MHCII-restricted antigen presentation by lymph node stromal cells is essential for regulatory T-cell proliferation and functions, and for the regulation of autoimmunity.
Abstract
How lymph node stromal cells (LNSCs) shape peripheral T-cell responses remains unclear. We have previously demonstrated that murine LNSCs, lymphatic endothelial cells (LECs), blood endothelial cells (BECs), and fibroblastic reticular cells (FRCs) use the IFN-γ–inducible promoter IV (pIV) of the MHC class II (MHCII) transactivator CIITA to express MHCII. Here, we show that aging mice (>1 yr old) in which MHCII is abrogated in LNSCs by the selective deletion of pIV exhibit a significant T-cell dysregulation in LNs, including defective Treg and increased effector CD4+ and CD8+ T-cell frequencies, resulting in enhanced peripheral organ T-cell infiltration and autoantibody production. The proliferation of LN-Tregs interacting with LECs increases following MHCII up-regulation by LECs upon aging or after exposure to IFN-γ, this effect being abolished in mice in which LECs lack MHCII. Overall, our work underpins the importance of LNSCs, particularly LECs, in supporting Tregs and T-cell tolerance.
Introduction
T-cell precursors undergo thymic negative selection, which ensures the elimination of developing T cells expressing TCR-recognizing self-Ags with excessive affinity. However, some autoreactive T cells escape this process of clonal deletion and exit the thymus to populate secondary lymphoid organs (SLOs). Therefore, additional mechanisms of T-cell tolerance are required in the periphery to avoid the development of autoimmunity. Among them, resting DCs, which constantly sample self-Ags in peripheral tissues and reach the draining LNs through the afferent lymph, present self-Ag–derived peptides to naive T cells. In the absence of danger, this phenomenon leads to clonal deletion, or anergy of autoreactive T cells (Steinman et al, 2003; Mueller, 2010). Alternatively, Tregs, by exhibiting suppressive immunoregulatory functions, can inhibit autoreactive T cells. Different subsets of Tregs have been described so far. Natural Tregs bear an autoreactive TCR, are induced in the thymus, and express the transcription factor Foxp3. Peripheral-induced Tregs can express Foxp3 or not, and differentiate in SLOs (Chen et al, 2003; Swee et al, 2009; Wirnsberger et al, 2011). Preservation of Treg function and biology is crucial for peripheral tolerance.
Lymph node stromal cells (LNSCs) have recently been promoted to the rank of new modulators of T-cell responses. After being considered for years as simple scaffolding, forming routes, and proper environment for Ag-lymphocyte encountering, we recently learned that they also impact both DC and T-cell functions. Lymphatic endothelial cells (LECs) promote DC entry into and T-cell egress from LNs (Sixt et al, 2005; Pham et al, 2010; Braun et al, 2011), whereas CCL19/CCL21–producing fibroblastic reticular cells (FRCs) control immune cells entry and proper localization into LNs (Link et al, 2007; Tomei et al, 2009). Blood endothelial cells (BECs) control T-cell homing to LNs (Bajenoff et al, 2003). In addition, LECs and FRCs are the major source of IL-7 in LNs, ensuring T-cell homeostasis. In inflammatory situations, however, LECs and FRCs produce nitric oxide to constrict T-cell expansion (Khan et al, 2011; Lukacs-Kornek et al, 2011; Siegert et al, 2011), whereas LECs further impair DC maturation in a contact-dependent fashion (Podgrabinska et al, 2009). In the context of peripheral tolerance, LNSCs, and in particular LECs and FRCs, ectopically express a large range of peripheral tissue Ags (PTAs), and further present PTA-derived peptides through MHC class I (MHCI) molecules to induce self-reactive CD8+ T-cell deletion (Cohen et al, 2010; Fletcher et al, 2010, 2011; Tewalt et al, 2012). We have previously demonstrated that, in addition to inducing CD4+ T-cell dysfunction by presenting peptide-MHC class II (MHCII) complexes acquired from DCs, LECs, BECs, and FRCs endogenously express MHCII molecules (Dubrot et al, 2014). Central tolerance of self-reactive CD4+ T cells is partially mediated by thymic epithelial cells (TECs), in which MHCII molecules are loaded with peptides derived either from phagocytosis and processing of extracellular Ags (Stern et al, 2006), or from autophagy and endocytosis of intracellular Ags (Adamopoulou et al, 2013; Aichinger et al, 2013). Whether these pathways can be involved in MHCII-restricted Ag presentation by LNSCs, and impact peripheral self-reactive T-cell responses, is currently unknown.
Here, we have used genetically modified mice in which MHCII expression by non-hematopoietic cells is abrogated. Upon aging, and compared with their control counterparts, these mice exhibit an enhancement of spontaneous autoimmune processes, with enhanced T-cell activation in SLOs and effector T-cell infiltration in peripheral tissues, as well as the production of autoantibodies. In contrast, the Treg compartment is significantly impaired in SLOs. Furthermore, Rag2−/− mice transferred with T cell isolated from LN of aging MHCII-deficient LNSC mice displayed similar immunological and clinical perturbations compared with recipient injected with age-matched control T cells, suggesting a direct link between MHCII expressed by LNSCs and the appearance of T cell–mediated signs of autoimmunity. Accordingly, upon aging or IFN-γ treatment, LECs up-regulate MHCII molecules, and interact with Treg to promote their proliferation. This phenotype is abolished in mice deficient for MHCII expression in LECs. Altogether, we prove that MHCII expression by LNSCs have a manifest impact in peripheral tolerance. Notably, LECs support self-Ag–specific T cell peripheral tolerance by promoting Treg proliferation through MHCII-restricted Ag presentation.
Results
Expression of MHCII by LNSCs
Our previous work has demonstrated that LNSCs impact CD4+ T-cell biology by presenting peptide-MHCII complexes acquired from DCs (Dubrot et al, 2014). Although these findings revealed a novel function of the lymphoid stroma as casual APC, whether endogenous MHCII Ag presentation by LNSCs can also impact T-cell responses remains to be determined. To abrogate cell-intrinsic pIV-mediated MHCII expression by LNSCs, we have used mice deficient for the IFN-γ–inducible pIV of CIITA (pIVKO) (Waldburger et al, 2001). As expected, steady-state MHCII expression by LECs, BECs, and FRCs was only partially abrogated in pIVKO mice compared with control mice (Fig 1A). Indeed, we recently described that MHCII expression by LNSCs partially results from a combination of both DC-acquired and CIITA promoter IV (pIV)–driven, endogenously expressed, MHCII molecules (Dubrot et al, 2014). DCs use the pI promoter of CIITA, and therefore, MHCII transfer to LNSCs is not abrogated following pIV deletion (Dubrot et al, 2014). However, pIV deletion led to the abrogation of endogenous MHCII expression by LECs, BECs, and FRCs. We confirmed this by analysing a second CTIIA-regulated gene, H2-M, the oligomorphic MHCII molecule involved in MHCII Ag loading. Ex vivo LECs, BECs, and FRCs express low H2-M and MHCII levels in the steady state, but substantially up-regulate H2-M and I-Ab molecules at both mRNA (Fig 1B) and protein (Fig 1C) levels after IFN-γ treatment (s.c. injection), suggesting a potential role for MHCII-restricted Ag presentation by LNSCs during inflammation. Both steady state and IFN-γ–inducible endogenous MHCII expressions were abrogated in pIVKO mice (Fig 1B and C).
Figure 1. LNSCs upte MHCII and MHCII-associated molecules in response to IFN-γ.
(A) Flow cytometry and plotted histograms showing MHCII expression (MFI) by LECs, BECs, and FRCs from LN of indicated naive mice. MHCII coming from DC (blue arrow)- or endogenous (red arrow)-origin, is indicated. (B) WT or pIV CIITA–deficient mice (pIVKO) mice were injected subcutaneously with PBS or 1 μg of IFN-γ; LNSCs were sorted by flow cytometry 24 h later. Histograms represent I-Aβ (upper panel) and H-2Mα (lower panel) relative mRNA expression by LECs, BECs, and FRCs from indicated mice, measured by qPCR and normalized to GAPDH. Data are representative of two experiments with 10 mice pooled/group. (C) LNSCs and DCs from draining LNs were analysed 24 h after IFN-γ injection (as previously) by flow cytometry. Dot plots represent the expression of MHCII and H-2M by LECs, BECs, FRCs, and DCs from indicated mice. Frequency of double positive fractions are highlighted in red. Data are representative of two experiments with three mice/group.
Abrogation of endogenous MHCII in LNSCs
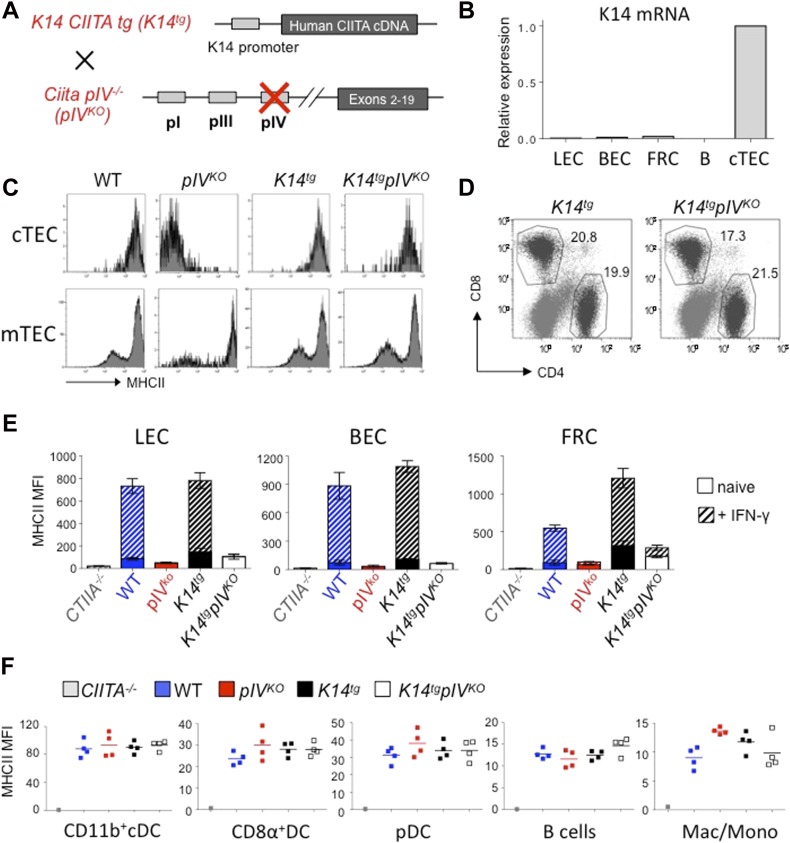
MHCII expression in cortical TECs (cTECs) depends on pIV. Because of the absence of MHCII expression by cTECs, pIVKO mice consequently exhibit a defect in CD4+ T-cell thymic positive selection, and lack peripheral CD4+ T cells (Waldburger et al, 2003). Therefore, pIVKO mice were crossed with transgenic mice expressing CIITA under the keratin 14 (K14) promoter, which is active in cTECs (Laufer et al, 1996), but not in LNSCs (Fig 2A and B). MHCII expression by cTECs was efficiently restored in K14 CIITA tg x Ciita pIV−/− (referred as K14tgpIVKO mice) mice compared with pIVKO mice, and to a similar extent compared with cTECs isolated from WT or control (K14 Ciita tg × Ciita pIV+/−, referred to as K14tg) mice (Fig 2C). As a consequence and as described before (Irla et al, 2008; Thelemann et al, 2014, 2016), K14tgpIVKO mice exhibit normal CD4+ T-cell frequencies in SLOs (Fig 2D). We did not observe any significant expression of the K14 transgene in LNSCs purified from these mice (Fig 2B). However, to avoid any off-target effect of the K14 Ciita transgene in LNSCs or other cells, K14tgpIVKO mice were always compared with K14tg controls in all experiments. Importantly, MHCII expression by mTECs is not altered in pIVKO and K14tgpIVKO mice (Fig 2C), for the, respective, following reasons: first, mTECs express the pIII of CIITA in addition to pIV (Irla et al, 2008), and second, the K14 promoter is not active in terminally differentiated mTECs (Sukseree et al, 2012). Therefore, negative CD4+ T-cell selection is unaffected in K14tgpIVKO mice. Accordingly, we have previously demonstrated that CD4+ thymocytes and peripheral CD4+ T cells that developed in K14tgpIVKO mice (6–10 wk-old) were indistinguishable from WT with respect to cell numbers and TCR V-β repertoire (Irla et al, 2008).
Figure 2. Selective abrogation of endogenous MHCII expression in LNSCs.
(A) Schematic representation of K14tgpIVKO mice. Briefly, pIVKO were crossed with transgenic mice expressing the full CIITA cDNA under the control of the keratin 14 (K14) promoter (K14tg). (B) Relative mRNA expression of K14 by LECs, BECs, FRCs, B cells and cTEC from K14tgpIVKO mice, measured by qPCR and normalized to GAPDH. Data are representative of two experiments with 10 mice pooled/group (C) Flow cytometry histograms showing the expression of MHCII molecules by cTECs (gated on CD45negEpCAM+Ly5.1hi cells) and mTECs (gated on CD45negEpCAM+Ly5.1intcells) from indicated mice. (D) Flow cytometry dot plots showing CD8+ and CD4+ T-cell frequencies in LN of indicated mice (gated on CD3+ cells). (C, D) Data are representative of two experiments with three mice/group. (E) Histograms showing MHCII expression (MFI) by LECs, BECs, and FRCs from LN of indicated mice, either naive (filled) or injected s.c. with IFN-γ 24 h before (hatched). (F) Graphs showing MHCII expression (MFI) by CD11b+ cDCs (CD11chiCD11b+), CD8α+ DCs (CD11chiCD8α+), pDCs (CD11cintPDCA-1+), B cells (CD19+), and monocytes/macrophages (CD11cneg CD11b+) isolated from LNs of indicated mice. (E, F) Data are representative of two experiments with 3-4 mice/group.
IFN-γ–induced endogenous and pIV-dependent MHCII up-regulation, which was efficiently abolished in LECs, BECs, and FRCs from K14tgpIVKO mice compared with control mice (Fig 2E). However, the expression of MHCII by other LN cells, i.e., different DC subtypes, B cells, or monocytes/macrophages, which rely on pI and/or pIII of CIITA (Waldburger et al, 2001), was found unaffected in K14tgpIVKO mice (Fig 2F). Altogether, our data demonstrate that endogenous MHCII expression is efficiently abrogated in LNSCs of K14tgpIVKO mice.
Mice deficient for MHCII expression in LNSCs develop spontaneous signs of T cell–mediated autoimmunity
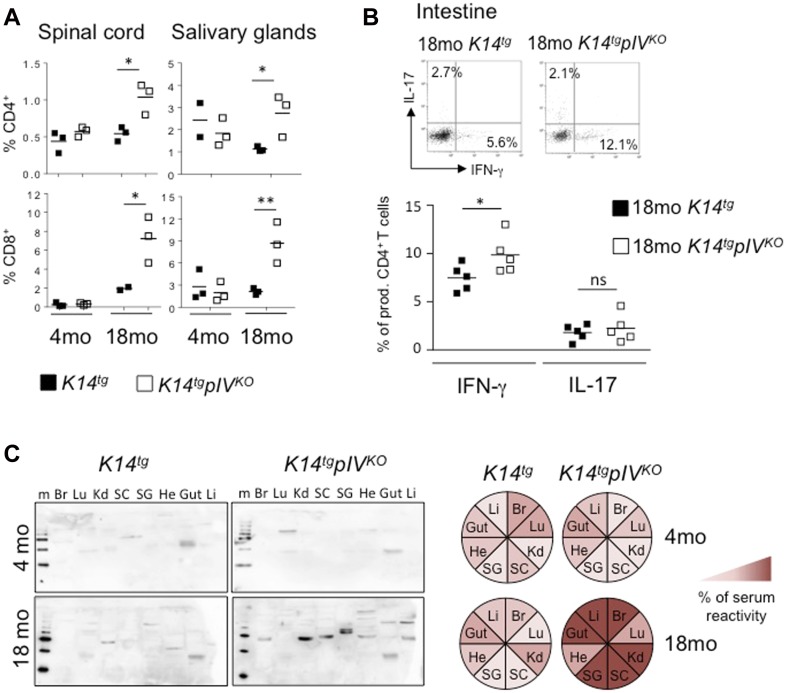
LNSCs have been so far described to function as tolerogenic Ag-presenting cells. Therefore, we tested whether the absence if MHCII expression by LNSCs would result in self-reactive T-cell tolerance breakdown and lead to immunopathology. Analysis of peripheral organs showed that the percentage of CD4+ and CD8+ T cells infiltrating the spinal cord (SC) and salivary glands (SGs) were markedly increased in K14tgpIVKO compared with K14tg control elderly (18 mo-old) mice (Fig 3A). No difference was observed in 4-mo-old mice (Fig 3A). In addition, intestines of 18-mo-old K14tgpIVKO exhibit increased frequencies of CD4+ T cells producing the proinflammatory cytokine IFN-γ compared with K14tg controls (Fig 3B). Other peripheral organs, such as the liver and the lungs, also exhibit a tendency of enhanced infiltrating CD4+ and CD8+ T cell numbers in 18-mo-old K14tgpIVKO mice, although not significant (not shown). Therefore, peripheral tissue T-cell infiltration was increased in a variety of non-lymphoid organs in elderly K14tgpIVKO mice. In agreement with a possible autoimmune syndrome, serum obtained from 18-mo-old K14tgpIVKO mice contained a broader spectrum of autoantibodies with enhanced reactivity to proteins from several tissues compared with K14tg control mice (Fig 3C), indicating an exacerbated development of systemic autoimmunity upon aging. Altogether, our data provide evidence that MHCII molecule expression by non-hematopoietic cells contribute to peripheral T-cell tolerance by providing a brake in the development of spontaneous signs of T cell–mediated autoimmune inflammation in peripheral tissues of elderly mice.
Figure 3. Aging K14tgpIVKO mice exhibit enhanced effector T-cell infiltration in peripheral organs and increased levels of autoantibodies.
(A) K14tgpIVKO (white) and K14tg control mice (black) were analysed at 4 and 18 mo. Percentages of infiltrating CD4+ (upper) and CD8+ (lower) T cells in SC and SGs from K14tgpIVKO and control K14tg mice at the indicated ages. Data are pooled from five individual mice. (B) Flow cytometry analyses of cytokine production by infiltrating CD4+ T cells in the intestine of 18-mo-old K14tgpIVKO and control K14tg mice. Data are pooled from four individual mice and are representative of two experiments. (C) Sera from 18-mo-old K14tgpIVKO and control mice were individually tested for antibody reactivity against organ-specific proteins in immunoblots loaded from left to right; the liver (Li), brain (Br), lung (Lu), kidney (Kd), SC, SGs, heart (He), and Gut. Representative examples are shown. Colored circles represent the intensity of serum reactivity in the immunoblots. Data are pooled from five individual mice. Two-way Anova; *P < 0.05; **P < 0.01; n.s., not significant. Error bars depict mean ± SEM.
MHCII abrogation in LNSCs enhances T-cell activation and impairs the Treg compartment in LNs
We next sought to determine whether exacerbated signs of T cell–mediated autoimmune inflammation in peripheral tissues of elderly K14tgpIVKO mice result from enhanced T-cell activation in SLOs. Analysis of CD4+ and CD8+ T cells in LN did not show any differences between 4-mo-old K14tgpIVKO and K14tg mice (Fig 4A). However, in aging mice (18 mo), we noticed a significant enhanced T-cell activation, with decreased frequencies of naive (CD62LhiCD44lo) and increased frequencies of activated (CD62LloCD44hi) CD4+ and CD8+ T cells in LNs of K14tgpIVKO compared with K14tg mice (Fig 4A). In addition, the frequency of effector CD4+ and CD8+ T cells expressing PD-1, and the frequency of CD4+ and CD8+ T cells producing IFN-γ, were enhanced in skin LNs from K14tgpIVKO compared with K14tg mice (Fig 4B). Because both CD4+ and CD8+ T-cell phenotypes and effector functions were affected, we postulated that an active mechanism of T-cell inhibition might have been lost in LNs of K14tgpIVKO mice upon aging. Accordingly, the frequency of Foxp3+ Tregs was significantly impaired (two-fold reduction) in LNs of aging K14tgpIVKO compared with K14tg control mice (Fig 4C). Foxp3+ staining on LN sections confirmed this phenotype (Fig 4D). In contrast, no difference in Treg frequencies was observed in 4-mo-old mice (Fig 4C). With Treg frequencies being reduced in aging K14tgpIVKO compared with K14tg controls, we next wondered whether this cellular population exhibited any phenotypic or functional alteration. We did not observe any alteration in the expression of several typical markers characterizing the Treg compartment, such as PD-1, CD44, CD25, or Foxp3 (not shown). In contrast, compared with control mice, LN Treg isolated from aging K14tgpIVKO mice exhibited an impaired ability to suppress the proliferation of naive CD4+ T cells in vitro at the Treg:Tnaive ratio of 1:5 (Fig 4E). The fact that no difference was observed when increased Treg numbers were used suggests that impaired Treg functions in knockout mice can be overcome with high Treg numbers.
Figure 4. K14tgpIVKO mice exhibit enhanced T-cell activation and impaired Treg frequencies in LNs upon aging.
K14tgpIVKO (white) and K14tg control mice (black) were analysed at 4 and 18 mo. (A) Frequencies of naive (CD62LhiCD44lo) and activated/memory (CD62LloCD44hi) CD4+ and CD8+ T cells. (B) Frequencies of PD-1+, IFN-γ, and IL-17 producing cells among CD4+ and/or CD8+ T cells. (C, D) Foxp3+ Treg identification by flow cytometry (CD4+ CD25+ Foxp3+) (C) and IHC staining (Foxp3+) (D) in lymph nodes of indicated mice. (A–D) Data are representative of three experiments with 3–7 mice/group. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant. Error bars depict mean ± SEM. (E) CD4+ CD25hi T cells (Treg) from total skin LNs of 18-mo-old K14tgpIVKO and K14tg mice were cultured (ratio 1:5, 1:3, and 1.1) with proliferation dye-labeled naive CD4+ CD25neg T cells. Labeled T-cell proliferation was assessed by flow cytometry after 5 d of co-culture. Data are representative of two experiments. Two-way Anova; **P < 0.01.
So far, we have accumulated convincing data that upon aging, K14tgpIVKO exhibits signs of spontaneous autoimmune defects. Organ-infiltrating T cells, and also the production of autoantibodies are increased, suggesting that the lack of MHCII expression by LNSCs in LNs of knockout elderly is likely to be responsible for this phenotype. However, these observations might also result from the lack of pIV of CIITA in the target organs, as other non-hematopoietic cells from peripheral tissues may up-regulate MHCII and contribute to this phenotype. Indeed, peripheral tissue-resident cells can up-regulate MHCII molecules in an IFN-γ–inducible pIV-dependent pathway and, by re-stimulating infiltrating T cells, possibly influence the outcome of T-cell responses (Duraes et al, 2013). Therefore, to determine whether enhanced signs of spontaneous autoimmunity in aging K14tgpIVKO mice were a direct consequence of MHCII deficiency in LNSCs, we adoptively transferred T cells isolated from LN of K14tgpIVKO or control K14tg mice into Rag2−/− mice. 4 mo later, recipients that received T cells from >18-mo-old K14tgpIVKO mice exhibited a significant loss of weight compared with mice injected with T cells isolated from age-matched K14tg controls (Fig 5A). Accordingly, immunofluorescence staining on small intestine sections revealed that Rag2−/− hosts that received LN T cells from K14tgpIVKO mice exhibited increased infiltrating CD45+ cells, and in particular in the CD3+ T-cell compartment (Fig 5B). In addition, frequencies of naive CD62LhiCD44lo CD4+ and CD8+ T cells were decreased in LN of mice injected with T cells from K14tgpIVKO donors (Fig 5C). Frequencies of effector CD4+ T cells producing IFN-γ or IL-17 were also augmented, as well as the frequency of IFN-γ producing CD8+ T cells, in LN of Rag2−/− mice transferred with T cells from K14tgpIVKO mice, (Fig 5D). Again, no significant difference was observed when adoptively transferred T cells were isolated from 4-mo-old K14tgpIVKO or control K14tg donors (not shown). As observed in donor LNs, the frequency of Foxp3+ Tregs was significantly reduced in LNs of recipient Rag2−/− mice injected with 18-mo-old K14tgpIVKO compared with control K14tg T cells (Fig 5E), suggesting that the impaired Treg compartment would account for autoimmune T-cell disorders. Although a role for MHCII expression by cells in peripheral organs cannot be formally excluded, our data show that both Treg and non-Treg cells were affected in the absence of MHCII-restricted Ag presentation by LNSCs.
Figure 5. T cells isolated from mice lacking MHCII expression in LNSCs induce autoimmunity upon transfer into immunodeficient mice.
(A–E) T cells isolated from LNs and spleen of 18-mo-old K14tgpIVKO (white) and control mice (black) were adoptively transferred into Rag2−/− recipient mice that were analysed 4 mo later. (A) Mouse weight. Data are pooled from four mice and representative of two experiments. (B) Sections of small intestines from recipient Rag2−/− mice were stained with antibodies against CD45 (green), CD3 (red), and with DAPI (blue). Pictures show representative tissue sections. Graphs represent the quantification of CD3+ T cells/area and are pooled from 12 tissue sections from three individual mice/group. (C–E) Flow cytometry dot plots showing the frequency of CD62LhiCD44lo CD4+ and CD8+ T cells (C), the frequency of CD4+ T cell–producing IFN-γ or IL-17 and the frequency of CD8+ T cell–producing IFN-γ in LNs (D) and Treg frequencies (E) in LNs of transferred Rag2−/− recipient mice. Data are representative of two experiments with 2–4 mice/group each. (C–E) *P < 0.05; **P < 0.01; ***P < 0.001; n.s. (A, B, D) unpaired t test, (C, E) two-way Anova.
Altogether, our data suggest that MHCII expression by LNSCs contributes to self-reactive T-cell tolerance. When abrogated, it results in impaired Treg frequencies, enhanced self-reactive T-cell activation, and subsequent peripheral tissue infiltration in elderly mice.
Deletion of MHCII on LNSCs decreases Treg proliferation
Interestingly, the levels of expression of MHCII molecules by LECs were significantly higher in elderly (18 mo) compared with young (4 mo) mice (Fig 6A). A slight, but not significant, increase in MHCII expression by BECs, but not by FRCs, was also observed. These observations can be a consequence of increased levels of IFN-γ in old versus young LNs, (although undetectable by ELISA, not shown) and/or an enhanced sensitivity to IFN-γ. Accordingly, LECs and BECs express higher levels of IFN-γ receptor in LNs from elderly compared with younger mice (Fig 6A). These results suggest a more substantial contribution of MHCII-restricted Ag presentation by LECs in aging mice, and provide a possible explanation of why the phenotype observed in K14tgpIVKO only appeared after several months.
Figure 6. Deletion of MHCII on LECs decreases Treg proliferation.
(A) MHCII and IFN-γ receptor (IFNγR) expression (MFI) by LECs, BECs, and FRCs from LN of WT mice of the indicated age. Data are representative of two experiments with three mice/group each. (B–D) LN sections depicting LECs (Lyve-1, white) and proliferating Tregs (red arrows) (Ki67, red; and Foxp3, green) from 18-mo-old K14tg and K14tgpIVKO mice (B), 3-mo-old WT and K14tg and K14tgpIVKO (C), and 3-mo-old Tamoxifen-treated Prox-1-CreERT2 MHCIIfl and MHCIIfl control mice (D). In (C) and (D), animals were treated with FTY720 every day for the last 6 d, and with IFN-γ 6 d before harvesting the LNs. Graphs represent the percentages of Ki67+ among Foxp3+ cells in contact with LECs from LN of the indicated mice. (B–D) Data are pooled from at least nine tissue sections from three individual mice/group. *P < 0.05; **P < 0.01; ***P < 0.001; n.s. (A, C) Two-way Anova, (B, D) unpaired t test.
Treg frequencies were found impaired in aging (18 mo), but not in young adult (4 mo) K14tgpIVKO mice lacking MHCII expression in LNSCs. Therefore, we tested whether Treg proliferation may be affected by the loss of MHCII expression in LECs. Immunofluorescence staining on LN sections from K14tgpIVKO and control elderly showed that the frequency of proliferating (red, Ki67+) Tregs (green, Foxp3+) in close interaction with LECs (Lyve-1+, in white) was significantly increased in aging controls compared with K14tgpIVKO mice (Fig 6B).
We next tested whether IFN-γ stimulation, which promotes MHCII up-regulation in LNSCs, could accelerate this phenotype. WT mice were injected or not with IFN-γ, together with FTY720, which inhibits T-cell egress from LNs (Cyster & Schwab, 2012), and LECs and Treg were analysed in LNs 6 d after IFN-γ injection. Immunofluorescence staining revealed that the frequency of proliferating (red, Ki67+) Tregs (green, Foxp3+) in close interaction with LECs (Lyve-1+, in white) was significantly increased in mice treated with IFN-γ compared with untreated mice (Fig 6C). Again, to determine whether increased Treg expansion in LNs following IFN-γ injection was dependent on MHCII expression by LNSC, most likely by LECs, we performed similar experiments in K14tgpIVKO and K14tg control mice. We observed an increased Treg proliferation in LNs of K14tg controls, and further demonstrated that proliferating (red, Ki67+) Tregs (green, Foxp3+) (red arrows) in close proximity of LECs (white, Lyve-1+) (Fig 6C). In contrast, Treg proliferation in LEC proximity (red arrows) was lower in LNs of K14tgpIVKO mice, demonstrating that the increase of proliferation of Tregs interacting with LECs was dependent on their expression of MHCII (Fig 6C). In contrast, we did not observe any increase in the proliferation of non-Treg (Foxp3neg) CD4+ T cells upon IFN-γ stimulation, nor difference between K14tg controls and K14tgpIVKO mice (not shown), suggesting that the Treg compartment is specifically affected. Finally, to examine a potential direct contribution of LECs as MHCII-restricted APCs in inducing Treg proliferation, we repeated the above experiments in mice in which MHCII expression was selectively abrogated in LECs. For that, we used Prox-1-CreERT2 mice, expressing the Tamoxifen-inducible Cre recombinase under the promoter Prox-1, which is selectively expressed in adult LECs (Bazigou et al, 2011). Prox-1-CreERT2 mice were crossed with MHCIIfl mice, allowing the selective deletion of MHCII molecules in LECs upon Tamoxifen treatment (not shown). Immunofluorescence analyses of LN sections demonstrated that the frequency of proliferating Tregs in contact with LECs 6 d after IFN-γ injection was significantly reduced in mice in which LECs do not express MHCII molecules (Prox-1-CreERT2 MHCIIfl) compared with control mice (MHCIIfl) (Fig 6D). Altogether, our results demonstrate that IFN-γ–mediated MHCII up-regulation by LNSCs promotes Treg proliferation, with a major contribution of LECs.
Discussion
Our results provide the first evidence for an in vivo role of LNSCs in impacting peripheral T-cell tolerance as MHCII-restricted APCs. We have recently published that subsets of murine LNSCs present MHCII–peptide complexes acquired from DCs to induce CD4+ T-cell dysfunction (Dubrot et al, 2014). Here, we further show that LNSCs inhibit autoreactive T-cell responses by directly presenting Ags through endogenous MHCII molecules. A recent study suggested that, although LECs express surface MHCII molecules, they cannot present a model Ag to CD4+ T cells presumably because of the lack of expression of H2-M in steady state, preventing the loading of endogenous antigenic peptides onto MHCII molecules (Rouhani et al, 2015). However, we show that LECs, BECs, and FRCs that express the IFN-γ–inducible-CIITA pIV, require IFN-γ to up-regulate H-2M molecules, as they do for MHCII expression, these two genes being co-regulated by CIITA (Reith & Mach, 2001). In addition, LNSCs were described to express considerable amounts of invariant chain (Ii) and cathepsin L (Rouhani et al, 2015), and therefore seem well equipped for the presentation of antigenic peptides through MHCII in situations involving the presence of IFN-γ.
Genetic abrogation of MHCII in LNSCs in vivo leads to impaired regulatory T-cell frequencies and in vitro functions, enhanced effector T cell differentiation LNs and peripheral tissue infiltration, and subsequent development of T cell–mediated autoimmunity in elderly mice. Indeed, mice lacking MHCII in LNSCs exhibit spontaneous signs of autoimmunity in elderly mice. LNSCs express a broad range of self-Ags. Therefore, our hypothesis is that abrogation of MHCII in these cells will lead to an inflation of polyclonal memory/effector T cells. In agreement, no difference was observed in the frequency of several TCR Vβ chains expressed by CD4+ and CD8+ T cells isolated from LNs of old control and knockout mice (not shown), suggesting that there is no restriction of T-cell clonality in mice lacking MHCII in LNSCs. Importantly, in this model there is no pre-existing early CD4+ T-cell bias, nor Treg function impairment. K14tgpIVKO young adult mice do not present any abnormality, excluding the possibility of a defect from thymic selection. In addition, T cells from 6- to 10-wk-old K14tgpIVKO mice were recently extensively characterized, and normal distribution of CD4+ naive, effector, and Foxp3+ T-cell subsets were observed in the thymus, spleen, and peripheral LNs (Thelemann et al, 2014).
In contrast to a recent study suggesting an activity of K14 promoter in LNSCs (Baptista et al, 2014), we did not observe any K14 mRNA expression in LECs, BECs, and FRCs (Fig 2B). Consistently, MHCII expression was similar in LNSCs isolated from pIVKO and K14tgpIVKO mice (Fig 2E), confirming that the K14 ciita transgene does not promote any MHCII expression in LNSCs. However, because peripheral non-hematopoietic tissue-resident cells can up-regulate MHCII molecules in an IFN-γ–inducible pIV-dependent manner (Duraes et al, 2013), this mouse model does not represent per se a valid mouse model to study the specific role of endogenous MHCII expression by LNSCs in shaping peripheral CD4+ T-cell responses. For instance, intestinal epithelial cells (IECs), hepatocytes and liver sinusoidal endothelial cells, pancreatic β cells, or astrocytes were postulated to be capable of MHCII-mediated Ag presentation, although very little in vivo data are available on the potential impact on peripheral CD4+ T-cell responses (reviewed in Duraes et al [2013]). Recently, using the mouse model we are presently exploiting, two studies have highlighted opposite roles for MHCII expression in distinct target tissues. First, IFN-γ–mediated MHCII up-regulation by IECs exerts anti-inflammatory effects and protect against colitis (Thelemann et al, 2014). In contrast, in the context of experimental autoimmune myocarditis, elevated IFN-γ–inducible cardiac endothelial MHCII expression exacerbates the disease (Thelemann et al, 2016). Together, these findings provide evidence of a critical role of pIV-mediated non-hematopoietic MHCII expression in peripheral tissues upon inflammation, the impact on T-cell responses being largely dependent on the target organ. Here, we add a distinct role of LN-resident stromal populations in shaping CD4+ T-cell responses, providing support to Treg homeostasis and maintaining peripheral tolerance. We cannot totally exclude a role for a lack of MHCII expression by cells in peripheral tissue on local T-cell reactivation in K14tgpIVKO mice. Indeed, it is possible that T cells infiltrating peripheral organs are maintained in a tolerogenic state by local MHCII+ cells, and that this phenomenon is abrogated in our mouse model. However, it is unlikely, for several reasons. First, a similar phenotype was obtained after adoptive transfer of T cells from LN of K14tgpIVKO mice into Rag2−/− with competent MHCII peripheral tissues. Importantly, T-cell compartments in the recipient Rag2−/− mice recapitulated the effects observed in the donor transgenic mice indicating a contribution of LNSCs to peripheral T-cell alterations. Second, although a contribution of MHCII expression by tissue-resident cells has been described before, it was always in inflammatory settings, in which these cells indeed up-regulate MHCII in contrast to our stud which was performed in the steady state (Duraes et al, 2013), minimizing their possible contribution as MHCII-restricted Ag presenting cells in the absence of model-induced inflammation. Finally, LN-resident Tregs exhibit impaired proliferation in LNs not only in K14tgpIVKO, but also in Prox-1-CreERT2 MHCIIfl mice, supporting a pro-tolerogenic role for MHCII-restricted Ag presentation by LNSCs, in particular LECs, in dampening T-cell autoimmune reactions.
We have previously shown that endogenous MHCII expression by LECs, BECs, and FRCs was significantly increased in WT mice compared to IFN-γ receptor–deficient mice (Dubrot et al, 2014), demonstrating that the presence of low levels of IFN-γ in naive mice drives pIV-mediated MHCII endogenous expression in steady-state LNSCs. This was confirmed by reduced MHCII expression by LNSCs in steady-state LNs from K14tgpIVKO compared with control mice. Therefore, basal (and presumably variable) levels of circulating steady-state IFN-γ promote endogenous MHCII expression by LNSCs, and confer them the ability to function as competent MHCII-mediated APCs. Increased inflammatory status over time might be due to the accumulation of punctual and mild infectious processes or altered gut microbiotic composition upon aging (Claesson et al, 2012).
Last, we provide evidence for a direct effect of MHCII-restricted Ag presentation by LECs in promoting Treg proliferation. Genetic selective abrogation of MHCII expression by LECs in mice results, similarly to what was observed in K14tgpIVKO mice, in impaired proliferation of Treg in contact with LECs. Therefore, among the LNSC subsets, LECs seems to be the main contributors in inducing Treg proliferation. Distinct sets of PTA expression, together with differential levels of expression of co-inhibitory molecules, such as PDL-1 (Tewalt et al, 2012), by LNSC populations might give rise to distinct impacts on T-cell responses, such as T-cell apoptosis, T-cell anergy, or Treg induction. Elevated PDL-1 expression by LECs compared with BECs and FRCs might explain a role for those cells in impacting Tregs, which express high levels of PD-1. Although we did not elucidate the molecular mechanisms, a selective effect on Tregs, which mostly express a self-reactive TCR, could result from the ability of LECs to present endogenously expressed PTAs. One explanation could be the use of the autophagy pathway by LECs to present endogenous PTAs through MHCII molecules. This hypothesis would be in accordance with previous results obtained in the thymic epithelium (Nedjic et al, 2008; Aichinger et al, 2013). Future experiments in the laboratory will use mice genetically deficient for autophagy to firmly demonstrate the implication of this pathway in the ability of the different LNSC subsets to present PTAs through MHCII molecules and to alter the Treg compartment. Our experiments do not rule out another possible mechanism of action by which MHCII expression in LNSC may support T-cell inactivation in an Ag-independent fashion. MHCII is a natural ligand for the molecule lymphocyte activation gene 3 (LAG-3), expressed on activated T cells and can be induced by IFN-γ stimulation. This immune checkpoint has an inhibitory role for LAG-3 in controlling both CD4 and CD8 T-cell proliferation in vitro and in vivo (Workman et al, 2004; Grosso et al, 2007). In addition, LAG-3 expression on Tregs has been shown to be important for their suppressive function (Grosso et al, 2007; Okamura et al, 2009). Moreover, the co-expression of LAG-3 with the inhibitory receptor PD-1 on exhausted T cells or Tregs correlates with a greater state of effector T-cell exhaustion and the suppressive function of Tregs. The relative contribution of both Ag-dependent and/or Ag-independent pathways remains to be addressed.
In conclusion, our work identifies novel in vivo functions of MHCII expression by LNSCs in the maintenance of peripheral T-cell tolerance by inhibiting autoreactive T cells. We also define the LECs as important MHCII expressing cells supporting Treg homeostasis.
Materials and Methods
Mice and treatments
C57BL/6 WT mice were purchased from Charles River. pIV−/− (Waldburger et al, 2003), Ciita−/− and K14tgpIVKO (Laufer et al, 1996), and Rag2−/− (Jackson Laboratories) Prox-1-CreERT2 MHCIIfl mice have been previously described (Bazigou et al, 2011). To selectively abrogate MHCII expression in LECs, Prox-1-CreERT2 MHCIIfl/fl (and MHCIIfl/fl control) mice were injected with tamoxifen (i.p) twice a day for four consecutive days (2 mg/mice/d). 2 wk after the last injection, mice were treated with IFN-γ and FTY720 as described below. These mouse strains are on a C57BL/6 background and were housed and maintained under SPF conditions at the Geneva Medical School animal facility and under EOPS conditions at Charles River, France. All animal husbandry and experiments were approved by and performed in accordance with guidelines from the Animal Research Committee of the University of Geneva. Genotyping was carried out by using PCR on ear biopsies with the Phire Tissue Direct PCR Master Mix (F170L; Thermo Fisher Scientific). When indicated, IFN-γ (Peprotech) was injected subcutaneously (both flanks and neck, 1 μg/50 μl), and FTY720 (Sigma-Aldrich) (20 μg) was injected every day for 6 d before the experiment. Mice from each group were randomized before treatments.
LNSC and cell tissue isolation
LNSCs were obtained as previously described (Dubrot et al, 2014). In brief, total skin or skin-draining LNs from individual or 10–15 pooled mice were cut into small pieces and digested in RPMI containing 1 mg/ml collagenase IV (Worthington Biochemical Corporation), 40 μg/ml DNase I (Roche), and 2% FBS. Undigested cells were further digested with 1 mg/ml collagenase d, and 40 μg/ml DNase I (Roche). The reaction was stopped by addition of 5 mM EDTA and 10% BSA. Samples were further disaggregated through a 70-μm cell strainer and blocked with anti-CD16/32 antibody. Single-cell suspensions were negatively selected using CD45 microbeads and a magnetic bead column separation (Miltenyi Biotec).
Cells from LN, the spleen, the SC, and SGs were isolated by digesting organ fragments with an enzymatic mix containing collagenase D (1 μg/ml) and DNAse I (10 μg/ml) (Roche) in HBSS [14]. For SC and SG, single-cell suspensions were further centrifuged through a discontinuous 30:70% percoll (Invitrogen) gradient. T cells were further analysed by flow cytometry.
Small intestines were excised and transferred into PBS 2% FCS. Peyer's patches and adipose tissue were carefully removed. The small intestines were cut longitudinally and cleaned of faecal content. The small intestines were next incubated in RPMI containing 10% FCS, 2 mM EDTA, and 1 mM DTT for 30 min at 37°C in a shaking incubator to remove epithelial cells, the supernatant being discarded. The remaining tissue was digested twice in RPMI containing 1 mg/ml of collagenase D (Roche) for 30 min at 37°C in a shaking incubator. Cells were recovered from the supernatant and filtered through a 70-μm cell strainer.
Antibodies, flow cytometry, and cell sorting
Anti-gp38 (8.1.1), anti-CD31 (390), anti-CD11c (N418), anti-CD44 (IM7), and anti-IAb (AF6.120.1) mAbs were from BioLegend. Anti-CD45 (30F11), anti-CD16/32 FcyRIII (2.4G2), anti-I-Ad/I-Ed (2G9), anti-IFNγR (GR20), and H2-M (2E5A) were from BD. Anti-CD19 (1D3), anti-CD8 (53–6.7), anti-CD4 (GK1.5), anti-CD11b (M1/70), anti-CD62L (MEL-14), anti-PDCA-1 (eBio927), anti-PD-1 (J43), anti-IFN-γ (XMG1.2), anti-IL-17 (eBio17B7), and anti-Foxp3 (FJK-16s) were from eBioscience. Anti-EpCAM (caa7-9G8) and anti-Ly5.1 (6C3) were from Miltenyi Biotec.
For LNSC flow cytometry sorting, enriched CD45neg cells were stained with mAbs against CD45, gp38, and CD31. In some experiments, cells were also stained with mAbs against MHCII or isotype control as indicated. For cTEC and mTEC sorting, enriched CD45neg cells were stained with mAbs against CD45, EpCAM, and Ly5.1.
Intracellular cytokine stainings were carried out with the Cytofix/Cytoperm kit (BD) for IFN-γ and IL-17 staining. Foxp3 staining was performed with the eBioscience kit, according to manufacturer's instructions. For IFN-γ and IL-17 staining, LN cells were cultured in RPMI containing 10% heat-inactivated fetal bovine serum, 50 mM 2-mercaptoethanol, 100 mM sodium pyruvate, and 100 μM penicillin/streptomycin at 37°C and 5% CO2. Cells were stimulated for 18 h with PMA/ionomycin and Golgi stop solution (BD) was added during the last 4 h of culture before the staining.
Cells were either acquired on a Gallios or sorted using a MoFlowAstrios (Beckman Coulter), and analysed using FlowJo (Tree Star) or Kaluza softwares.
Immunofluorescence microscopy
Mice were transcardiacally perfused with PBS, and intestines were fixed in paraformaldehyde before inclusion in paraffin. Preserved organs were cut into 7-μm-thick sections and deparaffinised in xylene–ethanol. Ag retrieval was performed in citrate buffer. Sections were then stained using labelled antibodies against CD45 (30-F11) and CD3 (17A2) and DAPI (Sigma-Aldrich) counter-staining. Sections were mounted with Mowiol fluorescent mounting medium (EMD). Images were acquired with a confocal microscope (LSM 700; Carl Zeiss Inc. and SP5; Leica).
Skin LNs from untreated mice or mice treated with IFN-γ and FTY720 were frozen in OCT medium. 10-μm-thick sections were cut and fixed with paraformaldehyde 4% for 20 min. After washing and permeabilization, the sections were stained overnight at 4°C using a rabbit anti-Lyve-1 antibody (Reliatech GmbH). Secondary staining was performed using a Alexafluor546-labelled donkey anti-rabbit antibody, Alexafluor488-labelled anti-Foxp3 (150D) antibodies and eF660-Ki67 (SolA15), for 2 h at room temperature. After DAPI (Sigma-Aldrich) staining, sections were mounted with Mowiol fluorescent mounting medium (EMD). Images were acquired with a confocal microscope (LSM 700; Carl Zeiss Inc. and SP5; Leica). Tregs in “close proximity” (<5 μm) of LECs were quantified as proliferating (Ki67+Foxp3+) or non-proliferating (Foxp3+) cells, and the ratio of proliferating Tregs was calculated. At least 20 images/condition were quantified.
Treg suppression assay
In vitro Treg suppressive assays were performed as follows: CD4+ CD25hi T cells (Treg) were purified by flow cytometry from total skin LNs of 18-mo-old K14tgpIVKO and K14tg control mice and cultured at the indicated ratio with 2 × 105 proliferation dye-labeled naive CD4+ CD25neg T cells in the presence of bone marrow–derived DC and anti-CD3 antibodies. Labeled T-cell proliferation was assessed by flow cytometry after 5 d of co-culture.
T-cell adoptive transfer into Rag2−/− recipients
LN and spleen of donor mice were harvested, and T cells were negatively selected from the cell suspension using a PAN T isolation kit (Mylteniy biotec) according to the manufacturer's instructions. Purity generally exceeded 95%. 2–5 × 106 T cells were injected intravenously into Rag2−/− mice. Recipient mice were randomized before transfer and co-housed during the experiment.
RNA isolation and quantitative RT-PCR
Total RNA was isolated using Tri-Reagent (Ambien) from sorted cells. cDNA was synthesized using random hexamers and M-MLV reverse transcriptase (Promega). PCRs were performed with the CFX Connect real-time PCR detection system and iQ SYBR green super mix (Bio-Rad Laboratories). The results were normalized with GAPDH or 60S ribosomal protein L32 mRNA expression and quantified with a standard curve generated with serial dilutions of a reference cDNA preparation. Primer sequences: I-Ab forward, 5′-CTG TGG TGG TGG TGA TGG T-3′ and reverse, 5′-CGT TGG TGA AGT AGC ACT CG-3′. H2-Mα forward, 5′-CTCGAAGCATCTACACCAGTG-3′ and reverse, 5′-TCCGAGAGCCCTATGTTGGG-3′. K14 orward, 5′-AGG GAG AGG ACG CCC ACC TT-3′ and reverse, 5′- CCT TGG TGC GGA TCT GGC GG-3′
Lysate preparation and immunoblotting
Tissues were homogenized in T-PER tissue protein extraction reagent (Thermo Fisher Scientific) supplemented with a cocktail of protease inhibitors (Complete, Roche). Protein extracts were incubated at 95°C for 5 min with of 4× SDS–PAGE–loading buffer (250 mmol Tris–HCl at pH = 6.8, 40% glycerol, 8% SDS, 0.57 mol β-mercaptoethanol, and 0.12% bromophenol blue). Equal amounts of protein were run on 12% SDS–PAGE gels and transferred onto a polyvinylidene difluoride membrane (Hybond-P; Amersham Biosciences). Sera from K14tgpIVKO or control mice were visualized with HRP-conjugated goat anti-mouse IgG (Bio-Rad Laboratories) and the ECL WesternBright Sirius (advansta).
Statistical analysis
Statistical significance was assessed by the two-tailed unpaired t test or two-way Anova, and Log-rank test using Prism software (GraphPad).
Supplementary Material
Acknowledgements
The authors thank JP Aubry-Lachainaye and C Gameiro for excellent assistance in flow cytometry. We also thank Claire-Anne Siegrist, Floriane Auderset, and Assunta Caruso for valuable discussions and/or technical help. This work was supported by the Swiss National Science Foundation (PP00P3_152951 and 310030_166541 to S Hugues) and the European Research Council (281365 to S Hugues).
Author Contributions
J Dubrot: conceptualization, formal analysis, investigation, methodology, and writing—original draft.
FV Duraes: conceptualization, formal analysis, investigation, and methodology.
G Harlé: formal analysis, investigation, and methodology.
A Schlaeppi: formal analysis, investigation, and methodology.
D Brighouse: formal analysis and methodology.
N Madelon: formal analysis, investigation, and methodology.
C Goepfert: formal analysis and investigation.
N Stokar-Regenscheit: formal analysis, investigation, and methodology.
H Acha-Orbea: conceptualization.
W Reith: conceptualization.
M Gannagé: conceptualization.
S Hugues: conceptualization, supervision, project administration, and writing—original draft, review, and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Adamopoulou E, Tenzer S, Hillen N, Klug P, Rota IA, Tietz S, Gebhardt M, Stevanovic S, Schild H, Tolosa E, et al. (2013) Exploring the MHC-peptide matrix of central tolerance in the human thymus. Nat Commun 4: 2039 10.1038/ncomms3039 [DOI] [PubMed] [Google Scholar]
- Aichinger M, Wu C, Nedjic J, Klein L (2013) Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. J Exp Med 210: 287–300. 10.1084/jem.20122149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M, Granjeaud S, Guerder S (2003) The strategy of T cell antigen-presenting cell encounter in antigen-draining lymph nodes revealed by imaging of initial T cell activation. J Exp Med 198: 715–724. 10.1084/jem.20030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista AP, Roozendaal R, Reijmers RM, Koning JJ, Unger WW, Greuter M, Keuning ED, Molenaar R, Goverse G, Sneeboer MM, et al. (2014) Lymph node stromal cells constrain immunity via MHC class II self-antigen presentation. eLife 3: 10.7554/elife.04433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Lyons OT, Smith A, Venn GE, Cope C, Brown NA, Makinen T (2011) Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest 121: 2984–2992. 10.1172/jci58050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, Munk A, Forster R (2011) Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol 12: 879–887. 10.1038/ni.2085 [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM (2003) Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198: 1875–1886. 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, et al. (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488: 178–184. 10.1038/nature11319 [DOI] [PubMed] [Google Scholar]
- Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH (2010) Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med 207: 681–688. 10.1084/jem.20092465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR (2012) Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 30: 69–94. 10.1146/annurev-immunol-020711-075011 [DOI] [PubMed] [Google Scholar]
- Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, Suter T, LeibundGut-Landmann S, Garbi N, Reith W, Swartz MA, et al. (2014) Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4+ T cell tolerance. J Exp Med 211: 1153–1156. 10.1084/jem.20132000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraes FV, Thelemann C, Sarter K, Acha-Orbea H, Hugues S, Reith W (2013) Role of major histocompatibility complex class II expression by non-hematopoietic cells in autoimmune and inflammatory disorders: Facts and fiction. Tissue Antigens 82: 1–15. 10.1111/tan.12136 [DOI] [PubMed] [Google Scholar]
- Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, Collier AR, Boyd RL, Turley SJ (2010) Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med 207: 689–697. 10.1084/jem.20092642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AL, Malhotra D, Turley SJ (2011) Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol 32: 12–18. 10.1016/j.it.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, et al. (2007) LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest 117: 3383–3392. 10.1172/jci31184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, et al. (2008) Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity 29: 451–463. 10.1016/j.immuni.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Khan O, Headley M, Gerard A, Wei W, Liu L, Krummel MF (2011) Regulation of T cell priming by lymphoid stroma. PLoS One 6: e26138 10.1371/journal.pone.0026138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH (1996) Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature 383: 81–85. 10.1038/383081a0 [DOI] [PubMed] [Google Scholar]
- Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA (2007) Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol 8: 1255–1265. 10.1038/ni1513 [DOI] [PubMed] [Google Scholar]
- Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, Turley SJ (2011) Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol 12: 1096–1104. 10.1038/ni.2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller DL. (2010) Mechanisms maintaining peripheral tolerance. Nat Immunol 11: 21–27. 10.1038/ni.1817 [DOI] [PubMed] [Google Scholar]
- Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L (2008) Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature 455: 396–400. 10.1038/nature07208 [DOI] [PubMed] [Google Scholar]
- Okamura T, Fujio K, Shibuya M, Sumitomo S, Shoda H, Sakaguchi S, Yamamoto K (2009) CD4+CD25-LAG3+ regulatory T cells controlled by the transcription factor Egr-2. Proc Natl Acad Sci USA 106: 13974–13979. 10.1073/pnas.0906872106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG (2010) Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 207: 17–27. 10.1084/jem.20091619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgrabinska S, Kamalu O, Mayer L, Shimaoka M, Snoeck H, Randolph GJ, Skobe M (2009) Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac-1/ICAM-1-dependent mechanism. J Immunol 183: 1767–1779. 10.4049/jimmunol.0802167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W, Mach B (2001) The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol 19: 331–373. 10.1146/annurev.immunol.19.1.331 [DOI] [PubMed] [Google Scholar]
- Rouhani SJ, Eccles JD, Riccardi P, Peske JD, Tewalt EF, Cohen JN, Liblau R, Makinen T, Engelhard VH (2015) Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat Commun 6: 6771 10.1038/ncomms7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S, Huang HY, Yang CY, Scarpellino L, Carrie L, Essex S, Nelson PJ, Heikenwalder M, Acha-Orbea H, Buckley CD, et al. (2011) Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS One 6: e27618 10.1371/journal.pone.0027618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L (2005) The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22: 19–29. 10.1016/j.immuni.2004.11.013 [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, et al. (2003) Dendritic cell function in vivo during the steady state: A role in peripheral tolerance. Ann N Y Acad Sci 987: 15–25. 10.1111/j.1749-6632.2003.tb06029.x [DOI] [PubMed] [Google Scholar]
- Stern LJ, Potolicchio I, Santambrogio L (2006) MHC class II compartment subtypes: Structure and function. Curr Opin Immunol 18: 64–69. 10.1016/j.coi.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Sukseree S, Mildner M, Rossiter H, Pammer J, Zhang CF, Watanapokasin R, Tschachler E, Eckhart L (2012) Autophagy in the thymic epithelium is dispensable for the development of self-tolerance in a novel mouse model. PLoS One 7: e38933 10.1371/journal.pone.0038933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swee LK, Bosco N, Malissen B, Ceredig R, Rolink A (2009) Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood 113: 6277–6287. 10.1182/blood-2008-06-161026 [DOI] [PubMed] [Google Scholar]
- Tewalt EF, Cohen JN, Rouhani SJ, Guidi CJ, Qiao H, Fahl SP, Conaway MR, Bender TP, Tung KS, Vella AT, et al. (2012) Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood 120: 4772–4782. 10.1182/blood-2012-04-427013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelemann C, Eren RO, Coutaz M, Brasseit J, Bouzourene H, Rosa M, Duval A, Lavanchy C, Mack V, Mueller C, et al. (2014) Interferon-gamma induces expression of MHC class II on intestinal epithelial cells and protects mice from colitis. PLoS One 9: e86844 10.1371/journal.pone.0086844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelemann C, Haller S, Blyszczuk P, Kania G, Rosa M, Eriksson U, Rotman S, Reith W, Acha-Orbea H (2016) Absence of nonhematopoietic MHC class II expression protects mice from experimental autoimmune myocarditis. Eur J Immunol 46: 656–664. 10.1002/eji.201545945 [DOI] [PubMed] [Google Scholar]
- Tomei AA, Siegert S, Britschgi MR, Luther SA, Swartz MA (2009) Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J Immunol 183: 4273–4283. 10.4049/jimmunol.0900835 [DOI] [PubMed] [Google Scholar]
- Waldburger JM, Rossi S, Hollander GA, Rodewald HR, Reith W, Acha-Orbea H (2003) Promoter IV of the class II transactivator gene is essential for positive selection of CD4+ T cells. Blood 101: 3550–3559. 10.1182/blood-2002-06-1855 [DOI] [PubMed] [Google Scholar]
- Waldburger JM, Suter T, Fontana A, Acha-Orbea H, Reith W (2001) Selective abrogation of major histocompatibility complex class II expression on extrahematopoietic cells in mice lacking promoter IV of the class II transactivator gene. J Exp Med 194: 393–406. 10.1084/jem.194.4.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirnsberger G, Hinterberger M, Klein L (2011) Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol 89: 45–53. 10.1038/icb.2010.123 [DOI] [PubMed] [Google Scholar]
- Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA (2004) Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol 172: 5450–5455. 10.4049/jimmunol.172.9.5450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.