Abstract
Patients with heart failure (HF) have a high prevalence of depression associated with a worse prognosis, particularly in older women. The present study evaluated whether sex and estrogens affect depression-like behavior and associated neuroinflammation induced by myocardial infarction (MI) in rats. MI was induced by occlusion of the left anterior descending artery in young adult male and female Wistar rats or in ovariectomized (OVX) female rats without and with estrogen [17β-estradiol (E2)] replacement. MI groups showed a comparable degree of cardiac dysfunction. Eight weeks post-MI, male rats with HF exhibited depression-like behaviors, including anhedonia and higher immobility in the sucrose preference and forced swim tests, which were not observed in female rats with HF. In the cued fear conditioning test, male but not female rats with HF froze more than sham rats. After OVX, female sham rats developed mild depression-like behaviors that were pronounced in OVX female rats post-MI and were largely prevented by E2 replacement. Cytokine levels in the plasma and paraventricular nucleus increased in both sexes with HF, but only male rats with HF showed an increase in cytokine levels in the prefrontal cortex. OVX alone did not affect cytokine levels, but OVX-MI caused significant increases in the prefrontal cortex, which were shifted to an anti-inflammatory pattern by E2 replacement. These results suggest that estrogens prevent depression-like behavior induced by HF post-MI in young adult female rats by inhibiting proinflammatory cytokine production and actions in the prefrontal cortex.
NEW & NOTEWORTHY In contrast to male rats, female rats with heart failure after myocardial infarction do not develop depression-like behavior or increases in prefrontal cortex cytokines. However, after ovariectomy, female rats exhibit similar changes, which are prevented by 17β-estradiol replacement. Neuroinflammation in the prefrontal cortex in male subjects may contribute to depression-like behavior, whereas its estrogen-dependent absence in female subjects may protect against depression.
Listen to this article's corresponding podcast at https://ajpheart.podbean.com/e/sex-differences-in-depression-like-behavior-post-myocardial-infarction/.
Keywords: depression, heart failure, myocardial infarction, neuroinflammation, sex
INTRODUCTION
Patients with heart failure (HF) have a high prevalence of depression, with a meta-analysis reporting an overall point estimate for the prevalence rate of 22%, two- to threefold higher than the rate in the general population (52). Moreover, patients with HF and depression, or patients with depression after myocardial infarction (MI), have a poorer quality of life and a greater than twofold increased risk of further cardiac events and mortality than those not depressed (22, 39, 52). The comorbidity of depression and HF may reflect an enhancement of inflammation. Patients with HF and major depressive disorder have high plasma proinflammatory cytokines (PIC) levels, specifically IL-2, IL-6, and TNF-α (11, 35). There are also sex differences in the rate of comorbidity and inflammation. Older women with heart disease, including HF, are at a greater risk of developing depression and have a worse prognosis than men (16, 52, 68). On the other hand, women at an age of 50 yr or less with HF have lower plasma TNF-α levels than men, possibly reflecting an inhibitory action of estrogens (11).
Despite these clinical sex differences in the interaction of depression and HF, preclinical studies have used only male rodents. These studies have shown that male rodents with HF induced by MI exhibit depression-like behavior and an increase in plasma PICs, such as TNF-α, IL-1β, and IL-6 (2, 14, 18, 19, 62). This inflammatory state extends to the brain. In rats with HF post-MI, microglia activation and increased IL-1β and TNF-α levels are found in the paraventricular nucleus (PVN) of the hypothalamus (29, 49). The PVN is the major hypothalamus nucleus for cardiovascular regulation in HF (47). We postulate that this neuroinflammation may extend to other brain areas, such as the prefrontal cortex (PFC), hippocampus, and amygdala, and contribute to depression-like behavior (65). PICs decrease serotonin concentration by activating indoleamine 2,3-dioxygenase (IDO) in microglia and shift tryptophan metabolism from serotonin to quinolinic acid (48). Quinolinic acid and its metabolites not only stimulate N-methyl-d-aspartate (NMDA) receptors but also induce oxidative damage (43), whereas activated microglia increase the release of PICs and glutamate (67). As a result, there is increased glutamatergic activation and decreased expression of brain-derived neurotrophic factor (BDNF), which may thereby contribute to depression (12).
The increased rates of depression in older women with HF are correlated with clinical and preclinical work that supports that estrogens protect against depression. For example, postmenopausal women have a higher incidence of depression compared with premenopausal women (66). Ovariectomized (OVX) rats exhibit depression-like behavior with a decrease of serotonin in the amygdala, hippocampus, and PFC (7). Hormonal replacement therapy reduces depression in menopause and postmenopausal women (59) and alleviates depression-like behavior in adult and aged OVX rats (31, 61, 69). Estrogens may protect against depression through several mechanisms, including promoting microglia resting phenotype (21) and increasing serotonin concentration by inhibiting IDO enzyme (69) and the expression of BDNF by genomic pathways (56). Considering the impact of estrogens, we hypothesized that young adult female rats would exhibit less depression-like behavior and have less neuroinflammation in the PVN, PFC, and amygdala than male rats with HF post-MI. To test this hypothesis, the present study first assessed the extent of depression-like behavior and neuroinflammation by assessing PIC levels in plasma, PVN, PFC, and amygdala in female and male rats with HF post-MI. These experiments showed clear sex differences in the extent of neuroinflammation in the PFC and in depression-like behavior. We then assessed the role of the ovaries and estrogens for protection against depression-like behavior by inducing HF post-MI in female rats after OVX without or with estrogen [17β-estradiol (E2)] replacement.
MATERIALS AND METHODS
Experimental Animals
Male and female Wistar rats (9–13 wk old, Charles River, Montreal, QC, Canada) weighing 200–250 g were housed in pairs at 21 ± 2°C and humidity of 46 ± 2% on a 12:12-h light-dark cycle, with the dark cycle starting at 3:00 AM. Rats were provided with standard chow and tap water ad libitum. All experimental procedures conformed with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Ottawa Animal Care Committee.
Experimental Groups and Design
Protocol 1: male versus female rats with HF post-MI.
After acclimatization for 5–7 days, rats in both sex groups were randomized into the following surgical groups (surgery to induce MI or sham surgery): male MI (n = 20), male sham (n = 8), female MI (n = 20), and female sham (n = 8). Eleven rats from the male MI group and fifteen rats from the female MI group survived. Rats from the male MI group (n = 3) and rats from the female MI group (n = 5) with MI size < 20% of the left ventricle (LV) were excluded from the study.
Protocol 2: HF post-MI in OVX female rats.
Female rats were randomized into the following groups: 1) sham OVX-sham pellet-sham MI (sham OVX-sham MI; n = 9), 2) OVX-sham pellet-sham MI (OVX-sham MI; n = 12), 3) OVX-E2 pellet-sham MI (OVX-E2-sham MI; n = 10), 4) OVX-sham pellet-MI (OVX-MI; n = 20), and 5) OVX-E2 pellet-MI (OVX-E2-MI; n = 20).
Estrogen pellets containing 1.5 mg E2 releasing 25 µg/day (Innovative Research of America, Sarasota, FL) were implanted subcutaneously in the dorsal neck area. This dose of E2 results in plasma E2 levels within the normal range during the estrous cycle of the rat (23, 41). Two weeks after OVX and E2 pellet implantation, rats underwent MI or sham MI surgery. Eight rats from the OVX-MI group and fifteen rats from the OVX-E2-MI group survived. Because of the unusual high mortality in the OVX-MI group, another 20 rats underwent OVX and MI surgery, of which 11 rats survived. Rats from the OVX-MI group (n = 8) and rats from the OVX-E2-MI group (n = 5) with MI size < 20% of the LV were excluded from the study.
Behavioral tests were carried out from 7 to 9 wk after MI or sham surgery between 7:00 AM and 2:00 PM. At the end, echocardiographic and hemodynamic parameters were obtained, and MI size was measured by planimetry. The final numbers of rats for each of the groups are shown in Tables 1 and 2.
Table 1.
Anatomic, echocardiographic, and hemodynamic measurements in male and female rats at 10 wk after MI
| Variables | Male Sham Group | Male MI Group | Female Sham Group | Female MI Group |
|---|---|---|---|---|
| Number of rats/group | 8 | 8 | 8 | 10 |
| Body weight, g | 605 ± 19.9 | 593 ± 13.2 | 368 ± 10.6‡ | 368 ± 11.2‡ |
| MI size, % | 26 ± 1.5 | 29 ± 1.5 | ||
| LV/body wt, mg/100 g | 170 ± 5.0 | 184 ± 4.5 | 200 ± 5.2 | 235 ± 11.2† |
| Right ventricle/body wt, mg/100 g | 42 ± 2.6 | 61 ± 12.9* | 42 ± 1.7 | 57 ± 9.4† |
| Echocardiographic parameters | ||||
| LV end-systolic volume, µl/100 g body wt | 9 ± 1.7 | 46 ± 4.3* | 5 ± 1.2 | 50 ± 3.8† |
| LV end-diastolic volume, µl/100 g body wt | 63 ± 7.5 | 99 ± 5.4* | 59 ± 4.5 | 102 ± 6.5† |
| Ejection fraction, % | 86 ± 1.6 | 49 ± 1.9* | 91 ± 1.6 | 52 ± 1.9† |
| Hemodynamic parameters | ||||
| LV peak systolic pressure, mmHg | 118 ± 1.9 | 107 ± 3.9* | 125 ± 2.0 | 112 ± 3.5† |
| LV end-diastolic pressure, mmHg | 3.6 ± 0.3 | 14.9 ± 1.6* | 3.5 ± 0.3 | 17.1 ± 2.1† |
| LV dP/dt(+), mmHg/s | 7,499 ± 176 | 5,864 ± 245* | 8,108 ± 105 | 6,027 ± 260† |
| LV dP/dt(−), mmHg/s | 6,466 ± 130 | 4,474 ± 245* | 7,092 ± 160 | 4,899 ± 315† |
Values are means ± SE. Two-way ANOVA was done for the sex and myocardial infarction (MI) interaction followed by the Bonferroni post hoc test. There was no significant sex × MI interaction for any variable. Body weight sex effect: F = 266.7, P < 0.001. Right ventricle/body weight MI effect: F = 10.2, P = 0.003. Left ventricle (LV)/body weight sex effect: F = 16.1, P < 0.0001. MI effect: F = 5.8, P = 0.022. LV end-systolic volume MI effect: F = 135.9, P < 0.0001. LV end-diastolic volume MI effect: F = 38.1, P < 0.0001. Ejection fraction sex effect: F = 4.4, P = 0.04. MI effect: F = 442.7, P < 0.0001. LV peak systolic pressure MI effect: F = 13.9, P = 0.001. LV end-diastolic pressure MI effect: F = 74.5, P < 0.0001. LV dP/dt(+) MI effect: F = 73.9, P < 0.0001. LV dP/dt(−) sex effect: F = 4.8, P = 0.036. MI effect: F = 76.5, P < 0.0001.
P < 0.05 vs. the male sham group;
P < 0.05 vs. the female sham group;
P < 0.05 vs. male groups.
Table 2.
Anatomic, echocardiographic, and hemodynamic measurements in OVX female rats without or with estrogen replacement at 10 wk after MI
| Variables | Sham OVX-Sham MI Group | OVX-Sham MI Group | OVX-E2 Sham MI Group | OVX-MI Group | OVX-E2-MI Group |
|---|---|---|---|---|---|
| Number of rats/group | 8 | 12 | 10 | 11 | 9 |
| Body weight, g | 362 ± 7.2 | 466 ± 14.4*† | 381 ± 12.7 | 437 ± 10.0*† | 365 ± 10.1 |
| Uterus/body weight, mg/100 g | 164 ± 13.1 | 38 ± 3.4*† | 162 ± 14.1 | 37 ± 1.1*† | 184 ± 14.6 |
| MI size, % | 27 ± 1.4 | 27 ± 1.7 | |||
| LV/body weight, mg/100 g | 204 ± 3.9 | 163 ± 3.7* | 204 ± 9.1 | 183 ± 5.8‡ | 230 ± 7.2‡ |
| Right ventricle/body weight, mg/100 g | 46 ± 1.7 | 37 ± 1.4† | 42 ± 2.3 | 40 ± 3.5† | 50 ± 2.4 |
| Echocardiographic parameters | |||||
| LV end-systolic volume, µl/100 g body wt | 7 ± 0.7 | 8 ± 0.7 | 6 ± 0.4 | 44 ± 4.1*‡ | 47 ± 3.4*‡ |
| LV end-diastolic volume, µl/100 g body wt | 68 ± 5.4 | 59 ± 1.9 | 59 ± 2.8 | 81 ± 6.0‡ | 103 ± 7.0*‡ |
| Ejection fraction, % | 89 ± 0.9 | 87 ± 0.9 | 90 ± 0.5 | 46 ± 2.5*‡ | 54 ± 2.4*‡ |
| Hemodynamic parameters | |||||
| LV peak systolic pressure, mmHg | 128 ± 1.5 | 127 ± 1.5 | 130 ± 3.2 | 121 ± 3.9‡ | 115 ± 3.9* |
| LV end-diastolic pressure, mmHg | 2.7 ± 0.4 | 4.1 ± 0.5 | 3.5 ± 0.3 | 10.1 ± 0.7*‡ | 13.3 ± 0.8*‡ |
| LV dP/dt(+), mmHg/s | 8,646 ± 261 | 8,773 ± 181 | 8,716 ± 181 | 6,445 ± 257*‡ | 6,064 ± 282*‡ |
| LV dP/dt(−), mmHg/s | 7,322 ± 280 | 7,366 ± 141 | 7,572 ± 145 | 5,425 ± 220*‡ | 5,390 ± 271*‡ |
Values are means ± SE. One-way ANOVA was done to compare all groups. Two-way ANOVA was performed for the ovariectomy (OVX) and myocardial infarction (MI) interaction followed by a Bonferroni post hoc test. Body weight OVX effect: F = 40.8, P < 0.0001. Uterus/body weight OVX effect: F = 222.5, P < 0.0001. Right venticle/body weight OVX effect: F = 15.3, P < 0.0001. Left ventricle (LV)/body weight MI effect: F = 12.8, P < 0.001. OVX effect: F = 45.8, P < 0.0001. LV end-systolic volume MI effect: F = 198.7, P < 0.0001. LV end-diastolic volume MI × OVX effect: F = 4.6, P = 0.031. Ejection fraction MI effect: F = 458.5, P < 0.0001. LV peak systolic pressure MI effect: F = 9.8, P = 0.001. LV end-diastolic pressure MI × OVX effect: F = 11.7, P < 0.001. LV dP/dt(+) MI effect: F = 131.1, P = 0.0001. LV dP/dt(−) MI effect: F = 104.3, P < 0.0001.
P < 0.05 vs. the sham OVX-sham MI group;
P < 0.05 vs. OVX-17β-estradiol (E2) groups;
P < 0.05 vs. the related sham MI group.
Surgical Procedures
Rats were premedicated with slow-release buprenorphine (1 mg/kg sc, Chiron Compounding Pharmacy, Guelph, ON, Canada) and anesthetized with 2% isoflurane. After intubation, the pericardium was opened via thoracotomy, and the left anterior descending coronary artery was ligated permanently. Sham rats underwent the same surgery but without ligation of the left anterior descending coronary artery.
Bilateral OVXs were performed through a longitudinal incision on each flank. Sham OVX rats underwent the same procedure except that the ovaries were exteriorized but not removed. For sham pellet implantation, a small incision was made but no pellet was implanted. Postsurgery rats were housed individually for 3–5 days and then returned to paired housing.
Behavior Tests
All behavioral tests except the sucrose preference test (SPT) were carried out in the dark cycle and were recorded by a digital camera linked to an EthoVision 11.5 xT video tracking system (Noldus Information Technology, Leesburg, VA). A dark holding room was used to minimize the duration of exposure to dim light in the Morris water maze (MWM) for the rats to see the visual cues and in the forced swim test (FST) and fear conditioning test (FCT) for accurate detection of immobility and freezing. The FST and FCT trials were scored both by hand and Ethovision software (v11.5). Hand score and software results were correlated >90%, and the presented results are calculated by the software. Behavior tests were performed in the following sequence for all rats with at least 1 day in between: SPT, MWM, FST, and FCT.
Tests to Assess Depression-Like Behavior
The SPT is used to evaluate anhedonia. Rats were habituated to the presence of two bottles, one bottle consisting of tap water and the other bottle consisting of 2% sucrose, for 3 days (58). Afterward, daily 1% sucrose and water consumption were measured for 7 days. The bottle location was switched on a daily basis to avoid location preference. Sucrose preference was calculated as the percent of sucrose intake over total daily fluid intake and is presented as the mean for the last 3 days.
The FST is performed to assess despair behavior. Each rat was placed in a 10-liter transparent plastic cylinder (height: 45 cm and diameter: 20 cm) containing 30 cm of tap water at 25 ± 2°C for 10 min. Immobility was defined as the lack of movements except those necessary to prevent the animal from drowning (55). Percent immobility was calculated as the percent of immobile behavior over the test duration.
Tests to Assess Learning and Memory
The MWM test is performed to evaluate spatial learning and memory. The water maze consists of a circular pool (diameter: 1.84 m) with a clear acrylic glass platform (diameter: 0.10 m) submerged in the center of the southwest quadrant of the pool 1 cm below the surface of water at 25°C. The maze was located in a dimly lit room with different black spatial cues mounted on the white wall in the south, west, and east quadrants of the room. The test consisted of 4 training days, with 2 sessions/day. On the first day, each session had four trials/session, whereas the following training days had 3 trials/session (60). For each trial during training, rats were randomly placed into one of the three quadrants that do not contain the platform. Each rat was allowed to swim for 60 s to find the hidden platform using extra maze visual cues. Probe trials were carried out 24 h after the last training session on days 3 and 5. The platform was removed, and rats were released on the opposite side of the platform location and allowed to swim for 60 s to evaluate the duration spent in the target quadrant where the platform was located. The learning curves for the training sessions and the times spent in the platform quadrant during the two probe sessions are presented.
The FCT is used to assess learning and associative memory. This test was performed using a PhenoTyper cage that is square in shape, has an electrifiable grid floor, a calibrated shock generator, and a sound source as well as a camera mounted in the top unit to allow for continuous video recording (45). During the acquisition day, rats were placed in the PhenoTyper chamber for 2 min for habituation and then heard a tone (conditioned stimulus) that coterminated with a shock (unconditioned stimulus) two times separated by a 1-min interval. The tone-shock pairing consisted of playing of a tone (~2,300 Hz, ~70 dB) for 30 s that ended concurrently with a 2-s foot shock (0.45 mA). Two minutes after receiving the last shock, the acquisition ended, and rats were placed back in their home cages. The second day, contextual fear conditioning was measured as the amount of freezing when the rat was in the same acquisition chamber for 6 min without shock or tone. On the third day, cued fear conditioning was measured in a different PhenoTyper cage with a different color, odor, flooring texture, and triangle shape instead of square to expose the rat to a novel context. To test that the rat did not recognize the context, freezing was measured for the first 3 min in the absence of the tone followed by 3 min in the presence of the tone. Freezing was defined as the absence of movement except those associated with breathing, and it was monitored across the whole trial length.
Echocardiography and Hemodynamic Measurements
An echocardiogram was obtained under 2% isoflurane anesthesia to measure cardiac diameters and ejection fraction (EF) using a Visual Sonics Vevo 770 System (Visual Sonics, Toronto, ON, Canada). A Millar catheter was then placed in the LV to measure LV peak systolic and end-diastolic pressures and dP/dtmax and dP/dtmin for 30–60 s.
Tissue Collection
After hemodynamics, trunk blood was collected into prechilled 50-ml tubes containing EDTA and centrifuged at 3,000 rpm for 30 min at 4°C to obtain plasma for cytokine levels. Hearts were removed and kept in cold saline followed by separation of the right ventricle (RV), whereas the LV was longitudinally dissected from the septum, and two to three cuts were performed on the apex to flatten the LV. The area of LV infarction was measured by planimetry, and infarct size was expressed as a percentage of the LV area. The brain was removed and snap frozen in methylbutane at −20°C. All tissues and plasma were kept at −80°C until further analysis.
Plasma and Brain Cytokines
Plasma cytokine levels were measured by a multiplexed bead-based immunoassay kit (no. 171K1002M, Bio-Rad, Mississauga, ON, Canada). In protocol 1, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, interferon-γ, and TNF-α were measured; in protocol 2, IL-1α, IL-1β, IL-2, IL-6, IL-10, and TNF-α were measured. Plasma samples were centrifuged at 13,000 g for 15 min at 4°C and diluted 1:4. The premixed conjugated beads interacted with targeted cytokines in plasma samples for 1.5 h at room temperature followed by incubation with biotinylated antibodies for 1 h at room temperature, which will interact with the cytokine of interest. The mixture was incubated with streptavidin-phycoerythrin for 10 min at room temperature. Finally, the Bioplex Protein Array System and related software (Bio-Plex Manager version 5.6_ were used to determine the levels of cytokines.
Brain punches were collected from the following 50-μm sections: the PFC from (2.8 to 1.8 mm), PVN (−0.8 to −2.3 mm), and amygdala from (−2.5 to −3.1 mm) and then homogenized in tissue lysis buffer [10 mM Tris (pH 7.4), 100 mM NaCl, 1% Triton X-100, 1 mM EDTA (pH 8.0), 1 mM EGTA, 10% glycerol, 0.1% SDS, and 0.5% deoxycholate containing 2 mM protease inhibitor cocktail, 2 mM Na3VO4, and 5 mM NaF]. The homogenate was centrifuged at 10,000 g for 20 min at 4°C, and the supernatant was collected. Protein concentration was determined by a Micro BCA protein assay kit (no. 113225, ThermoFisher Scientific, Rockford, IL). Two PVNs of each rat were pooled, providing ~100–130 μg total protein. Individual tissue samples with 0.5 mg/ml protein were assayed for IL-1α, IL-1β, IL-2, IL-6, IL-10, and TNF-α using a multiplexed bead-based immunoassay set with a cytokine reagent kit (no. 171304070M, Bio-Rad). Cytokine levels are expressed as picograms per milligram.
Western Blot Analysis
Immunoblot analysis was performed using 20 µg total protein from the PFC and amygdala. Proteins were separated by SDS-PAGE (12% gel) for mature BDNF (mBDNF) and transferred onto 0.2-µm PVDF membranes (Bio-Rad). Blots were blocked for 1 h at room temperature in 5% milk in Tris-buffered saline with 0.1% Tween 20 and then probed with rabbit monoclonal anti-BDNF (1:1,000, Ab1393, Abcam, Toronto, ON, Canada) at 4°C overnight. Blots were then incubated with goat anti-rabbit IgG- horseradish peroxidase (1:5,000, no. 111-035-144, Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. After a wash, the blot was developed with Luminata ECL reagent (MilliporeSigma, Etobicoke, ON, Canada) and visualized with the ChemiDoc XRS+ imager (Bio-Rad). Membranes were reprobed with monoclonal mouse anti-β-actin (1:10,000, A5316, Sigma, Oakville, ON, Canada) or GAPDH (1:10,000, MAB374, Millipore, Toronto, ON, Canada) as a protein loading control. Band densities were quantified using Image Laboratory software (Bio-Rad). The expression of mBDNF protein was calculated as the ratio of its band density relative to the density of β-actin in the same sample.
Statistical Analysis
To assess for sex × MI, two-way ANOVA was performed followed by the Bonferroni post hoc test. To determine differences between the five OVX female groups, one-way ANOVA was conducted, and, when significant, the OVX × MI interaction was assessed using two-way ANOVA followed by the Bonferroni post hoc test.
To compare freezing in context and cued test of FCT and spatial learning during the training days in the MWM, two-way repeated-measures ANOVA was performed with the Bonferroni post hoc test. P values of <0.05 were considered statistically significant. A priori power analyses suggested that n = 9/group would be sufficient to detect a 20% difference in the FST and n = 13/group to detect a 10% difference in the SPT.
RESULTS
Cardiac Function
Male versus female rats.
Cardiac function of male versus female rats is shown in Table 1. Final body weights showed the expected sex differences but no differences in MI versus sham groups. Male and female rats had similar MI sizes and developed a similar degree of cardiac dysfunction, with clear increases in end-systolic volume and end-diastolic volume compared with sham groups, and a significant decrease in EF. LV peak systolic pressure, LV dP/dtmax, and LV dP/dtmin significantly decreased, whereas LV end-diastolic pressure significantly increased. LV and RV weights significantly increased in female rats post-MI, whereas in male rats post-MI, only RV weight significantly increased.
OVX female rats.
Cardiac function of OVX female rats is shown in Table 2. Final body weights were higher in OVX rats but not in OVX-E2 rats compared with sham OVX rats. Uterus weights were markedly decreased in OVX rats but maintained in OVX-E2 rats. OVX without or with E2 replacement did not affect cardiac function in sham MI rats. OVX-MI and OVX-E2-MI rats showed similar MI sizes and a similar degree of cardiac dysfunction, as assessed by echocardiographic and hemodynamic parameters. LV and RV weights corrected for body weight were lower in OVX rats and showed modest increases post-MI.
Depression-Like Behavior
Male versus female rats.
the spt.
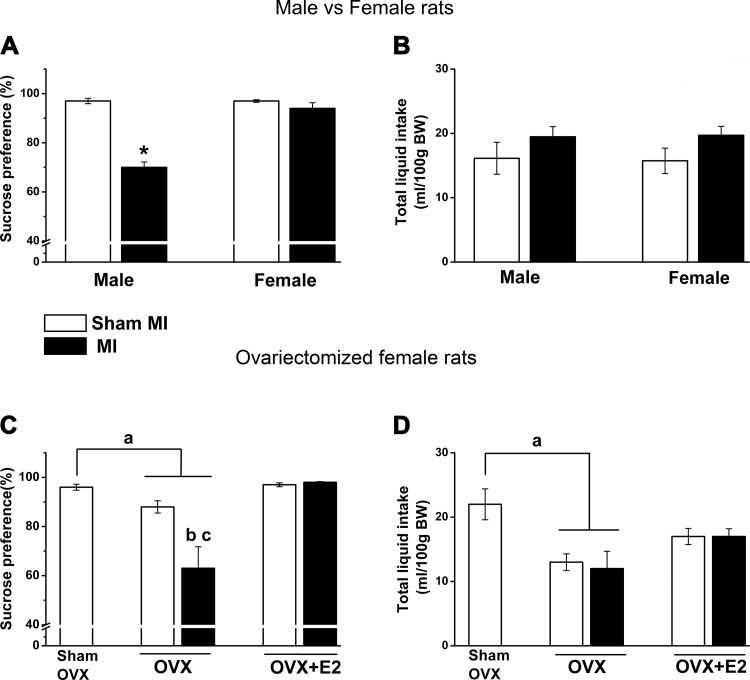
The sex and MI interaction was significant (F = 42.0, P < 0.0001), with male rats with HF post-MI having a significant decrease in preference for sucrose by 27% compared with sham male rats (Fig. 1A). No change in sucrose preference was observed in female MI versus sham rats (Fig. 1A), and there was no difference in total liquid between the four groups (Fig. 1B).
Fig. 1.
Sex × myocardial infarction (MI) and ovariectomy (OVX) × MI interactions for the percentage of sucrose intake and total liquid intake during the last 3 days of the test. Values are means ± SE; n = 8–11 rats/group. One-way ANOVA was done to compare all OVX groups with the sham OVX-sham MI group, and two-way ANOVA was done for sex × MI and OVX × MI interactions followed by a Bonferroni post hoc test. A: effects of sex and MI on sucrose intake as a percentage of total liquid intake. MI × sex effect: F = 42.4, P < 0.0001. B: total liquid intake of male and female rats. There was no MI or sex effect on liquid intake. C: effects of OVX and MI on sucrose preference in OVX female rats with or without 17β-estradiol (E2) replacement. OVX × MI effect: F = 5.7, P = 0.02. D: total liquid intake. OVX groups showed lower total liquid intake but not OVX-E2 groups compared with the sham OVX-sham MI group (F = 3.8, P = 0.009). *P < 0.0001 vs. the male sham group; aP < 0.05 vs. the sham OVX-sham MI group; bP < 0.05 vs. the OVX-sham MI group; cP < 0.05 vs. the OVX-E2-MI group. BW, body weight.
the fst.
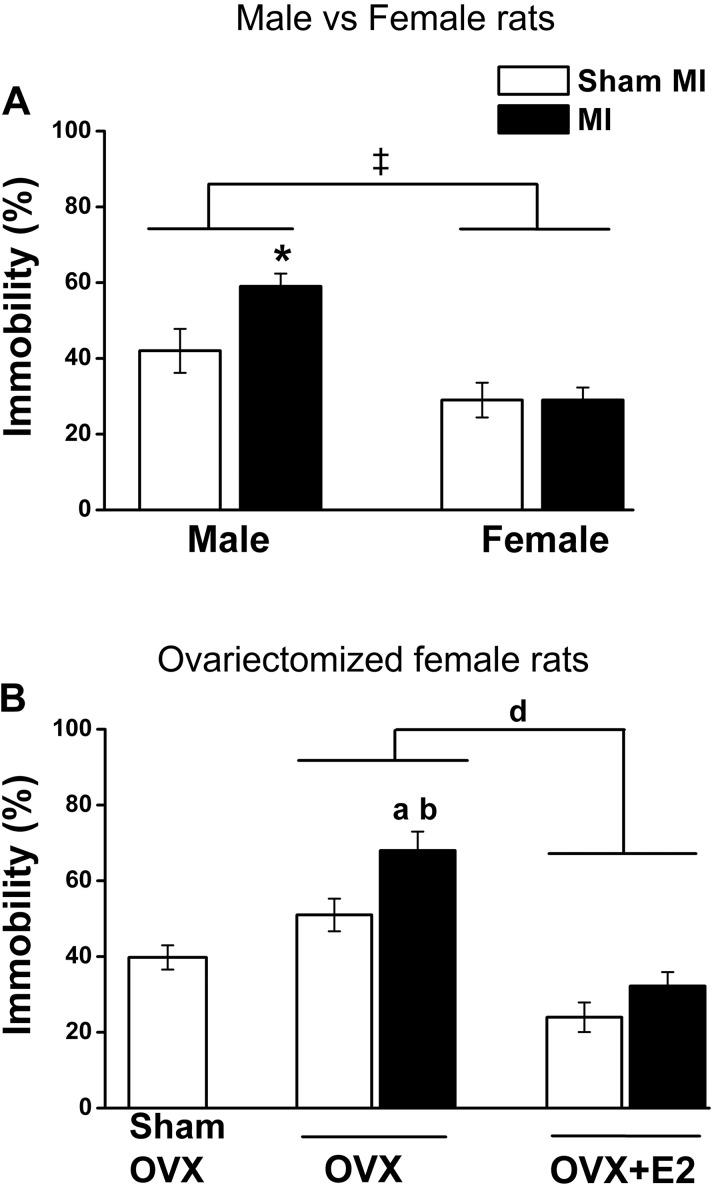
Two-way ANOVA showed a significant MI effect (F = 4.7, P < 0.05), with male rats with HF post-MI exhibiting a significant increase in immobility compared with sham males but not female rats with HF post-MI (Fig. 2A). Independent of MI, female rats were more mobile compared with male rats (sex effect: F = 26.7, P < 0.0001).
Fig. 2.
Sex × myocardial infarction (MI) and ovariectomy (OVX) × MI interactions for the percent immobility in the forced swim test. Values are means ± SE; n = 8–11 rats/group. One-way ANOVA was done to compare all OVX groups with the sham OVX-sham MI group, and two-way ANOVA was done for sex × MI and OVX × MI interactions followed by a Bonferroni post hoc test. A: effects of sex and MI. MI effect: F = 4.7, P = 0.039; sex effect: F = 26.7, P < 0.0001. B: effects of OVX and MI. MI × OVX interaction: F = 4.4, P = 0.04. OVX effect: F = 50.5, P < 0.0001. *P < 0.05 vs. the male sham group; ‡P < 0.0001 vs. male groups; aP < 0.05 vs. the sham OVX-sham MI group; bP < 0.05 vs. the OVX-sham MI group; dP < 0.05 vs. OVX-17β-estradiol (E2) groups.
OVX female rats.
the spt.
OVX decreased preference for sucrose by 8% in sham MI rats and by ~30% in MI rats (OVX × MI effect: F = 5.7, P = 0.02). E2 replacement prevented these decreases in both OVX-sham MI and OVX-MI rats (Fig. 1C). Total liquid intake per 100 g body wt was decreased in OVX groups (Fig. 1D).
the fst.
OVX alone caused a modest increase in immobility, whereas OVX-MI caused a marked increase (OVX × MI effect: F = 4.4, P = 0.04). E2 replacement prevented this increase (Fig. 2B).
Learning and Memory
Male versus female rats.
mwm test.
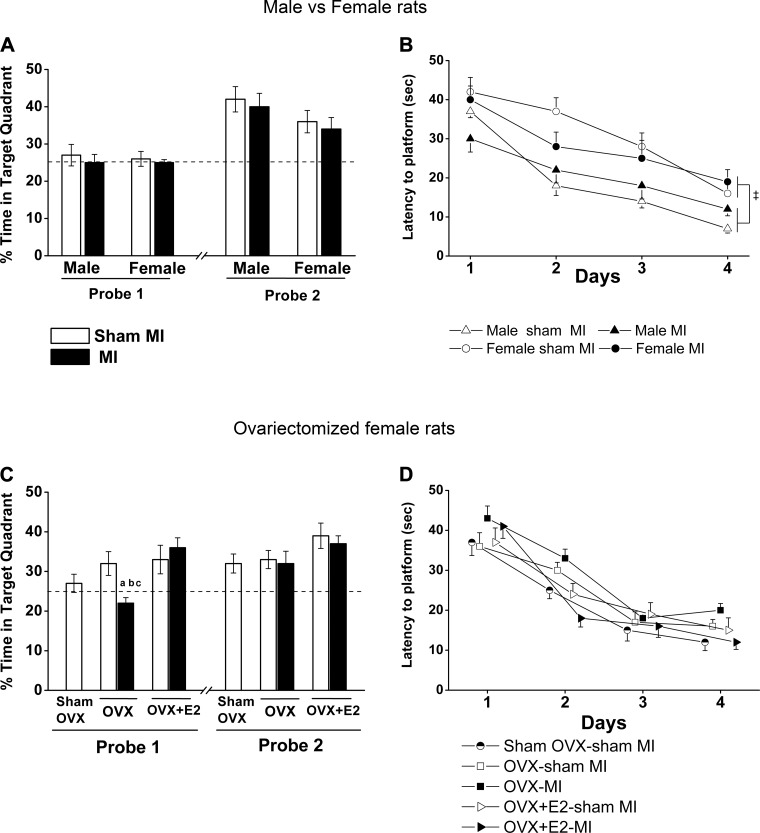
All groups had a similar significant decrease in their latency to the platform during the four days of training (Fig. 3B). Independent of MI, female rats needed more time to reach the platform compared with male rats (sex effect: F = 12.0, P < 0.05). For the probe trials, all groups increased their time in the target quadrant on day 5 (probe 2) compared with day 3 (probe 1). However, there was no MI or sex effect in either of the two probe trials (Fig. 3A). Similarly, there was no significant difference in locomotor activity as assessed by the speed of traveling and total path length (Table 3).
Fig. 3.
Sex × myocardial infarction (MI) and ovariectomy (OVX) × MI interactions in the Morris water maze test. Values are means ± SE; n = 8–10 rats/group. Repeated-measures ANOVA was performed to compare all groups during training days, and two-way ANOVA was done for sex × MI and OVX × MI interactions followed by a Bonferroni post hoc test. A: probes 1 and 2 were done on the third day and fifth day. Time spent in the target quadrant, where the platform was located in the training sessions, is expressed as a percentage. MI and sex interaction: F = 0.9, P = 0.35. B: latency to the platform on training days. Sex effect: F = 12.0, P < 0.05. C: probes 1 and 2. Probe 1, MI × OVX interaction: F = 9.2, P < 0.05. D: latency to the platform on training days. ‡P < 0.0001 vs. male groups; aP < 0.05 vs. the sham OVX-sham MI group; bP < 0.05 vs. the OVX-sham MI group; cP < 0.05 vs. the OVX-17β-estradiol (E2)-MI group.
Table 3.
Locomotor activity in the first training session of the Morris water maze
| Number of Rats/Group | Total Path Length, m | Speed, cm/s | |
|---|---|---|---|
| Male vs. female rats | |||
| Male sham group | 7 | 14 ± 2.0 | 26 ± 1.5 |
| Male MI group | 8 | 15 ± 0.8 | 26 ± 1.3 |
| Female sham group | 8 | 11 ± 1.8 | 25 ± 1.8 |
| Female MI group | 9 | 15 ± 0.6 | 28 ± 0.8 |
| OVX female rats | |||
| Sham OVX-sham MI group | 9 | 15 ± 1.0 | 28 ± 1.4 |
| OVX-sham MI group | 12 | 15 ± 1.5 | 28 ± 1.7 |
| OVX-E2-sham MI group | 10 | 15 ± 0.5 | 31 ± 0.8 |
| OVX-MI group | 9 | 14 ± 0.9 | 28 ± 0.8 |
| OVX-E2-MI group | 9 | 16 ± 0.7 | 27 ± 1.1 |
Values are means ± SE. Two-way ANOVA was performed for sex × myocardial infarction (MI) and ovariectomy (OVX) × MI interactions. E2, 17β-estradiol. No significant differences in locomotor activity were observed
the fct.
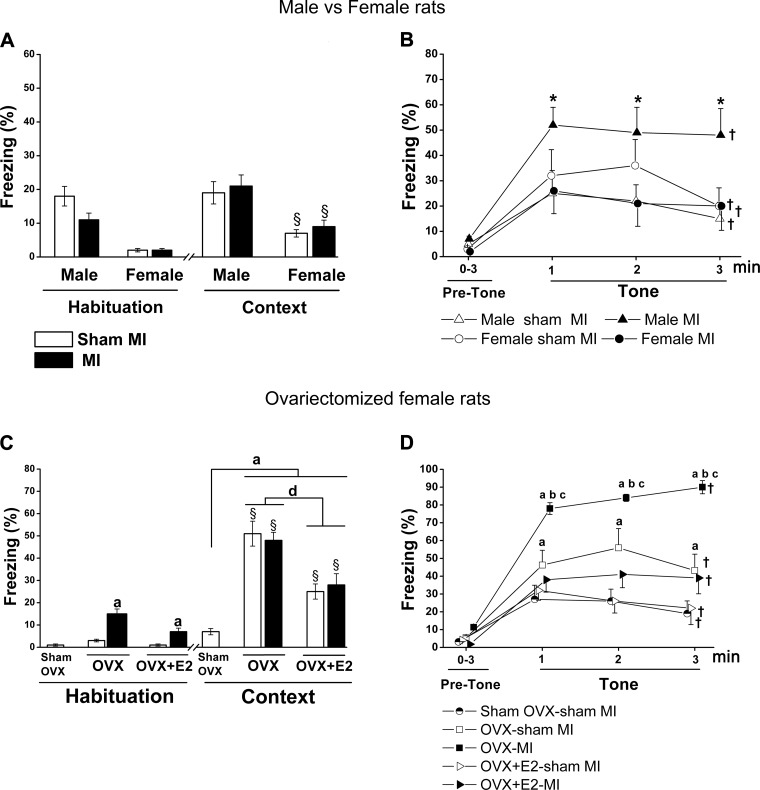
On day 1, during habituation, before presentation of the tone or shock, there was a significant higher freezing behavior in male rats compared with female rats, independent of MI (Fig. 4A). On day 2, on the context test, female rats showed a modest, but significant, increase in freezing compared with habituation on day 1 (F = 58.8, P < 0.0001), but there was no MI effect (Fig. 4A). On the cued FCT, all groups showed an increase in freezing behavior when hearing the tone. However, male rats with HF post-MI exhibited significantly more freezing behavior during the 3 min with the tone (Fig. 4B) compared with male sham rats, whereas there was no difference between the two female groups.
Fig. 4.
Sex × myocardial infarction (MI) and ovariectomy (OVX) × MI interactions in the fear conditioning test. Values are means ± SE; n = 7–11 rats/group. Freezing behavior is expressed as a percentage of time freezing over the specified period. One-way ANOVA was performed to compare all OVX groups with the sham OVX-sham MI group, and two-way ANOVA was performed for sex × MI and OVX × MI interactions. A: habituation on day 1 during the first 2 min of acquisition. Context on day 2 to assess contextual fear includes all 6 min of the test. Two-way ANOVA revealed a sex effect: F = 58.8, P < 0.0001. B: cued fear conditioning test consisted of 3-min habituation as the pretone period followed by the tone period for 3 min. Sex and MI interaction: F = 4.24, P = 0.049. C: habituation on day 1. MI effect: F = 39.2, P < 0.001. Context freezing on day 2: OVX effect, F = 21.1, P < 0.0001. D: freezing on cued conditioning. All groups froze significantly more during tone compared with their baseline on day 3 (F = 104.2, P < 0.0001). MI effect: F = 8.0, P = 0.008. OVX effect: F = 26.1, P = 0.0001. *P < 0.05 vs. the male sham group; §P < 0.05 vs. first day habituation; †P < 0.05 vs. pretone; aP < 0.05 vs. the sham OVX-sham MI group; bP < 0.05 vs. the OVX-sham MI group; cP < 0.05 vs. the OVX-17β-estradiol (E2)-MI group; dP < 0.05 vs. OVX-E2 groups.
OVX female rats.
mwm test.
All groups showed a similar significant decrease in latency to the platform during the 4 days of training (Fig. 3D). OVX alone or with E2 did not affect any component of the two probe trials (Fig. 3C). The OVX-MI group showed less time in the target quadrant in probe 1 (Fig. 3C), which improved in probe 2. Locomotor activity did not differ among the groups (Table 3).
the fct.
On day 1, freezing during habituation was very low in the sham groups but significantly higher in the OVX-MI group, which was partially prevented by E2 therapy (Fig. 4C). In contrast, OVX alone and OVX-MI caused a similar marked increase in freezing in the context test compared with the sham OVX-sham MI group, which was partially prevented by E2 therapy (OVX effect: F = 21.1, P < 0.001; Fig. 4C). During the cued conditioning test, all groups showed an increase in freezing during the tone. OVX alone caused a modest further increase in freezing, and OVX-MI caused a marked further increase. These increases were largely prevented in both groups by E2 treatment (Fig. 4D).
Cytokine Levels
Male versus female rats.
plasma.
Among the 11 cytokines, IL-1α, IL-4, IL-5, IL-6, IL-12, TNF-α, and interferon-γ showed a similar increase in male and female rats with HF post-MI. The anti-inflammatory cytokine IL-10 increased in both HF post-MI groups, but it was only significant in male rats with HF post-MI (Table 4).
Table 4.
Plasma cytokine levels increase in both male and female rats at 10 wk after MI
| Cytokine, pg/ml | Male Sham Group (n = 4) | Male MI Group (n = 4) | Female Sham Group (n = 5) | Female MI Group (n = 6) |
|---|---|---|---|---|
| IL-1α | 43 ± 5.4 | 96 ± 23.1* | 41 ± 7.4 | 75 ± 5.4† |
| IL-1β | 220 ± 32.9 | 223 ± 50.3 | 188 ± 44.3 | 145 ± 22.8 |
| IL-2 | 88 ± 11.1 | 123 ± 8.8 | 95 ± 20.9 | 142 ± 23.8 |
| IL-4 | 7 ± 0.6 | 12 ± 1.5* | 7 ± 1.1 | 11 ± 0.6† |
| IL-5 | 79 ± 21.7 | 180 ± 26.0* | 83 ± 19.9 | 155 ± 7.0† |
| IL-6 | 145 ± 17.8 | 492 ± 184.1* | 126 ± 46.3 | 279 ± 18.5† |
| IL-10 | 228 ± 35.8 | 442 ± 74.2* | 255 ± 46.7 | 370 ± 27.5 |
| IL-12 | 18 ± 3.4 | 47 ± 5.8* | 21 ± 3.8 | 42 ± 2.2† |
| IL-13 | 28 ± 5.7 | 49 ± 8.9 | 35 ± 12.5 | 41 ± 1.4 |
| TNF-α | 127 ± 7.5 | 290 ± 16.7.0* | 119 ± 15.9 | 262 ± 6.6† |
| Interferon-γ | 75 ± 14.8 | 169 ± 26.2* | 62 ± 9.2 | 141 ± 4.9† |
Values are means ± SE; n, number of rats/group. Two-way ANOVA was performed for the sex and myocardial infarction (MI) interaction followed by a Bonferroni post hoc test. There was no significant sex × MI interaction for any variable. IL-4 MI effect: F = 19.1, P = 0.001. IL-5 MI effect: F = 22.5, P < 0.001. IL-6 MI effect: F = 9.3, P = 0.003. IL-10 MI effect: F = 12.7, P = 0.003. IL-12 MI effect: F = 42.5, P < 0.001. TNF-α MI effect: F = 158.6, P < 0.001. Interferon-γ MI effect: F = 39.4, P < 0.001.
P < 0.05 vs. the male sham group;
P < 0.05 vs. the female sham group.
the pvn, pfc, and amygdala.
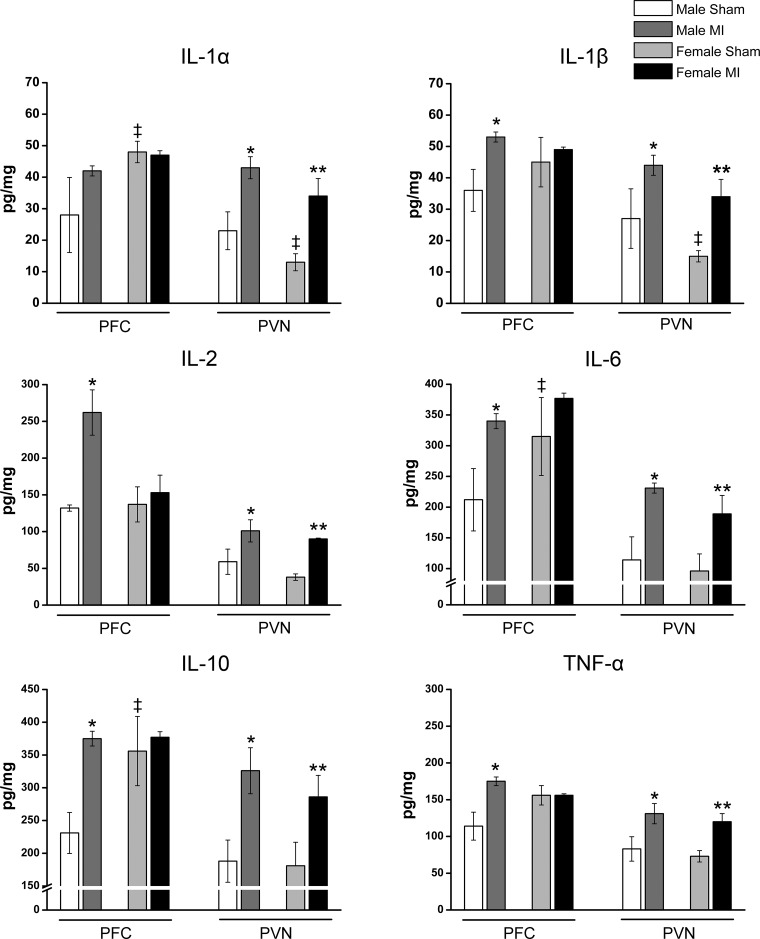
In sham rats, cytokine levels in the PVN were similar or somewhat lower in female versus male rats, but in the PFC, they were higher in female rats (Fig. 5). The PICs IL-1α, IL-1β, IL-2, IL-6, and TNF-α increased significantly in the PVN of the hypothalamus in both male and female rats with HF post-MI as well the anti-inflammatory cytokine IL-10 (Fig. 5). In contrast, PFC cytokines increased significantly in male rats with HF post-MI compared with male sham rats but for IL-2, only up to levels found in female sham rats, and showed no further increase in female rats with HF post-MI (Fig. 5). The amygdala showed no changes in any of the cytokines (Table 5).
Fig. 5.
Cytokine levels in the prefrontal cortex (PFC) and paraventricular nucleus (PVN) in male and female rats with heart failure at 10 wk postmyocardial infarction (post-MI). Values are means ± SE; n = 4–7 rats/group. Two-way ANOVA was performed for the sex and MI interaction followed by a Bonferroni post hoc test. In the PFC, MI × sex effect: IL-2, F = 4.7, P = 0.03; IL-10, F = 25.3, P = 0.0001; and TNF-α, F = 17.8, P = 0.001. MI effect: IL-6, F = 6.5, P = 0.02; and IL-1β, F = 4.6, P = 0.05. Sex effect: IL-1α, F = 5.6, P = 0.03; IL-6, F = 11.6, P = 0.003; and IL-10, F = 36.0, P = 0.001. In the PVN, MI effect: IL-1α, F = 17.7, P = 0.001; IL-1β, F = 10.7, P = 0.05; IL-2, F = 13.9, P = 0.002; IL-6, F = 13.1, P = 0.002; IL-10, F = 11.7, P = 0.003; and TNF-α, F = 14.3, P = 0.002. Sex effect: IL-1α, F = 4.1, P = 0.05; and IL-1β, F = 3.9, P = 0.05. *P < 0.05 vs. the male sham group; **P < 0.05 vs. the female sham group; ‡P < 0.0001 vs. the male sham group.
Table 5.
Cytokine levels in the amygdala of male and female rats are unchanged at 10 wk after MI
| Cytokine, pg/mg | Male Sham Group (n = 4) | Male MI Group (n = 5) | Female Sham Group (n = 5) | Female MI Group (n = 6) |
|---|---|---|---|---|
| IL-1α | 24 ± 6.0 | 29 ± 5.9 | 31 ± 2.3 | 29 ± 3.3 |
| IL-1β | 28 ± 7.5 | 36 ± 7.1 | 29 ± 6.0 | 33 ± 4.4 |
| IL-2 | 49 ± 8.4 | 41 ± 3.8 | 50 ± 6.3 | 41 ± 2.0 |
| IL-6 | 151 ± 45.0 | 205 ± 43.5 | 226 ± 16.8 | 201 ± 34.2 |
| IL-10 | 217 ± 51.5 | 259 ± 44.0 | 267 ± 15.0 | 250 ± 28.5 |
| TNF-α | 124 ± 33.2 | 150 ± 27.6 | 226 ± 16.8 | 201 ± 34.0 |
Values are means ± SE; n, number of rats/group. Two-way ANOVA was performed for the sex and myocardial infarction (MI) interaction. In the amygdala, there was no MI effect for all cytokines. MI effect: IL-1α, F = 0.02, P = 0.9; IL-1β, F = 0.5, P = 0.5; IL-2, F = 2.5, P = 0.1; IL-6, F = 0.0, P = 0.9; IL-10, F = 0.03, P = 0.9; TNF-α, F = 0.2, P = 0.7.
OVX female rats.
OVX with or without E2 with sham MI caused variable nonsignificant changes in plasma cytokines compared with the sham OVX-sham MI group (Table 6). Post-MI, plasma IL-2, IL-6, and TNF-α increased significantly in the OVX and OVX-E2 groups. The anti-inflammatory IL-10 also increased, the most being in the OVX-E2-MI group.
Table 6.
Plasma cytokine levels in OVX female rats with or without E2 replacement at 10 wk after MI
| Cytokine, pg/ml | Sham OVX-sham MI Group (n = 6) | OVX-Sham MI Group (n = 7) | OVX-E2-Sham MI Group (n = 7) | OVX-MI Group (n = 10) | OVX-E2-MI Group (n = 9) |
|---|---|---|---|---|---|
| IL-1α | 74 ± 9.8 | 70 ± 9.1 | 52 ± 11.3 | 82 ± 9.8 | 104 ± 19.9 |
| IL-1β | 144 ± 46.1 | 213 ± 27.5 | 399 ± 86.5 | 135 ± 20.3 | 207 ± 60.1 |
| IL-2 | 123 ± 16.4 | 131 ± 65.4 | 82 ± 9.0 | 193 ± 27.3* | 138 ± 9.8* |
| IL-6 | 479 ± 85.1 | 439 ± 109.3 | 357 ± 82.0 | 928 ± 173.6* | 630 ± 54.2* |
| IL-10 | 107 ± 26.2 | 82 ± 19.6 | 33 ± 5.9 | 131 ± 24.2* | 203 ± 34.9*† |
| TNF-α | 213 ± 49.1 | 149 ± 39.7 | 76 ± 6.9 | 288 ± 53.8* | 396 ± 64.4* |
Values are means ± SE; n, number of rats/group. E2, 17β-estradiol. One-way ANOVA was done to compare all groups, and two-way ANOVA was performed for the ovariectomy (OVX) and myocardial infarction (MI) interaction followed by a Bonferroni post hoc test. OVX × MI interaction for IL-10: F = 5.4, P = 0.027. MI effect: TNF-α, F = 19.7, P < 0.0001; IL-6, F = 5.0 P = 0.023; and IL-2, F = 5.1, P = 0.033. There was no MI or OVX effect for IL-1α or IL-1β.
P < 0.05 vs. the related sham MI group;
P < 0.05 vs. the OVX-MI group.
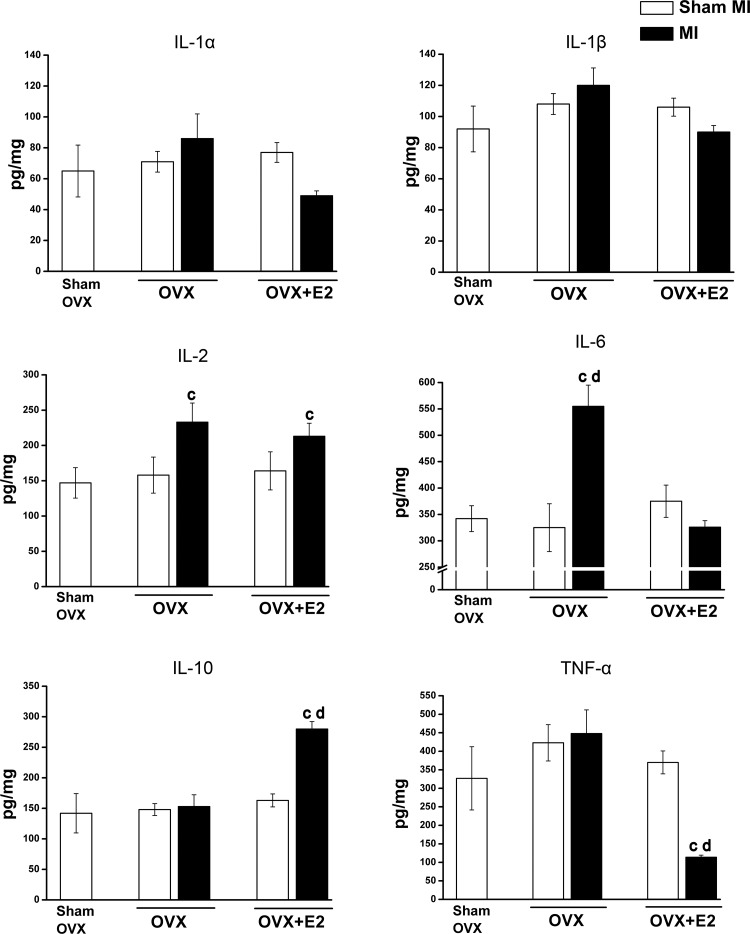
OVX with or witout E2 with sham MI did not affect cytokine levels in the PFC (Fig. 6). In the OVX-MI group, IL-2 and IL-6 increased significantly in the PFC. In contrast, in the PFC of the OVX-E2-MI group, IL-2 and anti-inflammatory IL-10 increased significantly, whereas TNF-α decreased markedly (Fig. 6).
Fig. 6.
Cytokine levels in the prefrontal cortex (PFC) of ovariectomized (OVX) female rats with or without 17β-estradiol (E2) replacement at 10 wk postmyocardial infarction (post-MI). Values are means ± SE; n = 6–11 rats/group. One-way ANOVA was done to compare all groups, and two-way ANOVA was performed for the OVX and MI interaction followed by a Bonferroni post hoc test. OVX × MI interaction: IL-6, F = 5.9, P = 0.024; IL-10, F = 12.5, P = 0.002; and TNF-α, F = 6.2, P = 0.02. MI effect: IL-2, F = 5.9, P = 0.023. OVX effect: IL-10, F = 16.7, P = 0.0001; and TNF-α, F = 12.8, P = 0.00. cP < 0.05 vs. the related sham MI group; dP < 0.05 vs. OVX-E2 groups.
BDNF Protein Levels
Male versus female rats.
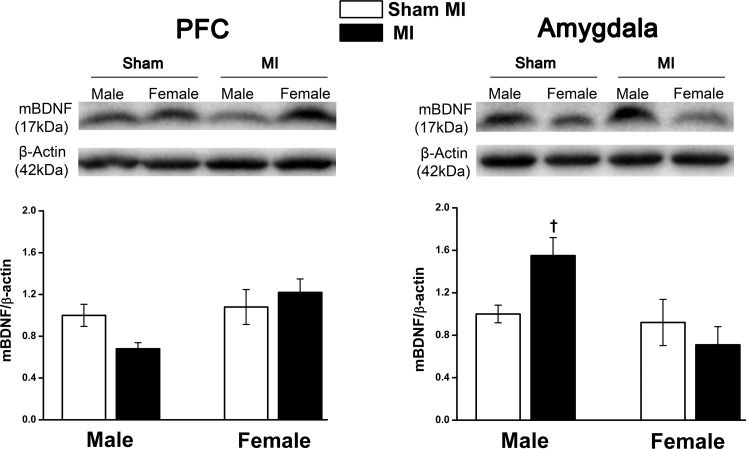
In the PFC, mBDNF protein expression had a nonsignificant trend (F = 2.8, P = 0.071) to decrease in the male post-MI group. The amygdala showed a significant MI and sex interaction (F = 5.4, P = 0.034), with mBDNF levels increasing significantly in male rats with HF post-MI compared with female rats with HF post-MI (Fig. 7).
Fig. 7.
Mature brain-derived neurotrophic factor (mBDNF) protein expression in the prefrontal cortex (PFC) and amygdala of male and female rats with heart failure at 10 wk postmyocardial infarction (post-MI). Values are means ± SE; n = 4–7 rats/group. Values for mBDNF/β-actin in the male sham group were normalized to 1. Two-way ANOVA was performed for the MI × sex interaction (F = 5.4, P = 0.034, in the amygdala and F = 2.8, P = 0.071, in the PFC). †P < 0.05 vs. the female MI group. Full blots were peer reviewed for validation.
OVX female rats.
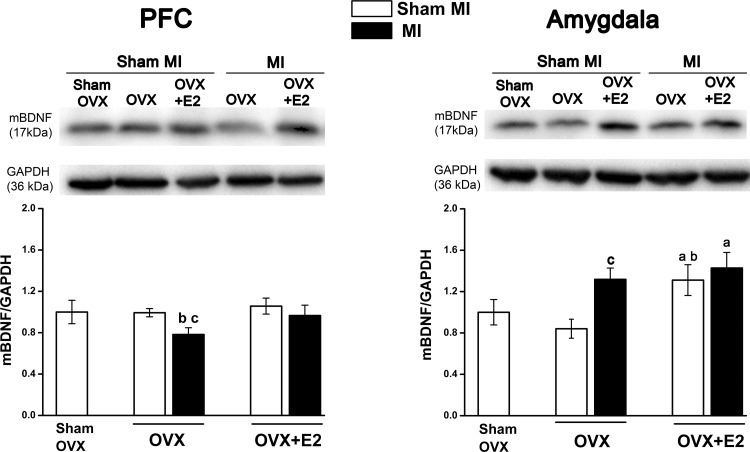
OVX with or without E2 did not affect mBDNF protein expression in the PFC of sham MI groups. In the OVX-MI group, mBDNF protein decreased significantly (F = 4.3, P < 0.05), which was prevented by E2 replacement (Fig. 8). In the amygdala, OVX did not effect mBDNF level, but E2 replacement increased mBDNF level significantly (F = 5.5, P = 0.024). The OVX-MI group showed a significant increase in mBDNF level compared with their sham group (F = 4.7, P = 0.03), but E2 replacement did not cause a further increase in the mBDNF level.
Fig. 8.
Mature brain-derived neurotrophic factor (mBDNF) protein expression in the prefrontal cortex (PFC) and amygdala of ovariectomized (OVX) female rats with heart failure at 10 wk postmyocardial infarction (post-MI). Values are means ± SE; n = 6–13 rats/group. Values for mBDNF/GAPDH in the sham OVX-sham MI group were normalized to 1. One-way ANOVA was done to compare all groups, and two-way ANOVA was performed for the OVX and MI interaction followed by a Bonferroni post hoc test. In the PFC, MI effect: F = 4.3, P = 0.046. OVX effect: F = 6.5, P = 0.016. In the amygdala, MI effect: F = 4.7, P = 0.038. OVX effect: F = 5.5, P = 0.024. aP < 0.05 vs. the sham OVX-sham MI group; bP < 0.05 vs. the OVX-sham MI group; cP < 0.05 vs. the related sham MI group. Full blots were peer reviewed for validation.
DISCUSSION
Our findings demonstrate that sex and estrogens influence neuroinflammation and depression-like behavior in rats with HF post-MI. In contrast with male rats, female rats with HF did not exhibit depression-like behaviors, even though male and female rats had a similar degree of HF and MI size. OVX female rats displayed mild depression-like behavior that was significantly more pronounced in OVX MI rats and largely prevented by E2 replacement. Plasma and PVN cytokine levels were likewise elevated in male and female rats with HF. PFC cytokine levels were higher in female versus male sham rats, and post-MI, they only increased in male rats. PFC cytokine levels were not affected by OVX per se, but after OVX, female rats post-MI also exhibited increases in PFC cytokines, which were changed to an anti-inflammatory pattern by E2 replacement. These findings suggest that the rise of neuroinflammation in the PFC in male subjects post-MI may contribute to the development of depression-like behavior, whereas in female subjects, estrogens may protect against depression by inhibiting cytokine production and actions.
HF in Male and Female Rats Post-MI
Male and female MI groups developed similar MI size (26% vs. 29% of the LV), associated with a similar clear increase in LV volumes, and showed a decrease in EF from ~90% to ~50% at 10 wk post-MI. In addition, LV end-diastolic pressure and RV weight increased and dP/dtmax decreased similarly in male and female MI groups. Altogether, the magnitude of the cardiac dysfunction was not affected by sex. These findings support previous studies in male and female rats showing similar cardiac dysfunction at 4–6 wk post-MI (10, 17, 34). On the other hand, male mice from 4 to 12 wk post-MI had worse EF and larger LV dimensions compared with female mice with similar MI size of ~30% (9). Two other studies in male and female mice with MI size of 30–40% showed an equivalent decline in cardiac function at 6 and 10 wk post-MI (8, 54). Collectively, these studies suggest that male and female rodents develop a similar degree of cardiac dysfunction for similar MI size.
OVX rats with or without E2 showed the expected pattern of changes in body weight and uterus weight but no significant changes in parameters of cardiac function in sham MI rats (Table 2). Consistent with a previous study (23), cardiac function was similarly decreased in OVX-MI and OVX-E2-MI rats.
Plasma and PVN Cytokines in Male and Female Rats Post-MI
This is the first study to compare the extent of peripheral and central inflammation in male and female rats post-MI. Female rats with HF post-MI had a similar pattern of increase in plasma cytokines compared with male rats with HF post-MI. At 10 wk post-MI, plasma levels of several PICs were increased two- to threefold, associated with an increase in the anti-inflammatory cytokine IL-10. A more variable pattern of changes was noted in OVX-MI-rats, whereas increases in plasma IL-6 and IL-10 were actually larger in OVX-E2-MI rats. Previous studies have shown that plasma IL-1β, IL-6, and TNF-α levels were significantly increased by two- to threefold in male rats at 4 wk post-MI (20, 28–30). Cytokine levels increase in the acute phase after MI as the immune system responds to the cardiac injury (40). Male and female mice post-MI showed equivalent trafficking of neutrophils and macrophages to initiate the wound repair process after MI (9). In the chronic phase, cytokines can further increase (25) and thus contribute to myocyte apoptosis and the progression of cardiac remodeling and dysfunction. A variety of studies have shown that inhibition of inflammation can inhibit cardiac dysfunction, for example, treatment of male mice with an IL-2 antibody for 14 days post-MI enhanced macrophage shifts from M1 to M2 and inhibited cardiac dysfunction (72). Treatment of male rats with the TNF-α inhibitor etanercept for 6 wk post-MI inhibited progression in cardiac dysfunction (6). Treatment of male rats with the anti-inflammatory cytokine IL-10 for 4 wk post-MI decreased IL-6 and TNF-α levels in the LV and plasma and improved cardiac function (57). These studies, therefore, highlight the growing recognition about the importance of cytokines in the progression of HF post-MI.
This is also the first study to show that female rats with HF post-MI develop inflammation in the PVN with 1.5- to 2-fold increases in the PICs IL-1α, IL-1β, IL-2, and TNF-α as well the anti-inflammatory IL-10, overall similar to the increases in male rats with HF post-MI. Microglia activation was observed by others in the PVN of male rats at 4 wk post-MI (49). Increases in plasma cytokines and ANG II may contribute to microglia activation, which then can produce local cytokines (71), which may enhance ANG II-ANG II type 1 receptor signaling with a decrease in GABA levels and an increase in glutamate levels (28, 30, 64). Central treatment with the cytokine synthesis inhibitor pentoxifylline decreased IL-1β and TNF-α levels in the PVN and attenuated cardiac decline post-MI (30). Similarly, SN50 (which inhibits nuclear translocation of NF-κB) administered centrally decreased IL-1β, IL-6, and TNF-α in the PVN and plasma, prevented an increase in plasma catecholamines, and was associated with less increase in LV end-diastolic pressure and less decline in EF in male rats post-MI (28). Altogether, the results suggest that in male and female rats, a similar increase of cytokines in the plasma and PVN is consistent with the similar cardiac dysfunction at 10 wk post-MI.
Depression-Like Behavior and Neuroinflammation in Male and Female Rats With HF Post-MI
This is the first study to demonstrate a clear difference in depression-like behavior in male and female rats with HF post-MI. Male rats with HF post-MI displayed clear depression-like behavior, such as anhedonia assessed by sucrose preference and behavioral despair assessed by the FST. This finding is similar to previous studies in male rats with HF post-MI (2, 3, 15, 18, 63) and occurred in the absence of any difference between control female and male rats. Spatial memory as assessed by MWM testing was conserved in both male and female rats with HF post-MI, consistent with a study in male rats by Wann et al. (63). Female rats had a slower learning rate in the MWM, which is consistent with previous MWM studies that compared male and female rats (32). HF post-MI had no effect on acquisition of fear conditioning in either sex group. In contrast, in the cued fear conditioning, male, but not female, rats with HF post-MI froze significantly more, suggesting that male rats with HF post-MI exhibit an enhanced adaptive response to stressful events (5). Taken together, male, but not female, rats with HF post-MI develop depression-like behavior.
Depression-like behavior has been linked to cytokines and neuroinflammation (13, 38, 69). Cytokines may contribute to depression-like behavior by several mechanisms, such as a decrease in the expression of BDNF through activating microglia and astrocytes, activating the IDO enzyme, which decreases the concentration of serotonin, and using tetrahydrobiopterin for nitric oxide synthesis, which is essential for dopamine production (13, 38). Indeed, treatment of male rats with pentoxifylline for 14 days alleviated anhedonia and immobility in the FST at 14 days post-MI (3). Similarly, treatment of male rats with etanercept for 7 days post-MI prevented anhedonia (18, 26). Consistent with a previous study in male rats (58a), in the present study, the PICs IL-1β, IL-2, IL-6, and TNF-α, but also IL-10, increased by 1.5-fold in the PFC of male rats with HF but not in the PFC of female rats with HF. The increase of PICs in the PFC of male rats was associated with a trend for reduction in mBDNF only in male rats with HF, similar to a previous study in male rats (27). However, cytokine levels were higher in female versus male sham rats and increased in male rats with HF, largely only to the levels of female control rats. If PICs in the PFC indeed contribute to depression-like behavior, these findings may suggest that female rats are protected against cytokine-induced depression-like behavior by, e.g., estrogens inhibiting downstream responses to cytokines as well as by inhibiting further increases in cytokines post-MI (see Depression-Like Behavior in OVX Female Rats Post-MI below). The high densities of estrogen receptor-α in the PFC compared with the PVN (33, 44) may play a role in this regard.
Finally, the amygdala revealed no significant changes in PICs or IL-10 in either sex group at 10 wk post-MI. Only male rats with HF showed an increase in cue-associated freezing, which is dependent on the amygdala (1), and an increase in mBDNF in the amygdala. An increase of mBDNF levels in the amygdala was also found in chronic stressed rats (37). Patients with depression showed an increase in the activity of the amygdala (70). High levels of BDNF in the amygdala might contribute to hyperexcitability of the fear circuit in the FCT (51) and enhance the expression of fear (42).
Depression-Like Behavior in OVX Female Rats Post-MI
Consistent with previous studies (7, 31, 69), after OVX, female rats developed depression-like behavior with a modest decrease in sucrose preference and an increase in immobility in the FST as well as a marked increase in context and cue-associated freezing in the FCT, whereas general locomotor activity was not affected. As expected (69), these behavior changes were largely prevented by E2 replacement, indicating that the adverse effects of OVX indeed mainly depend on estrogen deficiency. Enhanced cue-associated freezing was fully prevented by E2, but context-associated freezing was only partially prevented by E2, suggesting possible different effects of E2 in the amygdala versus the hippocampus (4, 46). Similar to the findings by Xu et al. (69), OVX alone or with E2 replacement did not affect cytokine levels, suggesting that an increase in neuroinflammation per se in the PFC does not contribute to the depression-like behavior by estrogen deficiency. Consistent with this finding, mBDNF levels in the PFC were not affected by OVX alone or with E2 replacement, similar to a previous study (36). In contrast, E2 replacement at physiological levels increased mBDNF levels in the whole amygdala. Altogether, OVX-induced estrogen deficiency may remove a protective effect against cytokine-induced actions on, e.g., IDO enzyme and serotonin production (48, 69) or on BDNF levels (12, 53, 56).
The present study is the first study to demonstrate the marked protection provided by the ovaries and estrogens against depression-like behavior in female rats post-MI. In contrast with intact female rats, OVX rats developed a marked decrease in sucrose preference and an increase in immobility in the FST post-MI, as similarly noted in intact male rats post-MI. Freezing behavior on the cued fear conditioning day of the FCT was markedly increased by MI in OVX rats, reaching ~80% versus ~50% in male rats post-MI. This finding may indicate a further activation of the fear circuit in the amygdala. Estrogen replacement prevented all of the OVX-MI-induced changes in behavior. This finding highlights the potent inhibitory effects of estrogens on mechanisms contributing to depression-like behavior in female rats with HF post-MI. In this regard, we evaluated the interaction of OVX, MI, and E2 replacement for cytokine levels in the PFC. In contrast with no changes in intact female rats post-MI, after OVX-MI, PICs IL-2 and IL-6 significantly increased, and TNF-α tended to increase in the PFC. E2 replacement shifted this pattern toward an anti-inflammatory one with a twofold increase in IL-10, possibly preventing an increase in IL-6 and causing a marked decrease in TNF-α (57). Consistent with the cytokines, mBDNF decreased in the PFC of the OVX-MI group, comparable to the changes in male rats post-MI. E2 replacement prevents this decrease of mBDNF in the PFC. Further studies with cytokine synthesis inhibitors, such as pentoxifylline (3), or with a specific cytokine inhibitor, such as etanercept (18, 26), are needed to determine the actual role of these cytokine changes for inducing depression-like behavior and the protective actions of estrogens in the setting of HF post-MI. Further studies are also needed to assess whether these same mechanisms play a role in psychological distress-induced depression.
In conclusion, in contrast with male rats, female rats with HF post-MI do not develop depression-like behavior; neither exhibit increases in cytokines in the PFC. However, female OVX-MI rats also develop marked depression-like behaviors that are prevented by E2 replacement, possibly by inhibiting production and actions of proinflammatory cytokines. Understanding the mechanisms contributing to these sex-specific and estrogen-dependent responses may contribute to new therapeutic strategies that may be sex specific (50).
GRANTS
F. H. H. Leenen holds the Pfizer Chair in Hypertension Research, an endowed chair supported by Pfizer Canada, University of Ottawa Heart Institute Foundation and Canadian Institutes of Health Research. This work was supported by University of Ottawa Heart Institute Grant 4571 and by Canadian Institutes of Health Research Operating Grant FRN: MOP-136923.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.A., D.L., and F.H.H.L. conceived and designed research; F.N. and M.A. performed experiments; F.N., M.A., and D.L. analyzed data; F.N., M.A., D.L., and F.H.H.L. interpreted results of experiments; F.N. prepared figures; F.N. and F.H.H.L. drafted manuscript; F.N., M.A., D.L., and F.H.H.L. edited and revised manuscript; F.N., M.A., D.L., and F.H.H.L. approved final version of manuscript.
REFERENCES
- 1.Armony JL, Quirk GJ, LeDoux JE. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. J Neurosci 18: 2592–2601, 1998. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bah TM, Benderdour M, Kaloustian S, Karam R, Rousseau G, Godbout R. Escitalopram reduces circulating pro-inflammatory cytokines and improves depressive behavior without affecting sleep in a rat model of post-cardiac infarct depression. Behav Brain Res 225: 243–251, 2011. doi: 10.1016/j.bbr.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 3.Bah TM, Kaloustian S, Rousseau G, Godbout R. Pretreatment with pentoxifylline has antidepressant-like effects in a rat model of acute myocardial infarction. Behav Pharmacol 22: 779–784, 2011. doi: 10.1097/FBP.0b013e32834d1385. [DOI] [PubMed] [Google Scholar]
- 4.Barha CK, Dalton GL, Galea LA. Low doses of 17α-estradiol and 17β-estradiol facilitate, whereas higher doses of estrone and 17α- and 17β-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology 35: 547–559, 2010. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckers T, Krypotos AM, Boddez Y, Effting M, Kindt M. What’s wrong with fear conditioning? Biol Psychol 92: 90–96, 2013. doi: 10.1016/j.biopsycho.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Berry MF, Woo YJ, Pirolli TJ, Bish LT, Moise MA, Burdick JW, Morine KJ, Jayasankar V, Gardner TJ, Sweeney HL. Administration of a tumor necrosis factor inhibitor at the time of myocardial infarction attenuates subsequent ventricular remodeling. J Heart Lung Transplant 23: 1061–1068, 2004. doi: 10.1016/j.healun.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Borrow AP, Cameron NM. Estrogenic mediation of serotonergic and neurotrophic systems: implications for female mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 54: 13–25, 2014. doi: 10.1016/j.pnpbp.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Bridgman P, Aronovitz MA, Kakkar R, Oliverio MI, Coffman TM, Rand WM, Konstam MA, Mendelsohn ME, Patten RD. Gender-specific patterns of left ventricular and myocyte remodeling following myocardial infarction in mice deficient in the angiotensin II type 1a receptor. Am J Physiol Heart Circ Physiol 289: H586–H592, 2005. doi: 10.1152/ajpheart.00474.2004. [DOI] [PubMed] [Google Scholar]
- 9.Cavasin MA, Tao Z, Menon S, Yang XP. Gender differences in cardiac function during early remodeling after acute myocardial infarction in mice. Life Sci 75: 2181–2192, 2004. doi: 10.1016/j.lfs.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Chen YF, Redetzke RA, Sivertson RM, Coburn TS, Cypher LR, Gerdes AM. Post-myocardial infarction left ventricular myocyte remodeling: are there gender differences in rats? Cardiovasc Pathol 20: e189–e195, 2011. doi: 10.1016/j.carpath.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 103: 2055–2059, 2001. doi: 10.1161/01.CIR.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 12.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med 5: 11–25, 2004. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 13.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246: 199–229, 2013. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis J, Chu Y, Johnson AK, Weiss RM, Felder RB. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am J Physiol Heart Circ Physiol 286: H2264–H2271, 2004. doi: 10.1152/ajpheart.01072.2003. [DOI] [PubMed] [Google Scholar]
- 15.Frey A, Popp S, Post A, Langer S, Lehmann M, Hofmann U, Sirén A, Hommers L, Schmitt A, Strekalova T, Ertl G, Lesch K, Frantz S. Experimental heart failure causes depression-like behavior together with differential regulation of inflammatory and structural genes in the brain. Front Behav Neurosci 8: 376, 2014. doi: 10.3389/fnbeh.2014.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb SS, Khatta M, Friedmann E, Einbinder L, Katzen S, Baker B, Marshall J, Minshall S, Robinson S, Fisher ML, Potenza M, Sigler B, Baldwin C, Thomas SA. The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol 43: 1542–1549, 2004. doi: 10.1016/j.jacc.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 17.Gouweleeuw L, Hovens IB, Liu H, Naudé PJW, Schoemaker RG. Differences in the association between behavior and neutrophil gelatinase-associated lipocalin in male and female rats after coronary artery ligation. Physiol Behav 163: 7–16, 2016. doi: 10.1016/j.physbeh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- 18.Grippo AJ, Francis J, Weiss RM, Felder RB, Johnson AK. Cytokine mediation of experimental heart failure-induced anhedonia. Am J Physiol Regul Integr Comp Physiol 284: R666–R673, 2003. doi: 10.1152/ajpregu.00430.2002. [DOI] [PubMed] [Google Scholar]
- 19.Grippo AJ, Johnson AK. Biological mechanisms in the relationship between depression and heart disease. Neurosci Biobehav Rev 26: 941–962, 2002. doi: 10.1016/S0149-7634(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 20.Guggilam A, Haque M, Kerut EK, McIlwain E, Lucchesi P, Seghal I, Francis J. TNF-α blockade decreases oxidative stress in the paraventricular nucleus and attenuates sympathoexcitation in heart failure rats. Am J Physiol Heart Circ Physiol 293: H599–H609, 2007. doi: 10.1152/ajpheart.00286.2007. [DOI] [PubMed] [Google Scholar]
- 21.Habib P, Beyer C. Regulation of brain microglia by female gonadal steroids. J Steroid Biochem Mol Biol 146: 3–14, 2015. doi: 10.1016/j.jsbmb.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Huffman JC. Review: depression after myocardial infarction is associated with increased risk of all-cause mortality and cardiovascular events. Evid Based Ment Health 16: 110, 2013. doi: 10.1136/eb-2013-101537. [DOI] [PubMed] [Google Scholar]
- 23.Hügel S, Reincke M, Strömer H, Winning J, Horn M, Dienesch C, Mora P, Schmidt HH, Allolio B, Neubauer S. Evidence against a role of physiological concentrations of estrogen in post-myocardial infarction remodeling. J Am Coll Cardiol 34: 1427–1434, 1999. doi: 10.1016/S0735-1097(99)00368-X. [DOI] [PubMed] [Google Scholar]
- 25.Irwin MW, Mak S, Mann DL, Qu R, Penninger JM, Yan A, Dawood F, Wen WH, Shou Z, Liu P. Tissue expression and immunolocalization of tumor necrosis factor-alpha in postinfarction dysfunctional myocardium. Circulation 99: 1492–1498, 1999. doi: 10.1161/01.CIR.99.11.1492. [DOI] [PubMed] [Google Scholar]
- 26.Johnson AK, Grippo AJ. Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol 57, Suppl 11: 5–29, 2006. [PubMed] [Google Scholar]
- 27.Kaloustian S, Wann BP, Bah TM, Girard SA, Apostolakis A, Ishak S, Mathieu S, Ryvlin P, Godbout R, Rousseau G. Apoptosis time course in the limbic system after myocardial infarction in the rat. Brain Res 1216: 87–91, 2008. doi: 10.1016/j.brainres.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Kang YM, Gao F, Li HH, Cardinale JP, Elks C, Zang WJ, Yu XJ, Xu YY, Qi J, Yang Q, Francis J. NF-κB in the paraventricular nucleus modulates neurotransmitters and contributes to sympathoexcitation in heart failure. Basic Res Cardiol 106: 1087–1097, 2011. doi: 10.1007/s00395-011-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circ Res 99: 758–766, 2006. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- 30.Kang YM, Zhang ZH, Xue B, Weiss RM, Felder RB. Inhibition of brain proinflammatory cytokine synthesis reduces hypothalamic excitation in rats with ischemia-induced heart failure. Am J Physiol Heart Circ Physiol 295: H227–H236, 2008. doi: 10.1152/ajpheart.01157.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiss A, Delattre AM, Pereira SI, Carolino RG, Szawka RE, Anselmo-Franci JA, Zanata SM, Ferraz AC. 17β-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res 227: 100–108, 2012. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 32.Kokras N, Dalla C. Sex differences in animal models of psychiatric disorders. Br J Pharmacol 171: 4595–4619, 2014. doi: 10.1111/bph.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERα and ERβ) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol 36: 357–378, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 34.Litwin SE, Katz SE, Litwin CM, Morgan JP, Douglas PS. Gender differences in postinfarction left ventricular remodeling. Cardiology 91: 173–183, 1999. doi: 10.1159/000006906. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord 139: 230–239, 2012. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 36.Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience 239: 34–45, 2013. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 41: 3–23, 2016. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65: 732–741, 2009. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moraska AR, Chamberlain AM, Shah ND, Vickers KS, Rummans TA, Dunlay SM, Spertus JA, Weston SA, McNallan SM, Redfield MM, Roger VL. Depression, healthcare utilization, and death in heart failure: a community study. Circ Heart Fail 6: 387–394, 2013. doi: 10.1161/CIRCHEARTFAILURE.112.000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94: 1543–1553, 2004. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 41.Nickenig G, Bäumer AT, Grohè C, Kahlert S, Strehlow K, Rosenkranz S, Stäblein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Böhm M. Estrogen modulates AT1 receptor gene expression in vitro and in vivo. Circulation 97: 2197–2201, 1998. doi: 10.1161/01.CIR.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 42.Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology 31: 287–296, 2006. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- 43.Parrott JM, Redus L, Santana-Coelho D, Morales J, Gao X, O’Connor JC. Neurotoxic kynurenine metabolism is increased in the dorsal hippocampus and drives distinct depressive behaviors during inflammation. Transl Psychiatry 6: e918, 2016. doi: 10.1038/tp.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pérez SE, Chen E-Y, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res 145: 117–139, 2003. doi: 10.1016/S0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- 45.Pham J, Cabrera SM, Sanchis-Segura C, Wood MA. Automated scoring of fear-related behavior using EthoVision software. J Neurosci Methods 178: 323–326, 2009. doi: 10.1016/j.jneumeth.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285, 1992. doi: 10.1037/0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 47.Pyner S. The paraventricular nucleus and heart failure. Exp Physiol 99: 332–339, 2014. doi: 10.1113/expphysiol.2013.072678. [DOI] [PubMed] [Google Scholar]
- 48.Raison CL, Rook GW, Miller AH, Begay TK. Role of inflammation in psychiatric disease. In: Neurobiology of Brain Disorders: Biological Basis of Neurological and Psychiatric Disorders. New York: Elsevier, 2015, p. 396–421. doi: 10.1016/B978-0-12-398270-4.00026-4 [DOI] [Google Scholar]
- 49.Rana I, Stebbing M, Kompa A, Kelly DJ, Krum H, Badoer E. Microglia activation in the hypothalamic PVN following myocardial infarction. Brain Res 1326: 96–104, 2010. doi: 10.1016/j.brainres.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 50.Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 97: 1–37, 2017. doi: 10.1152/physrev.00021.2015. [DOI] [PubMed] [Google Scholar]
- 51.Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev 105: 325–350, 1998. doi: 10.1037/0033-295X.105.2.325. [DOI] [PubMed] [Google Scholar]
- 52.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 48: 1527–1537, 2006. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 53.Şahin TD, Karson A, Balcı F, Yazır Y, Bayramgürler D, Utkan T. TNF-alpha inhibition prevents cognitive decline and maintains hippocampal BDNF levels in the unpredictable chronic mild stress rat model of depression. Behav Brain Res 292: 233–240, 2015. doi: 10.1016/j.bbr.2015.05.062. [DOI] [PubMed] [Google Scholar]
- 54.Shioura KM, Geenen DL, Goldspink PH. Sex-related changes in cardiac function following myocardial infarction in mice. Am J Physiol Regul Integr Comp Physiol 295: R528–R534, 2008. doi: 10.1152/ajpregu.90342.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 7: 1009–1014, 2012. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 56.Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol 27: 404–414, 2006. doi: 10.1016/j.yfrne.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stumpf C, Seybold K, Petzi S, Wasmeier G, Raaz D, Yilmaz A, Anger T, Daniel WG, Garlichs CD. Interleukin-10 improves left ventricular function in rats with heart failure subsequent to myocardial infarction. Eur J Heart Fail 10: 733–739, 2008. doi: 10.1016/j.ejheart.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry 15: 80–92, 2010. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58a.Ter Horst GJ. Central autonomic control of the heart, angina, and pathogenic mechanisms of post-myocardial infarction depression. Eur J Morphol 37: 257–266, 1999. doi: 10.1076/ejom.37.4.257.4722. [DOI] [PubMed] [Google Scholar]
- 59.Toffol E, Heikinheimo O, Partonen T. Hormone therapy and mood in perimenopausal and postmenopausal women: a narrative review. Menopause 22: 564–578, 2015. doi: 10.1097/GME.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 60.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 1: 848–858, 2006. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology 34: 909–916, 2009. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wann BP, Bah TM, Kaloustian S, Boucher M, Dufort AM, Le Marec N, Godbout R, Rousseau G. Behavioural signs of depression and apoptosis in the limbic system following myocardial infarction: effects of sertraline. J Psychopharmacol 23: 451–459, 2009. doi: 10.1177/0269881108089820. [DOI] [PubMed] [Google Scholar]
- 63.Wann BP, Bah TM, Boucher M, Courtemanche J, Le Marec N, Rousseau G, Godbout R. Vulnerability for apoptosis in the limbic system after myocardial infarction in rats: a possible model for human postinfarct major depression. J Psychiatry Neurosci 32: 11–16, 2007. [PMC free article] [PubMed] [Google Scholar]
- 64.Wei SG, Zhang ZH, Yu Y, Felder RB. Central SDF-1/CXCL12 expression and its cardiovascular and sympathetic effects: the role of angiotensin II, TNF-α, and MAP kinase signaling. Am J Physiol Heart Circ Physiol 307: H1643–H1654, 2014. doi: 10.1152/ajpheart.00432.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neurosci Biobehav Rev 37: 2331–2371, 2013. doi: 10.1016/j.neubiorev.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 66.Wise DD, Felker A, Stahl SM. Tailoring treatment of depression for women across the reproductive lifecycle: the importance of pregnancy, vasomotor symptoms, and other estrogen-related events in psychopharmacology. CNS Spectr 13: 647–662, 2008. doi: 10.1017/S1092852900013742. [DOI] [PubMed] [Google Scholar]
- 67.Wohleb ES. Neuron–microglia interactions in mental health disorders: “for better, and for worse”. Front Immunol 7: 544, 2016. doi: 10.3389/fimmu.2016.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright L, Simpson W, Van Lieshout RJ, Steiner M. Depression and cardiovascular disease in women: is there a common immunological basis? A theoretical synthesis. Ther Adv Cardiovasc Dis 8: 56–69, 2014. doi: 10.1177/1753944714521671. [DOI] [PubMed] [Google Scholar]
- 69.Xu Y, Sheng H, Tang Z, Lu J, Ni X. Inflammation and increased IDO in hippocampus contribute to depression-like behavior induced by estrogen deficiency. Behav Brain Res 288: 71–78, 2015. doi: 10.1016/j.bbr.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 70.Yu H, Chen ZY. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin 32: 3–11, 2011. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Y, Wei SG, Weiss RM, Felder RB. Angiotensin II type 1a receptors in the subfornical organ modulate neuroinflammation in the hypothalamic paraventricular nucleus in heart failure rats. Neuroscience 381: 46–58, 2018. doi: 10.1016/j.neuroscience.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zeng Z, Yu K, Chen L, Li W, Xiao H, Huang Z. Interleukin-2/anti-interleukin-2 immune complex attenuates cardiac remodeling after myocardial infarction through expansion of regulatory T cells. J Immunol Res 2016: 8493767, 2016. doi: 10.1155/2016/8493767. [DOI] [PMC free article] [PubMed] [Google Scholar]