Summary
Distal-less has been repeatedly co-opted for the development of many novel traits. Here, we document its curious role in the development of a novel abdominal appendage (“sternite brushes”) in sepsid flies. CRISPR/Cas9 deletions in the homeodomain result in losses of sternite brushes, demonstrating that Distal-less is necessary for their development. However, deletions in the upstream coding exon (Exon 2) produce losses or gains of brushes. A dissection of Exon 2 reveals that the likely mechanism for gains involves a deletion in an exon-splicing enhancer site that leads to exon skipping. Such contradictory phenotypes are also observed in butterflies, suggesting that mutations in the conserved upstream regions have the potential to generate phenotypic variability in insects that diverged 300 million years ago. Our results demonstrate the importance of Distal-less for the development of a novel abdominal appendage in insects and highlight how site-specific mutations in the same exon can produce contradictory phenotypes.
Subject Areas: Genetics, Molecular Biology, Evolutionary Biology, Developmental Biology
Graphical Abstract

Highlights
-
•
Distal-less is necessary for the development of a novel abdominal appendage
-
•
CRISPR/Cas9 editing produced both losses and gains of novel abdominal appendages
-
•
Gains of appendages result from mutations in exonic splicing enhancer (ESEs) sites
-
•
ESE mutations likely led to exon skipping and an altered Distal-less protein
Genetics; Molecular Biology; Evolutionary Biology; Developmental Biology
Introduction
Insects display a remarkable amount of morphological diversity. Such diversity can be generated through the co-option of existing gene modules in novel environments. One such repeatedly co-opted gene is Distal-less (Dll), which codes for a transcription factor that is essential for insect appendage patterning. Dll has been shown to be involved in the development of many morphological novelties that are essentially distal cuticular projections, like the grasping structures on antenna in male water striders (Khila et al., 2012), the nasus in termites (Toga et al., 2012), and the thoracic horns in scarab beetles (Moczek and Rose, 2009). Dll has even been co-opted for the development of novel pigmentation patterns in the wings of flies (Arnoult et al., 2013) and eyespots in butterflies (Zhang and Reed, 2016). Here, we look at its role in the development and evolution of another morphological novelty, an abdominal sternite brush in sepsids.
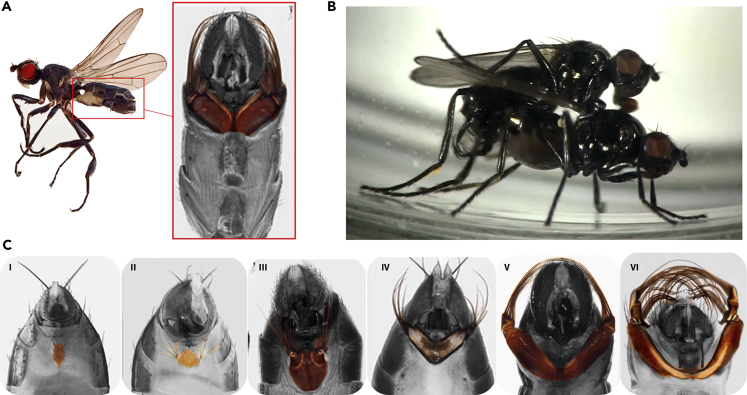
Males of some sepsid species (Sepsidae: Diptera) have evolved a moveable appendage on their fourth abdominal segment, the fourth sternite brush (Figure 1A). This novel appendage in sepsids is a modification of a sternite plate on the ventral fourth abdominal segment. In some species, the fourth sternite has been dramatically modified into a large, sclerotized leg-like structure that is complete with underlying musculature, articulation, and long distal brushes (Ang, 2013). Male sepsids use these elaborate appendages to stimulate females during copulation (Figure 1B, https://www.youtube.com/watch?v=BL9wffTKO50&feature=youtu.be). The fourth sternite brushes range from simple flat plates with few bristles to highly elaborate appendages (Figure 1C). A phylogenetic analysis reveals that the fourth sternite brush has a complex evolutionary history. It was acquired early in the radiation of this family, then lost multiple times, and then reacquired at least once (Bowsher et al., 2013).
Figure 1.
Sepsid Males of Some Species Have a Novel Appendage on Their Fourth Abdominal Segment Which Is a Modified Fourth Sternite (“Sternite Brush”)
(A–C) (A) Sternite brush of Themira biloba (highlighted in color). (B) Male Themira superba using the sternite brush to stimulate a female during mating. (C) Sternite diversity across Sepsidae: (i) Dicranosepsis bicolor without sternite modification and brushes in (ii) Microsepsis armillata, (iii) Themira flavicoxa, (iv) Perochaeta dikowi, (v) Themira putris, and (vi) Themira superba.
These structures also have an interesting developmental origin. Unlike typical dipteran appendages (e.g., legs and wings) that develop from imaginal discs, the novel sternite brush appendage in male sepsids develops from a cluster of ventral histoblast cells (Bowsher and Nijhout, 2007). In flies, histoblasts are clumps of imaginal cells that proliferate to form the adult abdominal epidermis, including the sternites and tergites. An earlier study characterized the expression pattern of Dll late in sepsid pupal development; they found that Dll was expressed only in the bristle cell nuclei of late-stage pupal tissues that give rise to the fourth sternite brush (Bowsher and Nijhout, 2009). Here, we develop and use CRISPR/Cas9 to show that Dll is involved in the development of these novel abdominal appendages in a non-model dipteran species, Themira biloba (Figure 1A). This is the first functionally characterized gene in a gene regulatory network involved in the development and evolution of a novel abdominal appendage. In the process, we discover an interesting phenomenon wherein deletions in a conserved Dll-coding region generated via CRISPR/Cas 9 produced not only the expected losses but also gains of novel appendages on different abdominal segments.
Results
Genome Editing at the Dll Locus Generates Loss-of-Function Mosaic Phenotypes
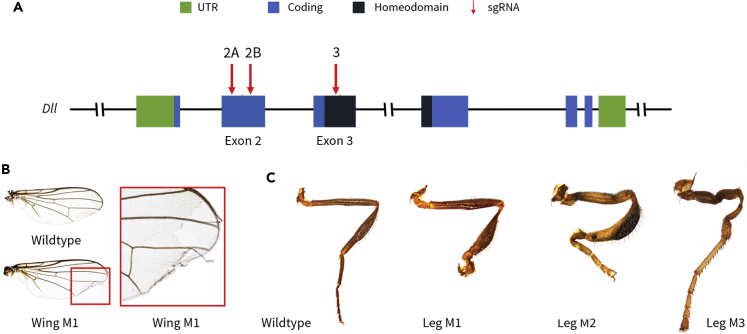
We designed three guides targeting two different exons within the Dll locus: the homeodomain (Exon 3) and the coding exon upstream of the homeodomain (Exon 2) (Figure 2A, Table S6). We screened for potential off-targets by conducting a local BLASTN search comparing the transcriptome of T. biloba (Melicher et al., 2014) against the three guide sequences.
Figure 2.
Deletions in Dll-Coding Region Generate Leg and Wing Mutants
(A–C) (A) The Homeodomain (Exon 3) and upstream coding exon (Exon 2) of Dll were targeted using CRISPR/Cas9. Three guides were designed: two within the coding regions of Exon 2 and one within the coding region of Exon 3. (B) Mosaic mutant with a disrupted wing margin (Exon 2, sgRNA-2B mutant). (C) Mosaic mutant males with leg deformations (Leg M1 and M2: Exon 2 sgRNA-2B mutants, Leg M3: Exon 3 mutant)
We injected 4-hr-old T. biloba embryos with Cas9 protein and guides targeting these coding regions. We observe that Dll loss-of-function mutations are highly lethal; this result is similar to what has been shown in Drosophila melanogaster (Cohen and Jürgens, 1989). After optimization, we find that 20%–30% of embryos survive microinjection with 22%–37% of larvae developing into adults. In total, we obtained 80 mosaic mutants (Table 1). A total of 58 mutants were observed with malformed or missing sternites; monomorphic sternite malformations were observed in both males and females. However, sternite losses were only observed in males. We observed a higher proportion of abdominal or posterior phenotypes; this is likely due to the injection of CRISPR/Cas9 and single guide RNA (sgRNA) complex into the posterior end of the embryo. Even so, one in eight of the recovered mutants exhibited leg or wing phenotypes consistent with known Dll mutants in D. melanogaster: mosaic mutants with wing margin deformities, hindleg deformities, and loss of tarsal structures were observed (Figures 2B and 2C). All mutants were successfully verified with next-generation sequencing (NGS) on an Illumina MiSeq 2 x 300-bp platform with tagged amplicon sequencing (7-bp tags). The read counts and three most abundant mutant haplotypes for each mutant specimen can be found in Table S3. Only the most abundant mutant haplotype for each specimen was used for downstream alignments and analyses.
Table 1.
Summary of CRISPR/Cas9 Injection Results
| Injected Eggs | Larvae | Adults | Mutants |
||||
|---|---|---|---|---|---|---|---|
| Leg/Wing | Sternite Malformations | Ectopic Structures | Others (e.g., Clasper Malformations) | ||||
| Exon 2 (sgRNA 2A) | 1,715 | 605 | 151 | 1 | 8 | 1 | 0 |
| Exon 2 (sgRNA 2B) | 4,631 | 957 | 363 | 8 | 29 | 3 | 6 |
| Exon 3 (sgRNA3) | 4,003 | 1,187 | 261 | 1 | 21 | 0 | 2 |
| Control | 1,004 | 153 | 109 | 0 | 0 | 0 | 0 |
Control injections were carried out using only Cas9 protein without any sgRNA.
As a control, we injected approximately 1,000 embryos with Cas9 alone without obtaining mutant phenotypes (Table 1). The surviving adults from the control injections were genotyped through sequencing with NGS and found to have no mutations in the Dll-coding region. We also sequenced injected individuals displaying a wild-type phenotype and found no mutations in the Dll-coding region.
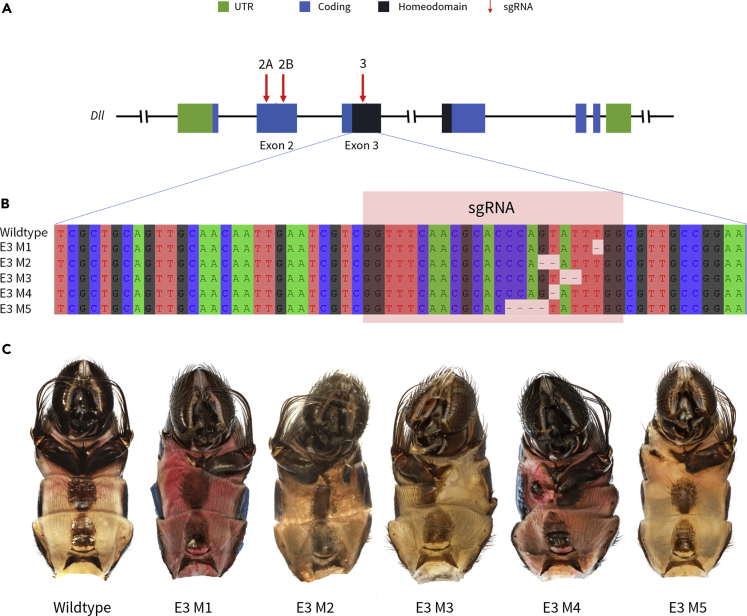
A Functional Dll Homeodomain Is Necessary for Fourth Sternite Brush Development
Targeted genome editing at the Dll homeodomain produced mosaic mutant males with loss of the fourth sternite appendage. The fourth sternite appendage in T. biloba consists of a bifurcated sternite with a pair of cuticular protrusions or lobes that each terminate in a tuft of bristles. Of the 21 mosaic mutants with sternite malformations, five exhibited malformed or missing fourth sternite appendages (Figure 3C): mutants E3 M1 and E3 M3 display a complete loss of the sternite, lobe, and bristles in one-half of the fourth sternite appendage, whereas in the others (E3 M2, E3 M4, and E3 M5) the lobe and bristles of one-half of the fourth sternite appendage were lost, leaving behind small misshapen remnants of the sternite. Characterization of these mutants with NGS showed deletions in the target region, which disrupted the reading frame (Figure 3B). These mosaic mutant phenotypes confirm that a functional Dll gene product is necessary for the development of this appendage in T. biloba males.
Figure 3.
Dll Homeodomain Is Necessary for the Development of the Fourth Sternite Brush
(A) Dll locus.
(B) Disruptive mutations of Exon 3 yielding mosaic mutants in (C).
(C) Corresponding mosaic mutants with losses of the fourth sternite brush.
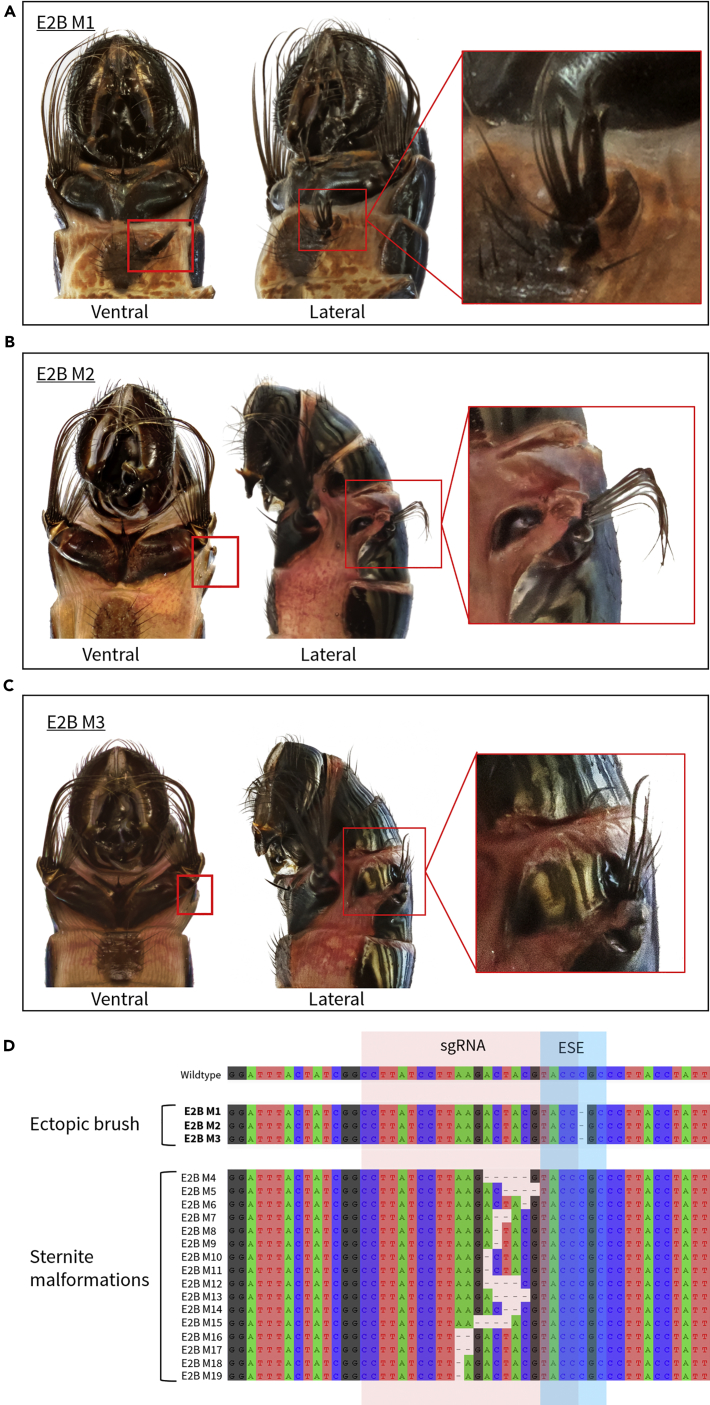
Genome Editing at the Coding Exon Upstream of the Homeodomain Produces Ectopic Structures
Targeting Exon 2, using sgRNA-2B, which is immediately upstream of the homeodomain, produced mosaic mutants with sternite malformations as expected (Figure S1). However, we also recover unexpected mutant males with small ectopic sternite brushes on the third ventral segment (Figure 4A) and the fourth dorsal segment (Figures 4B and 4C). In mutant E2B M1, we observed a small cuticular protrusion with a tuft of bristles on the top right margin of the third ventral sternite (Figure 4A). A similar ectopic cuticular protrusion with bristles was also observed in E2B M2 and E2B M3, wherein the protrusion disrupts the right margin of the fourth tergite (Figures 4B and 4C). To rule out the possibility that the ectopic phenotypes were a result of off-target gene editing, we screened for potential off-target effects (see Methods and Table S2 for additional details) and found no good matches (>65% identity).
Figure 4.
Ectopic Sternite Brush Phenotypes Obtained with Genome Editing of Exon 2
(A–D) (A) Ectopic mutant with ectopic brush on third ventral abdominal segment (E2BM1). (B and C) Ectopic mutants with ectopic brushes on the fourth dorsal abdominal segment (E2BM2 and E2BM3). (D) Sequences confirm that mutations underlying the ectopic brush phenotypes (E2BM1, E2BM2, E2BM3) lie within a predicted exonic splicing enhancer (ESE), whereas mutations underlying sternite malformation phenotypes (see Table S1) lie outside of the predicted ESE. The alignment is reverse complemented to display the putative ESE site sequence as predicted by ESEfinder 3.0 (Cartegni et al., 2003).
Such exon-specific gain and loss phenotypes have similarly been observed in Lepidoptera wherein deletions in the Dll homeodomain result in losses of eyespots in Bicyclus anynana (Connahs et al., 2017), whereas deletions in the region upstream of the homeodomain can produce ectopic eyespots in B. anynana (Connahs et al., 2017) and Vanessa cardui (Zhang and Reed, 2016) as well as enlarged eyespots in Junonia coenia (Zhang and Reed, 2016). Comparative sequence analysis between Lepidoptera and Diptera reveals that this region of Dll is almost as highly conserved as the Dll functional homeodomain. Further investigation shows that it is conserved even across Holometabola (Figure S2).
To understand the genetic mechanism underlying the development of the ectopic brush phenotype, we sequenced all mosaic mutants using NGS. We find that a single point mutation underlies all ectopic mutant phenotypes (Figure 4D). A bioinformatics analysis of Exon 2 using ESEfinder 3.0 (Cartegni et al., 2003) revealed that this single point mutation lies within a predicted exonic splicing enhancer (ESE) site for the SR protein, splicing factor 2 (SF2) (Table S1). SF2 is an RNA-binding, sequence-specific splice factor that binds to ESE sites to promote inclusion of the exon during alternative splicing at the pre-mRNA level. In contrast, all other mutant phenotypes observed were due to mutations that lie outside of this predicted ESE site (Figure 4D). Previous studies of naturally occurring and artificially induced mutations in ESEs have shown that such mutations can lead to exon skipping (Hong-Xiang et al., 2001, Wang et al., 2002). This naturally occurring form of cellular RNA splicing occurs when defective portions of exons are “skipped” over to restore the reading frame. This produces an altered but sometimes still functional protein, which is in some instances over-expressed (Chang et al., 2007). It has also been recently shown in adenocarcinoma cells that CRISPR/Cas9-induced mutations lead to exon skipping (Mou et al., 2017).
Genome Editing at Exon 2 (sgRNA-2b) Results in Exon Skipping and the Production of an Altered Dll Protein with an Intact Homeodomain
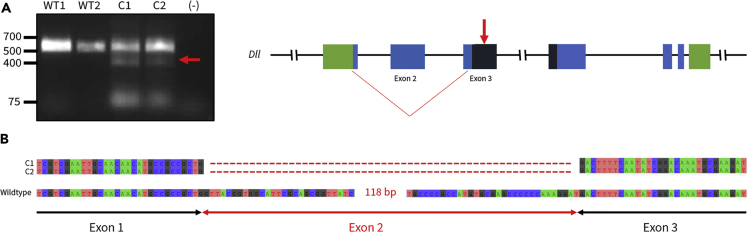
We hypothesized that the ectopic phenotypes in sepsids may be gain-of-function mutants that resulted from an altered Dll protein that retains partial or full function. To test this, we investigated whether mutations in Exon 2 (sgRNA-2B) generate exon-skipped Dll transcripts in embryos. We injected two replicates of 160 embryos with Cas9 protein, and sgRNA-2B. RNA was extracted from the two mutant replicates (C1 and C2) as well as two wild-type replicates. RT-PCR was performed on 12-hr-old mutant and wild-type embryos. Primers were anchored in Exon 1 and Exon 3 of Dll (see Table S4 for primers). We found an alternatively spliced transcript lacking Exon 2 in both mutant embryo replicates, but not in the wild-type replicates (Figure 5).
Figure 5.
CRISPR/Cas9-Induced Deletions in Exonic Splicing Enhancer (ESE) of Exon 2 (sgRNA-2B) Generate Exon-Skipped Dll Transcripts
(A) Primers flanking sgRNA-2B were used to amplify cDNA obtained from the two mosaic mutant embryo replicates (C1 and C2) and wild-type embryos. C1 and C2 mosaic mutants produced a mixture of a short PCR product (∼500 bp) and the wild-type PCR product (726 bp).
(B) Sanger sequencing of the shorter PCR product obtained from C1 and C2 confirms that Exon 2 has been excluded; an altered Dll transcript is present in both replicates of Exon 2 mutant embryos. Sanger sequencing of the longer product yields a wild-type Dll sequence.
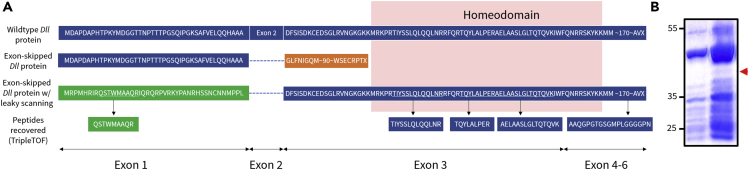
Sanger sequencing of the shorter PCR product obtained from C1 and C2 confirms that Exon 2 was skipped. Based on sequence predictions, we find that with leaky scanning this exon-skipped transcript could produce an altered Dll protein with an intact homeodomain (Figure 6A). If translation of the exon-skipped transcript is initiated at the original start codon, the downstream homeodomain is disrupted, yielding a non-functional protein. However, examination of the Dll translation initiation site identified a putative alternative start site 1 bp immediately downstream of the Kozak sequence. Translation initiation at this alternative start codon would produce an exon-skipped protein with a functional homeodomain (Figure 6A). To show the feasibility of this mechanism, we performed an in vitro protein synthesis assay using a plasmid containing the Dll-coding sequence lacking Exon 2 (see Methods for details). We expressed a protein (>30 kDa) in vitro using the PURExpress kit (New England Biolabs) (Figure 6B). An analysis with the TripleTOF 5600 detected the presence of the bioinformatically predicted protein that is obtained only if the alternative initiation codon is utilized. The TripleTOF obtained peptides that correspond to Exon 1 of the predicted leaky-scanned protein, the intact Dll homeodomain, as well as Exon 5 (Figures 6A and S3).
Figure 6.
Protein Predictions
(A) The exon-skipped Dll transcript generates an altered protein with a disrupted homeodomain. However, with leaky scanning, the exon-skipped Dll transcript can generate an altered protein that recapitulates the wild-type protein sequence from Exon 3 onward with an intact homeodomain. Analysis with a TripleTOF 5600 recovered peptides that matched to the predicted “Dll protein under leaky scanning.”
(B) The in vitro-synthesized truncated Dll protein and control reaction were separated on a 10% SDS-polyacrylamide gel and visualized with Coomassie blue staining. The exon-skipped Dll transcript was translated into a >30-kDa protein product.
These experiments suggest that exon skipping occurs and that the ectopic sternite brush phenotypes could arise from a change in expression of an altered Dll transcript with full or partial function. However, Dll expression was previously observed only in the developing fourth sternite brush during the late pupal stage (48 hr after puparium formation) (Bowsher and Nijhout, 2009), which could not explain the presence of ectopic brushes on the third abdominal segment as observed in mutant E2B M1. To discern whether the underlying predicted change in expression levels was a spatial gain of expression or a misregulation of an already expressed gene, we investigated Dll expression in the developing histoblast clusters in the late larval stage in T. biloba and D. melanogaster. RT-PCR was performed on dissected epidermal larval segments; the epidermis includes the histoblast clusters that eventually develop into the adult sternites and tergites. We found that Dll expression in D. melanogaster was detected in all abdominal segments as well as in the thoracic segment (Figure S5). To rule out any artifacts from imprecise dissections, another gene, Abdominal-B (Abd-B), was tested. As expected in D. melanogaster, Abd-B was only detected in the fifth to eighth abdominal segments. Similar to D. melanogaster, in T. biloba the RT-PCR results indicate that Dll is present in all abdominal segments in the larval stages (Figure S4 and Table S5).
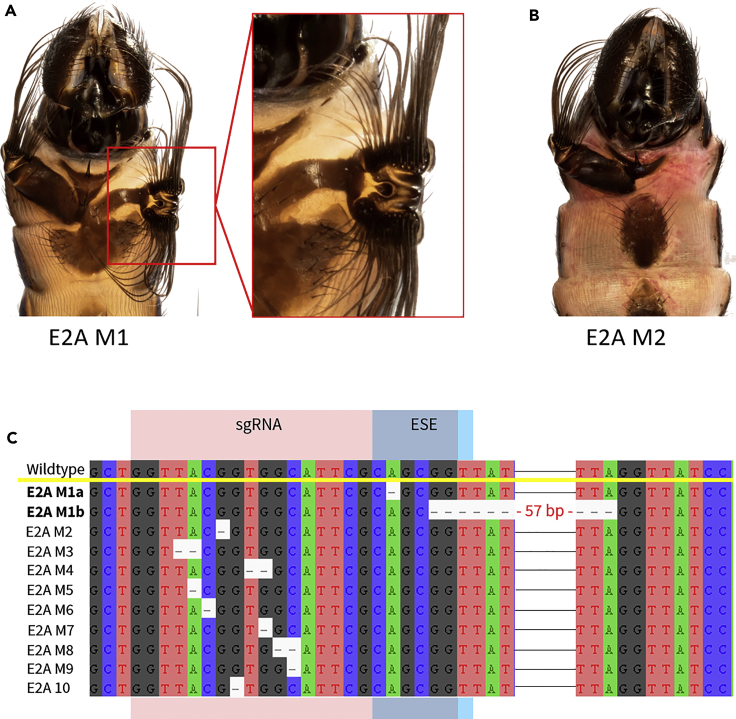
Genome Editing of Another Putative ESE Site (sgRNA-2A) within Exon 2 Again Produces Both Gain and Loss Phenotypes
To test the hypothesis that specific mutations within ESE sites produce mutants with ectopic phenotypes, we designed a guide targeting another predicted ESE site within Exon 2 (sgRNA-2A, Table S1) and found comparable results. We observed nine mutants with sternite malformations with one mutant exhibiting a complete loss of one-half of the fourth sternite appendage (E2A M2). Interestingly, we also observed one mutant male (E2A M1) with an ectopic fourth sternite brush: on the right half of the fourth sternite appendage, the third sternite is expanded, whereas the fourth sternite is misshapen and ends in two bristled lobes instead of the expected one (Figure 7A). Upon characterization of all the mutants using NGS, we observed that only the mutations underlying the ectopic brush mutant disrupted the targeted ESE site. The most abundant mutant haplotype was a single point deletion within the ESE site, whereas the second most abundant mutant haplotype was a 57-bp deletion that disrupts the targeted ESE site as well as an additional downstream putative ESE site. As predicted, the mutations underlying the loss and other sternite malformations lay outside of the ESE site. These results lend further support to the hypothesis that specific CRISPR/Cas9-induced mutations that disrupt ESE sites have the potential to yield ectopic phenotypes, either through an over-expression or a prolonged expression of an altered but functional Dll protein.
Figure 7.
Mutant Phenotypes Obtained with Genome Editing of Second Putative ESE Site in Exon 2 (sgRNA-2A)
(A–C) (A) Mutant with ectopic brush on fourth ventral abdominal segment (E2A M1). (B) Mutant with loss of half of the fourth sternite brush (E2A M2). (C) Sequences confirm that mutations underlying the ectopic brush phenotype (E2A M1) lie within a predicted exonic splicing enhancer (ESE), whereas mutations underlying sternite malformation phenotypes lie outside of the predicted ESE. The two most abundant mutant haplotypes for E2A M1 are shown in this alignment (E2A M1a is a single-point deletion, whereas E2A M1b is a 57-bp deletion that disrupts this target ESE site as well as another downstream ESE site).
To discern if this exon-skipped transcript was exclusively the result of targeted genome editing at Exon 2 (sgRNA-2A), we performed targeted long-read isoform sequencing on Pacbio Sequel to qualitatively characterize the alternative splice forms of Dll present in both exon-skipped mutants and wild-type individuals. Using a guide targeting Exon 2, we injected 405 embryos and screened the surviving third instar larvae. RNA was extracted from 12 injected and 2 wild-type larvae and used to synthesize cDNA. Using an in vitro cleavage assay, 2 of the 12 larvae were identified as exon-skipped mutants (M4 and M6). We then designed Dll-specific tagged primers anchored in Exon 1 and Exon 7 (see Table S4 for tagged primer sequences) to amplify Dll splice forms, which were then sent in for Pacbio Sequel targeted isoform circular consensus sequencing. This generates a consensus sequence from multiple passes of a single circular molecule. The reads were analyzed using the Pacbio SMRT Link 5.1.0 analysis software (see Methods for parameters).
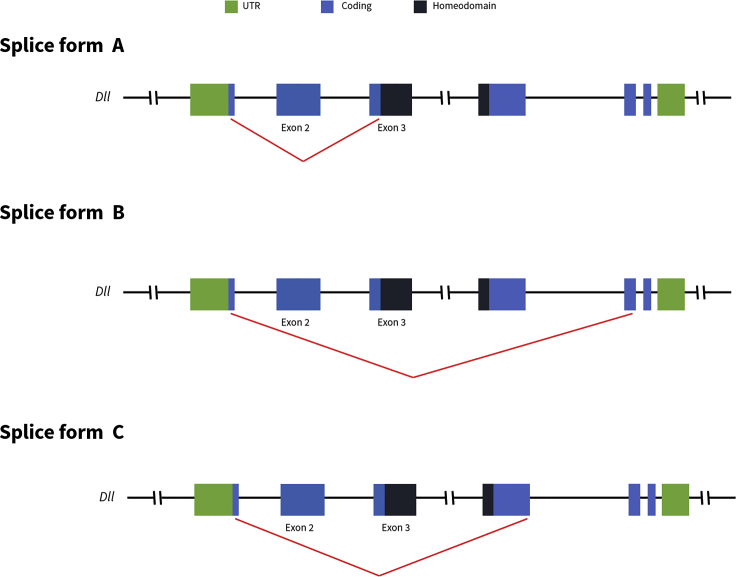
From the sequencing data, we identified three splice forms alongside the wild-type splice form (Figure 8). Splice forms B and C were detected in M6 and both wild-type larvae. However, splice form A, which excluded Exon 2, was only detected in the exon-skipped mutants, M4 and M6. These results suggest that the exon-skipped transcript (splice form A) does not naturally occur and is instead produced only when Exon 2 (sgRNA-2A) is disrupted with CRISPR/Cas9.
Figure 8.
Alternative Splice Forms Identified from Pacbio Isoform Sequencing of Two Exon-Skipped Mutant Individuals (M4 and M6) and Two Wild-Type Individuals
Splice form A excludes Exon 2 and is found only in the two mutant individuals, M4 and M6. Splice form B, which excludes Exon 2 to Exon 4, is found in both wild-type individuals and M6. Splice form C, which excludes Exon 2, Exon 3, and part of Exon 4 is found in M6 and the two wild-type individuals.
To test if changed expression levels of Dll can be detected in ectopic mutants, we carried out a quantitative PCR assay on injected mosaic mutant larvae despite the high risk of such an experiment yielding a false-negative result because (1) mosaic mutant larvae have a mixture of wild-type, mutant, and exon-skipped transcripts; (2) only mosaic mutants with a high proportion of wild-type cells are predicted to be viable; and (3) the natural expression levels of Dll at this stage are low. The results of the qPCR assay were inconclusive (see Methods for details).
Discussion
The overall body plan of winged insects has remained remarkably conserved with regard to the position of appendages, which are restricted to the head, thorax, and posteriormost segments of the 11-segmented abdomen (genitalia). Occasional exceptions to this body plan are losses or reductions of structures, such as the reduction of maxillary and mandibular structures in D. melanogaster (Angelini and Kaufman, 2006). However, gains of articulated appendages, especially in the pregenital abdominal segments, are rare. Sepsids are one of the few exceptions (Hoch et al., 2014), having very recently evolved a novel abdominal appendage. This makes sepsids an attractive model system for understanding how a gene regulatory network is assembled for the development of novel appendages.
Morphological novelties are often hypothesized to arise from either existing or de novo genetic machinery (True and Carroll, 2002, Wagner and Lynch, 2010). Genes can either be co-opted along with their existing network or be assembled differently into a de novo network. In addition, novel phenotypes may also arise from the evolution of de novo or orphan genes. Recent studies have shown that the genetic architecture underlying morphological novelties in insects is more complex than expected; in some instances, a combination of both de novo genes and the co-option of an existing gene regulatory network underly a novel phenotype (Santos et al., 2017, Hilgers et al., 2018), whereas in others, existing gene regulatory networks are modified and/or partially co-opted (Hu et al., 2018, Moczek, 2009, Moczek and Rose, 2009, Glassford et al., 2015). We illustrate in this study another instance whereby an important gene in the appendage-patterning gene regulatory network, Dll, is co-opted in the development of a novel morphological structure: the fourth sternite brush.
Here, we show conclusively that Dll is necessary for the development of a novel abdominal appendage in T. biloba; disruptions in the homeodomain and in the coding region upstream lead to losses of the fourth sternite brush. We also observed malformed monomorphic sternites in both males and females, raising the question of how Dll fits into a gene regulatory network that patterns a sexually dimorphic trait. To obtain a better understanding of the underlying gene architecture responsible for building this morphological novelty, functional characterization of more appendage and sex-patterning genes (e.g., doublesex) would have to be carried out.
Moreover, we also reveal that specific deletions in Exon 2 can lead not only to the expected losses but also to unexpected gains of the novel fourth sternite brush. Through a detailed dissection of the upstream coding exon, we show that deletions in ESE sites can generate the ectopic phenotypes observed and go further to prove that the ensuing exon-skipped transcripts can be translated in vitro into an altered Dll protein that retains an intact homeodomain. These findings highlight how small modifications of Dll have the potential to generate very different phenotypes. This would be a single observation in a fly species if it were not for the fact that a similarly diverse set of phenotypes can be generated by mutations in the same coding region in butterflies, which diverged from flies nearly 300 million years ago (Misof et al., 2014). In both sepsid flies and several species of butterflies (V cardui and B. anynana), disruption of the region upstream of the Dll homeodomain produces ectopic structures (Zhang and Reed, 2016, Connahs et al., 2017) and exon-skipped Dll transcripts (Connahs et al., 2017). Moreover, a comparative analysis across Holometabola reveals that the region of Dll immediately upstream of the homeodomain is highly conserved. The conserved nature of this protein region as well as the appearance of ectopic traits in two divergent lineages suggests that mutations at this conserved region may have had the potential to generate morphological variation for at least 300 million years. Although we find in T. biloba that the exon-skipped transcript does not occur naturally, further investigation into B. anynana and other holometabolan lineages might provide more insight into the possible role of this conserved region in the evolution and development of structural novelties.
Based on our results, we propose that Dll genome editing studies should target multiple exons and include the exon upstream of the homeodomain. Such screening may yield particularly interesting results for those species that have novel traits whose development involves Dll. Our study suggests that this gene may not only be important for the origin of novel traits but also has the potential for generating morphological diversity via splicing regulation. These findings also have implications beyond Dll as CRISPR/Cas9 is now extensively adopted for genome editing purposes from single cells to whole organisms. Future CRISPR/Cas9 studies should target multiple exons, particularly functional domains, to identify phenotypes generated by exon skipping. Bioinformatics tools should be also used to predict ESE sites in potential target regions, which should either be avoided to reduce the chances of generating conflicting results or targeted with CRISPR/Cas9 to test whether gain-of-function mutations can be produced.
Limitations of Study
Our study suggests that deletions in ESE sites result in exon skipping and the development of ectopic structures. However, the process by which exon-skipped transcripts produce the ectopic structures remains unclear because quantifying gene expression of mutated cells within a mosaic mutant is difficult, i.e., we were not able to isolate the signal from mutant cells alone. We predict that the exon-skipped transcript is functional because the homeodomain is intact. However, in vivo tests for protein folding and functionality would be desirable.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by a Lee Kuan Yew Postdoctoral Fellowship grant (R-154-000-646-112) and an MOE AcRF Tier 2 grant (R-154-000-A62-112). The authors would like to thank Amrita Srivathsan and Ang Yuchen for their assistance with bioinformatics and imaging, respectively. We also thank Zhang Yizhong and Lee Zheng Fen for their assistance with microinjections. We extend our gratitude to Mindy Tuan for providing us with the mating behavior of Themira superba, Dacotah Melicher for providing us with Dll scaffolds, Fong Siao Wan for her assistance with the RNA extractions and RT-PCR experiments, Antonia Monteiro and Heidi Connahs for the stimulating discourse, and Sunita Subramaniam, Lim Teck Kwang, Anjali Gupta, and Thorsten Wohland for assistance with the protein experiments. We would also like to thank Liou Yih-Cherng for feedback on the protein experiments. For help with our qPCR experiment, we would like to thank Hiong Kum Chew and Ip Yuen Kwong. We also thank Julia H. Bowsher for her assistance with the design of the RT-PCR experiments and her insightful feedback on the manuscript.
Author Contributions
K.F.Y.S. and G.R. planned the study, performed most of the experiments, and wrote the paper with feedback from the other authors. K.F.Y.S., G.R., and A.S. performed the microinjection experiments, and G.R. conducted the imaging, characterization, and analysis of CRISPR/Cas9 mutants. K.F.Y.S. designed the guides for CRISPR experiments and performed the in vitro protein synthesis assay, reverse-transcriptase PCR, qPCR experiments, and alignments of Dll across Holometabola. G.R. performed the SDS protein gels and the ProteinPilot analysis. K.F.Y.S. designed the Dll isoform experiment, and K.F.Y.S. and G.R. processed and analyzed the results. R.M. supervised and supported the research; all authors discussed the results and provided comments for the manuscript.
Declaration of Interests
The authors declare no competing financial interests.
Published: December 21, 2018
Footnotes
Supplemental Information includes Transparent Methods, five figures, and six tables and can be found with this article online at https://doi.org/10.1016/j.isci.2018.11.036.
Contributor Information
Rudolf Meier, Email: meier@nus.edu.sg.
Kathy F.Y. Su, Email: kathysufy@gmail.com.
Data and Software Availability
Most of the processed sequencing data files are available on a Mendeley database https://doi.org/10.17632/ps3p7jnb5t.1.
Supplemental Information
The three most dominant mutant haplotypes per individual are recorded here. The dominant haplotype (in bold) per individual is used for downstream analyses.
References
- Ang Y. National University of Singapore; 2013. Modern Morphological Techniques and the Evolutionary Biology and Taxonomy of Sepsidae (Diptera) PhD thesis. [Google Scholar]
- Angelini D.R., Kaufman T.C. Insect appendages and comparative ontogenetics. Dev. Biol. 2006;286:57–77. doi: 10.1016/j.ydbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Arnoult L., Su K.F.Y., Manoel D., Minervino C., Magriña J., Gompel N., Prud’homme B. Emergence and diversification of fly pigmentation through evolution of a gene regulatory module. Science. 2013;339:1423–1426. doi: 10.1126/science.1233749. [DOI] [PubMed] [Google Scholar]
- Bowsher J.H., Ang Y., Ferderer T., Meier R. Deciphering the evolutionary history and developmental mechanisms of a complex sexual ornament: the abdominal appendages of Sepsidae (Diptera) Evolution. 2013;67:1069–1080. doi: 10.1111/evo.12006. [DOI] [PubMed] [Google Scholar]
- Bowsher J.H., Nijhout H.F. Evolution of novel abdominal appendages in a sepsid fly from histoblasts, not imaginal discs. Evol. Dev. 2007;9:347–354. doi: 10.1111/j.1525-142X.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Bowsher J.H., Nijhout H.F. Partial co-option of the appendage patterning pathway in the development of abdominal appendages in the sepsid fly Themira biloba. Dev. Genes Evol. 2009;219:577–587. doi: 10.1007/s00427-010-0319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartegni L., Wang J., Zhu Z., Zhang M.Q., Krainer A.R. ESEfinder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31:3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y.-F., Chan W.-K., Imam J.S., Wilkinson M.F. Alternatively spliced T-cell receptor transcripts are up-regulated in response to disruption of either splicing elements or reading frame. J. Biol. Chem. 2007;282:29738–29747. doi: 10.1074/jbc.M704372200. [DOI] [PubMed] [Google Scholar]
- Cohen S.M., Jürgens G. Proximal—distal pattern formation in Drosophila: cell autonomous requirement for Distal-less gene activity in limb development. EMBO J. 1989;8:2045–2055. doi: 10.1002/j.1460-2075.1989.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connahs H., Tlili S., van Creij J., Loo T.Y.J., Banerjee T., Saunders T.E., Monteiro A. Disrupting different Distal-less exons leads to ectopic and missing eyespots accurately modeled by reaction-diffusion mechanisms. bioRxiv. 2017 [Google Scholar]
- Glassford W.J., Johnson W.C., Dall N.R., Smith S.J., Liu Y., Boll W., Noll M., Rebeiz M. Co-option of an ancestral hox-regulated network underlies a recently evolved morphological novelty. Dev. Cell. 2015;34:520–531. doi: 10.1016/j.devcel.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers L., Hartmann S., Hofreiter M., von Rintelen T. Novel genes, ancient genes, and gene co-option contributed to the genetic basis of the radula, a molluscan innovation. Mol. Biol. Evol. 2018;35:1638–1652. doi: 10.1093/molbev/msy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch H., Wessel A., Asche M., Baum D., Beckmann F., Bräunig P., Ehrig K., Mühlethaler R., Riesemeier H., Staude A. Non-sexual abdominal appendages in adult insects challenge a 300 million year old bauplan. Curr. Biol. 2014;24:R16–R17. doi: 10.1016/j.cub.2013.11.040. [DOI] [PubMed] [Google Scholar]
- Hong-Xiang L., Cartegni L., Zhang M.Q., Krainer A.R. A mechanism for exon skipping caused by nonsense or missense mutations in BRCA1 and other genes. Nat. Genet. 2001;27:55–58. doi: 10.1038/83762. [DOI] [PubMed] [Google Scholar]
- Hu Y., Schmitt-Engel C., Schwirz J., Stroehlein N., Richter T., Majumdar U., Bucher G. A morphological novelty evolved by co-option of a reduced gene regulatory network and gene recruitment in a beetle. Proc. Biol. Sci. 2018;285 doi: 10.1098/rspb.2018.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khila A., Abouheif E., Rowe L. Function, developmental genetics, and fitness consequences of a sexually antagonistic trait. Science. 2012;336:585–589. doi: 10.1126/science.1217258. [DOI] [PubMed] [Google Scholar]
- Melicher D., Torson A.S., Dworkin I., Bowsher J.H. A pipeline for the de novo assembly of the Themira biloba (Sepsidae: Diptera) transcriptome using a multiple k-mer length approach. BMC Genomics. 2014;15:188. doi: 10.1186/1471-2164-15-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misof B., Liu S., Meusemann K., Peters R.S., Donath A., Mayer C., Frandsen P.B., Ware J., Flouri T., Beutel R.G. Phylogenomics resolves the timing and pattern of insect evolution. Science. 2014;346:763–767. doi: 10.1126/science.1257570. [DOI] [PubMed] [Google Scholar]
- Moczek A.P. On the origins of novelty and diversity in development and evolution: a case study on beetle horns. Cold Spring Harb. Symp. Quant. Biol. 2009;74:289–296. doi: 10.1101/sqb.2009.74.010. [DOI] [PubMed] [Google Scholar]
- Moczek A.P., Rose D.J. Differential recruitment of limb patterning genes during development and diversification of beetle horns. Proc. Natl. Acad. Sci. U S A. 2009;106:8992–8997. doi: 10.1073/pnas.0809668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H., Smith J.L., Peng L., Yin H., Moore J., Zhang X.-O., Song C.-Q., Sheel A., Wu Q., Ozata D.M. CRISPR/Cas9-mediated genome editing induces exon skipping by alternative splicing or exon deletion. Genome Biol. 2017;18:108. doi: 10.1186/s13059-017-1237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M.E., Le Bouquin A., Crumière A.J.J., Khila A. Taxon-restricted genes at the origin of a novel trait allowing access to a new environment. Science. 2017;358:386–390. doi: 10.1126/science.aan2748. [DOI] [PubMed] [Google Scholar]
- Toga K., Hojo M., Miura T., Maekawa K. Expression and function of a limb-patterning gene Distal-less in the soldier-specific morphogenesis in the nasute termite Nasutitermes takasagoensis. Evol. Dev. 2012;14:286–295. doi: 10.1111/j.1525-142X.2012.00545.x. [DOI] [PubMed] [Google Scholar]
- True J.R., Carroll S.B. Gene co-option in physiological and morphological evolution. Annu. Rev. Cell Dev. Biol. 2002;18:53–80. doi: 10.1146/annurev.cellbio.18.020402.140619. [DOI] [PubMed] [Google Scholar]
- Wagner G.P., Lynch V.J. Evolutionary novelties. Curr. Biol. 2010;20:R48–R52. doi: 10.1016/j.cub.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Wang J., Hamilton J.I., Carter M.S., Li S., Wilkinson M.F. Alternatively spliced TCR mRNA induced by disruption of reading frame. Science. 2002;297:108–110. doi: 10.1126/science.1069757. [DOI] [PubMed] [Google Scholar]
- Zhang L., Reed R.D. Genome editing in butterflies reveals that spalt promotes and Distal-less represses eyespot colour patterns. Nat. Commun. 2016;7:11769. doi: 10.1038/ncomms11769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The three most dominant mutant haplotypes per individual are recorded here. The dominant haplotype (in bold) per individual is used for downstream analyses.