Abstract
Introduction
Subjective cognitive decline (SCD) is the earliest stage on the continuum toward Alzheimer's disease. This study examined (1) differences in white matter integrity between individuals with SCD and healthy control subjects and (2) how white matter integrity related to memory and executive function.
Methods
Diffusion tensor imaging and neuropsychological assessment data were retrieved from the Alzheimer's Disease Neuroimaging Initiative database for 30 individuals with SCD and 44 control subjects.
Results
Results revealed significantly lower white matter integrity in individuals with SCD relative to control subjects in widespread regions, including the bilateral corticospinal tracts, superior and inferior longitudinal fasciculi, fronto-occipital fasciculi, corpus callosum, forceps major and minor, hippocampi, anterior thalamic radiations, and the cerebellum. There was a widespread relationship between diffusion tensor imaging metrics and executive function in SCD, but not healthy control subjects, and no relationship with memory for either group.
Discussion
Relatively lower white matter integrity in SCD may be a useful early biomarker for risk of future cognitive decline. Future research should better characterize the SCD group longitudinally and in individuals at risk for Alzheimer's disease.
Keywords: Subjective cognitive decline, Diffusion tensor imaging, Executive function, Memory, Alzheimer's disease
Highlights
-
•
Individuals with SCD had lower FA and higher MD relative to healthy controls.
-
•
Lower FA and higher mean MD were related to lower executive function in SCD only.
-
•
White matter integrity was unrelated to memory in SCD and healthy control subjects.
-
•
DTI metrics may be a sensitive biomarker for early Alzheimer's disease.
1. Introduction
Currently, 5.7 million older adults in the United States are living with Alzheimer's disease (AD), an incurable neurodegenerative disorder that includes primary deficits in memory and other cognitive skills (e.g., executive function). With the aging population, the prevalence of AD is expected to increase dramatically; by 2050, the number of AD cases are expected to double, with a corresponding cost of $1.1 trillion annually for health care, long-term care, and hospice care for individuals with AD and related dementias [1]. A goal of emerging research is to identify those at greatest risk for AD before cognitive symptoms are manifest, using biomarkers and other risk factors [2].
Most of the current research in this area focuses on individuals with the amnestic variant of mild cognitive impairment (MCI), who are at elevated risk to decline to AD [3]. However, individuals who subjectively report cognitive decline and who have normal performance on objective neuropsychological assessment measures have been recognized as earlier in the continuum toward dementia (i.e., between healthy aging and MCI) [4], [5], [6]. To date, there has been a paucity of research on individuals with subjective cognitive decline (SCD), likely because of varying terminology (e.g., subjective memory complaints, subjective memory impairment, and significant memory concerns) and lack of common classification standards [7]. Recently, increasing attention has been given to SCD as an International Working Group was formed to define a conceptual framework for SCD [8]. Notably, the term SCD reached consensus given that individuals tend to present with concerns regarding memory and/or other forms of cognitive impairment [8]. This renewed focus on SCD has been an important development in AD research given the desire to identify individuals at risk for AD before significant decline on clinical assessment [9]. Emerging research has suggested that objective, physiologically based neuroimaging measures may be able to differentiate individuals with SCD from healthy control subjects [7], [10].
The ideal method for detecting preclinical biomarkers for AD would be noninvasive, easily repeatable, and widely available, as is magnetic resonance imaging (MRI). Most MRI-based biomarker research has focused on visualizing neuronal loss in specific gray matter structures. To date, hippocampus volumetry is the first-tier imaging biomarker for AD [11]. In line with this, there have been investigations focused on hippocampal volumetry in individuals with SCD. For example, Ryu et al. [12] used voxel-based morphometry to examine differences in gray matter between individuals with SCD and healthy control subjects. They found that Individuals with SCD had lower entorhinal cortical volumes than control participants, but there were no significant differences in hippocampal volume between groups. Interestingly, this group also used diffusion tensor imaging (DTI) and detected microstructural differences in both entorhinal and hippocampal regions between groups, suggesting that DTI may be a more sensitive method. Indeed, it is possible that microstructural changes (as measured by DTI) occur early on in SCD, although these changes have been less investigated.
DTI is a MRI-based acquisition method that measures water diffusion to estimate the integrity of white matter tissue. Specifically, fractional anisotropy (FA) provides an index of the degree of directionality of water diffusion and mean diffusivity (MD) is a measure of the mean rate of water diffusion [13], such that decreases in FA and increases in MD are considered indicative of demyelination and axonal deterioration, as a result of neurodegeneration [14], [15], [16].
DTI has previously been used to identify early changes in white matter metrics in individuals with AD (e.g., see Mayo et al. [17]; for a review, see Amlien and Fjell [18]) and MCI (e.g., see Liu et al. [19]). However, to date there have only been a handful of studies published that have compared individuals with SCD relative to control subjects using tract-based spatial statistics (TBSS). One initial study by Wang et al. [20] compared individuals with MCI and SCD to healthy control subjects. The results indicated that DTI metrics fell along a continuum, such that the SCD group was an intermediary to the MCI and control subjects. Selnes et al. [21] took a similar approach to comparing DTI metrics in individuals with SCD to healthy control subjects and found widespread differences in MD in several tracts underlying the posterior cingulate, retrosplenial, and middle temporal cortices. Additional analyses suggested that the degeneration observed in white matter was independent of gray matter atrophy. Selnes et al. [22] then followed up to examine whether baseline DTI metrics predict cognitive decline and medial temporal lobe atrophy in participants with SCD, MCI, and healthy control subjects. They found that baseline MD, but not FA was different between participants with different cognitive outcomes (including decline) and that both MD and FA at baseline were related to atrophy in the medial temporal lobe. Recently Li et al. [23] examined white matter integrity in individuals with SCD compared with healthy control subjects and found that the SCD group had decreased FA and increased MD in widespread white matter tracts. Furthermore, correlations between DTI parameters and a measure of verbal memory indicated lower FA and higher MD were correlated with poorer cognitive performance. Although this relationship was not explored directly in the TBSS pipeline and the neuropsychological measures included short cognitive screeners and a verbal memory test, it highlights the importance of considering DTI metrics in the context of cognition.
The main objectives of the present study were (1) to investigate microstructural differences in white matter between a group of individuals with SCD and matched healthy control subjects and (2) to extend previous research by investigating how white matter microstructure in these groups relates to indices of memory and executive function, using a whole brain, TBSS approach. It was hypothesized that individuals with SCD would have lower FA and higher MD values than healthy control subjects, particularly in the medial temporal lobes. It was also expected that measures of memory and executive function would show a positive relationship with FA and a negative relationship with MD in both groups.
2. Methods
Data used in the preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, positron emission tomography, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD.
2.1. Participants
Full eligibility criteria for the ADNI are described in the procedures manual found on the ADNI website (http://adni.loni.usc.edu/methods/documents/). Individuals with SCD (termed significant memory concerns) and healthy control subjects both had normal memory function on the Logical Memory II subscale (≥9 for 16 or more years of education; ≥5 for 8–15 years of education; ≥3 for 0–7 years of education), a Mini-Mental State Examination (MMSE) score between 24 and 30, a Clinical Dementia Rating score of 0, and an absence of impairment in activities of daily living. Individuals with SCD differed from healthy control subjects in having a significant concern reported by the subject, study partner, or clinician, as confirmed by a Cognitive Change Index score (≥16).
Neuroimaging and neuropsychological assessment data were retrieved from the first available (screening) time point. Thirty individuals with SCD (F = 20, mean age = 72.9 years, SD = 4.8) and 44 similarly aged control subjects (F = 25, mean age = 72.5 years, SD = 6.4) were included.
All participants provided informed written consent to participate in the ADNI study (approved by the sites Institutional Review Board). The Human Research Ethics Board at the University of Victoria approved the secondary data analyses for the present study.
2.2. Image acquisition
According to ADNI protocol, images were acquired from 3 T MRI scanners (GE Medical Systems). Axial diffusion-weighted image data were acquired with a spin echo planar imaging sequence. Scan parameters are as follows: acquisition matrix = 256 × 256, voxel size = 1.4 × 1.4 × 2.7 mm3, flip angle = 90°, number of slices = 59. There were 46 images acquired for each scan: 41 diffusion-weighted images (b = 1000 seconds/mm2) and five non–diffusion-weighted images (b = 0 seconds/mm2). Repetition time varied across scanning sites, but was approximately 13,000 milliseconds.
2.3. Neuropsychological assessment data
The composite scores for memory (ADNI-MEM) [24] and executive function (ADNI-EF) [25] were retrieved from the ADNI database. ADNI-MEM represents a composite score derived from a single factor model with the Rey Auditory Verbal Learning Test, AD Assessment Schedule—Cognition, Logical Memor,y and MMSE data. ADNI-EF represents a composite score derived using item response theory methods from digit symbol substitution, digit span backwards, trails A and B, category fluency, and clock drawing.
2.4. Data analyses
All data analyses were performed in Functional MRI of the Brain Software Library version 5.0 (available at http://fsl.fmrib.ox.ac.uk/) [26]. Diffusion-weighted images were corrected for eddy current distortions and head movement using Eddy correct tool and nonbrain tissue was removed using Brain Extraction tool [27]. FA and MD maps were created using DTIfit and input into TBSS [28]. All data were nonlinearly aligned to FMRIB58_FA space. Then, the mean FA image was created and thresholded (FA > 0.2) to create the mean FA skeleton. Voxelwise statistical analyses were performed using Randomize with threshold-free cluster enhancement to correct for multiple comparisons (P < .05). Between-group comparisons were made between individuals with SCD and healthy control subjects. Additional statistical models were used to examine the relationship between white matter structures and ADNI-MEM and ADNI-EF scores in each group, controlling for age. White matter regions were identified with Johns Hopkins University's atlases available in Functional MRI of the Brain Software Library [29], [30].
3. Results
3.1. Participant characteristics
There were no significant differences between the groups in terms of age, sex, education level, or cognitive scores (see Table 1).
Table 1.
Participant characteristics
| Variable | Subjective cognitive decline (n = 30) | Healthy control subjects (n = 44) | P value∗ |
|---|---|---|---|
| Age (y) | 72.94 (±4.79) | 72.49 (±6.37) | .84 |
| Education (y) | 16.40 (±2.59) | 16.21 (±2.78) | .78 |
| Gender ratio (F:M) | 20:10 | 25:18 | .46 |
| ADNI–memory composite | 1.11 (±0.54) | 0.44 (±0.78) | .50 |
| ADNI–executive function composite | 0.43 (±0.78) | 0.74 (±0.72) | .09 |
| APOE Status (total) | |||
| APOE ε2-3 | 2 | 3 | |
| APOE ε3-3 | 18 | 23 | |
| APOE ε3-4 | 9 | 12 | |
| APOE ε4-4 | 1 | 0 | |
| Not available | 0 | 6 |
Abbreviations: APOE, apolipoprotein E; SD, standard deviation.
NOTE. Mean (SD)
∗t tests and χ2 test obtained P values.
3.2. Microstructural white matter differences between individuals with SCD and healthy control subjects
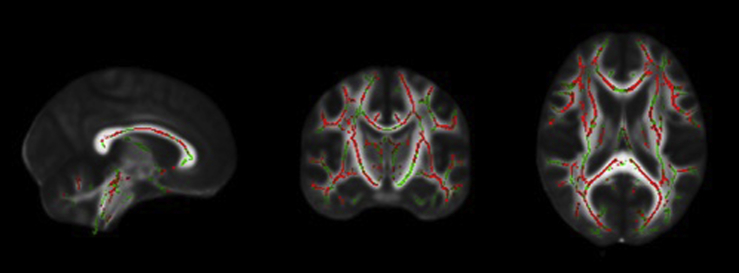
The between-group FA analyses revealed diffuse reductions in the SCD group relative to the healthy control group in regions including bilateral corticospinal tracts, superior and inferior longitudinal fasciculi, fronto-occipital fasciculi, corpus callosum, forceps major and minor, hippocampi, anterior thalamic radiations, and the cerebellum (Fig. 1). There were no regions in which FA was lower in healthy control subjects relative to SCD.
Fig. 1.
From left to right: sagittal, coronal, and axial slices of the standard FMRIB_FA_58 brain displaying results of TBSS analyses showing regions that have significantly lower FA (red) overlaid on the white matter skeleton (green) in individuals with SCD compared with healthy control subjects (P < .05, corrected for multiple comparisons, radiological view). Abbreviations: FA, fractional anisotropy; SCD, subjective cognitive decline; TBSS, tract-based spatial statistics.
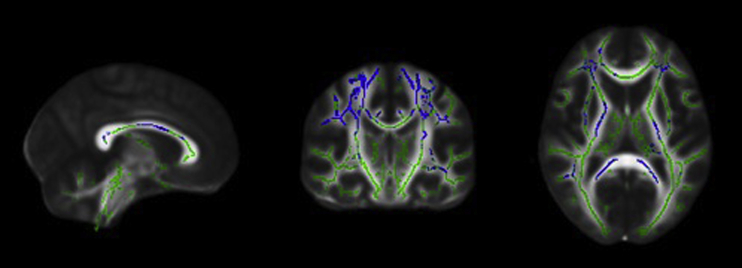
The between-group MD analyses showed diffusely higher MD in the SCD group compared with the control group in regions including bilateral corticospinal tracts, superior and inferior longitudinal fasciculi, superior corona radiata, and corpus callosum (Fig. 2). There were no regions identified where healthy control subjects had higher MD than individuals with SCD.
Fig. 2.
From left to right: sagittal, coronal, and axial slices of the standard FMRIB_FA_58 brain displaying results of TBSS analyses showing regions that have significantly higher MD (blue) overlaid on the white matter skeleton (green) in individuals with SCD compared with healthy control subjects (P < .05, corrected for multiple comparisons, radiological view). Abbreviations: MD, mean diffusivity; SCD, subjective cognitive decline; TBSS, tract-based spatial statistics.
3.3. Relationship between microstructural white matter and cognitive performance
Analyses investigating the relationship between FA/MD and ADNI-MEM composite scores did not detect significant relationships in healthy control subjects or individuals with SCD.
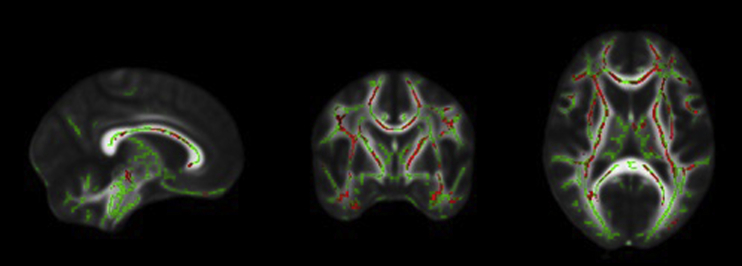
No significant relationship between FA and executive function, as measured by the ADNI-EF composite, was detected in the healthy control subjects. In contrast, FA was significantly related to executive function in individuals with SCD. Specifically, the SCD group showed a significant positive relationship between FA and executive function in bilateral corticospinal tracts, superior longitudinal fasciculi, corpus callosum, forceps major and minor, hippocampi, and anterior thalamic radiations (Fig. 3).
Fig. 3.
From left to right: sagittal, coronal, and axial slices of the standard FMRIB_FA_58 brain displaying results of TBSS analyses showing a positive relationship between FA and ADNI–executive function composite scores (red) overlaid on the white matter skeleton (green) in individuals with SCD (P < .05, corrected for multiple comparisons, radiological view). Abbreviations: FA, fractional anisotropy; SCD, subjective cognitive decline; TBSS, tract-based spatial statistics.
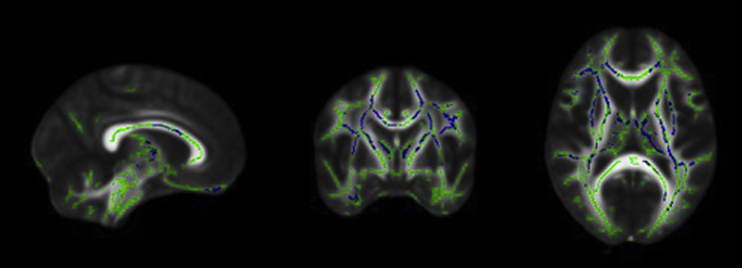
Similar to FA, there was no significant relationship between MD and ADNI-EF composite scores for control subjects, whereas there was a significant negative relationship in diffuse regions for the SCD group. Specifically, significant regions included bilateral corticospinal tracts, superior longitudinal fasciculus, superior corona radiata, cingulate, corpus callosum, anterior thalamic radiations, and right hippocampus (Fig. 4).
Fig. 4.
From left to right: sagittal, coronal, and axial slices of the standard FMRIB_FA_58 brain displaying results of TBSS analyses showing a negative relationship between MD and ADNI–executive function composite scores (blue) overlaid on the white matter skeleton (green) in individuals with SCD (P < .05, corrected for multiple comparisons, radiological view). Abbreviations: MD, mean diffusivity; SCD, subjective cognitive decline; TBSS, tract-based spatial statistics.
4. Discussion
The present study represents a key step in characterizing individuals with SCD—an increasingly recognized risk factor for the development of AD. The primary hypothesis was that individuals with SCD would have lower FA and higher MD values than healthy control subjects, bored out, in regions including and beyond the medial temporal lobes. The secondary hypothesis that measures of memory and executive function would show a positive relationship with FA and a negative relationship with MD in both groups was as predicted for executive function in individuals with SCD, but not healthy control subjects. Neither group showed relationships between FA or MD and an index of memory. These results are further discussed in the context of the extant literature subsequently.
4.1. Microstructural white matter differences between individuals with SCD and healthy control subjects
The present study revealed lower FA and higher MD scores in the SCD group relative to healthy control subjects in diffuse regions including the bilateral corticospinal tracts, superior and inferior longitudinal fasciculi, fronto-occipital fasciculi, corpus callosum, forceps major and minor, hippocampi, anterior thalamic radiations, and the cerebellum. These findings are quite consistent with a recent study by Li et al. [23], who also discovered widespread differences in similar regions between individuals with SCD and healthy control subjects. The current findings are also consistent with other previous studies in detecting group differences between those with SCD and healthy control subjects in middle temporal cortices [20], [21], [22], although the present study detected more widespread differences.
A main objective in detecting differences between those with SCD and healthy control subjects is to characterize possible biomarkers that could be used to identify individuals that may eventually develop AD; an especially important aim, given that both groups perform equally on neuropsychological assessment. The present study demonstrates that there are microstructural differences in individuals with SCD relative to healthy control subjects, which suggests that there has been a breakdown of myelin and/or axons in this group. In this context, DTI appears to offer sensitive metrics as a biomarker, although it is imperative to consider whether white matter degeneration is a result of retrogenesis (primary breakdown of myelin and axons), or anterograde (Wallerian) degeneration (secondary loss of myelin and axons because of gray matter degeneration) [31]. In the present results, we observe quite diffuse differences in FA, but an anterior to posterior gradient of degeneration is suggested based on the MD results, which reveal higher MD in frontal and anterior regions, rather than the temporal lobes. The pattern of progression from late myelinating tracts toward earlier myelinating tracts is in keeping with the retrogenesis hypothesis and suggestive of white matter degeneration as a primary process that is independent of gray matter. These findings are in keeping with work by Jung et al. [32], who examined individuals with SCD, MCI, and AD and discovered a progressive decrease in FA in bilateral anterior corona radiata, cingulate gyrus, hippocampi, fornix, corpus callosum, and the left uncinate fasciculus; a pattern that they suggested was evidence for the retrogenesis hypothesis as well.
4.2. Relationship between microstructural white matter and cognitive performance
Relationships between FA/MD and ADNI-EF composite scores in individuals with SCD were revealed in widespread regions, including the bilateral corticospinal tracts, superior longitudinal fasciculi, corpus callosum, forceps major and minor, hippocampi, and anterior thalamic radiations. Specifically, lower FA and higher MD (indicative of decreased white matter integrity) in these regions were associated with lower executive function scores. There were no significant relationships detected between FA/MD and executive function in the control group or with ADNI-MEM composite scores in either group.
The present study is the first to examine the relationship between cognitive performance and DTI metrics in SCD using TBSS. Before this, only a few studies had examined the relationship between cognition and microstructural white matter characteristics in individuals with SCD, with correlative or predictive approaches. Previous research on SCD has found a relationship between cognitive decline (as measured by the MMSE) and medial temporal lobe atrophy [22], that poorer verbal memory scores are associated with higher FA and lower MD [23] and that white matter diffusivity predicted memory performance in a combined group of individuals with SCD/MCI and normal cerebral spinal fluid tau levels [33]. In particular, rather than utilizing individual test scores, our findings extend previous work [22], [23] by utilizing two separate composite indices of memory [24] and executive function [25] that comprised well validated neuropsychological assessment measures. These composite scores have addressed several psychometric concerns with the use of the individual constituent tests and have been especially recommended for investigations using neuroimaging [24], [25]. Furthermore, the present study examined the relationships between cognition and white matter microstructure in individuals from a large multisite study in North America, which adds to the generalizability of findings from other global sites, such as Norway [22], [33] and China [23].
It is noteworthy that although the criteria that separated the individuals with SCD from healthy control subjects were memory complaints (as measured by the first 12 items on the Cognitive Change Index), there were no significant relationships found between memory performance (as measured by the ADNI-MEM composite) and DTI metrics. Interestingly, only the SCD group showed a relationship between DTI metrics and performance on executive function tasks (as indexed by the ADNI-EF composite) in quite congruent regions to where differences in DTI metrics were observed between the SCD and healthy control groups. These results suggest that the microstructural differences in white matter between the groups may relate to executive function.
It is possible that individuals may be able to functionally compensate for structural changes, until frontal degeneration reaches a critical point, and cognitive declines become measurable.
Notably, a recent study by Valech et al. [34] assessed how complaints in SCD may discriminate preclinical AD from healthy aging and found complaints about language and executive function to be key. Other studies have shown cross-sectional differences between individuals with SCD and healthy control subjects in on experimental-cognitive tests of executive function (e.g. [35], [36]). Unfortunately, although there are items on the Cognitive Change Index that assess complaints about executive function, the items are not included as part of the ADNI protocol, and therefore it is not possible to assess whether the individuals in this study had concerns about their executive functioning or to consider how such concerns may relate to the present findings. These results underscore the importance of broadening the conceptualization of subjective decline from solely memory concerns to cognitive complaints, echoing the recommendations of the SCD International Working Group.
4.3. Limitations and future directions
There were several limitations to the present study that may offer directions for future research. The present study examined microstructural differences in white matter between individuals with SCD and healthy control subjects, but did not adjust for macrostructural brain characteristics, such as white matter lesion load or atrophy. Part of the reason that the present study did not incorporate these factors was the strict exclusion criteria used by the ADNI database, which leads to a relatively low white matter hyperintensity lesion burden across participants [37]. However, future work could compare white matter hyperintensity lesion burden and whole brain and temporal lobe atrophy between groups directly, using more broadly defined samples.
Future studies could also further investigate and follow up on the current findings. On the basis of the pattern of results observed here, white matter integrity may decline independently of atrophy in gray matter. Future work could aim to better distinguish between the influences of retrograde and anterograde degeneration. Although it is likely that these degenerative mechanisms occur in some combination, it is imperative to determine which process is primary, given the ultimate objective of determining the earliest biomarker for AD.
Similarly, with this ultimate objective in mind, it is necessary to find ways to distinguish which individuals with SCD are most likely to eventually develop AD. Individuals with SCD currently represent a mixed group—some may remain stable, and others may develop various types of dementia, including AD. In the current article, we report apolipoprotein E (APOE ε) status; however, the composition of genetic markers within the present study limited our ability to examine genetic effects. To better characterize SCD, future studies should more fully evaluate the nature of cognitive concerns, take a longitudinal approach, and also incorporate other biomarkers for AD, such as APOE ε4 genotype.
Another limitation of this study was the small sample size. However, the size of the sample was commensurate, if not larger than many other neuroimaging studies in SCD and in line with the estimated number of participants needed to detect differences in FA and MD [38]. We included the maximum number of participants possible from one of the largest neuroimaging initiatives focused on AD to date. Promisingly, future research addressing these limitations will eventually become possible with the ADNI database as the number of participants and data points for the SCD group increases over time.
Finally, our goal was to extend previous research by investigating the relationship between more specific cognitive domains (i.e., executive function and memory) and white matter microstructure. To this end, we chose to limit our analyses to the most commonly used metrics (FA and MD) as they have been shown to better attain the relationship between cognition and white matter integrity in MCI and AD [14]. Many studies have limited their analyses to these metrics [12], [17], [19], [32]. However, like others [20], [23], [33], future work of DTI in SCD should utilize other metrics in DTI, such as radial and axial diffusivity, which may provide more specific information regarding demyelination and axonal degeneration, respectively. Future work should aim to investigate other DTI indices in individuals with SCD.
5. Conclusions
The present findings that individuals with SCD have significantly lower white matter integrity than healthy control subjects indicates that DTI metrics may be a sensitive biomarker for early AD. The present study was also the first to examine the relationship between DTI metrics and indices of cognitive performance, which demonstrated a differential relationship between executive function and microstructural white matter in individuals with SCD compared with healthy control subjects. This study is one of only a few that have focused on microstructural characteristics of white matter in SCD, a group that is thought to be earliest on the continuum toward AD. Given that age is the largest risk factor for AD and the aging population is growing at an exponential rate, globally, it is imperative that more studies focus on characterizing those with SCD to identify individuals who will go on to develop AD before symptom onset so that neuroprotective interventions can be delivered.
Research in context.
-
1.
Systematic review: The authors reviewed the literature using publication databases (e.g., PubMed), focusing on samples equivalent to subjective cognitive decline (SCD).
-
2.
Interpretation: Our finding of widespread decreased white matter integrity in individuals with SCD shows potential for diffusion tensor imaging metrics as biomarkers for future cognitive decline. Lower white matter integrity was related to lower executive function in individuals with SCD, which affirms recommendations from SCD International Working Group to include cognitive complaints beyond memory in the definition of SCD.
-
3.
Future directions: Longitudinal approaches should be used to (1) further characterize SCD, (2) determine whether loss of integrity in white matter is primary or secondary to gray matter atrophy, and (3) explore how other biomarkers for Alzheimer's disease (e.g., APOE ε4 genotype) are related to SCD. The Alzheimer's Disease Neuroimaging Initiative database will be an ideal source to investigate SCD as the number of participants and data points increase.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer's Association; Alzheimer's Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer's Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding for secondary analyses of the ADNI database was provided by a Catalyst Grant from the Canadian Institute for Health Research.
References
- 1.Alzheimer's Association 2018 Alzheimer's disease facts and figures. Alzheimers Dement. 2018;3:367–429. [Google Scholar]
- 2.Jack C.R., Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;4:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;3:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reisberg B., Ferris S.H., de Leon M.J., Crook T. The global deterioration scale for assessment of primary degenerative. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 5.Reisberg B., Gauthier S. Current evidence for subjective cognitive impairment as the pre-mild cognitive impairment stage of subsequently manifest Alzheimer's disease. Int Psychogeriatr. 2008;1:1–16. doi: 10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- 6.Reisberg B., Prichep L., Mosconi L., John E.R., Glodzik-Sobanska L., Boksay I. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimers Dement. 2008;1:S98–S108. doi: 10.1016/j.jalz.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 7.Rabin L.A., Smart C.M., Amariglio R.E. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13:369–396. doi: 10.1146/annurev-clinpsy-032816-045136. [DOI] [PubMed] [Google Scholar]
- 8.Jessen F., Amariglio R.E., van Boxtel M., Breteler M., Ceccaldi M., Chételat G. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;6:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smart C.M., Karr J.E., Areshenkoff C.N., Rabin L.A., Hudon C., Gates N. Non-pharmacologic interventions for older adults with subjective cognitive decline: systematic review, meta-analysis, and preliminary recommendations. Neuropsychol Rev. 2017;3:245–257. doi: 10.1007/s11065-017-9342-8. [DOI] [PubMed] [Google Scholar]
- 10.Hu X., Harzem J., Huang B., Weber B., Jessen F. Abnormal functional connectivity within default mode network in persons with subjective cognitive decline: self-reflection of own memory deficits? Alzheimers Dement. 2016;7:39. [Google Scholar]
- 11.Sheikh-Bahaei N., Sajjadi S.A., Manavaki R., Gillard J.H. Imaging biomarkers in Alzheimer's disease: a practical guide for clinicians. J Alzheimers Dis Rep. 2017;1:71–88. doi: 10.3233/ADR-170013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryu S.Y., Lim E.Y., Na S., Shim Y.S., Cho J.H., Yoon B. Hippocampal and entorhinal structures in subjective memory impairment: a combined MRI volumetric and DTI study. Int Psychogeriatr. 2017;5:785–792. doi: 10.1017/S1041610216002349. [DOI] [PubMed] [Google Scholar]
- 13.Le Bihan D., Mangin J.F., Poupon C., Clark C.A., Pappata S., Molko N. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;4:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 14.Bosch B., Arenaza-Urquijo E.M., Rami L., Sala-Llonch R., Junqué C., Solé-Padullés C. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol Aging. 2012;1:61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Kantarci K. Fractional anisotropy of the fornix and hippocampal atrophy in Alzheimer's disease. Front Aging Neurosci. 2014;6:1–4. doi: 10.3389/fnagi.2014.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serra L., Cercignani M., Lenzi D., Perri R., Fadda L., Caltagirone C. Grey and white matter changes at different stages of Alzheimer's disease. J Alzheimers Dis. 2010;1:147–159. doi: 10.3233/JAD-2010-1223. [DOI] [PubMed] [Google Scholar]
- 17.Mayo C.D., Mazerolle E.L., Ritchie L., Fisk J.D., Gawryluk J.R. Alzheimer's Disease Neuroimaging Initiative. longitudinal changes in microstructural white matter metrics in Alzheimer's disease. Neuroimage Clin. 2017;13:330–338. doi: 10.1016/j.nicl.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amlien I.K., Fjell A.M. Diffusion tensor imaging of white matter degeneration in Alzheimer's disease and mild cognitive impairment. Neuroscience. 2014;276:206–215. doi: 10.1016/j.neuroscience.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Spulber G., Lehtimäki K.K., Könönen M., Hallikainen I., Gröhn H. Diffusion tensor imaging and tract-based spatial statistics in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2011;9:1558–1571. doi: 10.1016/j.neurobiolaging.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., West J.D., Flashman L.A., Wishart H.A., Santulli R.B., Rabin L.A. Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim Biophys Acta. 2012;3:423–430. doi: 10.1016/j.bbadis.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selnes P., Fjell A.M., Gjerstad L., Bjørnerud A., Wallin A., Due-Tønnessen P. White matter imaging changes in subjective and mild cognitive impairment. Alzheimers Dement. 2012;5:S112–S121. doi: 10.1016/j.jalz.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Selnes P., Aarsland D., Bjørnerud A., Gjerstad L., Wallin A., Hessen E. Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. J Alzheimers Dis. 2013;3:723–736. doi: 10.3233/JAD-2012-121603. [DOI] [PubMed] [Google Scholar]
- 23.Li X., Tang Z., Sun Y., Tian J., Liu Z., Han Y. White matter degeneration in subjective cognitive decline: a diffusion tensor imaging study. Oncotarget. 2016;34:54405–54414. doi: 10.18632/oncotarget.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crane P.K., Carle A., Gibbons L.E., Insel P., Mackin R.S., Gross A. Development and assessment of a composite score for memory in the Alzheimer's Disease Neuroimaging Initiative. Brain Imaging Behav. 2012;4:502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbons L.E., Carle A.C., Mackin R.S., Harvey D., Mukherjee S., Insel P. A composite score for executive functioning, validated in Alzheimer's Disease Neuroimaging Initiative participants with baseline mild cognitive impairment. Brain Imaging Behav. 2012;4:517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E.J., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 27.Smith S.M. Fast robust automated brain extraction. Hum Brain Mapp. 2002;3:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;4:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Mori S., Crain B.J. 2nd ed. Academic Press; Amsterdam: 2006. MRI atlas of human white matter. [Google Scholar]
- 30.Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;3:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alves G.S., Oertel Knöchel V., Knöchel C., Carvalho A.F., Pantel J., Engelhardt E. Integrating retrogenesis theory to Alzheimer's disease pathology: insight from DTI-TBSS investigation of the white matter microstructural integrity. Biomed Res Int. 2015;2015:1–11. doi: 10.1155/2015/291658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung W.B., Lee Y.M., Kim Y.H., Mun C.-W. Automated classification to predict the progression of Alzheimer's disease using whole-brain volumetry and DTI. Psychiatry Investig. 2015;1:92–102. doi: 10.4306/pi.2015.12.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grambaite R., Stenset V., Reinvang I., Walhovd K.B., Fjell A.M., Fladby T. White matter diffusivity predicts memory in patients with subjective and mild cognitive impairment and normal CSF total tau levels. J Int Neuropsychol Soc. 2010;1:58–69. doi: 10.1017/S1355617709990932. [DOI] [PubMed] [Google Scholar]
- 34.Valech N., Tort-Merino A., Coll-Padrós N., Olives J., León M., Rami L. Executive and language subjective cognitive decline complaints discriminate preclinical Alzheimer's disease from normal aging. J Alzheimers Dis. 2018;2:689–703. doi: 10.3233/JAD-170627. [DOI] [PubMed] [Google Scholar]
- 35.Smart C.M., Krawitz A. The impact of subjective cognitive decline on Iowa Gambling Task performance. Neuropsychology. 2015;6:971–987. doi: 10.1037/neu0000204. [DOI] [PubMed] [Google Scholar]
- 36.Mulligan B.P., Smart C.M., Ali J.I. Relationship of subjective and objective performance indicators in subjective cognitive decline. Psychol Neurosci. 2016;3:362–378. [Google Scholar]
- 37.Scarapicchia V., Mazerolle E.L., Fisk J.D., Ritchie L.J., Gawryluk J.R. Resting state BOLD variability in Alzheimer's disease: a marker of cognitive decline or cerebrovascular status? Front Aging Neurosci. 2018;10:1–13. doi: 10.3389/fnagi.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Santis S., Drakesmith M., Bells S., Assaf Y., Jones D.K. Why diffusion tensor MRI does well only some of the time: variance and covariance of white matter tissue microstructure attributes in the living human brain. Neuroimage. 2014;89:35–44. doi: 10.1016/j.neuroimage.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]