Abstract
Purpose:
β-Blockers (BBs) have been associated with a reduced cardiorespiratory fitness (CRF). This is possibly caused by inhibition of β2-receptors in the airways. However, there are limited data available on β-receptor selectivity and CRF. We therefore aimed to assess the association between BB use and CRF and to assess the association between β-receptor selectivity and CRF.
Methods:
Participants in the Maastricht Study were aged between 40 and 75 years. Exposure to BB use was determined by use of pharmacy records. General linear models were used to obtain adjusted means of 2 proxies for CRF: covered distance during the 6-minute walk test (6MWT) and estimated maximum power output adjusted for body mass (Wmax kg−1) during the submaximal cycle ergometer test. Adjusted means were compared between current, past, and never BB users. Current users were subsequently stratified by β-receptor selectivity and dose.
Results:
Compared to never use, current use was associated with a lower CRF, based on the 6MWT (current use: 569.7 m; never use: 580.4 m [P = .010]), but not based on the cycling test (current use: 2.14 W kg−1; never use: 2.13 W kg−1 [P = .690]). There was no difference between current selective and current nonselective BB use.
Conclusion:
β-Blockers use was associated with CRF based on the 6MWT but not the cycling test. There was no difference between current selective and nonselective BB users, possibly due to the small number of nonselective BB users, differential underlying diseases, other pharmacological properties, and limitations related to the proxies of the outcome.
Keywords: β-blockers, cardiorespiratory fitness, work load
Introduction
For decades, β-adrenoceptor antagonists or β-blockers (BBs) have been used to treat a wide variety of cardiovascular diseases. The main pharmacological effect of BBs with respect to cardiovascular diseases is blocking cardiac and vascular β-receptors. Blockade of β1-receptors results in reduced chronotropic and inotropic effects. However, pulmonary side effects may occur by unintentional antagonism of the β2-receptors in the airways.1 In order to minimize these side effects, cardioselective or β1-selective BBs such as metoprolol were developed. Previous studies have shown that the affinity for the β1-receptorcompared to the β2-receptor differs between BBs.2–5 Therefore, pulmonary side effects are expected to occur less with highly β1-selective BBs compared to less selective BBs. Furthermore, β1-selectivity is known to decrease at higher doses.6 Consequently, side effects are more likely to occur at higher doses.
The ability of the circulatory and respiratory systems to supply oxygen to skeletal muscles during sustained physical activity is often referred to as cardiorespiratory fitness (CRF). Previously, the effect of BB selectivity on CRF has been assessed in small crossover trials.7–10 During a submaximal endurance test in healthy volunteers, the use of nonselective BBs resulted in a larger reduction in CRF compared to β1-selective BBs.7 Furthermore, the use of bisoprolol tended to reduce CRF less than atenolol.8 This is possibly due to the fact that atenolol has a lower selectivity for the β1-receptor compared to bisoprolol.5 Additionally, the use of nebivolol, a highly β1-selective BB, was associated with no effect on CRF, whereas this was the case with the use of atenolol, a less β1-selective BB.9
The most prominent mechanism proposed to explain the difference between selective and nonselective BBs on CRF is related to the fact that β2-receptors are predominantly present in respiratory tract tissues such as the alveoli epithelium, where they influence gas exchange efficacy.11 Consequently, bisoprolol, a β1-selective BB, has been found to affect carbon monoxide diffusing capacity and respiratory function less when compared to carvedilol.12 By reducing respiratory function, nonselective BBs may limit CRF more than β1-selective BBs. Furthermore, other factors, such as underlying morbidities, pulmonary function, drug use, and physical activity, may also be involved when assessing the association between BB use and CRF.
To our knowledge, the effect of β1-versus β2 affinity on CRF has only been assessed by comparing 2 or 3 separate BBs in small controlled study populations.7–10 However, in order to evaluate whether these results can be generalized to BB use in clinical practice, a wider range of BBs should be compared in a real-life setting. Furthermore, the effect of the loss of selectivity of β1-selective BBs on CRF at higher doses is largely unknown. Therefore, the aim of this study was to assess the association between BB use and CRF in a large population-based observational study and to assess the association between BB selectivity and CRF. Additionally, we aimed to assess the association between current selective BB use, stratified by dose, and CRF. More elaborate information on these topics may provide support for prescribers to decide which type of BB, and in what dose, is preferably used by patients with a limited CRF a priori.
Methods
Data Source
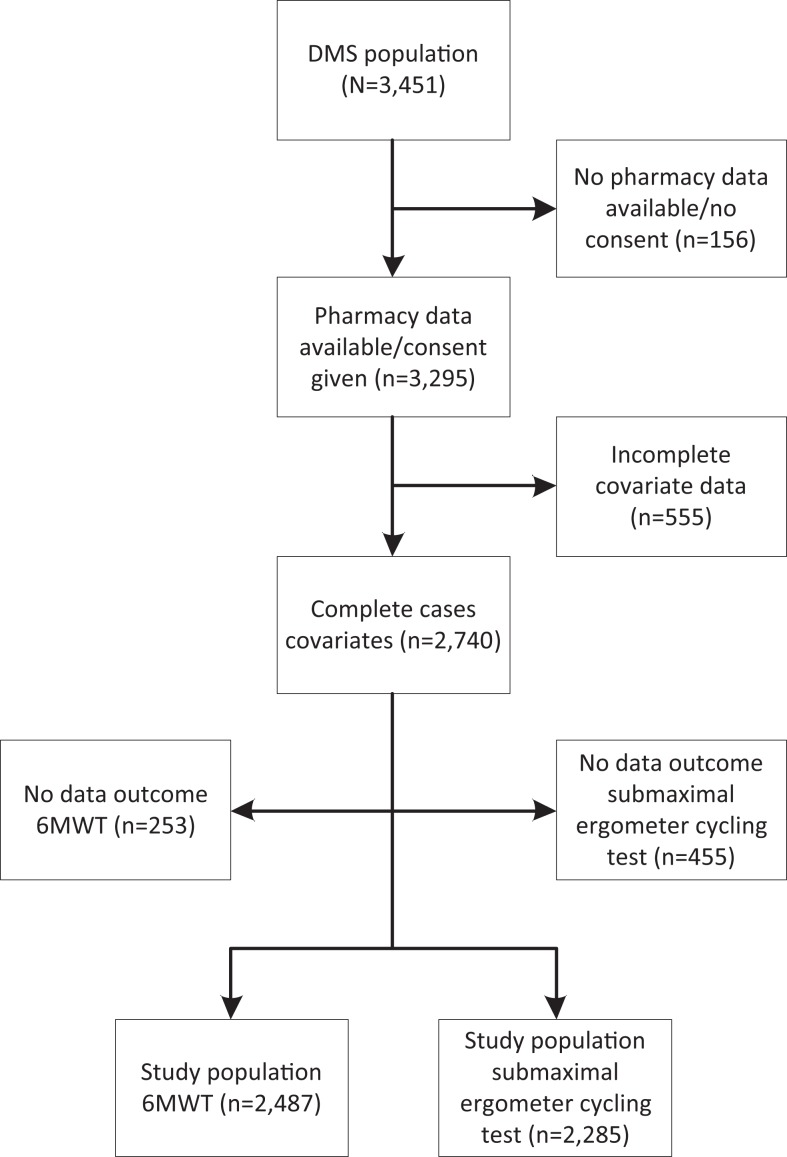
The Maastricht Study is an observational prospective population-based cohort study. The study focuses on the etiology, pathophysiology, complications, and comorbidities of type 2 diabetes mellitus (T2DM) and is characterized by an extensive phenotyping approach. Eligibility for participation were all individuals aged between 40 and 75 years and living in the southern part of the Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known T2DM status, with an oversampling of individuals with T2DM, for reasons of efficiency. The present report includes cross-sectional data from the first 3451 participants who completed the baseline survey between November 2010 and September 2013. The examinations of each participant were performed within a time window of 3 months. The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (Permit 131088-105234-PG).13 Drug use data were available from electronic dispensing records obtained from community pharmacies. Since these data are also used by the pharmacists to get reimbursed by the health insurers, it is expected to be highly accurate. If no consent was given for collection of pharmacy data, then those participants were excluded. Furthermore, we performed complete case analyses. Therefore, in case of missing data for any of the variables, participants were excluded (remaining: n = 2740; Figure 1).
Figure 1.
Flowchart exclusion criteria study populations complete cases.
Abbreviation: DMS=The Maastricht Study.
Exposure
At the date of outcome assessment (index date), current BB use was determined by an active prescription of a BB according to pharmacy data (ie, if the most recent prescription prior to index date was expected to end after the index date). Past BB use was defined as a prescription of a BB prior to the index date that was discontinued before index date. Additionally, for each current user, the prescribed daily dose was determined by combining dose (eg, 150 mg) and frequency (eg, once daily) data. If the frequency was unknown, the median frequency of all users of the specific BB was imputed. Next, for each current BB user the defined daily dose (DDD) and metoprolol equivalents were calculated (eg, 1 DDD = 150 mg metoprolol equivalent). Selective BBs included nebivolol, bisoprolol, atenolol, and metoprolol. Nonselective BBs included pindolol, carvedilol, propranolol, and sotalol. Preparations in which these drugs were combined with other substances were included. Anatomical Therapeutic Chemical (ATC) coding was used for classification (Supplemental Table 1).
Outcomes
Cardiorespiratory fitness was determined by use of 2 measurements: covered distance in meters during a fast-paced 6-minute walk test (6MWT)14 and estimated maximum power output adjusted for body mass (Wmax kg−1) during a submaximal cycle ergometer test.15
Six-minute walk test
Participants with a myocardial infarction, heart surgery, or angioplasty in the 3 months prior to examination were ineligible to start the 6MWT. Participants with recent symptoms of angina pectoris, a high (systolic or diastolic) blood pressure, or tachycardia could not take part either (remaining: n = 2487; Figure 1). Participants were instructed to walk as many 40-m laps as possible in 6 minutes at a fast pace without running. After 6 minutes or when the participant was unwilling or unable to continue, the covered distance was measured.
Submaximal cycle ergometer test
For safety reasons, participants with a myocardial infarction, cerebrovascular event, pneumonia, angina pectoris, or a venous thromboembolic event in the 3 months prior to examination or a history of other cardiovascular comorbidities were ineligible to start the test (remaining: n = 2285; Figure 1). Details regarding the submaximal cycle ergometer test are described in Supplemental Text 1. In short, the estimated maximum power output (Wmax) was used as an objective measure of CRF.16–18 Wmax was calculated by extrapolation of power output achieved at submaximal levels of heart rate (HR; ≥75% of age predicted maximum HR) or submaximal rate of perceived exertion (RPE ≥ 15) to maximum levels (100% HR and RPE = 20, respectively). Rate of perceived exertion was measured using the 15-point Borg scale, an interval scale ranging from 6 (“no exertion at all”) up to 20 (“maximal exertion”).19,20 In order to limit the effect of additional muscle mass in patients with higher weight, Wmax was subsequently adjusted for body mass (Wmax kg−1).15 Although the submaximal cycling test was completed at ≥85% of age predicted maximum HR or RPE ≥17, participants with incomplete tests were also included if they reached ≥75% of age predicted maximum HR or RPE ≥15.
Covariates
Factors including age, sex, body mass index (BMI), education (low, medium, and high), smoking status (never, former, and current), self-reported moderate-to-vigorous physical activity (MVPA),21 and T2DM status were used as confounders. Use of β-sympathomimetics, calcium channel blockers, diuretics, angiotensin-converting enzyme (ACE) inhibitors, angiotensin-II (AT-II) receptor blocker, statins, benzodiazepines, opioids, or antipsychotics in the 6 months prior index date were also included as confounders.
Statistical Analyses
General linear models (GLMs) were used to obtain and compare adjusted means (or estimated marginal means) of CRF outcomes (GLM procedure, SAS 9.4). Adjusted means of current and past users were compared to never users. Three models were used: model 1 included age and sex and model 2 (lifestyle) included model 1 + BMI, smoking status, education, and MVPA. Model 3 (comorbidities and drug use) included model 2 + T2DM status, and use of β-sympathomimetics, calcium channel blockers, diuretics, ACE-inhibitors, AT-II receptor blockers, statins, benzodiazepines, opioids, or antipsychotics in the 6-months prior to index date. Furthermore, current users were compared to past users, and current selective BB users were compared to current nonselective BB users by means of post hoc pairwise comparisons (t-test). Additionally, adjusted means of CRF were calculated in current selective BB users stratified by metoprolol equivalents. Adjustments were made as above, and significance was set at P < .05 for all analyses.
Since BBs are known to reduce HR, the estimation of CRF from the submaximal exercise test may be biased. Therefore, sensitivity analyses comparing the various exercise test completion criteria were conducted. Three completion criteria were compared: estimated Wmax kg−1 based on (1) both HR and RPE criteria (same as main analysis), (2) the HR criterion only, and (3) the RPE criterion only. These analyses were adjusted according to the above-mentioned model 3.
In an additional analysis, we divided the participants, with data on both outcomes, into tertiles based on the covered distance during the 6MWT and on the achieved output during the cycle ergometer test in order to determine correlation between these outcomes.
Results
Baseline characteristics of never, current (selective and nonselective), and past BB users are depicted in Table 1. Current and past BB users were older compared to never BB users. Compared to never users, the percentage of women was lower in the current BB users. Furthermore, current BB users had a higher BMI, less hours of MVPA, and a lower education when compared to never users. Current nonselective BB users were more often current smokers. Current and past BB users used more drugs compared to never users. Participants who were excluded due to missing data were 0.6 to 0.9 years older, BMI was 0.6 to 0.7 kg m−1 higher, smoked more often, had a lower education, and were more often diagnosed with T2DM than the study population prior to exclusions (Supplemental Table 2).
Table 1.
Characteristics of Study Population by β-Blocker Exposure Status.
| Characteristic | Never BB use, n = 1919 | Current selective BB use, n = 391 | Current nonselective BB use, n = 29 | Past BB use, n = 401 | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Age, years, mean, SD | 58.6 | 8.2 | 63.1 | 6.9 | 64.7 | 7.6 | 60.9 | 8.0 |
| Sex, (% women) | 994 | 51.8 | 138 | 35.3 | 13 | 44.8 | 203 | 50.6 |
| BMI, kg m−1 mean, SD | 26.4 | 4.3 | 29.4 | 4.5 | 29.6 | 5.4 | 27.5 | 4.7 |
| MVPA,a median, IQR | 5.0 | 5.3 | 3.0 | 4.5 | 3.0 | 3.8 | 4.5 | 5.0 |
| Education (%)b | ||||||||
| Low | 541 | 28.2 | 186 | 47.6 | 14 | 48.3 | 144 | 35.9 |
| Med | 559 | 29.1 | 107 | 27.4 | 8 | 27.6 | 107 | 26.7 |
| High | 819 | 42.7 | 98 | 25.1 | 7 | 24.1 | 150 | 37.4 |
| Smoking status (%) | ||||||||
| Never | 713 | 37.2 | 119 | 30.4 | 10 | 34.5 | 132 | 32.9 |
| Former | 978 | 51.0 | 227 | 58.1 | 14 | 48.3 | 218 | 54.4 |
| Current | 228 | 11.9 | 45 | 11.5 | 5 | 17.2 | 51 | 12.7 |
| T2DMc | 385 | 20.1 | 207 | 52.9 | 15 | 51.7 | 111 | 27.7 |
| History of drug use (6 months prior to date of inclusion, %) | ||||||||
| β-Blockers | 0 | 0.0 | 391 | 100.0 | 29 | 100.0 | 66 | 16.5 |
| Nebivolol | 0 | 0.0 | 17 | 4.3 | 0 | 0.0 | 2 | 0.5 |
| Bisoprolol | 0 | 0.0 | 30 | 7.7 | 0 | 0.0 | 10 | 2.5 |
| Atenolol | 0 | 0.0 | 23 | 5.9 | 0 | 0.0 | 2 | 0.5 |
| Metoprolol | 0 | 0.0 | 325 | 83.1 | 1 | 3.4 | 40 | 10.0 |
| Carvedilol | 0 | 0.0 | 1 | 0.3 | 6 | 20.7 | 2 | 0.5 |
| Propranolol | 0 | 0.0 | 1 | 0.3 | 9 | 31.0 | 9 | 2.2 |
| Sotalol | 0 | 0.0 | 0 | 0.0 | 13 | 44.8 | 2 | 0.5 |
| Pindolol | 0 | 0.0 | 0 | 0.0 | 2 | 6.9 | 0 | 0.0 |
| Calcium channel blockers | 77 | 4.0 | 98 | 25.1 | 3 | 10.3 | 61 | 15.2 |
| Diuretics | 175 | 9.1 | 152 | 38.9 | 10 | 34.5 | 91 | 22.7 |
| AT-II receptor antagonists | 198 | 10.3 | 156 | 39.9 | 10 | 34.5 | 114 | 28.4 |
| ACE-inhibitors | 113 | 5.9 | 99 | 25.3 | 5 | 17.2 | 78 | 19.5 |
| Organic nitrates | 4 | 0.2 | 57 | 14.6 | 1 | 3.4 | 16 | 4.0 |
| Statins | 419 | 21.8 | 294 | 75.2 | 18 | 62.1 | 180 | 44.9 |
| Benzodiazepines | 117 | 6.1 | 49 | 12.5 | 3 | 10.3 | 45 | 11.2 |
| Opioids | 54 | 2.8 | 22 | 5.6 | 1 | 3.4 | 23 | 5.7 |
| Anti-psychotics | 11 | 0.6 | 6 | 1.5 | 0 | 0.0 | 3 | 0.7 |
| Anti-hyperglycemic drugs | 260 | 13.5 | 158 | 40.4 | 11 | 37.9 | 80 | 20.0 |
| Oral | 244 | 12.7 | 149 | 38.1 | 10 | 34.5 | 73 | 18.2 |
| Insulin | 62 | 3.2 | 49 | 12.5 | 3 | 10.3 | 23 | 5.7 |
| Asthma/COPD medication | 122 | 6.4 | 46 | 11.8 | 4 | 13.8 | 41 | 10.2 |
| Inhaled corticosteroidsd | 25 | 1.3 | 6 | 1.5 | 3 | 10.3 | 12 | 3.0 |
| β-sympathomimeticd | 48 | 2.5 | 17 | 4.3 | 1 | 3.4 | 22 | 5.5 |
| Combinationsd | 60 | 3.1 | 25 | 6.4 | 1 | 3.4 | 12 | 3.0 |
| Other asthma/COPD medicationd | 34 | 1.8 | 21 | 5.4 | 1 | 3.4 | 11 | 2.7 |
| Ocular β-blockers | 18 | 0.9 | 1 | 0.3 | 0 | 0.0 | 3 | 0.7 |
Abbreviations: ACE, angiotensin converting enzyme; AT-II, angiotensin-II; BB, β-blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; MVPA, moderate-to-vigorous physical activity; T2DM, type 2 diabetes mellitus; SD, standard deviation.
a Hours per week, according to the modified Community Health Activities Model Program for Seniors (CHAMPS) questionnaire.18 Activities accounted as MVPA were fast walking, fast cycling, heavy gardening, heavy household work, jogging/running, swimming, tennis, team sport, and intensive exercise.
b Low, no education, primary education not completed, primary education, lower vocational education; medium, intermediate vocational education, higher secondary education; high, higher professional education, university education.
c Determined by an oral glucose tolerance test. A fasting plasma glucose level of ≥7.0 mmol/L (126 mg/dL) or a 2-hour plasma glucose level ≥11.1 mmol/L (200 mg/dL) were defined as T2DM according to the World Health Organization guidelines. Others were defined as non-T2DM.
d May not add up to total asthma/COPD medication as patients could use drugs from multiple subclasses.
In the 6MWT, current use of BBs was associated with a significantly lower CRF (adjusted mean distance = 569.7 m) compared to never use (adjusted mean distance = 580.4 m; P = .010) and past use (adjusted mean distance = 582.2 m; P = .010; Table 2). While CRF was also reduced in current users of selective BBs (adjusted mean distance = 570.6 m) compared to never users (P = .022), there was no statistically significant difference between current selective and current nonselective BB users. Among users of selective BBs, CRF did not further decrease with a higher daily dose.
Table 2.
Adjusted Means of Cardiorespiratory Fitness Based on Distance (Meters) Walked During 6MWT Between BB Users Stratified by Exposure Status and Metoprolol Equivalents.a
| Exposure Status | Crude Mean (SD) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| Mean | P Value | Mean | P Value | Mean | P Value | ||
| Never BB use (n = 1775) | 597.6 (77.1) | 595.3 | Ref | 582.3 | Ref | 580.4 | Ref |
| Past BB use (n = 356) | 578.0 (80.7) | 582.6 | .003 | 578.6 | .321 | 582.2 | .642 |
| Current BB use (n = 356) | 542.7 (81.0) | 549.7 | <.001 | 562.2 | <.001 | 569.7 | .010 |
| Current nonselective BB use (n = 26) | 522.0 (79.2) | 537.4 | <.001 | 554.0 | .026 | 557.4 | .067 |
| Current selective BB use (n = 330) | 544.3 (81.1) | 550.5 | <.001 | 562.7 | <.001 | 570.6 | .022 |
| 0.1-75 mg (n = 132)b | 549.7 (83.5) | 557.8 | <.001 | 566.9 | .009 | 573.5 | .257 |
| 75-100 mg (n = 133)b | 541.5 (76.4) | 543.9 | <.001 | 556.7 | <.001 | 565.3 | .012 |
| >100 mg (n = 65)b | 539.1 (85.7) | 550.1 | <.001 | 567.1 | .065 | 577.0 | .693 |
Abbreviations: 6MWT, 6-minute walk test; ACE, angiotensin converting enzyme; AT-II, angiotensin-II; BB, β-blocker; BMI, body mass index; MVPA, moderate-to-vigorous physical activity; T2DM, type 2 diabetes mellitus.
a Model 1 includes: age, sex. Model 2 includes model 1 + BMI, smoking status, education, MVPA. Model 3 includes: model 2 + T2DM status, drug use in 6 months prior to index date: β-sympathomimetic, calcium channel blockers, diuretics, ACE-inhibitors, AT-II receptor blockers, statins, benzodiazepines, opioids, or antipsychotics.
b Metoprolol equivalents: 150 mg metoprolol = 5 mg nebivolol = 10 mg bisoprolol = 75 mg atenolol = 37.5 mg carvedilol = 160 mg propranolol = 160 mg sotalol = 15 mg pindolol.
In the submaximal cycling ergometer test, current BB use was not associated with a significantly lower CRF (adjusted mean = 2.14 W kg−1; P = .690) compared to never use (adjusted mean = 2.13 W kg−1; Table 3). Results remained similar after stratification by BB selectivity. After adjustment, none of the dosage strata was associated with a statistically significant difference in CRF compared to never use. Given the potential effect of BBs on the assumed linear relation between HR and power output, additional analyses (Table 4) were conducted in which the analyses were stratified by the 2 different approaches to estimate Wmax (ie, HR and RPE). When using only Wmax estimated from HR values (WmaxHR), a statistically significantly higher CRF in current BB use (adjusted mean = 2.39 W kg−1; P < .001) was observed compared to never use (adjusted mean = 2.25 W kg−1). On the other hand, when only using Wmax estimated from RPE values (WmaxRPE), no difference in CRF was observed between current use (adjusted mean = 2.18 W kg−1; P = .429) and never use (adjusted mean = 2.22 W kg−1). In addition, Wmax in current BB users (adjusted mean = 2.39 W kg−1) was significantly (t test, P < .05) higher than in past users (adjusted mean = 2.20 W kg−1).
Table 3.
Adjusted Means of Cardiorespiratory Fitness Based on Cycling Test (Wmax kg−1) Between BB Users Stratified by Exposure Status and Metoprolol Equivalents.a
| Exposure Status | Crude Mean (SD) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| Mean | P Value | Mean | P Value | Mean | P Value | ||
| Never BB use (n = 1663) | 2.22 (0.58) | 2.21 | Ref | 2.14 | Ref | 2.13 | Ref |
| Past BB use (n = 335) | 2.02 (0.54) | 2.05 | <.001 | 2.06 | .005 | 2.08 | .048 |
| Current BB use (n = 287) | 1.94 (0.61) | 1.97 | <.001 | 2.10 | .205 | 2.14 | .690 |
| Current nonselective BB use (n = 22) | 1.76 (0.56) | 1.85 | .002 | 2.01 | .176 | 2.03 | .313 |
| Current selective BB use (n = 265) | 1.95 (0.62) | 1.98 | <.001 | 2.11 | .318 | 2.15 | .503 |
| 0.1-75 mg (n = 102)b | 1.98 (0.55) | 2.04 | .002 | 2.12 | .723 | 2.14 | .774 |
| 75-100 mg (n = 116)b | 1.98 (0.62) | 1.99 | <.001 | 2.12 | .725 | 2.18 | .273 |
| >100 mg (n = 47)b | 1.82 (0.73) | 1.85 | <.001 | 2.05 | .172 | 2.11 | .770 |
Abbreviations: ACE, angiotensin converting enzyme; AT-II, angiotensin-II; BB, β-blocker; BMI, body mass index; MVPA, moderate-to-vigorous physical activity; T2DM, type 2 diabetes mellitus; Wmax kg−1, estimated maximal power output adjusted for body mass.
a Model 1 includes: age, sex. Model 2 includes model 1 + BMI, smoking status, education, MVPA. Model 3 includes: model 2 + T2DM status, drug use in 6 months prior to index date: β-sympathomimetic, calcium channel blockers, diuretics, ACE-inhibitors, AT-II receptor blockers, statins, benzodiazepines, opioids, or antipsychotics.
b Metoprolol equivalents: 150 mg metoprolol = 5 mg nebivolol = 10 mg bisoprolol = 75 mg atenolol = 37.5 mg carvedilol = 160 mg propranolol = 160 mg sotalol = 15 mg pindolol.
Table 4.
Adjusted Means of Cardiorespiratory Fitness Based on Cycling Test (Wmax kg−1) Between BB Users Stratified by Exposure Status and Metoprolol Equivalents, Using Different Criteria to Complete Cycling Test.a
| Any Criterionb (n = 2285) | HR Criterion (n = 2025) | RPE Criterion (n = 1429) | ||||
|---|---|---|---|---|---|---|
| Exposure Status | Mean | P Value | Mean | P Value | Mean | P Value |
| Never BB use | 2.13 | Ref | 2.25 | ref | 2.22 | Ref |
| Past BB use | 2.08 | .048 | 2.20 | .154 | 2.21 | .789 |
| Current BB use | 2.14 | .690 | 2.39 | <.001 | 2.18 | .429 |
| Current nonselective BB use | 2.03 | .313 | 2.25 | .983 | 2.09 | .324 |
| Current selective BB use | 2.15 | .503 | 2.40 | <.001 | 2.19 | .499 |
| 0.1-75 mgc | 2.14 | .774 | 2.30 | .356 | 2.18 | .605 |
| 75-100 mgc | 2.18 | .273 | 2.50 | <.001 | 2.22 | .975 |
| >100 mgc | 2.11 | .770 | 2.46 | .052 | 2.14 | .369 |
Abbreviations: ACE, angiotensin converting enzyme; AT-II, angiotensin-II; BB, β-blocker; BMI, body mass index; HR, heart rate; MVPA, moderate-to-vigorous physical activity; RPE, rate of perceived exertion; T2DM, type 2 diabetes mellitus; Wmax kg−1, estimated maximal power output adjusted for body mass.
a All analyses adjusted for: age, sex, BMI, smoking status, education, MVPA, T2DM status, and drug use in 6 months prior to index date: β-sympathomimetic, calcium channel blockers, diuretics, ACE-inhibitors, AT-II receptor blockers, statins, benzodiazepines, opioids, or antipsychotics.
b Main analysis: Table 3, model 3.
c Metoprolol equivalents: 150 mg metoprolol = 5 mg nebivolol = 10 mg bisoprolol = 75 mg atenolol = 37.5 mg carvedilol = 160 mg propranolol = 160 mg sotalol = 15 mg pindolol.
Additional analyses (Supplemental Table 3) showed that the outcomes correlated rather well, as the majority of the participants in the lowest 6MWT distance category were also in the lowest cycle ergometer test tertile (59.13%). Similarly, the majority of the participants in the highest 6MWT category were in the highest cycle test category (55.73%).
Discussion
This study shows that, in a population aged between 40 and 75 years, current use of BBs was associated with a lower CRF when measured with the 6MWT. However, there was no association between BB use and CRF determined with the submaximal cycle ergometer test. For both proxies of the outcome, there was no difference between current use of selective versus nonselective BBs and CRF. Furthermore, there was no clear association between dose and CRF among users of selective BBs.
To our knowledge, a 6MWT has not been used previously as a proxy for CRF when assessing the association between BB use and CRF. Several earlier studies have assessed this association using a cycle ergometer test to obtain proxies for CRF.7–9 Vanhees et al reported a significant decrease in CRF in BB users (atenolol or bisoprolol) compared to placebo.8 Van Bortel and van Baak found that nebivolol, a highly β1-selective BB, was associated with no effect on CRF, whereas this was the case with the use of atenolol, a less β1-selective BB.9 Gullestad et al found a significantly larger reduction in working capacity during a continuous exercise when using timolol (a nonselective BB) compared to metoprolol (a selective BB).7 In contrast to our study, the authors of these studies found a difference between BB users and nonusers, and they found a difference between the use of selective versus nonselective or less selective BBs. The results presented in these studies may be different from those in our study due to the use of different exercise protocols. Furthermore, the results in these studies may be different from those in our study due to the fact that in the previous studies, healthy volunteers were included in placebo-controlled, double-blind, crossover designs; whereas, in the Maastricht Study, a more diverse population was included in a population-based setting. The designs used in the previous studies may limit the effect of confounding, but the generalizability of the results may be limited.
When the 6MWT was used as a proxy for CRF, a lower CRF in current BB users was found compared to never users. The crude difference between current and never users was approximately 55 m. However, after adjustment (model 3) the difference between current and never users was approximately 11 m. As a recent systematic review indicated that a minimal clinically important difference ranges from 14 to 30.5 m, the clinical relevance of the difference found in this study may be limited.22 Furthermore, the difference in CRF between nonselective and selective BB users was not statistically significant. This may be due to a relatively small sample size in group of nonselective BB users (n = 26). Conversely, it could be due to the fact that the 6MWT is accepted as a proxy for CRF in moderately impaired or older populations.14,23 It may, however, not be an appropriate proxy in the relatively young and healthy population included in the Maastricht Study. This may have led to a limited discriminatory ability between nonselective and selective BB users. Stratification of current BB use by increasing doses was not associated with significant decrease in CRF compared to never use. We expected to see a lower CRF with increasing dose, followed by a strong drop in CRF with the highest dose. In this study, the highest dosage stratum may not have been high enough for this effect.
In contrast to the 6MWT, the submaximal ergometer cycle test is considered to be an appropriate proxy for CRF in a generally healthy population.24 However, the usefulness of the cycling proxy may be limited for another reason. After adjustment, CRF based on the submaximal cycling ergometer test was similar in current and never BB users. This may have been due to the effect of BB use on one of the completion criteria of the cycling test (ie, HR ≥ 85%). Current BB users are likely to have an ‘artificially’ reduced HR due to the effect on the β1-receptors. Therefore, in order to reach an HR ≥85% during the cycling test, an individual will need to put in more effort and power, leading to an overestimation of CRF. This was confirmed by the higher estimated CRF of current BB users compared to nonusers in the cycling test when only the HR criterion was used. This suggests that the HR criterion is biased when assessing the association between BB use and CRF, and consequently, the use of the RPE criterion might be more appropriate when evaluating this association. Previous studies have shown that a submaximal, RPE-guided, graded exercise protocol can provide estimates of maximal power that are as valid and reliable as established methods based upon HR response.19,25 However, the use of RPE may also have some disadvantages: It requires understanding of the Borg scale and may be affected by socially desirable responding. Furthermore, RPE may be indirectly affected by BB use. Due to HR suppression, BB users may experience a certain exercise level as more exhausting. Cardiorespiratory fitness based on the RPE criterion in the cycle ergometer test tended to be lower in nonselective BB users compared to selective BB users. However, the difference between nonselective and selective BB users was not statistically significant. Furthermore, stratification of current BB use by increasing doses was not associated with significant decrease in CRF compared to never use.
A strength of this study was that we conducted our analyses in a large population of which >450 patients were currently using a BB in a real-life setting. This study therefore reflects BB use in clinical practice rather than in a controlled research environment. There are some relevant limitations as well. First, confounding by indication may have affected the results. β-Blockers are most likely prescribed to treat an underlying (cardiovascular) disease. This underlying diagnosis is likely to affect CRF regardless of BB use. Stratification by indication could have provided insight into the effect of the different underlying diseases. However, data on indications were not available. We have, therefore, attempted to minimize the effect of the underlying diagnosis by adjusting for other cardiovascular drugs, such as calcium channel blockers, diuretics, ACE inhibitors, and AT-II receptor blockers. However, residual confounding may still be present. Second, due to the lack of data, we were unable to take into account the effect of pulmonary function on the association between BB use and CRF. This may be relevant as BB users may have a poorer pulmonary function compared to nonusers and a reduced pulmonary function may subsequently result in a reduced CRF. However, use of BBs was significantly associated with a lower forced expiratory volume in 1 second and a lower forced vital capacity.26 Consequently, pulmonary function as a covariate lies within the causal pathway from exposure to outcome and therefore does not meet the criteria of a confounder, and adjustment for this factor is therefore not justified. Third, BBs are known to have other pharmacological effects besides the antagonism of β-receptors. For instance, pindolol has intrinsic sympathomimetic activity (ISA). This phenomenon is sometimes referred to as partial agonism. Because of this property, pindolol has limited negative chronotropic effects in a resting state compared to BB without ISA.27 However, in an active state, for example, during the cycle ergometer test, the negative chronotropic effects may still be present. It is therefore unlikely that this partial agonism affected the results of this study. Moreover, there were only 2 pindolol users in this study. Furthermore, carvedilol and nebivolol exhibit vasodilation through nitric oxide release.28,29 Carvedilol may also cause vasodilation by blocking the α-1 receptor.23 These pharmacological effects on the cardiovascular system may affected CRF and interfered with the effect of β-receptor selectivity. Third, the cross-sectional design of the study requires caution with regard to causal inferences. Fourth, misclassification of exposure may also be of concern as dispensing data were used to determine exposure. The nature of this data does not allow us to confirm medication adherence. Furthermore, due to the fact that fasting blood samples were taken prior to the 6MWT, participants were not allowed to take their daily medication. After the sampling, however, participants were allowed to take their pills and approximately 1 hour later the 6MWT was conducted. This may have resulted in sub-therapeutical levels of BB exposure, potentially explaining why we found no difference in CRF between selective and nonselective BB users. Finally, selection bias may have occurred due to the exclusion of patients with missing data or those with a history of severe cardiovascular comorbidities. This suggests that especially BB users with severe cardiovascular illness, who were likely to achieve poorly on the CRF tests, were excluded, whereas relatively healthy BB users remained included. This is also reflected by the fact that compared to the full study population the excluded patients were older, had a higher BMI, smoked more often, and were more often diagnosed with T2DM. Consequently, this may explain why we found no difference between current BB users and never users during the cycling test.
In conclusion, current BB use was associated with a lower CRF based on the 6MWT but not the cycling test compared to never use. There was no difference between current selective and nonselective BB users for both outcomes. These data from clinical practice suggest that, with regard to their effect on CRF, there is no indication for prescribers to choose a selective BB over a nonselective BB or that prescribers already make correct choices. However, as several limitations are present in this study, more research is required. Future studies, with larger sample sizes, stratified by substance or including cardiorespiratory outcomes, may be considered.
Supplemental Material
Supplemental_file for The Association Between β-Blocker Use and Cardiorespiratory Fitness: The Maastricht Study by Johannes T. H. Nielen, Frank de Vries, Jeroen. H. P. M. van der Velde, Hans H. C. M. Savelberg, Nicolaas C. Schaper, Pieter C. Dagnelie, Ronald M. A. Henry, Miranda Schram, Coen D. A. Stehouwer, Annelies Boonen, Annemarie Koster, and Bart J. F. van den Bemt in Journal of Cardiovascular Pharmacology and Therapeutics
Acknowledgment
Authors thank all participants of the Maastricht Study, their community pharmacists, the Apothekers Vereniging Maastricht, and the Verenigde Apotheken Limburg for their cooperation.
Footnotes
Author contributions: J.N., F.V., J.V., A.K., and B.B. contributed to the conception and design of the study. J.N., F.V., J.V., H.S., N.S., P.D., R.H., M.S., C.S., and A.K. contributed to the acquisition of data. J.N. and J.V. contributed to analysis of data. All authors contributed to interpretation of data. J.N., F.V., J.V., A.K., and B.B. drafted the manuscript and all authors contributed intellectual content in revising the article. All authors gave final approval of the version to be submitted and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. B.B. receives research grants to his department from Pfizer and Roche and occasionally speakers honoraria from Pfizer, Roche, Abbvie, and MSD. A.B. receives research grants to her department from Amgen Abbvie, Pfizer, and Merck and occasionally speakers honoraria from Pfizer, UCB, and Sandoz. P.D. has received unrestricted grants from The Netherlands Organisation for Scientific Research (NWO), the European Union (EU), and nutritional industry for research unrelated to this topic.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Maastricht Study was supported by the European Regional Development Fund via OP-Zuid, the Province of Limburg, the Dutch Ministry of Economic Affairs (grant 31O.041), Stichting De Weijerhorst (Maastricht, the Netherlands), the Pearl String Initiative Diabetes (Amsterdam, the Netherlands), the Cardiovascular Center (Maastricht, the Netherlands), CARIM School for Cardiovascular Diseases (Maastricht, the Netherlands), CAPHRI School for Public Health and Primary Care (Maastricht, the Netherlands), NUTRIM School for Nutrition and Translational Research in Metabolism (Maastricht, the Netherlands), Stichting Annadal (Maastricht, the Netherlands), Health Foundation Limburg (Maastricht, the Netherlands), and by unrestricted grants from Janssen-Cilag B.V. (Tilburg, the Netherlands), Novo Nordisk Farma B.V. (Alphen aan den Rijn, the Netherlands), and Sanofi-Aventis Netherlands B.V. (Gouda, the Netherlands).
ORCID iD: Johannes T. H. Nielen  http://orcid.org/0000-0002-3633-2504
http://orcid.org/0000-0002-3633-2504
Jeroen. H. P. M. van der Velde  http://orcid.org/0000-0002-2904-4598
http://orcid.org/0000-0002-2904-4598
References
- 1. Lewis RV, Lofthouse C. Adverse reactions with beta-adrenoceptor blocking drugs. An update. Drug Saf. 1993;9(4):272–279. [DOI] [PubMed] [Google Scholar]
- 2. Harms HH. Isoproterenol antagonism of cardioselective beta adrenergic receptor blocking agents: a comparative study of human and guinea-pig cardiac and bronchial beta adrenergic receptors. J Pharmacol Exp Ther. 1976;199(2):329–335. [PubMed] [Google Scholar]
- 3. Abrahamsson T. The beta 1- and beta 2-adrenoceptor stimulatory effects of alprenolol, oxprenolol and pindolol: a study in the isolated right atrium and uterus of the rat. Br J Pharmacol. 1986;87(4):657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bundkirchen A, Brixius K, Bölck B, Nguyen Q, Schwinger RH. Beta 1-adrenoceptor selectivity of nebivolol and bisoprolol. A comparison of [3H]CGP 12.177 and [125I]iodocyanopindolol binding studies. Eur J Pharmacol. 2003;460(1):19–26. [DOI] [PubMed] [Google Scholar]
- 5. Baker JG. The selectivity of beta-adrenoceptor antagonists at the human beta1, beta2 and beta3 adrenoceptors. Br J Pharmacol. 2005;144(3):317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dorow P, Tönnesmann U. Dose-response relationship of the beta-adrenoceptor antagonist bisoprolol in patients with coronary heart disease and chronic obstructive bronchitis. Eur J Clin Pharmacol. 1984;27(2):135–139. [DOI] [PubMed] [Google Scholar]
- 7. Gullestad L, Dolva LO, Søyland E, Kjekshus J. Difference between beta-1-selective and non-selective beta-blockade during continuous and intermittent exercise. Clin Physiol. 1988;8(5):487–499. [DOI] [PubMed] [Google Scholar]
- 8. Vanhees L, Defoor JG, Schepers D, et al. Effect of bisoprolol and atenolol on endurance exercise capacity in healthy men. J Hypertens. 2000;18(1):35–43. [DOI] [PubMed] [Google Scholar]
- 9. Van Bortel LM, van Baak MA. Exercise tolerance with nebivolol and atenolol. Cardiovasc Drugs Ther. 1992;6(3):239–247. [DOI] [PubMed] [Google Scholar]
- 10. Gullestad L, Hallen J, Medbø JI, Grønnerød O, Holme I, Sejersted OM. The effect of acute vs chronic treatment with beta-adrenoceptor blockade on exercise performance, haemodynamic and metabolic parameters in healthy men and women. Br J Clin Pharmacol. 1996;41(1):57–67. [DOI] [PubMed] [Google Scholar]
- 11. Mutlu GM, Koch WJ, Factor P. Alveolar epithelial beta2-adrenergic receptors. Am J Respir Crit Care Med. 2004;170(12):1270–1275. [DOI] [PubMed] [Google Scholar]
- 12. Agostoni P, Contini M, Cattadori G, et al. Lung function with carvedilol and bisoprolol in chronic heart failure: is beta selectivity relevant? Eur J Heart Fail. 2007;9(8):827–833. [DOI] [PubMed] [Google Scholar]
- 13. Schram MT, Sep SJS, van der Kallen CJ, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29(6):439–451. [DOI] [PubMed] [Google Scholar]
- 14. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 15. van der Velde JH, Koster A, van der Berg JD, et al. Sedentary behaviour, physical activity, and fitness—The Maastricht Study. Med Sci Sport Exerc. 2017. [DOI] [PubMed] [Google Scholar]
- 16. Storer TW, Davis JA, Caiozzo VJ. Accurate prediction of VO2max in cycle ergometry. Med Sci Sports Exerc. 1990;22(5):704–712. [DOI] [PubMed] [Google Scholar]
- 17. Sartor F, Vernillo G, de Morree HM, et al. Estimation of maximal oxygen uptake via submaximal exercise testing in sports, clinical, and home settings. Sports Med. 2013;43(9):865–873. doi:10.1007/s40279-013-0068-3. [DOI] [PubMed] [Google Scholar]
- 18. Billat V, Lopes P. Indirect methods for estimation of aerobic power. In: Maud PJ, Foster C, eds. Physiological Assessment of Human Fitness. Champaign, IL: Human Kinetics; 2006:19–38. [Google Scholar]
- 19. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–98. [PubMed] [Google Scholar]
- 20. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 21. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. [DOI] [PubMed] [Google Scholar]
- 22. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377–381. [DOI] [PubMed] [Google Scholar]
- 23. Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54(1):127–132. [DOI] [PubMed] [Google Scholar]
- 24. Coquart JB, Garcin M, Parfitt G, Tourny-Chollet C, Eston RG. Prediction of maximal or peak oxygen uptake from ratings of perceived exertion. Sports Med. 2014;44(5):563–578. [DOI] [PubMed] [Google Scholar]
- 25. Eston RG, Lamb KL, Parfitt G, King N. The validity of predicting maximal oxygen uptake from a perceptually-regulated graded exercise test. Eur J Appl Physiol. 2005;94:221–227. [DOI] [PubMed] [Google Scholar]
- 26. Loth DW, Brusselle GG, Lahousse L, Hofman A, Leufkens HG, Stricker BH. β-Adrenoceptor blockers and pulmonary function in the general population: the Rotterdam Study. Br J Clin Pharmacol. 2014;77(1):190–200. doi:10.1111/bcp.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aellig WH. Pindolol a beta-adrenoceptor blocking drug with partial agonist activity: clinical pharmacological considerations. Br J Clin Pharmacol. 1982;13(suppl 2):187S–192S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107(21):2747–2752. [DOI] [PubMed] [Google Scholar]
- 29. Bristow MR. Beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101(5):558–569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_file for The Association Between β-Blocker Use and Cardiorespiratory Fitness: The Maastricht Study by Johannes T. H. Nielen, Frank de Vries, Jeroen. H. P. M. van der Velde, Hans H. C. M. Savelberg, Nicolaas C. Schaper, Pieter C. Dagnelie, Ronald M. A. Henry, Miranda Schram, Coen D. A. Stehouwer, Annelies Boonen, Annemarie Koster, and Bart J. F. van den Bemt in Journal of Cardiovascular Pharmacology and Therapeutics