.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39: 275–280, 2002. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 4.Arnold AC, Gallagher PE, Diz DI. Brain renin-angiotensin system in the nexus of hypertension and aging. Hypertens Res 36: 5–13, 2013. doi: 10.1038/hr.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badoer E. Neurons in the hypothalamic paraventricular nucleus that project to the rostral ventrolateral medulla are not activated by hypotension. Brain Res 801: 224–227, 1998. doi: 10.1016/S0006-8993(98)00560-5. [DOI] [PubMed] [Google Scholar]
- 6.Badoer E, McKinley MJ, Oldfield BJ, McAllen RM. A comparison of hypotensive and non-hypotensive hemorrhage on Fos expression in spinally projecting neurons of the paraventricular nucleus and rostral ventrolateral medulla. Brain Res 610: 216–223, 1993. doi: 10.1016/0006-8993(93)91403-F. [DOI] [PubMed] [Google Scholar]
- 7.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regul Integr Comp Physiol 268: R625–R633, 1995. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- 8.Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res 15: 254–263, 2005. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- 9.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 63: 572–579, 2014. doi: 10.1161/HYPERTENSIONAHA.113.01743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco JH, Gastaldi AC, Gardim CB, Araujo JE, Simões MV, Oliveira LF, Carvalho EE, Souza HC. Chronic cholinergic stimulation promotes changes in cardiovascular autonomic control in spontaneously hypertensive rats. Auton Neurosci 193: 97–103, 2015. doi: 10.1016/j.autneu.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15: 453–462, 1995. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 12.Braga DC, Mori E, Higa KT, Morris M, Michelini LC. Central oxytocin modulates exercise-induced tachycardia. Am J Physiol Regul Integr Comp Physiol 278: R1474–R1482, 2000. doi: 10.1152/ajpregu.2000.278.6.R1474. [DOI] [PubMed] [Google Scholar]
- 13.Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 299: R439–R451, 2010. doi: 10.1152/ajpregu.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks VL, Freeman KL, Clow KA. Excitatory amino acids in rostral ventrolateral medulla support blood pressure during water deprivation in rats. Am J Physiol Heart Circ Physiol 286: H1642–H1648, 2004. doi: 10.1152/ajpheart.01004.2003. [DOI] [PubMed] [Google Scholar]
- 15.Brooks VL, Kane CM, Van Winkle DM. Altered heart rate baroreflex during pregnancy: role of sympathetic and parasympathetic nervous systems. Am J Physiol Regul Integr Comp Physiol 273: R960–R966, 1997. doi: 10.1152/ajpregu.1997.273.3.R960. [DOI] [PubMed] [Google Scholar]
- 16.Brooks VL, Quesnell RR, Kane CM, Keil LC. Hemodynamic and hormonal responses to hemorrhage in conscious rabbits at mid- and late gestation. Am J Physiol Regul Integr Comp Physiol 275: R1082–R1090, 1998. doi: 10.1152/ajpregu.1998.275.4.R1082. [DOI] [PubMed] [Google Scholar]
- 17.Buttler L, Jordão MT, Fragas MG, Ruggeri A, Ceroni A, Michelini LC. Maintenance of blood-brain barrier integrity in hypertension: a novel benefit of exercise training for autonomic control. Front Physiol 8: 1048, 2017. doi: 10.3389/fphys.2017.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carillo BA, Oliveira-Sales EB, Andersen M, Tufik S, Hipolide D, Santos AA, Tucci PJ, Bergamaschi CT, Campos RR. Changes in GABAergic inputs in the paraventricular nucleus maintain sympathetic vasomotor tone in chronic heart failure. Auton Neurosci 171: 41–48, 2012. doi: 10.1016/j.autneu.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Carrive P, Gorissen M. Premotor sympathetic neurons of conditioned fear in the rat. Eur J Neurosci 28: 428–446, 2008. doi: 10.1111/j.1460-9568.2008.06351.x. [DOI] [PubMed] [Google Scholar]
- 20.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol 589: 1643–1662, 2011. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavalleri MT, Burgi K, Cruz JC, Jordão MT, Ceroni A, Michelini LC. Afferent signaling drives oxytocinergic preautonomic neurons and mediates training-induced plasticity. Am J Physiol Regul Integr Comp Physiol 301: R958–R966, 2011. doi: 10.1152/ajpregu.00104.2011. [DOI] [PubMed] [Google Scholar]
- 22.Cechetto DF, Saper CB. Neurochemical organization of the hypothalamic projection to the spinal cord in the rat. J Comp Neurol 272: 579–604, 1988. doi: 10.1002/cne.902720410. [DOI] [PubMed] [Google Scholar]
- 23.Chaar LJ, Alves TP, Batista Junior AM, Michelini LC. Early training-induced reduction of angiotensinogen in autonomic areas-the main effect of exercise on brain renin-angiotensin system in hypertensive rats. PLoS One 10: e0137395, 2015. doi: 10.1371/journal.pone.0137395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen A, Huang BS, Wang HW, Ahmad M, Leenen FH. Knockdown of mineralocorticoid or angiotensin II type 1 receptor gene expression in the paraventricular nucleus prevents angiotensin II hypertension in rats. J Physiol 592: 3523–3536, 2014. doi: 10.1113/jphysiol.2014.275560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Li L, Chen SR, Chen H, Xie JD, Sirrieh RE, MacLean DM, Zhang Y, Zhou MH, Jayaraman V, Pan HL. The α2δ-1-NMDA receptor complex is critically involved in neuropathic pain development and gabapentin therapeutic actions. Cell Rep 22: 2307–2321, 2018. doi: 10.1016/j.celrep.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen QH, Toney GM. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 118: 797–807, 2003. doi: 10.1016/S0306-4522(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 27.Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol 103: 4–15, 2010. doi: 10.1152/jn.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HA, Jeon SB, Samuel S, Allison T, Lee K. Paroxysmal sympathetic hyperactivity after acute brain injury. Curr Neurol Neurosci Rep 13: 370, 2013. doi: 10.1007/s11910-013-0370-3. [DOI] [PubMed] [Google Scholar]
- 29.Cohen WR, Galen LH, Vega-Rich M, Young JB. Cardiac sympathetic activity during rat pregnancy. Metabolism 37: 771–777, 1988. doi: 10.1016/0026-0495(88)90013-3. [DOI] [PubMed] [Google Scholar]
- 30.Coldren KM, Li DP, Kline DD, Hasser EM, Heesch CM. Acute hypoxia activates neuroendocrine, but not presympathetic, neurons in the paraventricular nucleus of the hypothalamus: differential role of nitric oxide. Am J Physiol Regul Integr Comp Physiol 312: R982–R995, 2017. doi: 10.1152/ajpregu.00543.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol 90: 169–173, 2005. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- 32.Cork SC, Chazot PL, Pyner S. Altered GABAA α5 subunit expression in the hypothalamic paraventricular nucleus of hypertensive and pregnant rats. Neurosci Lett 620: 148–153, 2016. doi: 10.1016/j.neulet.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Cruz JC, Bonagamba LG, Machado BH, Biancardi VC, Stern JE. Intermittent activation of peripheral chemoreceptors in awake rats induces Fos expression in rostral ventrolateral medulla-projecting neurons in the paraventricular nucleus of the hypothalamus. Neuroscience 157: 463–472, 2008. doi: 10.1016/j.neuroscience.2008.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dampney RA. Central mechanisms regulating coordinated cardiovascular and respiratory function during stress and arousal. Am J Physiol Regul Integr Comp Physiol 309: R429–R443, 2015. doi: 10.1152/ajpregu.00051.2015. [DOI] [PubMed] [Google Scholar]
- 35.Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol 29: 261–268, 2002. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- 36.Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog Neurobiol 71: 359–384, 2003. doi: 10.1016/j.pneurobio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Dange RB, Agarwal D, Teruyama R, Francis J. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflammation 12: 31, 2015. doi: 10.1186/s12974-015-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daubert DL, Chung MY, Brooks VL. Insulin resistance and impaired baroreflex gain during pregnancy. Am J Physiol Regul Integr Comp Physiol 292: R2188–R2195, 2007. doi: 10.1152/ajpregu.00614.2006. [DOI] [PubMed] [Google Scholar]
- 39.Davern PJ, Head GA. Fos-related antigen immunoreactivity after acute and chronic angiotensin II-induced hypertension in the rabbit brain. Hypertension 49: 1170–1177, 2007. doi: 10.1161/HYPERTENSIONAHA.106.086322. [DOI] [PubMed] [Google Scholar]
- 40.Doleman B, Sherwin M, Lund JN, Williams JP. Gabapentin for the hemodynamic response to intubation: systematic review and meta-analysis [in French]. Can J Anaesth 63: 1042–1058, 2016. doi: 10.1007/s12630-016-0668-0. [DOI] [PubMed] [Google Scholar]
- 41.Donella-Deana A, Cesaro L, Sarno S, Ruzzene M, Brunati AM, Marin O, Vilk G, Doherty-Kirby A, Lajoie G, Litchfield DW, Pinna LA. Tyrosine phosphorylation of protein kinase CK2 by Src-related tyrosine kinases correlates with increased catalytic activity. Biochem J 372: 841–849, 2003. doi: 10.1042/bj20021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci USA 92: 9298–9302, 1995. doi: 10.1073/pnas.92.20.9298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erdos B, Backes I, McCowan ML, Hayward LF, Scheuer DA. Brain-derived neurotrophic factor modulates angiotensin signaling in the hypothalamus to increase blood pressure in rats. Am J Physiol Heart Circ Physiol 308: H612–H622, 2015. doi: 10.1152/ajpheart.00776.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fassoulaki A, Melemeni A, Paraskeva A, Petropoulos G. Gabapentin attenuates the pressor response to direct laryngoscopy and tracheal intubation. Br J Anaesth 96: 769–773, 2006. doi: 10.1093/bja/ael076. [DOI] [PubMed] [Google Scholar]
- 45.Felix JV, Michelini LC. Training-induced pressure fall in spontaneously hypertensive rats is associated with reduced angiotensinogen mRNA expression within the nucleus tractus solitarii. Hypertension 50: 780–785, 2007. doi: 10.1161/HYPERTENSIONAHA.107.094474. [DOI] [PubMed] [Google Scholar]
- 46.Ferrario CM. ACE2: more of Ang-(1–7) or less Ang II? Curr Opin Nephrol Hypertens 20: 1–6, 2011. doi: 10.1097/MNH.0b013e3283406f57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Freeman KL, Brooks VL. AT1 and glutamatergic receptors in paraventricular nucleus support blood pressure during water deprivation. Am J Physiol Regul Integr Comp Physiol 292: R1675–R1682, 2007. doi: 10.1152/ajpregu.00623.2006. [DOI] [PubMed] [Google Scholar]
- 48.Fuchs E, Jöhren O, Flügge G. Psychosocial conflict in the tree shrew: effects on sympathoadrenal activity and blood pressure. Psychoneuroendocrinology 18: 557–565, 1993. doi: 10.1016/0306-4530(93)90033-H. [DOI] [PubMed] [Google Scholar]
- 49.Furlong TM, McDowall LM, Horiuchi J, Polson JW, Dampney RA. The effect of air puff stress on c-Fos expression in rat hypothalamus and brainstem: central circuitry mediating sympathoexcitation and baroreflex resetting. Eur J Neurosci 39: 1429–1438, 2014. doi: 10.1111/ejn.12521. [DOI] [PubMed] [Google Scholar]
- 50.Gabor A, Leenen FH. Cardiovascular effects of angiotensin II and glutamate in the PVN of Dahl salt-sensitive rats. Brain Res 1447: 28–37, 2012. doi: 10.1016/j.brainres.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 51.Gabor A, Leenen FH. Central mineralocorticoid receptors and the role of angiotensin II and glutamate in the paraventricular nucleus of rats with angiotensin II-induced hypertension. Hypertension 61: 1083–1090, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00797. [DOI] [PubMed] [Google Scholar]
- 52.Geerling JC, Shin JW, Chimenti PC, Loewy AD. Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol 518: 1460–1499, 2010. doi: 10.1002/cne.22283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glass MJ, Wang G, Coleman CG, Chan J, Ogorodnik E, Van Kempen TA, Milner TA, Butler SD, Young CN, Davisson RL, Iadecola C, Pickel VM. NMDA receptor plasticity in the hypothalamic paraventricular nucleus contributes to the elevated blood pressure produced by angiotensin II. J Neurosci 35: 9558–9567, 2015. doi: 10.1523/JNEUROSCI.2301-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenwood JP, Scott EM, Stoker JB, Walker JJ, Mary DA. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation 104: 2200–2204, 2001. doi: 10.1161/hc4301.098253. [DOI] [PubMed] [Google Scholar]
- 55.Gupta PD, Henry JP, Sinclair R, Von Baumgarten R. Responses of atrial and aortic baroreceptors to nonhypotensive hemorrhage and to tranfusion. Am J Physiol 211: 1429–1437, 1966. doi: 10.1152/ajplegacy.1966.211.6.1429. [DOI] [PubMed] [Google Scholar]
- 56.Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol 433: 222–238, 2001. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- 57.Hanssens M, Keirse MJ, Spitz B, van Assche FA. Angiotensin II levels in hypertensive and normotensive pregnancies. Br J Obstet Gynaecol 98: 155–161, 1991. doi: 10.1111/j.1471-0528.1991.tb13361.x. [DOI] [PubMed] [Google Scholar]
- 58.Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Syst 50: 1–11, 1994. doi: 10.1016/0165-1838(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 59.Heesch CM, Zheng H, Foley CM, Mueller PJ, Hasser EM, Patel KP. Nitric oxide synthase activity and expression are decreased in the paraventricular nucleus of pregnant rats. Brain Res 1251: 140–150, 2009. doi: 10.1016/j.brainres.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higa-Taniguchi KT, Felix JV, Michelini LC. Brainstem oxytocinergic modulation of heart rate control in rats: effects of hypertension and exercise training. Exp Physiol 94: 1103–1113, 2009. doi: 10.1113/expphysiol.2009.049262. [DOI] [PubMed] [Google Scholar]
- 61.Higa KT, Mori E, Viana FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary-vagal complex. Am J Physiol Regul Integr Comp Physiol 282: R537–R545, 2002. doi: 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- 62.Hines T, Beauchamp D, Rice C. Baroreflex control of sympathetic nerve activity in hypertensive pregnant rats with reduced uterine perfusion. Hypertens Pregnancy 26: 303–314, 2007. doi: 10.1080/10641950701415598. [DOI] [PubMed] [Google Scholar]
- 63.Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience 80: 1209–1224, 1997. doi: 10.1016/S0306-4522(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 64.Holbein WW, Bardgett ME, Toney GM. Blood pressure is maintained during dehydration by hypothalamic paraventricular nucleus-driven tonic sympathetic nerve activity. J Physiol 592: 3783–3799, 2014. doi: 10.1113/jphysiol.2014.276261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science 252: 851–853, 1991. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 66.Horiuchi J, Atik A, Iigaya K, McDowall LM, Killinger S, Dampney RA. Activation of 5-hydroxytryptamine-1A receptors suppresses cardiovascular responses evoked from the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 301: R1088–R1097, 2011. doi: 10.1152/ajpregu.00144.2011. [DOI] [PubMed] [Google Scholar]
- 67.Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54: 859–871, 2007. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Jackson K, Silva HM, Zhang W, Michelini LC, Stern JE. Exercise training differentially affects intrinsic excitability of autonomic and neuroendocrine neurons in the hypothalamic paraventricular nucleus. J Neurophysiol 94: 3211–3220, 2005. doi: 10.1152/jn.00277.2005. [DOI] [PubMed] [Google Scholar]
- 69.Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science 270: 644–646, 1995. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 70.Jarvis SS, Shibata S, Bivens TB, Okada Y, Casey BM, Levine BD, Fu Q. Sympathetic activation during early pregnancy in humans. J Physiol 590: 3535–3543, 2012. doi: 10.1113/jphysiol.2012.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jhanwar-Uniyal M, Beck B, Jhanwar YS, Burlet C, Leibowitz SF. Neuropeptide Y projection from arcuate nucleus to parvocellular division of paraventricular nucleus: specific relation to the ingestion of carbohydrate. Brain Res 631: 97–106, 1993. doi: 10.1016/0006-8993(93)91192-U. [DOI] [PubMed] [Google Scholar]
- 72.Jiang N, Shi P, Desland F, Kitchen-Pareja MC, Sumners C. Interleukin-10 inhibits angiotensin II-induced decrease in neuronal potassium current. Am J Physiol Cell Physiol 304: C801–C807, 2013. doi: 10.1152/ajpcell.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson M, Gao SA, Friberg P, Annerstedt M, Carlström J, Ivarsson T, Jensen G, Ljungman S, Mathillas O, Nielsen FD, Strömbom U. Baroreflex effectiveness index and baroreflex sensitivity predict all-cause mortality and sudden death in hypertensive patients with chronic renal failure. J Hypertens 25: 163–168, 2007. doi: 10.1097/01.hjh.0000254377.18983.eb. [DOI] [PubMed] [Google Scholar]
- 74.Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature 330: 163–165, 1987. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- 75.Kaila K, Voipio J, Paalasmaa P, Pasternack M, Deisz RA. The role of bicarbonate in GABAA receptor-mediated IPSPs of rat neocortical neurones. J Physiol 464: 273–289, 1993. doi: 10.1113/jphysiol.1993.sp019634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanbar R, Oréa V, Barrès C, Julien C. Baroreflex control of renal sympathetic nerve activity during air-jet stress in rats. Am J Physiol Regul Integr Comp Physiol 292: R362–R367, 2007. doi: 10.1152/ajpregu.00413.2006. [DOI] [PubMed] [Google Scholar]
- 77.Kapusta DR, Pascale CL, Kuwabara JT, Wainford RD. Central nervous system Gαi2-subunit proteins maintain salt resistance via a renal nerve-dependent sympathoinhibitory pathway. Hypertension 61: 368–375, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kapusta DR, Pascale CL, Wainford RD. Brain heterotrimeric Gαi2-subunit protein-gated pathways mediate central sympathoinhibition to maintain fluid and electrolyte homeostasis during stress. FASEB J 26: 2776–2787, 2012. doi: 10.1096/fj.11-196550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karim F, Kidd C, Malpus CM, Penna PE. The effects of stimulation of the left atrial receptors on sympathetic efferent nerve activity. J Physiol 227: 243–260, 1972. doi: 10.1113/jphysiol.1972.sp010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kenney MJ, Weiss ML, Patel KP, Wang Y, Fels RJ. Paraventricular nucleus bicuculline alters frequency components of sympathetic nerve discharge bursts. Am J Physiol Heart Circ Physiol 281: H1233–H1241, 2001. doi: 10.1152/ajpheart.2001.281.3.H1233. [DOI] [PubMed] [Google Scholar]
- 81.Kim YB, Kim YS, Kim WB, Shen FY, Lee SW, Chung HJ, Kim JS, Han HC, Colwell CS, Kim YI. GABAergic excitation of vasopressin neurons: possible mechanism underlying sodium-dependent hypertension. Circ Res 113: 1296–1307, 2013. doi: 10.1161/CIRCRESAHA.113.301814. [DOI] [PubMed] [Google Scholar]
- 82.King TL, Heesch CM, Clark CG, Kline DD, Hasser EM. Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol 302: R1219–R1232, 2012. doi: 10.1152/ajpregu.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.King TL, Kline DD, Ruyle BC, Heesch CM, Hasser EM. Acute systemic hypoxia activates hypothalamic paraventricular nucleus-projecting catecholaminergic neurons in the caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 305: R1112–R1123, 2013. doi: 10.1152/ajpregu.00280.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kleiber AC, Zheng H, Schultz HD, Peuler JD, Patel KP. Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure. Am J Physiol Regul Integr Comp Physiol 294: R1863–R1872, 2008. doi: 10.1152/ajpregu.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kleiber AC, Zheng H, Sharma NM, Patel KP. Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure. Am J Physiol Heart Circ Physiol 298: H1546–H1555, 2010. doi: 10.1152/ajpheart.01006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kopp UC, Smith LA, DiBona GF. Facilitatory role of efferent renal nerve activity on renal sensory receptors. Am J Physiol Renal Physiol 253: F767–F777, 1987. doi: 10.1152/ajprenal.1987.253.4.F767. [DOI] [PubMed] [Google Scholar]
- 87.Kozicz T, Bordewin LA, Czéh B, Fuchs E, Roubos EW. Chronic psychosocial stress affects corticotropin-releasing factor in the paraventricular nucleus and central extended amygdala as well as urocortin 1 in the non-preganglionic Edinger-Westphal nucleus of the tree shrew. Psychoneuroendocrinology 33: 741–754, 2008. doi: 10.1016/j.psyneuen.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 88.Kunkler PE, Hwang BH. Lower GABAA receptor binding in the amygdala and hypothalamus of spontaneously hypertensive rats. Brain Res Bull 36: 57–61, 1995. doi: 10.1016/0361-9230(94)00164-V. [DOI] [PubMed] [Google Scholar]
- 89.Kuo CD, Chen GY, Yang MJ, Lo HM, Tsai YS. Biphasic changes in autonomic nervous activity during pregnancy. Br J Anaesth 84: 323–329, 2000. doi: 10.1093/oxfordjournals.bja.a013433. [DOI] [PubMed] [Google Scholar]
- 90.Kvochina L, Hasser EM, Heesch CM. Pregnancy decreases GABAergic inhibition of the hypothalamic paraventricular nucleus. Physiol Behav 97: 171–179, 2009. doi: 10.1016/j.physbeh.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Laffer CL, Scott RC III, Titze JM, Luft FC, Elijovich F. Hemodynamics and salt-and-water balance link sodium storage and vascular dysfunction in salt-sensitive subjects. Hypertension 68: 195–203, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.LaGrange LP, Toney GM, Bishop VS. Chronic angiotensin II infusion attenuates the renal sympathoinhibitory response to acute volume expansion. Am J Physiol Regul Integr Comp Physiol 284: R1098–R1107, 2003. doi: 10.1152/ajpregu.00165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li DP, Byan HS, Pan HL. Switch to glutamate receptor 2-lacking AMPA receptors increases neuronal excitability in hypothalamus and sympathetic drive in hypertension. J Neurosci 32: 372–380, 2012. doi: 10.1523/JNEUROSCI.3222-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49: 916–925, 2007. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- 95.Li DP, Pan HL. Glutamatergic regulation of hypothalamic presympathetic neurons in hypertension. Curr Hypertens Rep 19: 78, 2017. doi: 10.1007/s11906-017-0776-4. [DOI] [PubMed] [Google Scholar]
- 96.Li DP, Pan HL. Increased group I metabotropic glutamate receptor activity in paraventricular nucleus supports elevated sympathetic vasomotor tone in hypertension. Am J Physiol Regul Integr Comp Physiol 299: R552–R561, 2010. doi: 10.1152/ajpregu.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol 290: H1110–H1119, 2006. doi: 10.1152/ajpheart.00788.2005. [DOI] [PubMed] [Google Scholar]
- 98.Li DP, Pan HL. Role of γ-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther 320: 615–626, 2007. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- 99.Li DP, Yang Q, Pan HM, Pan HL. Plasticity of pre- and postsynaptic GABAB receptor function in the paraventricular nucleus in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 295: H807–H815, 2008. doi: 10.1152/ajpheart.00259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol 586: 1637–1647, 2008. doi: 10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li DP, Zhou JJ, Pan HL. Endogenous casein kinase-1 modulates NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Physiol 593: 4439–4452, 2015. doi: 10.1113/JP270831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li DP, Zhou JJ, Zhang J, Pan HL. CaMKII regulates synaptic NMDA receptor activity of hypothalamic presympathetic neurons and sympathetic outflow in hypertension. J Neurosci 37: 10690–10699, 2017. doi: 10.1523/JNEUROSCI.2141-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li DP, Zhu LH, Pachuau J, Lee HA, Pan HL. mGluR5 Upregulation increases excitability of hypothalamic presympathetic neurons through NMDA receptor trafficking in spontaneously hypertensive rats. J Neurosci 34: 4309–4317, 2014. doi: 10.1523/JNEUROSCI.4295-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res 93: 990–997, 2003. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- 105.Lieberman DN, Mody I. Casein kinase-II regulates NMDA channel function in hippocampal neurons. Nat Neurosci 2: 125–132, 1999. doi: 10.1038/5680. [DOI] [PubMed] [Google Scholar]
- 106.Lohmeier TE, Hildebrandt DA, Hood WA. Renal nerves promote sodium excretion during long-term increases in salt intake. Hypertension 33: 487–492, 1999. doi: 10.1161/01.HYP.33.1.487. [DOI] [PubMed] [Google Scholar]
- 107.Lovick TA, Coote JH. Effects of volume loading on paraventriculo-spinal neurones in the rat. J Auton Nerv Syst 25: 135–140, 1988. doi: 10.1016/0165-1838(88)90018-5. [DOI] [PubMed] [Google Scholar]
- 108.Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res 454: 123–130, 1988. doi: 10.1016/0006-8993(88)90810-4. [DOI] [PubMed] [Google Scholar]
- 109.Lovick TA, Malpas S, Mahony MT. Renal vasodilatation in response to acute volume load is attenuated following lesions of parvocellular neurones in the paraventricular nucleus in rats. J Auton Nerv Syst 43: 247–255, 1993. doi: 10.1016/0165-1838(93)90331-N. [DOI] [PubMed] [Google Scholar]
- 110.Ludbrook J, Ventura S. Roles of carotid baroreceptor and cardiac afferents in hemodynamic responses to acute central hypovolemia. Am J Physiol Heart Circ Physiol 270: H1538–H1548, 1996. doi: 10.1152/ajpheart.1996.270.5.H1538. [DOI] [PubMed] [Google Scholar]
- 111.Ma H, Chen SR, Chen H, Li L, Li DP, Zhou JJ, Pan HL. α2δ-1 is essential for sympathetic output and NMDA receptor activity potentiated by angiotensin II in the hypothalamus. J Neurosci 38: 6388–6398, 2018. doi: 10.1523/JNEUROSCI.0447-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma H, Chen SR, Chen H, Zhou JJ, Li DP, Pan HL. α2δ-1 couples to NMDA receptors in the hypothalamus to sustain sympathetic vasomotor activity in hypertension. J Physiol 596: 4269–4283, 2018. doi: 10.1113/JP276394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav 73: 147–158, 2002. doi: 10.1016/S0091-3057(02)00791-8. [DOI] [PubMed] [Google Scholar]
- 114.Martins AS, Crescenzi A, Stern JE, Bordin S, Michelini LC. Hypertension and exercise training differentially affect oxytocin and oxytocin receptor expression in the brain. Hypertension 46: 1004–1009, 2005. doi: 10.1161/01.HYP.0000175812.03322.59. [DOI] [PubMed] [Google Scholar]
- 115.Masilamani S, Heesch CM. Effects of pregnancy and progesterone metabolites on arterial baroreflex in conscious rats. Am J Physiol Regul Integr Comp Physiol 272: R924–R934, 1997. doi: 10.1152/ajpregu.1997.272.3.R924. [DOI] [PubMed] [Google Scholar]
- 116.Masson GS, Costa TS, Yshii L, Fernandes DC, Soares PP, Laurindo FR, Scavone C, Michelini LC. Time-dependent effects of training on cardiovascular control in spontaneously hypertensive rats: role for brain oxidative stress and inflammation and baroreflex sensitivity. PLoS One 9: e94927, 2014. doi: 10.1371/journal.pone.0094927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masson GS, Nair AR, Silva Soares PP, Michelini LC, Francis J. Aerobic training normalizes autonomic dysfunction, HMGB1 content, microglia activation and inflammation in hypothalamic paraventricular nucleus of SHR. Am J Physiol Heart Circ Physiol 309: H1115–H1122, 2015. doi: 10.1152/ajpheart.00349.2015. [DOI] [PubMed] [Google Scholar]
- 118.McKinley MJ, Pennington GL, Oldfield BJ. Anteroventral wall of the third ventricle and dorsal lamina terminalis: headquarters for control of body fluid homeostasis? Clin Exp Pharmacol Physiol 23: 271–281, 1996. doi: 10.1111/j.1440-1681.1996.tb02823.x. [DOI] [PubMed] [Google Scholar]
- 119.McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, Martelli D. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 214: 8–32, 2015. doi: 10.1111/apha.12487. [DOI] [PubMed] [Google Scholar]
- 120.Michelini LC. Differential effects of vasopressinergic and oxytocinergic pre-autonomic neurons on circulatory control: reflex mechanisms and changes during exercise. Clin Exp Pharmacol Physiol 34: 369–376, 2007. doi: 10.1111/j.1440-1681.2007.04589.x. [DOI] [PubMed] [Google Scholar]
- 121.Michelini LC, Stern JE. Exercise-induced neuronal plasticity in central autonomic networks: role in cardiovascular control. Exp Physiol 94: 947–960, 2009. doi: 10.1113/expphysiol.2009.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mifflin S, Cunningham JT, Toney GM. Neurogenic mechanisms underlying the rapid onset of sympathetic responses to intermittent hypoxia. J Appl Physiol 119: 1441–1448, 2015. doi: 10.1152/japplphysiol.00198.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyajima E, Yamada Y. Reduced sympathetic inhibition in salt-sensitive Japanese young adults. Am J Hypertens 12: 1195–1200, 1999. doi: 10.1016/S0895-7061(99)00122-3. [DOI] [PubMed] [Google Scholar]
- 124.Müller CJ, Quintino-Dos-Santos JW, Schimitel FG, Tufik S, Beijamini V, Canteras NS, Schenberg LC. On the verge of a respiratory-type panic attack: selective activations of rostrolateral and caudoventrolateral periaqueductal gray matter following short-lasting escape to a low dose of potassium cyanide. Neuroscience 348: 228–240, 2017. doi: 10.1016/j.neuroscience.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 125.Nishi H, Hashimoto K, Panchenko AR. Phosphorylation in protein-protein binding: effect on stability and function. Structure 19: 1807–1815, 2011. doi: 10.1016/j.str.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.O’Callaghan EL, Choong YT, Jancovski N, Allen AM. Central angiotensinergic mechanisms associated with hypertension. Auton Neurosci 175: 85–92, 2013. doi: 10.1016/j.autneu.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 127.O’Donohue TL, Jacobowitz DM. Studies of alpha-MSH-containing nerves in the brain. Prog Biochem Pharmacol 16: 69–83, 1980. [PubMed] [Google Scholar]
- 128.O’Hagan KP, Casey SM. Arterial baroreflex during pregnancy and renal sympathetic nerve activity during parturition in rabbits. Am J Physiol Heart Circ Physiol 274: H1635–H1642, 1998. doi: 10.1152/ajpheart.1998.274.5.H1635. [DOI] [PubMed] [Google Scholar]
- 129.Olivan MV, Bonagamba LG, Machado BH. Involvement of the paraventricular nucleus of the hypothalamus in the pressor response to chemoreflex activation in awake rats. Brain Res 895: 167–172, 2001. doi: 10.1016/S0006-8993(01)02067-4. [DOI] [PubMed] [Google Scholar]
- 130.Ormezzano O, Cracowski JL, Quesada JL, Pierre H, Mallion JM, Baguet JP. EVAluation of the prognostic value of BARoreflex sensitivity in hypertensive patients: the EVABAR study. J Hypertens 26: 1373–1378, 2008. doi: 10.1097/HJH.0b013e3283015e5a. [DOI] [PubMed] [Google Scholar]
- 131.Pardillo-Díaz R, Carrascal L, Muñoz MF, Ayala A, Nunez-Abades P. Time and dose dependent effects of oxidative stress induced by cumene hydroperoxide in neuronal excitability of rat motor cortex neurons. Neurotoxicology 53: 201–214, 2016. doi: 10.1016/j.neuro.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 132.Patel KP, Salgado HC, Liu X, Zheng H. Exercise training normalizes the blunted central component of the baroreflex in rats with heart failure: role of the PVN. Am J Physiol Heart Circ Physiol 305: H173–H181, 2013. doi: 10.1152/ajpheart.00009.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Patel KP, Zheng H. Central neural control of sympathetic nerve activity in heart failure following exercise training. Am J Physiol Heart Circ Physiol 302: H527–H537, 2012. doi: 10.1152/ajpheart.00676.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci 26: 199–206, 2003. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 135.Peers C, Boyle JP. Oxidative modulation of K+ channels in the central nervous system in neurodegenerative diseases and aging. Antioxid Redox Signal 22: 505–521, 2015. doi: 10.1089/ars.2014.6007. [DOI] [PubMed] [Google Scholar]
- 136.Polson JW, Mrljak S, Potts PD, Dampney RA. Fos expression in spinally projecting neurons after hypotension in the conscious rabbit. Auton Neurosci 100: 10–20, 2002. doi: 10.1016/S1566-0702(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 137.Potts PD, Ludbrook J, Gillman-Gaspari TA, Horiuchi J, Dampney RA. Activation of brain neurons following central hypervolaemia and hypovolaemia: contribution of baroreceptor and non-baroreceptor inputs. Neuroscience 95: 499–511, 2000. doi: 10.1016/S0306-4522(99)00426-1. [DOI] [PubMed] [Google Scholar]
- 138.Pyner S. The paraventricular nucleus and heart failure. Exp Physiol 99: 332–339, 2014. doi: 10.1113/expphysiol.2013.072678. [DOI] [PubMed] [Google Scholar]
- 139.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000. doi: 10.1016/S0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- 140.Pyner S, Deering J, Coote JH. Right atrial stretch induces renal nerve inhibition and c-fos expression in parvocellular neurones of the paraventricular nucleus in rats. Exp Physiol 87: 25–32, 2002. doi: 10.1113/eph8702279. [DOI] [PubMed] [Google Scholar]
- 141.Qi J, Zhang DM, Suo YP, Song XA, Yu XJ, Elks C, Lin YX, Xu YY, Zang WJ, Zhu Z, Kang YM. Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovasc Toxicol 13: 48–54, 2013. doi: 10.1007/s12012-012-9184-9. [DOI] [PubMed] [Google Scholar]
- 142.Qiao X, Zhou JJ, Li DP, Pan HL. Src kinases regulate glutamatergic input to hypothalamic presympathetic neurons and sympathetic outflow in hypertension. Hypertension 69: 154–162, 2017. doi: 10.1161/HYPERTENSIONAHA.116.07947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49: 647–652, 2007. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 144.Ramchandra R, Hood SG, Frithiof R, McKinley MJ, May CN. The role of the paraventricular nucleus of the hypothalamus in the regulation of cardiac and renal sympathetic nerve activity in conscious normal and heart failure sheep. J Physiol 591: 93–107, 2013. doi: 10.1113/jphysiol.2012.236059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reddy MK, Patel KP, Schultz HD. Differential role of the paraventricular nucleus of the hypothalamus in modulating the sympathoexcitatory component of peripheral and central chemoreflexes. Am J Physiol Regul Integr Comp Physiol 289: R789–R797, 2005. doi: 10.1152/ajpregu.00222.2005. [DOI] [PubMed] [Google Scholar]
- 146.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397: 251–255, 1999. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 147.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol Heart Circ Physiol 256: H1060–H1065, 1989. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- 148.Sanz-Clemente A, Gray JA, Ogilvie KA, Nicoll RA, Roche KW. Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep 3: 607–614, 2013. doi: 10.1016/j.celrep.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res 117: 305–312, 1976. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 150.Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol Heart Circ Physiol 260: H305–H318, 1991. doi: 10.1152/ajpheart.1991.260.2.H305. [DOI] [PubMed] [Google Scholar]
- 151.Schaich CL, Wellman TL, Koi B, Erdos B. BDNF acting in the hypothalamus induces acute pressor responses under permissive control of angiotensin II. Auton Neurosci 197: 1–8, 2016. doi: 10.1016/j.autneu.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev 24: 855–872, 2000. doi: 10.1016/S0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 153.Scrogin KE, Grygielko ET, Brooks VL. Osmolality: a physiological long-term regulator of lumbar sympathetic nerve activity and arterial pressure. Am J Physiol Regul Integr Comp Physiol 276: R1579–R1586, 1999. doi: 10.1152/ajpregu.1999.276.6.R1579. [DOI] [PubMed] [Google Scholar]
- 154.Scrogin KE, McKeogh DF, Brooks VL. Is osmolality a long-term regulator of renal sympathetic nerve activity in conscious water-deprived rats? Am J Physiol Regul Integr Comp Physiol 282: R560–R568, 2002. doi: 10.1152/ajpregu.00780.2000. [DOI] [PubMed] [Google Scholar]
- 155.Sermasi E, Howl J, Wheatley M, Coote JH. Localisation of arginine vasopressin V1a receptors on sympatho-adrenal preganglionic neurones. Exp Brain Res 119: 85–91, 1998. doi: 10.1007/s002210050322. [DOI] [PubMed] [Google Scholar]
- 156.Shi P, Martinez MA, Calderon AS, Chen Q, Cunningham JT, Toney GM. Intra-carotid hyperosmotic stimulation increases Fos staining in forebrain organum vasculosum laminae terminalis neurones that project to the hypothalamic paraventricular nucleus. J Physiol 586: 5231–5245, 2008. doi: 10.1113/jphysiol.2008.159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shi Z, Cassaglia PA, Gotthardt LC, Brooks VL. Hypothalamic paraventricular and arcuate nuclei contribute to elevated sympathetic nerve activity in pregnant rats: roles of neuropeptide Y and α-melanocyte-stimulating hormone. Hypertension 66: 1191–1198, 2015. doi: 10.1161/HYPERTENSIONAHA.115.06045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 203: 289–297, 2011. doi: 10.1111/j.1748-1716.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 159.Sladek CD, Michelini LC, Stachenfeld NS, Stern JE, Urban JH. Endocrine-Autonomic Linkages. Compr Physiol 5: 1281–1323, 2015. doi: 10.1002/cphy.c140028. [DOI] [PubMed] [Google Scholar]
- 160.Smith DW, Buller KM, Day TA. Role of ventrolateral medulla catecholamine cells in hypothalamic neuroendocrine cell responses to systemic hypoxia. J Neurosci 15: 7979–7988, 1995. doi: 10.1523/JNEUROSCI.15-12-07979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol 563: 249–263, 2005. doi: 10.1113/jphysiol.2004.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regul Integr Comp Physiol 286: R719–R725, 2004. doi: 10.1152/ajpregu.00494.2003. [DOI] [PubMed] [Google Scholar]
- 163.Stocker SD, Simmons JR, Stornetta RL, Toney GM, Guyenet PG. Water deprivation activates a glutamatergic projection from the hypothalamic paraventricular nucleus to the rostral ventrolateral medulla. J Comp Neurol 494: 673–685, 2006. doi: 10.1002/cne.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res 742: 219–224, 1996. doi: 10.1016/S0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- 165.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491: 156–162, 1989. doi: 10.1016/0006-8993(89)90098-X. [DOI] [PubMed] [Google Scholar]
- 166.Taniguchi S, Nakazawa T, Tanimura A, Kiyama Y, Tezuka T, Watabe AM, Katayama N, Yokoyama K, Inoue T, Izumi-Nakaseko H, Kakuta S, Sudo K, Iwakura Y, Umemori H, Inoue T, Murphy NP, Hashimoto K, Kano M, Manabe T, Yamamoto T. Involvement of NMDAR2A tyrosine phosphorylation in depression-related behaviour. EMBO J 28: 3717–3729, 2009. doi: 10.1038/emboj.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tong G, Shepherd D, Jahr CE. Synaptic desensitization of NMDA receptors by calcineurin. Science 267: 1510–1512, 1995. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- 168.Venerando A, Ruzzene M, Pinna LA. Casein kinase: the triple meaning of a misnomer. Biochem J 460: 141–156, 2014. doi: 10.1042/BJ20140178. [DOI] [PubMed] [Google Scholar]
- 169.Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96, Pt A: 70–82, 2015. doi: 10.1016/j.neuropharm.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 170.Wang G, Coleman CG, Chan J, Faraco G, Marques-Lopes J, Milner TA, Guruju MR, Anrather J, Davisson RL, Iadecola C, Pickel VM. Angiotensin II slow-pressor hypertension enhances NMDA currents and NOX2-dependent superoxide production in hypothalamic paraventricular neurons. Am J Physiol Regul Integr Comp Physiol 304: R1096–R1106, 2013. doi: 10.1152/ajpregu.00367.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang HW, Huang BS, Chen A, Ahmad M, White RA, Leenen FH. Role of brain aldosterone and mineralocorticoid receptors in aldosterone-salt hypertension in rats. Neuroscience 314: 90–105, 2016. doi: 10.1016/j.neuroscience.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 172.Wang LY, Orser BA, Brautigan DL, MacDonald JF. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature 369: 230–232, 1994. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- 173.Watkins ND, Cork SC, Pyner S. An immunohistochemical investigation of the relationship between neuronal nitric oxide synthase, GABA and presympathetic paraventricular neurons in the hypothalamus. Neuroscience 159: 1079–1088, 2009. doi: 10.1016/j.neuroscience.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 174.Yang Z, Wheatley M, Coote JH. Neuropeptides, amines and amino acids as mediators of the sympathetic effects of paraventricular nucleus activation in the rat. Exp Physiol 87: 663–674, 2002. doi: 10.1113/eph8702439. [DOI] [PubMed] [Google Scholar]
- 175.Ye ZY, Li DP, Byun HS, Li L, Pan HL. NKCC1 upregulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J Neurosci 32: 8560–8568, 2012. doi: 10.1523/JNEUROSCI.1346-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ye ZY, Li DP, Li L, Pan HL. Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J Neurosci 31: 8271–8279, 2011. doi: 10.1523/JNEUROSCI.1147-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ye ZY, Li L, Li DP, Pan HL. Casein kinase 2-mediated synaptic GluN2A up-regulation increases N-methyl-D-aspartate receptor activity and excitability of hypothalamic neurons in hypertension. J Biol Chem 287: 17438–17446, 2012. doi: 10.1074/jbc.M111.331165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zahner MR, Pan HL. Role of paraventricular nucleus in the cardiogenic sympathetic reflex in rats. Am J Physiol Regul Integr Comp Physiol 288: R420–R426, 2005. doi: 10.1152/ajpregu.00563.2004. [DOI] [PubMed] [Google Scholar]
- 179.Zhang K, Li YF, Patel KP. Blunted nitric oxide-mediated inhibition of renal nerve discharge within PVN of rats with heart failure. Am J Physiol Heart Circ Physiol 281: H995–H1004, 2001. doi: 10.1152/ajpheart.2001.281.3.H995. [DOI] [PubMed] [Google Scholar]
- 180.Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol 282: R1006–R1015, 2002. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- 181.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol Regul Integr Comp Physiol 275: R728–R734, 1998. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 182.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: an emerging concept for neurogenic hypertension. Hypertension 57: 1026–1033, 2011. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]