Abstract
Background
Early identification of patients with chronic viral hepatitis coinfected with human immunodeficiency virus (HIV) is essential for optimal care. The objectives of this study were to estimate the prevalence of HIV coinfection among patients newly diagnosed with chronic viral hepatitis, HIV testing prevalence, and identify factors associated with coinfection.
Methods
Patients with chronic viral hepatitis newly enrolled in The Danish Database for Hepatitis B and C between 2002 and 2015 were identified. The HIV coinfection prevalence was calculated, and risk factors associated with HIV coinfection were estimated by logistic regression.
Results
In total, 8490 patients were included: 3091 had chronic hepatitis B (CHB), 5305 had chronic hepatitis C (CHC), and 94 had CHB and CHC. The prevalence of HIV coinfection was 4.4% (95% confidence interval [CI], 4.0–4.9) and was higher among CHC and CHB-CHC patients than CHB patients with a prevalence of 5.3% (95% CI, 4.7–5.9), 6.4% (95% CI, 2.4–13.4), and 2.9 (95% CI, 2.3–3.5), respectively (P < .0001). The HIV testing prevalence increased from 65% to 88% between 2002 and 2014 concurrently with a decrease in the HIV coinfection prevalence from 7.8% (95% CI, 5.5–10.7) to 1.6% (95% CI, 0.7–3.2). Age 35–50 years, male sex, and sexual route of viral hepatitis transmission were associated with HIV coinfection with odds ratios of 4.42 (95% CI, 1.40–13.94), 2.21 (95% CI, 1.74–2.81), and 8.81 (95% CI, 6.30–12.33), respectively.
Conclusions
The prevalence of HIV coinfection among patients with newly diagnosed chronic viral hepatitis decreased concurrently with an increase in HIV testing prevalence.
Keywords: chronic viral hepatitis, HIV, nationwide cohort study, prevalence
Human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV) are all blood-borne pathogens and thus share routes of transmission, such as sexual contact, from mother to child during pregnancy (vertical), intravenous drug use (IDU), or blood transfusion [1–3]. Consequently, a small but significant population of coinfected patients with HIV and chronic viral hepatitis exists [4, 5]. It has long been known that patients with HBV or HCV and HIV coinfection have a more rapidly progressing course of liver disease [6–12] and increased risk of chronic kidney disease [13]. In addition, early initiation of highly active antiretroviral therapy (HAART) is essential to avoid serious acquired immune deficiency syndrome (AIDS)-related events among HIV-infected individuals [14]. As a result, early identification of patients with HIV coinfection among patients with viral hepatitis is crucial. In line with international guidelines, the Danish clinical practice guidelines recommend that all patients newly diagnosed with chronic hepatitis B (CHB) and/or chronic hepatitis C (CHC) are tested for HIV [2, 15, 16]. Since the Danish clinical practice CHB and CHC guidelines were introduced, a great effort has been made to increase HIV testing of chronic viral hepatitis patients [15, 17].
Denmark is a low-prevalence country with regards to HIV, CHB, and CHC with estimated prevalences of approximately 0.2% [18], 0.2% [19], and 0.4% [20], respectively. A previously published Danish regional study suggested an HIV coinfection prevalence of 5% among patients with CHC [21]. However, no peer-reviewed, nationwide studies on Danish patients have been published with regards to the HIV prevalence among chronic viral hepatitis patients.
Previous studies have primarily focused on viral hepatitis coinfection among patients infected with HIV, and nationwide information is lacking on the HIV coinfection prevalence among patients with chronic viral hepatitis and risk factors associated with HIV coinfection.
Therefore, in this nationwide cohort study, we aimed to estimate the prevalence of HIV coinfection among patients newly diagnosed with CHB and CHC in Denmark and to describe changes in the HIV testing prevalence and prevalence of HIV coinfection over time. Moreover, we wished to investigate whether any baseline characteristics of patients newly diagnosed with CHB and/or CHC were associated with HIV coinfection.
MATERIALS AND METHOD
Setting
All citizens in Denmark are provided with a unique, 10-digit person identification number that permits linkage between multiple data sources. Healthcare services are publicly funded and free of charge to the individual in Denmark. The prospectively collected data used in this nationwide cohort study were extracted from The Danish Database for Hepatitis B and C (DANHEP). The DANHEP is a nationwide database, initiated on January 1, 2002, with ongoing enrollment of all patients over the age of 15 years attending specialized care for CHB and/or CHC in Denmark. Currently, all 18 hospital departments of either infectious diseases, internal medicine with infectious diseases specialists, or gastroenterology are responsible for care of patients with CHB and CHC contribute to DANHEP. The database contains data on patient demographics and clinical and laboratory results.
Study Population
Data from DANHEP were extracted in July 2015. Chronic hepatitis B was defined as a positive hepatitis B surface antigen (HBsAg) before or at least 6 months after enrollment in DANHEP. Chronic hepatitis C was defined as the detection of HCV-ribonucleic acid (RNA) before or within 6 months after enrollment in DANHEP. Chronic infection with HBV and HCV (CHB-CHC) was defined as fulfilling the criteria for both CHB and CHC. All patients enrolled in DANHEP after January 1, 2002 who met the criteria for CHB and/or CHC and who were at least 15 years of age were included in this study. At the date of enrollment in DANHEP, the patient was considered newly diagnosed with chronic viral hepatitis.
We extracted results of HIV antibody/antigen tests performed before or within 6 months of enrollment in DANHEP with viral hepatitis (HIV positive, HIV negative, or unknown HIV status [Supplementary Material]). An HIV test performed at any point before July 2015, regardless of its relation to enrollment in DANHEP, was defined as “ever HIV tested”.
In the presence of conflicting results with regards to HCV-RNA, HBsAg, and HIV antigen/antibody test results registered in DANHEP at enrollment, an ad hoc assessment in each case was performed to determine the hepatitis and HIV status. All laboratory analyses were performed locally at the hospital responsible for care of the patient.
Definition of Covariates
We categorized the following patient characteristic covariates at enrollment:
• Country of birth (Western Europe [including Greenland], Eastern Europe, Africa, Asia, North America, South America, and other)
• Alcohol abuse was defined as the self-reported weekly consumption of more than 14 units of alcohol for women and 21 units of alcohol for men.
• Route of transmission was defined as self-reported suspected route of viral hepatitis transmission (IDU, tattoo/piercing, vertical transmission, sexual transmission, blood/blood product exposure [including needle injury], multiple, and other reasons).
• Reason for testing for viral hepatitis (clinical symptoms [including elevated liver function tests]; screening during pregnancy; screening for other reasons [including screening of persons with known risk factors defined by the Danish Health and Medicines Authorities {Supplementary Material}] [15]; and other reasons)
• Sex (male, female)
• Age at enrollment
• Clinical cirrhosis (yes/no) was defined as the presence of clinical signs of cirrhosis before or up to 6 months after the enrollment date (oesophageal varices, ascites, and hepatic encephalopathy).
• HCV genotype
Statistical Analyses
Baseline characteristics are presented as median with interquartile range or absolute and relative frequencies. Differences in patient characteristics between hepatitis groups (CHB, CHC, and CHB-CHC) were tested for statistical significance by applying the Kruskal-Wallis, Fisher’s exact test, χ2, or χ2 test with Montecarlo simulation using 10000 samples, whenever appropriate. The prevalence of HIV coinfection with 95% confidence interval (CI) was calculated using the Clopper-Pearson [22] exact method as the number of HIV-positive individuals divided by the total number of patients including patients with unknown HIV status. To estimate the risk of HIV coinfection (HIV positive vs HIV nonpositive) associated with different hepatitis groups, univariate logistic regression was applied. To estimate the change in HIV coinfection prevalence over time between 2002 and 2015, we used multiple logistic regression analysis with year of enrollment in DANHEP as a continuous covariate and adjusted for sex, age, and country of birth. Year of enrollment and age were tested for log linearity, and because age did not show log linearity it was introduced as a categorical covariate. To estimate the risk of HIV coinfection associated with sex, age, and country of birth (Western Europe as reference), we used univariate logistic regression models. The risk of HIV coinfection associated with route of transmission was estimated using multiple logistic regression models adjusting for confounding factors. Further details on the handling of the substantial number of missing values for route of transmission can be found in the Supplementary Material. All logistic regression models were determined on the basis of directed acyclic graphs [23] to reduce bias (Supplementary Figure 1). Human immunodeficiency virus testing and HIV coinfection prevalence were compared between departments of gastroenterology and hepatology versus departments with infectious diseases specialists using the χ2 test.
Multiple logistic regression models were tested for goodness-of-fit by applying the Hosmer-Lemeshow test [24]. Statistical tests were 2-sided, and P < .05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC), and figures were created using R studio [25].
Ethical Approval
This study was approved by the Danish Data Protection agency in accordance with Danish law (J.nr. 2012-58-0023).
RESULTS
Study Population
As of July 2015, 10784 patients had been enrolled in DANHEP. We excluded 2294 patients in total, 1715 of whom patients were enrolled before January 1, 2002, 517 patients did not fulfill the definitions of either CHB or CHC, and 62 patients were <15 years. Of the 8490 patients included, 3091 had CHB, 5305 had CHC, and 94 had both CHB and CHC. Baseline characteristics stratified by hepatitis group are presented in Table 1. Patients with CHB were younger than CHC and CHB-CHC patients, and the distribution of sex differed between the hepatitis groups with more female CHB patients than CHC and CHB-CHC patients. The overall prevalence of HIV was 4.4% (95% CI, 4.0–4.9), and the prevalence was 2.9% (95% CI, 2.3–3.5) among CHB patients, 5.3% (95% CI, 4.7–5.9) among CHC patients, and 6.4% (95% CI, 2.4–13.4) among CHB-CHC patients (P < .0001).
Table 1.
Baseline Characteristics
| Variable | Chronic Hepatitis B | Chronic Hepatitis C | Chronic Hepatitis B and C | Totala |
|---|---|---|---|---|
| Number (%) | 3091 (36.4) | 5305 (62.5) | 94 (1.1) | 8490 |
| Age at diagnosis (median, q1, q3) | 34.9 (27.6–44.8) | 45.0 (36.6–52.4) | 41.3 (35.0–49.2) | 41.4 (32.4–50.5), P < .0001 |
| Women, N (%) | 1654 (53.5) | 1768 (33.3) | 27 (28.7) | 3449 (40.6) |
| P < .0001 | ||||
| HIV status | ||||
| Positive | 89 (2.9) | 281 (5.3) | 6 (6.4) | 376 (4.4) |
| Negative | 2198 (71.1) | 4119 (77.6) | 67 (71.3) | 6384 (75.2) |
| Unknown | 804 (26.0) | 905 (17.1) | 21 (22.3) | 1730 (20.4) |
| P < .0001b | ||||
| Origin/country of birth | 450 (14.6) | 129 (2.4) | 4 (4.3) | 583 (6.9) |
| Africa | 1592 (51.5) | 409 (7.7) | 20 (21.3) | 2021 (23.8) |
| Asia | 285 (9.2) | 262 (4.9) | 4 (4.3) | 551 (6.5) |
| Eastern Europe | 2 (0.1) | 21 (0.4) | - | 23 (0.3) |
| North America | 13 (0.4) | 32 (0.6) | - | 45 (0.5) |
| South America | 509 (16.5) | 4279 (80.7) | 61 (64.9) | 4849 (57.1) |
| Western Europec | 47 (1.5) | 16 (0.3) | - | 63 (0.7) |
| Otherd | 193 (6.2) | 157 (3.0) | 5 (5.3) | 355 (4.2) |
| Unknown | P < .0001 | |||
| Route of transmission | 61 (2.0) | 268 (5.1) | - | 329 (3.9) |
| Blood/blood product exposuree | 67 (2.2) | 3238 (61.0) | 53 (56.4) | 3358 (39.6) |
| IDU | 126 (4.1) | 164 (3.1) | 1 (1.1) | 291 (3.4) |
| Sexual contact | 14 (0.5) | 107 (2.0) | 2 (2.1) | 123 (1.5) |
| Tattoo/piercing | 886 (28.7) | 25 (0.5) | 7 (7.5) | 918 (10.8) |
| Vertical | - | 13 (0.3) | - | 13 (0.2) |
| Multiple | 74 (2.4) | 70 (1.3) | 4 (4.3) | 148 (1.7) |
| Other reason | 1863 (60.3) | 1420 (26.8) | 27 (28.7) | 3310 (39.0) |
| Unknown | P < .0001 | |||
| Diagnostic test reason | 544 (17.6) | 1585 (29.9) | 28 (29.8) | 2157 (25.4) |
| Clinical symptoms | 372 (12.0) | 590 (11.1) | 13 (13.8) | 975 (11.5) |
| Other reason | 450 (14.6) | 52 (1.0) | 1 (1.1) | 503 (5.9) |
| Screening pregnancy | 1063 (34.4) | 1624 (30.6) | 20 (21.3) | 2707 (31.9) |
| Screening other | 662 (21.4) | 1454 (27.4) | 32 (34.0) | 2148 (25.3) |
| Unknown | P < .0001 | |||
| History of alcohol abuse | 128 (4.1) | 1348 (25.4) | 17 (18.1) | 1493 (17.6) P < .0001 |
| HCV genotype | ||||
| Genotype 1 | - | 2086 (39.3) | 33 (35.1) | 2119 (39.3) |
| Genotype 2 | - | 388 (7.3) | 4 (4.3) | 392 (7.3) |
| Genotype 3 | - | 2049 (38.6) | 25 (26.6) | 2074 (38.4) |
| Genotype 4–6 | - | 140 (2.6) | 2 (2.1) | 142 (2.6) |
| Multiple genotypes | - | 54 (1.0) | 2 (2.1) | 56 (1.0) |
| Unknown | - | 588 (11.1) | 28 (29.8) | 616 (11.4) |
| P = .49 | ||||
| Clinical cirrhosis | 31 (1.0) | 219 (4.1) | 2 (2.1) | 252 (3.0) |
| P < .0001 |
Abbreviations: HIV, human immunodeficiency virus; IDU, intravenous drug use.
aKruskal-Wallis, Fisher’s exact, χ2 test, or χ2 test with Montecarlo simulation using 10000 samples when appropriate.
bHIV positive vs not HIV positive.
cIncluding Greenland.
dIncluding Australia, New Zealand, and Pacific Islands.
eIncluding needle stick injuries.
Human Immunodeficiency Virus-Coinfected Patients
Baseline characteristics of patients with HIV coinfection can be seen in Table 2 and Supplementary Table 1. The majority of HIV-coinfected patients had CHC (75%), and their demographic characteristics were akin to that of the CHC population with regards to the distribution of sex and country of birth. Sexual route of viral hepatitis transmission was more prevalent among HIV-positive patients (22.1% vs 2.7%), and vertical transmission of viral hepatitis was rare in HIV-coinfected patients (0.8% vs 9.8%).
Table 2.
Patient Characteristics of HIV-Positive Patients, HIV-Negative Patients, and Patients With Unknown HIV Status
| Patient Characteristic | HIV Positive | HIV Negative | HIV Unknown | P Valuea |
|---|---|---|---|---|
| Number | 376 | 6384 | 1730 | |
| Age, median (IQR) | 40.3 (34.7–47.7) | 41.4 (32.5–50.5) | 41.9 (31.4–51.3) | .8 |
| Women, N (%) | 91 (24.2) | 2550 (40.0) | 808 (46.7) | <.0001 |
| Hepatitis type | ||||
| Hepatitis B | 89 (23.7) | 2198 (34.4) | 804 (46.5) | <.0001 |
| Hepatitis C | 281 (74.7) | 4119 (64.5) | 905 (52.3) | |
| Hepatitis B and C | 6 (1.6) | 67 (1.1) | 21 (1.2) | |
| Country of Birth, N (%) | ||||
| Africa | 30 (8.0) | 431 (6.8) | 122 (7.1) | <.0001 |
| Asia | 37 (9.8) | 1484 (23.3) | 500 (28.9) | |
| Eastern Europe | 19 (5.1) | 437 (6.9) | 95 (5.5) | |
| North America | 1 (0.3) | 15 (0.2) | 7 (0.4) | |
| South America | 8 (2.1) | 32 (0.5) | 5 (0.3) | |
| Western Europeb | 273 (72.6) | 3728 (58.4) | 848 (49.0) | |
| Otherc | 3 (0.8) | 39 (0.6) | 21 (1.2) | |
| Unknown | 5 (1.3) | 218 (3.4) | 132 (7.6) | |
| Route of Transmission, N (%) | ||||
| Blood/blood product exposured | 11 (2.9) | 235 (3.7) | 83 (4.8) | <.0001 |
| IDU | 166 (44.2) | 2708 (42.4) | 484 (28.0) | |
| Sexual contact | 83 (22.1) | 172 (2.7) | 36 (2.1) | |
| Tattoo/piercing | 2 (0.5) | 96 (1.5) | 25 (1.5) | |
| Vertical | 3 (0.8) | 625 (9.8) | 290 (16.8) | |
| Multiple | 0 (-) | 11 (0.2) | 2 (0.1) | |
| Other Reason | 2 (0.5) | 112 (1.8) | 34 (2.0) | |
| Unknown | 109 (29.0) | 2425 (38.0) | 776 (44.9) | |
| Diagnostic Test Reason, N (%) | ||||
| Clinical symptoms | 77 (20.5) | 1565 (24.5) | 515 (29.8) | <.0001 |
| Other reason | 34 (9.0) | 773 (12.1) | 168 (9.7) | |
| Screening pregnancy | 2 (0.5) | 402 (6.3) | 99 (5.7) | |
| Screening other | 137 (36.4) | 2025 (31.7) | 545 (31.5) | |
| Unknown | 126 (33.5) | 1619 (25.4) | 403 (23.3) | |
| History of alcohol abuse, N (%) | 45 (12.0) | 1205 (18.9) | 243 (14.1) | <.0001 |
| HCV Genotype, N (%)e | ||||
| Genotype 1 | 133 (46.3) | 1626 (38.8) | 360 (38.9) | <.0001 |
| Genotype 2 | 17 (5.9) | 290 (6.9) | 85 (9.2) | |
| Genotype 3 | 81 (28.2) | 1670 (39.9) | 323 (34.9) | |
| Genotype 4–6 | 22 (7.7) | 89 (2.1) | 31 (3.4) | |
| Multiple genotypes | 5 (1.7) | 41 (1.0) | 10 (1.1) | |
| Unknown | 29 (10.1) | 470 (11.2) | 117 (12.6) | |
| Clinical cirrhosis, N (%) | 5 (1.3) | 196 (3.1) | 51 (3.0) | .15 |
Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, intravenous drug use; IQR, interquartile range.
aKruskal-Wallis, χ2 test, or χ2 test with Montecarlo simulation using 10000 samples when appropriate.
bIncluding Greenland.
cIncluding Australia, New Zealand, and Pacific Islands.
dIncluding needle stick injuries.
eGenotype distribution among patients with chronic hepatitis C.
Human Immunodeficiency Virus Testing
In the entire study population, 79.6% were tested for HIV as newly diagnosed with viral hepatitis at enrollment in DANEHP and 87.8% of patients were ever tested for HIV coinfection before July 2015. In addition to HIV-positive patients identified at enrollment in DANHEP, an additional 20 patients were diagnosed with HIV coinfection a median of 44 months (range, 9–116) after enrollment. Seven of these 20 patients were not HIV tested at enrollment and had their first positive HIV test performed a median of 41 months after enrollment (range, 9–116). The remaining 13 patients were HIV negative at enrollment in DANHEP and subsequently became HIV positive after a median of 47 months (range, 11–101).
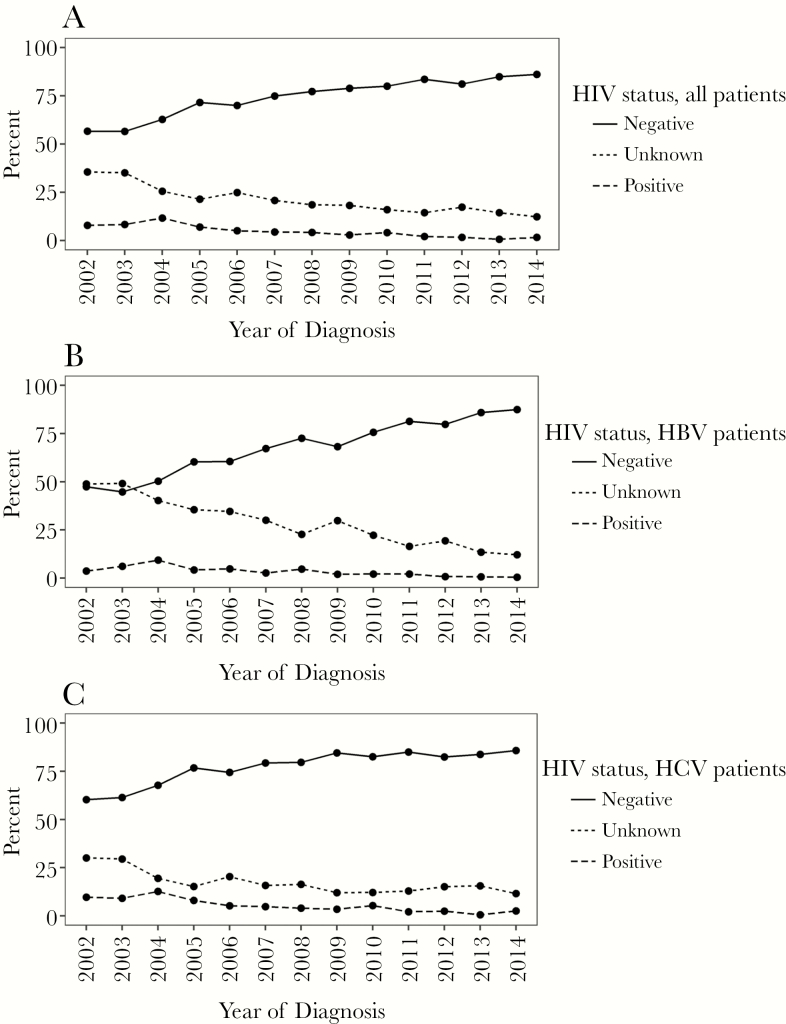
The HIV testing prevalence among newly diagnosed chronic viral hepatitis patients increased from 64.5% in 2002 to 87.7% in 2014, respectively, whereas the prevalence of HIV coinfection among newly enrolled patients during the same period fell from 7.8% (95% CI, 5.5–10.7) in 2002 to 1.6% (95% CI, 0.7–3.2) in 2014. Human immunodeficiency virus coinfection status (positive, negative, unknown) for all patients, CHB and CHC patients, in relative and absolute frequencies, for each year of enrollment between 2002 and 2014 (the last year with complete data), are shown in Figure 1A–C and Supplementary Figure 2A–C, respectively. Departments of gastroenterology and hepatology were less likely to test for HIV than departments with infectious disease specialists (70.4% vs 82.8%, P < .0001) (Supplementary Table 2).
Figure 1.
Human immunodeficiency virus (HIV)-status in percentage among all newly diagnosed patients with chronic viral hepatitis between 2002 and 2014. (A) All patients. (B) Patients with chronic hepatitis B virus (HBV) infection. (C) Patients with chronic hepatitis C virus (HCV) infection.
Patient Characteristics Associated With Human Immunodeficiency Virus Coinfection
We found that CHC was associated with HIV coinfection with an odds ratio (OR) of 1.89 (95% CI, 1.48–2.41) compared with CHB patients. After adjusting for sex, age and country of birth, we found that the prevalence of HIV coinfection decreased over time between 2002 and 2015 with an OR of HIV coinfection of 0.84 (95% CI, 0.82–0.87) per year. Estimates of OR for HIV coinfection associated with baseline characteristics are listed in Table 3. We found that age 35–50 years (OR = 4.42; 95% CI, 1.40–13.94), male sex (OR = 2.21; 95% CI, 1.74–2.81), birth in South America (OR = 3.62; 95% CI, 1.67–7.86), and sexual transmission of viral hepatitis (OR = 8.81; 95% CI, 6.30–12.33) were associated with HIV coinfection. We were surpised to find that all 8 HIV-coinfected patients from South America had CHC, 7 of whom reported sexual route of viral hepatitis transmission. Birth in Asia (OR = 0.31; 95% CI, 0.22–0.44) and vertical transmission of viral hepatitis (OR = 0.09; 95% CI, 0.03–0.29) were negatively associated with HIV coinfection.
Table 3.
Risk Factors Associated With HIV Coinfection Among Patients With Newly Diagnosed Chronic Viral Hepatitis B and/or C
| Risk Factor | Odds Ratio (95% CI) | N |
|---|---|---|
| Patients with CHC vs CHBa | 1.89 (1.48–2.41) | 8490 |
| Patients with CHB-CHC vs CHBa | 2.3 (0.98–5.40) | 8490 |
| Ageb, years | ||
| ≤35 | 2.64 (0.83–8.39) | 8490 |
| >35–≤50 | 4.42 (1.40–13.94) | |
| >50–≤65 | 2.41 (0.75–7.73) | |
| >65 | Reference | |
| Sex, men vs womenc | 2.21 [1.74; 2.81] | 8490 |
| Year of enrollmentd | 0.84 [0.82; 0.87] | 8135 |
| Country of birth, Africa vs WEe | 0.91 [0.62; 1.34] | 8135 |
| Country of birth, Asia vs WEe | 0.31 [0.22; 0.44] | 8135 |
| Country of birth, Eastern Europe vs WEe | 0.60 [0.37; 0.96] | 8135 |
| Country of birth, North America vs WEe | 0.76 [0.10; 5.67] | 8135 |
| Country of birth, South America vs WEe | 3.62 [1.67; 7.86] | 8135 |
| Route of transmission, blood exposure vs IDUf | 0.67 [0.34; 1.29] | 5023 |
| Route of transmission, sexual transmission vs IDUf | 8.81 [6.30; 12.33] | 5023 |
| Route of transmission, tattoo/piercing vs IDUf | 0.30 [0.07; 1.26] | 5023 |
| Route of transmission, vertical transmission vs IDUf | 0.09 [0.03; 0.29] | 5023 |
Abbreviations: CHB, chronic hepatitis B virus; CHC, chronic hepatitis C virus; CI, confidence interval; IDU, intravenous drug use; OR, odds ratio; WE, Western Europe.
aUnivariate, type 3 test (P < .0001).
bUnivariate, type 3 test (P < .0001).
cUnivariate.
dAdjusted for sex, age, and country of birth.
eUnivariate, type 3 test (P < .0001).
fAdjusted for age, sex, year of enrollment, country of birth, type 3 (P < .0001).
DISCUSSION
In this nationwide cohort study of patients with newly diagnosed chronic viral hepatitis attending specialized care, we found an overall prevalence of HIV coinfection of 4.4% and an increase in HIV testing prevalence coinciding with a decrease in the prevalence of HIV coinfection since 2002. Chronic hepatitis C or CHB-CHC, younger age, male sex, birth in South America, and sexual transmission of viral hepatitis were associated with a higher prevalence of HIV coinfection. In contrast, patients of Asian origin and patients vertically infected with viral hepatitis showed a lower prevalence HIV coinfection.
The prevalence of HIV coinfection among CHB patients found in our study is slightly lower than the estimates published from other low HIV-prevalence settings (United States) of 5.2%–6.3% among CHB patients [26, 27], whereas the overall HIV coinfection prevalence of 5.3% among CHC patients found in our study was similar to estimates found in larger CHC patient cohorts from the United Kingdom (UK) and the United States at 5.0% and 4.3%, respectively [27, 28].
Demographics of CHB patients in our study differed from CHB patients in the US-based studies [26, 27] with fewer from Asia, fewer women, and more were sexually infected with HIV, whereas the demographic characteristics of CHC patients in our study were similar to patients with CHC included in the UK study [28]. However, no previous nationwide study has been conducted, and a systematic review found considerable variation in the prevalence of HIV coinfection among European patients with CHC living with intravenous drug use, ranging from 0% to 70% [29]. The low HIV prevalence in the Danish population [18], the nationwide design with inclusion of viral hepatitis patients from various risk groups, may partly explain differences between the HIV coinfection prevalence found in the Danish viral hepatitis population and those described elsewhere [26, 30, 31]. Moreover, the universal access to healthcare services and drug addiction treatment with needle exchange programs (all free of charge) in Denmark [32] may be partly responsible.
Although we have no data to draw any definitive conclusions as to why the HIV coinfection prevalence is declining in the Danish chronic viral hepatitis population, one could speculate that access to HAART (which reduces sexual HIV transmission [33]), needle exchange programs, and/or oral opioid substitution therapy of intravenous drug users may play a role. Our data showed a steady increase in the proportion of patients tested for HIV throughout the study period, which is most likely the result of a national effort to increase HIV testing in risk groups [15], the process of making HIV testing routine in all patients newly diagnosed with chronic viral hepatitis, and the introduction of screening for HIV in pregnant women. The importance of knowledge about HIV by the physician may be indicated by the fact that patients attending departments of infectious diseases appeared to be more likely to be tested for HIV than patients attending departments of gastroenterology.
Approximately 60% of the Danish CHC cohort is infected with HCV through IDU, and the majority are males and infected in Denmark [20, 34]. In contrast, the population of CHB patients in Denmark comprises primarily relatively younger women, many of whom were vertically infected with HBV in their country of origin, often Asia, Africa, and the Middle East [35]. Immigration, and the introduction of nationwide screening of pregnant women for HBV in 2005 and HIV infection in 2010 in Denmark [36, 37], may have influenced the demographics of the chronic viral hepatitis cohort and their HIV coinfection prevalence because the prevalence of HIV infection is very low among pregnant women in Denmark (0.06% HIV infected among all pregnant women tested in 2016 [38]). Only 1 of 203 (0.5%) HBsAg-positive pregnant women were also HIV positive (M. Wessman, written personal communication, 15 December 2017). The majority of individuals infected with HIV in Denmark are male, with a median age of 34 years at HIV diagnosis, approximately half of whom have been infected through homosexual transmission, and only approximately 10% are infected through IDU [39, 40]. Hence, it was not surprising to find that younger age, male sex, and sexual transmission of viral hepatitis were associated with a higher prevalence of HIV coinfection and that Asian origin and vertical transmission were associated with a lower HIV coinfection prevalence. The higher risk of HIV coinfection associated with HCV seen in this study is presumed to be due to underlying differences in behavioral risks (eg, IDU, men who have sex with men [MSM] [41]) and not due to a causal link between HCV and HIV per se. However, our data limit further conclusions on whether sexual transmission was in fact more likely to be among MSM or sex workers. A more surprising finding was the association between South American origin and HIV coinfection. Our study included very few patients from South America with a surprisingly high HIV coinfection prevalence among CHC patients—approximately all of whom had been sexually infected with HCV. The HIV epidemic in South and Central America varies, but in many countries it is driven by transmission among MSM [42], with a median HCV seroprevalence of 11% among people living with HIV/AIDS, with considerable variation throughout the region [43]. Thus, the association could represent a selected group of South American patients with high-risk behavior but is most likely the result of a spurious finding.
A major strength of our study is the nationwide design with comprehensive data. However, the fact that Denmark is a low-prevalence country with regards to HBV, HCV, and HIV limits the generalizability to other settings. Despite the nationwide design, DANHEP only includes patients with CHB or CHC attending specialized care. There are a substantial number of patients in Denmark with either undiagnosed chronic viral hepatitis or diagnosed viral hepatitis but not enrolled in specialized care [19, 20]. In addition, DANHEP does not include detailed information on homosexual and heterosexual behavior or sex work, and a large proportion of patients had unknown route of transmission. Whether this is due to genuine ignorance about the route of transmission or an unwillingness to reveal behavioral risks (such as MSM, sex work, or IDU) is unknown.
Because HIV, HBV, and HCV are blood-borne viruses and share routes of transmission, patients diagnosed with viral hepatitis have at least 1 risk factor for HIV coinfection (ie, IDU, vertical transmission etc). An HIV test is cheap, easy to perform, and associated with negligible health risks, and because the consequences of missing an HIV diagnosis can have detrimental consequences, we strongly recommend HIV testing all viral hepatitis patients regardless of their risk profile. Our results also indicate that there is still room for improvement in HIV testing among chronic viral hepatitis patients in Denmark, but a continuous focus on this area may well lead to improvements in HIV testing frequencies.
CONCLUSIONS
In conclusion, in this nationwide study including patients with newly diagnosed chronic viral hepatitis, the prevalence of HIV coinfection in Denmark decreased between 2002 and 2014 concurrently with an observed increase in HIV testing prevalence. The overall prevalence of HIV coinfection was approximately 4%, but it was higher among patients with CHC and CHB-CHC coinfection than patients with CHB and higher among patients of male sex, younger age, and with sexual transmission of viral hepatitis.
Supplementary Material
Acknowledgments
Disclaimer. None of the funding sources were involved in the design of the study, data collection or analysis, or writing of the final manuscript.
Financial support. This work funded by the Bonén Foundation. N. W. received financial support from the Danish Council for Research and Innovation (Grant Number 12-127717).
Potential conflicts of interest. S. H. served as an unpaid speaker at an MSD-sponsored event, received a young researcher bursary from EASL for participation in International Liver Conference, Barcelona, 2016, and received an honorarium for being invited speaker at the HIV and Hepatitis Nordic Conference, Stockholm, 2018. A. L. L. served as an advisory board member for MSD, Abbvie, Gilead, and BMS and has received honoraria from Abbvie and BMS and grants from Gilead. J. G.’s institution has received grants and payment for speeches and advisory boards from Gilead, Viiv, Abbott, BMS, MSD, Janssen, Bohringer, Sanofi-Pasteur, and Medivir. S. L. is employed at Novo Nordisk. N. W. received lecture honoraria from Abbvie, BMS, Gilead, Janssen, and MSD, served as an advisory board member for AbbVie, BMS, Gilead, Medivir, and MSD, and worked as a clinical Investigator for Abbvie, BMS, and MSD. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Holmberg S, Syryaprasad A, Ward J. Updated CDC recommendations for the management of hepatitis B virus-infected health-care providers and students. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr6103a1.htm. Accessed 27 June 2018. [PubMed]
- 2. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol 2011; 55:245–64. [DOI] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–85. [DOI] [PubMed] [Google Scholar]
- 4. Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol 2007; 13:2436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim JH, Psevdos G, Suh J, Sharp VL. Co-infection of hepatitis B and hepatitis C virus in human immunodeficiency virus-infected patients in New York City, United States. World J Gastroenterol 2008; 14:6689–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thio CL, Seaberg EC, Skolasky R Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS). Lancet 2002; 360:1921–6. [DOI] [PubMed] [Google Scholar]
- 7. Di Martino V, Rufat P, Boyer N, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology 2001; 34:1193–9. [DOI] [PubMed] [Google Scholar]
- 8. Pineda JA, Romero-Gómez M, Díaz-García F, et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology 2005; 41:779–89. [DOI] [PubMed] [Google Scholar]
- 9. Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut 2003; 52:1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macías J, Berenguer J, Japón MA, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology 2009; 50:1056–63. [DOI] [PubMed] [Google Scholar]
- 11. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001; 33:562–9. [DOI] [PubMed] [Google Scholar]
- 12. López-Diéguez M, Montes ML, Pascual-Pareja JF, et al. The natural history of liver cirrhosis in HIV-hepatitis C virus-coinfected patients. AIDS 2011; 25:899–904. [DOI] [PubMed] [Google Scholar]
- 13. Mocroft A, Neuhaus J, Peters L, et al. Hepatitis B and C co-infection are independent predictors of progressive kidney disease in HIV-positive, antiretroviral-treated adults. PLoS One 2012; 7:e40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sundhedsstyrelsen. [Vejledning om HIV (Human Immundefekt Virus), Hepatitis B og C virus: Forebyggelse af Blodbåren i smitte, Diagnostik og HåNdtering i Sundhedsvæsnet og På Andre Arbejdspladser.] Available at: https://sundhedsstyrelsen.dk/publ/Publ2013/03mar/HIVogHepBogCvejl.pdf. Accessed 28 November 2013. [Google Scholar]
- 16. Weis N, Rye Clausen M, Brehm Christensen P, Krarup H, Lund A, Schlichting P.[Behandling af Hepatitis B Virus (HBV) og Hepatitis C Virus (HCV) Infektion.] Copenhagen: Danish society of Infectious diseases and Danish society of gastroenterology and hepatology, 2014. [Google Scholar]
- 17. Weis N.[Den Danske Database for Hepatitis B og C, Årsrapport 2005–2009.] Copenhagen: The DANHEP group, 2005. https://www.sundhed.dk/content/cms/78/1878_aarsrapport-2009-hepatitis.pdf. Accessed February 2015. [Google Scholar]
- 18. UNAIDS. UNAIDS country factsheet. Available at: http://www.unaids.org/en/regionscountries/countries/denmark. Accessed 12 May 2016.
- 19. Hansen N, Hay G, Cowan S, et al. Hepatitis B prevalence in Denmark—an estimate based on nationwide registers and a national screening programme, as on 31 December 2007. Euro Surveill Bull 2013; 18(47) pii: 20637. [DOI] [PubMed] [Google Scholar]
- 20. Christensen PB, Hay G, Jepsen P, et al. Hepatitis C prevalence in Denmark -an estimate based on multiple national registers. BMC Infect Dis 2012; 12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bolther M, Dalgaard LS, Kristensen LH, et al. Testing for hepatitis B virus and HIV in patients with chronic hepatitis C: screening performance and outcome. Scand J Infect Dis 2014; 46:686–92. [DOI] [PubMed] [Google Scholar]
- 22. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26:404–13. [Google Scholar]
- 23. Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol 2008; 8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hosmer DW, Lemeshow S.. Applied Logistic Regression. 3rd ed New York: John Wiley & Sons Inc; 2013. [Google Scholar]
- 25. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; R Lang Environ Stat Comput Available at: https://www.r-project.org/. Accessed 21 January 2016. [Google Scholar]
- 26. Sanchez MA, Scheer S, Shallow S, et al. Epidemiology of the viral hepatitis-HIV syndemic in San Francisco: a collaborative surveillance approach. Public Health Rep 2014; 129(Suppl 1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bosh KA, Coyle JR, Hansen V, et al. HIV and viral hepatitis coinfection analysis using surveillance data from 15 US states and two cities. Epidemiol Infect 2018; 146:920–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ireland G, Delpech V, Kirwan P, et al. Prevalence of diagnosed HIV infection among persons with hepatitis C virus infection: England, 2008–2014. HIV Med 2018; nov 10(19), 708–715. doi: 10.1111/hiv.12662. Epub 2018 Jul 26. [DOI] [PubMed] [Google Scholar]
- 29. Wiessing L, Ferri M, Grady B, et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One 2014; 9:e103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Curcio F, Villano G, Masucci S, et al. Epidemiological survey of hepatitis C virus infection in a cohort of patients from a ser.T in naples, Italy. J Addict Med 2011; 5:43–9. [DOI] [PubMed] [Google Scholar]
- 31. Flores GL, de Almeida AJ, Miguel JC, et al. A cross section study to determine the prevalence of antibodies against HIV infection among hepatitis B and C infected individuals. Int J Environ Res Public Health 2016; 13:314. doi: 10.3390/ijerph13030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fouchard J.[National Handlingsplan Til Forebyggelse af Hepatitis C Blandt Stofmisbrugere.] Copenhagen: The Danish Health Authority, Sundhedsstyrelsen; 2007. http://www.sst.dk [Google Scholar]
- 33. Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wessman M, Cowan S.[EPI-NYT uge 11 2017: Akut og Kronisk Hepatitis C, 2016.] Available at: https://www.ssi.dk/Aktuelt/Nyhedsbreve/EPI-NYT/2017/Uge%2011%20-%202017.aspx. Accessed 15 December 2017. [Google Scholar]
- 35. Wessman M, Cowan S.[EPI-NYT uge 9 2017: Akut og Kronisk Hepatitis B 2016.] Available at: https://www.ssi.dk/Aktuelt/Nyhedsbreve/EPI-NYT/2017/Uge%209%20-%202017.aspx. Accessed 15 December 2017. [Google Scholar]
- 36.[Vejledning om Generel Screening af Gravide for Infektion Med Hepatitis B Virus, Human Immundefekt Virus (HIV) og Syfilis.] Available at: http://sundhedsstyrelsen.dk/publ/Publ2010/CFF/Graviditet/Vejl_screening_hep_b_hiv_syf.pdf. Accessed 18 November 2014. [Google Scholar]
- 37.[Generel Screening af Gravide for Hepatitis B.] Available at: https://sundhedsstyrelsen.dk/da/sundhed/smitsomme-sygdomme/hepatitis-leverbetaendelse/screening-af-gravide. Accessed 5 November 2014. [Google Scholar]
- 38. Wessman M, Christiansen AH, Mellerup N, Cowan S, Hoffman S.[EPI-NYT uge 35 2017: Screening af Gravide for Hepatitis B, hiv og syfilis, 2016.] Available at: https://www.ssi.dk/Aktuelt/Nyhedsbreve/EPI-NYT/2017/Uge%2035%20-%202017.aspx. Accessed 15 December 2017. [Google Scholar]
- 39. Christiansen AH, Cowan S, Peternsen A, Fonager J.[EPI-NYT uge 36 2017: HIV 2016.] 2017. Available at: https://www.ssi.dk/Aktuelt/Nyhedsbreve/EPI-NYT/2017/Uge%2036%20-%202017.aspx. Accessed 15 December 2017. [Google Scholar]
- 40. Obel N, Engsig FN, Rasmussen LD, et al. Cohort profile: the Danish HIV cohort study. Int J Epidemiol 2009; 38:1202–6. [DOI] [PubMed] [Google Scholar]
- 41. Barfod TS, Omland LH, Katzenstein TL. Incidence and characteristics of sexually transmitted acute hepatitis C virus infection among HIV-positive men who have sex with men in Copenhagen, Denmark during four years (2006–2009): a retrospective cohort study. Scand J Infect Dis 2011; 43:145–8. [DOI] [PubMed] [Google Scholar]
- 42. García PJ, Bayer A, Cárcamo CP. The changing face of HIV in Latin America and the Caribbean. Curr HIV/AIDS Rep 2014; 11:146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tengan FM, Ibrahim KY, Dantas BP, et al. Seroprevalence of hepatitis C virus among people living with HIV/AIDS in Latin America and the Caribbean: a systematic review. BMC Infect Dis 2016; 16:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.