Highlights
-
•
Gentamicin induced dose dependent and temporal change in urinary biomarkers.
-
•
Histological changes were minimal to severe on Day 4 & 8 respectively at both doses.
-
•
Several fold increase in urinary biomarkers on Day 4 and 8 at both doses.
-
•
On Day 8, increase in urinary and serum markers and histological changes.
-
•
Clusterin is highly sensitive urinary biomarkers.
Keywords: Gentamicin, Nephrotoxicity, Blood urea nitrogen, Creatinine, Renal biomarkers
Abstract
Consistent, sensitive biomarkers of acute kidney injury in animal models and humans have historically represented a poorly met need for investigators and clinicians. Detection of early kidney damage using urinary biomarkers is essential to assess the adversity in preclinical toxicology studies, which will help in reducing attrition of lead candidates in drug development. This study was undertaken to evaluate recently identified urinary biomarkers use in identifying acute kidney injury compared to traditional serum markers in experimentally induced nephrotoxicity in male Sprague Dawley (SD) rats. Gentamicin induced nephrotoxicity in Sprague Dawley rats is commonly detected using serum markers and histological evaluation of kidneys. Gentamicin, an aminoglycoside was administered at 30 and 100 mg/kg/day dose (subcutaneous) for seven consecutive days to induce nephrotoxicity. On day 4 and day 8 post treatment, serum and urine samples from these rats were analyzed for traditional serum/urine and novel urinary biomarkers and microscopic evaluation of kidneys.
On Day 4, no statistically significant change in serum BUN and creatinine level, but increase in urinary microalbumin (mALB) and urinary protein (UP) noticed in both doses of Gentamicin treated rats. On Day 8 significant increase in serum blood urea nitrogen (BUN), serum creatinine, UP and urinary mALB at 100 mg/kg/day, increase in total protein and decrease in albumin in 30 and 100 mg/kg/day and decrease in BUN and creatinine at 100 mg/kg of Gentamicin treated rats. The BUN and creatinine levels or fold change was comparable between control and 30 mg/kg of Gentamicin on Day 8, however, there was 5.6 and 3.4 fold change in BUN and Creatinine level noticed at 100 mg/kg/day of Gentamicin. On Day 4 and 8, significant increase in urinary levels of Clusterin was noted with animals administered both doses of Gentamicin. Similarly, significant increase in urinary levels of kidney injury molecule 1 (Kim-1), Cystatin C and neutrophil gelatinase-associated lipocalin (NGAL) were noticed with animals administered Gentamicin at 100 mg/kg/day on both Day 4 and 8. All these markers have shown dose-dependent change.
Histological changes seen on Day 4 and Day 8 were of minimal to mild and moderate to severe in nature at both doses, respectively. The results demonstrated the sensitiveness and accuracy of detecting acute renal damage with novel urinary biomarkers, and their use in diagnosing early kidney damage. This helps in adversity assessment in animal toxicology studies and advocating right treatment to patients who have early renal injury which otherwise can only be diagnosed by elevated levels of traditional biomarkers in blood only after >30% of kidneys is damaged.
1. Introduction
The process of drug discovery and development is challenging, rewarding, time consuming and complex. The attrition in drug development is not uncommon due to unpredicted toxicities and adverse effects in clinical studies. Hence, there is a need to identify, validate and evaluate various biomarkers that aid in decision making for the advancement of compound to next nonclinical or clinical stages of development. An emerging approach to achieve this objective is the use of biomarkers early in the lead optimization stage or Phase 1 clinical trial. Safety assessment of kidney is mandatory component of preclinical and in clinical trials of new test substance. Drug-induced kidney injury is one among the reasons for the compound attrition in drug development [[1], [2], [3]]. Kidney damage may be acute (sudden reduction in renal function or urine output) or chronic (persistent structural and functional alterations) and is traditionally diagnosed using serum markers like BUN and creatinine levels which gets elevated only after significant (approximately 30%) kidney damage [4]. As these markers are insensitive and lack specificity, the novel renal biomarkers have been used recently as a tool to evaluate early kidney damage both in preclinical species and human.
Gentamicin is an effective aminoglycoside antibiotic that is widely prescribed drug for treating patients with infections, but its associated adverse effects of oxidative stress and kidney injury limits its long-term clinical use [[5], [6], [7], [8], [9], [10]]. The onset of renal failure is usually slower and the daily rise of serum creatinine tends to be lower than other causes of acute renal failure. Serum creatinine and blood urea nitrogen characteristically increase 7–10 days after initiation of aminoglycoside therapy [11]. In more than half of the cases with nephrotoxicity, the decline in renal function occurs only after the therapy has been completed [12]. Long duration is required to recover from gentamicin-induced nephrotoxicity, particularly in elderly individuals. Although the vast majority of patients do recover, the presence of several risk factors may alter the clinical presentation or the course, resulting in the early appearance of acute renal failure as well as a protracted course. In patients with underlying chronic kidney disease, recovery in renal function may be incomplete in some. In addition, various tubular dysfunction and electrolyte abnormalities may also occur (Donald [13]). Spirulina platensis, Trigonella foenum-graecum, Cupressuflavone, Ceftriaxone, Allicin, ascorbic acid, polyphenols, sesame oil, alpha lipoic acid, Terminalia muelleri, rosuvastatin, and Vitamin E have been demonstrated to protect against nephrotoxicity or oxidative stress induced by deltamethrin, Carbontetrachloride, Diazinon, Cisplatin and Fipronil. [[14], [15], [16], [17], [18], [19], [20], [21], [22]]. Similarly, Nigella sativa oil (NSO) and/or ascorbic acid (AA) protected against Oxytetracycline-induced hepatonephrotoxicity in rabbits through their free radical-scavenging and potent antioxidant activities [18,23].
Clusterin is a disulfide-linked heterodimeric protein of 75 kDa, consisting of α- and β-subunits [24,25]. It is a glycoprotein isolated from sheep’s testis responsible for clearance of cellular debris and apoptosis [26]. Clusterin is present in many physiologic fluids and over expressed in kidney and urine of various species of animals in pre-clinical acute kidney injury (AKI), tissue remodeling, unilateral ureter obstruction, or subtotal nephrectomy models of kidney injury. Although clusterin is expressed in several tissues, its molecular size prevents a filtration in the kidney, thus rendering its urinary levels specific to kidney injury [27]. Clusterin has also been proposed as potential markers of kidney toxicity [28]. Expression of clusterin is noticed in necrotic tubules with high-dose of paraminophenol administration in rats, often with higher intensity at the apical pole of the epithelium [27,29,30]. Clusterin has been shown to exhibit a protective gene function against gentamicin-induced renal tubular cell injury [31]. Paradoxically, up-regulation of clusterin suggests the occurrence of renal injury. Therefore, clusterin is considered as potential biomarker of nephrotoxicity. Increased levels of clusterin protein in urine have been detected following ischemic or chemically induced injury in rats [30,32].
Kidney injury molecule, (Kim-1 and KIM-1 in rodents and humans, respectively) is a 104 kDa transmembrane glycoprotein with an immunoglobulin domain and mucin that is undetectable in routine techniques in healthy kidney tissue or in the urine but is expressed at substantial level on the apical surface of proximal convoluted tubule (PCT) following injury or in certain types of renal carcinoma [33]. KIM-1 is found in S1, S2 and S3 segments of PCT in case of ischemic and toxic AKI in human. Basal expression of KIM-1 is undetectable or very negligible amount in healthy kidney, but it significantly gets elevated in a dedifferentiated and regenerating PCT [33]. The mucin ectodomain shed into urine during tubular injury and is stable for long time. Significantly high level of Kim-1 has been detected in urine in various models of nephrotoxicity [34]. Vaidya et al. [35] found that high level of urinary Kim-1 seen in Cisplatin induced nephrotoxicity or ischemic injury in rats with concurrent no change in blood based traditional markers of renal function demonstrating sensitivity and use of adopting Kim-1 to monitor tubular injury. Irrespective of differences in mechanism of renal injury, Kim-1 has been considered as highly sensitive and specific biomarker of PCT injury based on few studies conducted [[36], [37], [38]]. Elevated KIM-1 level is noticed in patients with acute tubular necrosis, thus KIM-1 helpful in early detection of renal injury [39]. Both the Food and Drug Administration (FDA) and European Medicines Agency (EMA) consider KIM-1 to be a highly sensitive biomarker for detecting acute drug induced injury during drug development.
Cystatin C (CysC) is a small molecule of 13.3 kDa, 122 amino acid non-glycosylated basic cysteine protease (lysosomal proteinases) inhibitor, preventing breakdown of certain intracellular and extracellular proteins within the body, constitutively expressed by all nucleated cells, and is synthesized and secreted to the plasma at a steady rate [40]. In healthy state, negligible amount of CysC is found in urine. Urinary CysC is a validated and qualified biomarker of acute tubular and glomerular injury in rats and it increases during impaired reabsorptive capacity of PCT [41]. Blood levels of Cystatin C is unaltered with muscle mass, exercise, sex, age of the individual etc. Hence, it is considered as more realistic marker for glomerular function assessment even in cachexia or early AKI, where serum creatinine could underestimate the true renal function. CysC is considered as specific marker for glomerular filtration rate (GFR) assessment than primary marker for AKI although it can be used to detect AKI. Many independent studies demonstrated the superiority of serum Cystatin C compared with serum creatinine, especially to detect minor alterations in GFR reduction. Investigators started using serum CysC as GFR marker recently in toxicology studies although it has been widely used in Clinic. Ozer et al. [42] demonstrated that serum CysC is superior to other traditional markers in detecting site specific nephrotoxicity in rats.
NGAL (human neutrophil lipocalin, lipocalin-2, siderocalin, or LCN2) is a 25 KDa glycoprotein which is covalently bound to neutrophil-gelatinase of the lipocalin family, and it contains eight beta strands forming a β-barrel in a closed cup. It is minimally expressed in lungs, stomach, colon and epithelial cells located in the PCT and neutrophils [43,44]. NGAL will increase if the thick ascending limb of the loop of Henle, distal tubule and collecting duct is damaged in rodents and humans. NGAL precedes changes in serum creatinine [45,46]. In general, low NGAL is detected in plasma. It is completed reabsorbed in the glomerular filtrate by the megalin-cubilin transporter complex present in PCT. In case of protein overload nephropathy, saturation of the re-absorption capacity of this complex can lead to increased urinary NGAL, and tubular back-leak can result in increases in plasma NGAL. In addition, drug induced kidney injury (DIKI) can cause increased expression and release of NGAL as a protective mechanism as shown for other “tubular stress” proteins such as KIM-1 [47]. As a consequence, conditions which lead either to saturation or impairment of the re-absorption complex or to increased de novo expression of NGAL in kidney are expected to demonstrate the utility of NGAL as a kidney biomarker in the context of drug-development [46,47]. EMA and US FDA encouraged the conduct of nonclinical and exploratory clinical analyses to evaluate the translational relevance of changes in urinary NGAL values and the magnitude of change in urinary NGAL that could be considered clinically meaningful in the determination of kidney injury when observed in an individual subject for the data submitted by PSTC in 2014. EMA recommend evaluating urinary Osteopontin (OPN) and NGAL in early clinical studies and prospectively discuss proposed application of the clinical biomarker to decisions during the course of the study for clinical trial authorization [48]. In conclusion, NGAL was found to be a useful early predictor of AKI, with urine or plasma/serum NGAL levels functioning as well. Additionally, NGAL level had prognostic value for clinical endpoints, such as initiation of dialysis and mortality [49].
Hence, in the current experiment, the early detection of gentamicin induced nephrotoxicity was assessed in male Sprague Dawley rats using recently identified urinary biomarkers and the changes were compared with traditional serum biomarkers and microscopic changes in kidneys.
2. Materials and method
2.1. Materials
Gentamicin (Genticyn Abbott, 80 mg/2 ml) was administered at 30 and 100 mg/kg/day subcutaneously for 7 consecutive days. Doses were selected based on the results obtained in an exploratory dose-finding toxicity study (results not discussed in this manuscript), where dose- dependent increase in serum blood urea nitrogen, creatinine and histological changes were seen in kidneys. Two dose levels of Gentamicin, 30 and 100 mg/kg/day (single daily dose, subcutaneous) were selected in this study in order to establish clear kidney toxicity without lethality.
2.2. Animals and study design
In brief, 48 male Sprague Dawley rats supplied by Glenmark Pharmaceuticals Limited, Navi Mumbai, were acclimatized for at least 10 days prior to dosing. The rats were aged between 7–8 weeks, weighing between 175–190 g at the start of dosing. These 48 rats were randomized into 2 experiments, with 18 rats in Experiment 1 and 30 rats in Experiment 2. In the Experiment 1, 18 rats were assigned to control (sterile water, intraperitoneal), 30 and 100 mg/kg/day of Gentamicin (single daily dose, subcutaneous) groups, as 6 rats in each group. These animals were euthanized using isoflurane and oxygen on Day 4 for assessing kidney weight, gross and histological assessment. In the Experiment 2, 10 animals each were assigned to control (sterile water, intraperitoneal), 30 and 100 mg/kg/day of Gentamicin (single daily dose, subcutaneous) groups. Urine and blood (serum) samples were collected on Day -4 (4 days before start of dosing) for baseline data collection, and on Day 4 and 8. On Day 8, these animals were euthanized using isoflurane and oxygen for assessing kidney weight, gross and histological assessment of kidneys. The experimental animals were observed daily for clinical signs and mortality if any. Body weight and feed consumption were recorded once in 3 days and until the day of sacrifice.
All animals were maintained in an individually ventilated cages (IVC) with Corn cob (BioCobb, AT&T) as the bedding material. Commercial pellet diet (Altromin, Germany) and community tap water passed through a RO system (Millipore) were given. Feed and water was provided ad libitum throughout the study period unless restricted by experimental requirements. All animals were identified by tail marking for individual identification throughout the study period. Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guideline was followed regarding animal welfare practices and the experimental protocol was approved by Glenmark’s Institutional Animal Ethics Committee (IAEC).
2.3. Blood sample collection and analysis
Blood samples (∼1.0 ml) were collected from all Experiment 2 rats under light isoflurane anesthesia after overnight fasting from the retro-orbital plexus and serum was separated by centrifugation for 10 min at approximately 1000g after a firm clot was formed. Serum samples were analysed for various biochemical parameters using Rx Daytona (Randox Laboratories Limited) either on the day of collection or stored at −80 °C for subsequent analysis.
2.4. Urine collection and analysis
Urine sample were collected for about 15–16 h by keeping animals individually in rat metabolic cages. During the period of urine collection, access to food was restricted but water was offered ad libitum. Urine was collected in chilled 25 ml conical polypropylene tubes kept in ice container throughout the collection period. After low speed centrifugation (400g) at 4 °C for 5 min, multiple aliquots of the supernatant urine were prepared and stored in polypropylene tubes at −80 °C until analysis. Urine samples were thawed completely, mixed well by vortexing and centrifuged prior to use in the assay to remove particulates. Not more than 2 freeze/thaw cycles were followed. Urine samples were analyzed for traditional markers like total protein, urinary protein, BUN, creatinine and micro albumin using Rx Daytona (Randox Laboratories Limited) and novel biomarkers (Clusterin, Kim-1, Cystatin C and Lipcalin-2/NGAL) were analysed (immunoassay) using EMD Millipore’s MILLIPLEX MAP Rat Kidney Toxicity Magnetic Bead Panels with Luminex X MAP technology. Performance of the different analyzers used in the study was verified by analyzing the respective quality controls before sample analysis. Urine samples were diluted to 1:5000 in the assay buffer provided in the kit for the biomarkers estimation. All the biomarkers levels were expressed considering urine concentration of creatinine (divide urinary biomarker concentration by urinary creatinine concentration). In the result section, the term “level” is used to describe the biomarker values expressed in this way. Fold differences in the normalized values were calculated relative to the contemporary control mean value.
2.5. Statistical analysis
Statistical analysis was performed on body weights, body weight gain, clinical chemistry, selected urinalysis parameters, organ weights and organ to body and brain weight percentages and kidney biomarkers. Results for all these parameters were expressed as mean ± standard deviation of the mean (SD). All the comparisons were made between treatment groups and vehicle control group. The data was evaluated using one-way ANOVA with Dunnett’s post test (multiple comparison test). A p < 0.05 was considered significant in all evaluations. The complete analysis was performed using GraphPad Prism statistical software version 5.02 for Windows, GraphPad Software, San Diego California USA.
2.6. Histological evaluation
On the day of terminal sacrifice i.e. on day 4 and day 8 all the experimental animals were subjected to inhalation of isoflurane: oxygen for euthanasia followed by exsanguination. The rats were observed for gross necropsy examinations of kidney and other organs. Weight of brain and kidneys (paired) were recorded. Kidneys from all animals was fixed in 10% neutral buffered formalin as processed further for histological evaluations. For histological evaluations left and right kidneys were trimmed transversely and longitudinally, respectively to ensure optimal examination of all parts of the kidney i.e. renal pelvis, renal papilla and the junction with the ureter. Post processing of kidney samples in various grades of isopropyl alcohol and xylene, they were embedded in paraffin and sectioned at a thickness of 4–5 μm and either stained with Hematoxylin and Eosin (H&E, egg albumin coated slides) for histological changes. Microscopic observations were described according to distribution (focal, multifocal and diffuse), severity (minimal, mild, moderate and severe) and morphologic characteristics. Representative images of microscopic lesions were taken using Leica DFC 295 camera. Changes in all treatment groups were compared with concurrent vehicle control group.
3. Result
The purpose of this study was to evaluate the time course of both recently identified urinary biomarkers and traditional markers of nephrotoxicity and compare with histopathology evaluation of Gentamicin induced nephrotoxicity in male Sprague Dawley rats. Urinary protein, albumin, mALB and biomarkers, and serum biochemical parameters were measured prior to start of treatment to establish a preliminary reference range and to assess the physiological and biological variation of these parameters. Despite known differences in immunoassay results due to differences in sensitivity and specificity of antibodies, differences in standardization, and the inherent variation between animals the normal range was obtained for the different parameters including biomarkers which were generally in line with other published data [42,50,51]. For practical reasons and ease of comparing the effect post dosing of Gentamicin, these baseline data is not included in this manuscript. No clinical signs noticed in any of the treated animals during the entire period of dosing. Slightly reduced body weight and food consumption was noticed on Day 7 in rats treated with 100 mg/kg of Gentamicin.
3.1. Serum clinical chemistry
On Day 4, no statistically significant change in serum BUN and creatinine level noticed in both doses of Gentamicin treated rats. On Day 8 significant increase in creatinine and BUN were noticed at 100 mg/kg/day Gentamicin treated rats. Statistically significant decrease was observed in albumin with Gentamicin at 30 and 100 mg/kg/day. The BUN and creatinine levels or fold change was comparable between control and 30 mg/kg of Gentamicin on Day 8, however, there was 5.6 and 3.4 fold change in BUN and Creatinine level noticed at 100 mg/kg/day of Gentamicin (Table 1). Some of the urinary biomarkers have increased to several folds compared to control although there were no significant change in BUN and creatinine at 30 mg/kg/day in doses level of Gentamicin on Day 4 or Day 8.
Table 1.
Serum markers and urinary biomarkers levels on Day 4 and Day 8 (n = 10).
| Day | Treatment groups | Urinary biomarkers (ng/mg creatinine) |
Serum markers (mg/dL) |
|||||
|---|---|---|---|---|---|---|---|---|
| Clusterin | Kim-1 | Cystatin C | NGAL | Creatinine | BUN | |||
| 4 | Control | Mean | 39.44 | 0.29 | 0.57 | 41.69 | 0.57 | 17.90 |
| SD | 6.49 | 0.06 | 0.22 | 29.29 | 0.05 | 2.48 | ||
| Gentamicin 30 mg/kg | Mean | 74.63** | 0.32 | 0.94 | 45.41 | 0.55 | 17.52 | |
| SD | 24.87 | 0.11 | 0.46 | 15.94 | 0.09 | 1.98 | ||
| Gentamicin 100 mg/kg | Mean | 148.95*** | 0.80*** | 2.03*** | 80.11* | 0.63 | 20.28 | |
| SD | 34.75 | 0.32 | 1.19 | 40.75 | 0.06 | 2.70 | ||
| 8 | Control | Mean | 43.10 | 0.30 | 0.63 | 43.34 | 0.59 | 18.46 |
| SD | 13.20 | 0.10 | 0.27 | 20.13 | 0.07 | 2.39 | ||
| Gentamicin 30 mg/kg | Mean | 336.34*** | 0.96 | 1.67 | 97.11 | 0.59 | 18.93 | |
| SD | 182.31 | 0.73 | 0.91 | 46.73 | 0.06 | 1.67 | ||
| Gentamicin 100 mg/kg | Mean | 402.14*** | 4.39*** | 20.19*** | 395.03*** | 1.99*** | 103.18*** | |
| SD | 147.80 | 1.49 | 4.98 | 352.26 | 0.86 | 36.31 | ||
| Fold Change as compared to control on Day 4 | ||||||||
| Gentamicin 30 mg/kg | 1.9 | 1.1 | 1.7 | 1.1 | 1.0 | 1.0 | ||
| Gentamicin 100 mg/kg | 3.8 | 2.8 | 3.6 | 1.9 | 1.1 | 1.1 | ||
| Fold Change as compared to control on Day 8 | ||||||||
| Gentamicin 30 mg/kg | 7.8 | 3.2 | 2.6 | 2.2 | 1.0 | 1.0 | ||
| Gentamicin 100 mg/kg | 9.3 | 14.6 | 31.9 | 9.1 | 3.4 | 5.6 | ||
One way ANOVA followed by Dunnett’s multiple comparison test (P < 0.05).
One way ANOVA followed by Dunnett’s multiple comparison test (P < 0.01).
One way ANOVA followed by Dunnett’s multiple comparison test (P < 0.001); Kim-1=kidney injury molecule; NGAL = Neutrophil gelatinase associated lipocalin; BUN = blood urea nitrogen.
3.2. Urine clinical chemistry and biomarkers
On Day 4, increase in mALB and UP was observed with Gentamicin at 30 and 100 mg/kg/day. On Day 8, increase in total protein was seen at both dose levels of Gentamicin; increase in urinary protein and mALB, and decrease in BUN and creatinine at 100 mg/kg of Gentamicin (Table 2). On Day 4 and 8, significant increase in urinary levels of Clusterin was noted with animals administered both doses of Gentamicin. Similarly, significant increase in urinary levels of Kim-1, Cystatin C and NGAL were noticed with animals administered Gentamicin at 100 mg/kg/day on both Day 4 and 8. All these markers have shown dose-dependent change.
Table 2.
Urine chemistry markers on Day 4 and Day 8 (n = 10).
| Treatment | CREA | BUN | Total protein | mALB | Urinary protein | |
|---|---|---|---|---|---|---|
| (mg/dL) | (mg/dL) | (g/dl) | (mg/l) | (g/l) | ||
| Day 4 | ||||||
| Control | Mean | 32.03 | 784.12 | 0.16 | 7.32 | 0.36 |
| SD | 20.32 | 373.23 | 0.11 | 3.34 | 0.25 | |
| N | 10 | 10 | 10 | 10 | 10 | |
| Gentamicin 30 mg/kg |
Mean | 26.27 | 733.65 | 0.16 | 13.41 | 0.47 |
| SD | 6.81 | 255.98 | 0.05 | 11.23 | 0.20 | |
| N | 10 | 10 | 10 | 10 | 10 | |
| Gentamicin 100 mg/kg |
Mean | 21.96 | 605.60 | 0.14 | 8.85 | 0.51 |
| SD | 5.86 | 139.32 | 0.06 | 2.14 | 0.20 | |
| N | 10 | 10 | 10 | 10 | 10 | |
| Day 8 | ||||||
| Control | Mean | 29.78 | 781.31 | 0.16 | 7.12 | 0.36 |
| SD | 6.80 | 193.08 | 0.04 | 4.02 | 0.15 | |
| N | 10 | 10 | 10 | 10 | 10 | |
| Gentamicin 30 mg/kg |
Mean | 29.98 | 630.37 | 0.24** | 17.73 | 0.66 |
| SD | 5.20 | 253.93 | 0.05 | 16.76 | 0.29 | |
| N | 10 | 10 | 10 | 10 | 10 | |
| Gentamicin 100 mg/kg |
Mean | 14.85*** | 392.06*** | 0.31*** | 77.74*** | 1.80*** |
| SD | 2.87 | 108.23 | 0.09 | 11.12 | 0.55 | |
| N | 10 | 10 | 10 | 10 | 10 | |
One way ANOVA followed by Dunnett’s multiple comparison test (P < 0.01).
One way ANOVA followed by Dunnett’s multiple comparison test (P < 0.001); CREA = Creatinine, BUN = Blood urea nitrogen, mALB = microalbumin.
3.3. Gross pathology, weight and histological evaluations
On Day 4 and 8, kidneys were pale, discolored and enlarged in animals treated with Gentamicin at 30 and 100 mg/kg. Additionally, perirenal edema in animals treated with Gentamicin at 100 mg/kg on Day 8. These findings were correlated with organ weight increase and tubular epithelial cell degeneration/necrosis and tubular epithelial cell regeneration/basophilia in the cortex and the medulla on Day 8.
Incidence and severity of histopathological changes seen in kidneys were as mentioned below. All control animals showed normal structure and architecture of all areas of kidneys. On Day 4, no pathological changes noticed with Gentamicin at 30 mg/kg except solitary incidence of minimal tubular basophilia/regeneration (Fig. 1) indicating minimal damage without any change in serum BUN and creatinine levels but increase in urinary Clusterin level. Gentamicin at 100 mg/kg showed multifocal areas of minimal tubular dilatation (2/6), minimal tubular degeneration (2/6), minimal tubular epithelial vacuolation (2/6) and minimal and mild interstitial inflammation (1/6, 5/6 respectively) (Fig. 1).
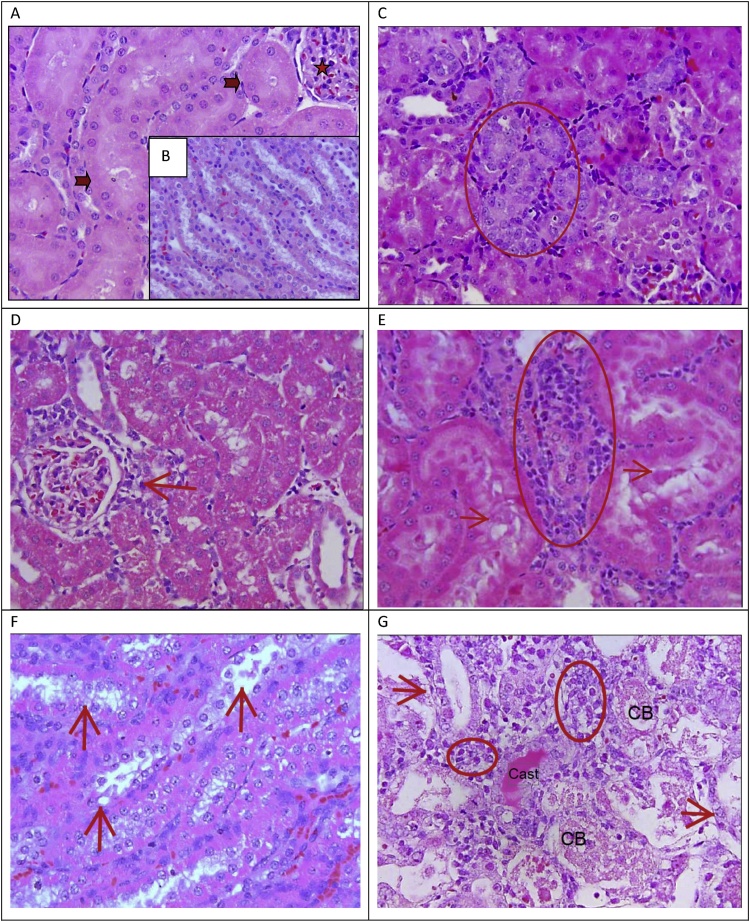
Fig. 1.
A: Normal structure of glomeruli (star) and tubules (arrow); B: Normal structure of collecting ducts; C: Minimal tubular basophilia (circle) with Gentamicin at 30 mg/kg/day on Day 4. D: Minimal focal inflammatory cell collection around glomeruli (arrow) with Gentamicin at 100 mg/kg/day on Day 4. E: Mild inflammatory cell (interstitial) infiltration (circle) and tubular degeneration with Gentamicin at 30 mg/kg/day on Day 8. F: Mild degeneration and necrosis (arrow) of collecting ducts with Gentamicin at 30 mg/kg/day on Day 8. G: Severe distal tubular degeneration and necrosis (arrow), presence of cast, cellular debris (CB) and inflammatory cells/interstitial nephritis (circle) with Gentamicin at 100 mg/kg/day on Day 8. H & E, lens x40.
On Day 8, Gentamicin at 30 mg/kg/day showed multifocal areas of minimal and mild tubular dilatation (6/10 and 4/10), minimal and mild tubular degeneration (3/10 and 2/10 respectively), minimal and mild tubular epithelial vacuolation (1/10 and 3/10 respectively), minimal and mild interstitial inflammation (4/10 each), minimal tubular necrosis (2/10), presence of hyaline cast (2/10 minimal), and, minimal and mild accumulation of proteineous material in tubular lumen (4/10 and 2/10 respectively). Gentamicin at 100 mg/kg/day showed multifocal areas of moderate and severe tubular dilatation (4/10 and 6/10), mild and moderate tubular degeneration (3/10 and 7/10 respectively), minimal and mild tubular epithelial vacuolation (3/10 each), minimal, mild and moderate interstitial inflammation (1/10, 4/10 and 5/10 respectively), moderate and severe tubular necrosis (3/10 and 7/10 respectively), presence of hyaline cast (4/10 each minimal and moderate), granular cast (4/4 each mild and moderate), mild and moderate accumulation of cellular debris within the tubules (2/10 and 6/10 respectively), mild accumulation of proteineous material in tubular lumen (8/10) and presence of tubular epithelial hyaline droplets (3/10 mild, 1/10 moderate) (Fig. 1). These histological changes were dose and time dependent. Hyaline droplets are eosinophilic homogeneous cytoplasmic droplets present normally in the proximal tubule represent alpha2 μglobulin sequestered in secondary lysosomes. Tubular degeneration and hyaline cast were confined to proximal convoluted tubules, the distal convoluted tubules and/or the collecting ducts.
4. Discussion
No clinical signs noticed in any of the treated animals during the entire period of dosing. Slightly reduced body weight and food consumption was noticed on Day 7 in rats treated with 100 mg/kg of Gentamicin. There is paucity of reported information on change in body weight or food consumption with similar type of experiments/doses of Gentamicin, however, the reduction in body weight on Day 7 at 100 mg/kg/day dose level is related to nephrotoxicity as demonstrated in histological changes.
No significant elevation in serum BUN and creatinine level noticed in both doses of Gentamicin treated rats on Day 4 and in low dose of Gentamicin on Day 8. However, both these parameters were increased by 5.6 and 3.4 fold over control at 100 mg/kg on Day 8. No alterations in albumin noticed on Day 4 but it was significantly reduced at 30 and 100 mg/kg/day. Although there were no significant change in BUN and creatinine at 30 mg/kg/day in doses level of Gentamicin on Day 4 or Day 8, some of the urinary biomarkers have increased to several folds compared to control.
On Day 4, increase in mALB and UP was observed with Gentamicin at 30 and 100 mg/kg/day. On Day 8, increase in total protein was seen at both dose levels of Gentamicin; increase in urinary protein and mALB, and decrease in BUN and creatinine at 100 mg/kg of Gentamicin. A microalbumin in urine determines the presence of the albumin in urine. In a properly functioning body, albumin is not normally present in urine because it is retained in the bloodstream by the kidneys. It occurs when the kidney leaks small amounts of albumin into the urine, in other words, when there is an abnormally high permeability for albumin in the glomerulus of the kidney. Normally, filtered albumin is largely reabsorbed via endocytosis in the proximal tubule. Increases in urinary albumin could be due to impaired reabsorption of albumin by the proximal tubules. Hence, in the absence of glomerular injury, which can cause an increased filtered load that can saturate proximal tubular reabsorption mechanisms resulting in frank proteinuria, the presence of small amounts of albumin (microalbumin) is considered indicative of reduced functional (reabsorptive) capacity in the proximal tubules. In this study, it is noteworthy that significant increase in urinary mALB (albuminuria) but lack of changes in serum albumin (data not presented) levels in Gentamicin at 100 mg/kg on Day 8 is due to impaired reabsorption in the proximal tubules indicating effect of these nephrotoxicants on glomerular filtration rate or tubular injury which correlated with variety of histological changes in proximal convoluted tubules.
Creatinine has the mainstay for assessing renal function and GFR clinically. Creatinine is secreted by the liver and proximal tubules of kidneys, then being transported to the muscles via the blood. Creatinine is subject to glomerular filtration and, to a lesser extent tubular secretion. Creatinine concentrations can vary with individual body muscle mass and measurement source (serum versus urine). In the current study, urinary creatinine excretion in Gentamicin treated animals on Day 4 were comparable to control. However, decrease in creatinine at 100 mg/kg of Gentamicin was seen on Day 8. This was associated with severe histopathological changes in kidneys indicating decline in kidney function.
Blood urea nitrogen is a waste product of metabolism that is excreted by the kidneys in urine. During glomerular filtration, urea passes from blood to the glomerular filtrate, the fluid that is the precursor of urine. The concentration of urea in the filtrate as it is formed is similar to that in plasma so the amount of urea entering the proximal tube of the nephron from the glomerulus is determined by the GFR. Urea is both reabsorbed and secreted (recycled back into the filtrate) during passage of the filtrate through the rest of the tubule of the nephron; the net effect of these two processes results in around 30–50% of the filtered urea appearing in urine. In case of kidney damage, the urea will not properly get filtered into urine. This was exactly reflected in the current study where there were no significant changes in urinary BUN excretion on Day 4 with Gentamicin that correlated with minimal histological changes in kidneys. However, decrease in urinary BUN excretion seen in 100 mg/kg/day of Gentamicin that correlated with severe histopathological findings.
The traditional parameters like serum creatinine and BUN were significantly increased only on Day 8 at 100 mg/kg of Gentamicin. There were no significant change in serum creatinine and BUN levels on Day 4 at both doses and Day 8 at 30 mg/kg/day of Gentamicin. At 100 mg/kg of Gentamicin, the percentage change in serum creatinine and BUN level was around 3.4 and 5.6 fold, respectively over control, whereas the urinary biomarkers were found to 9.1 to 31.9 fold over respective control animals indicating sensitiveness of these urinary biomarkers. It was noted that the increase in BUN and creatinine at 100 mg/kg/day of Gentamicin was approximately 5.6 fold (for BUN) and 3.4 fold (for creatinine), on Day 8. This has correlated with severe degree of histological changes in kidneys with nephrotoxicant. Although the change in the BUN and creatinine were minimal but several fold change in some of the urinary biomarkers at 30 mg/kg of Gentamicin on Day 8 with the minimal to moderate histopathological changes in kidneys indicating sensitiveness of urinary biomarkers over traditional markers like BUN and creatinine.
The tubular proteins released during tubular injury have garnered much attention as better biomarkers as they can potentially be true, early, real time and proportionate to the injury as compared to conventional or traditional markers/endpoints. Current experimental data suggests Clusterin is highly sensitive urinary biomarkers of nephrotoxicity with Gentamicin in male Sprague Dawley rats. The level of this biomarker was increased at the low doses of Gentamicin, and was duration dependent. Similarly, Kim-1, Cystatin and NGAL shown to be highly sensitive urinary biomarker for Gentamicin (only at 100 mg/kg) as the levels were increased on Day 4 and reproduced on Day 8 too. The magnitude change in urinary biomarkers with Gentamicin treated (30 and 100 mg/kg on Day 4 and 8) animals compared to serum creatinine and BUN is in accordance with Ozer et al. [42] and Zhou et al. [51].
Clusterin is a disulfide-linked heterodimeric protein of 75 kDa, consisting of α- and β-subunits [24,25]. Clusterin is a glycoprotein responsible for clearance of cellular debris and apoptosis [26]. Changes of protein levels of clusterin have been observed in kidneys and in the urine in animal studies, as well as in human. Although clusterin is expressed in several tissues, its molecular size prevents a filtration of clusterin in the kidney, thus rendering its urinary levels specific to kidney injury and proposed as potential markers of kidney toxicity [28]. Clusterin was among those urinary proteins recently shown to outperform serum BUN and creatinine for detection of chemical-induced tubular injury [41]. The dose-dependent and duration dependent increase in Clusterin in the current experiment on Day 4 and Day 8 with Gentamicin treated animals is corroborated with previous reports and histological changes suggesting it as sensitive urinary biomarker to detect early renal damage [42,[52], [53], [54]].
Kim-1 is a type 1 cell membrane glycoprotein, and its mRNA levels will be markedly elevated after initiation of kidney injury. Kim-1 rapidly synthesized and the protein generated is found in high levels at the apical membrane of the proximal tubule [33]. The data generated by Vaidya et al. [37] and Ozer et al. [42] demonstrated which Kim-1 out-performed traditional markers (serum creatinine and BUN), were reviewed by regulatory authorities before deciding that Kim-1 was qualified for the detection of acute drug-induced nephrotoxicity in rats. The current study result of Kim-1 changes for Gentamicin treated animals both in Day 4 and 8 is in parallel to reports from various authors [28,[36], [37], [38],42,50,51,55]. Both the FDA and EMA consider Kim-1 to be a highly sensitive biomarker for detecting acute drug induced injury during drug development [48]. The evidence from the current study further confirms that urinary Kim-1 is a highly sensitive and specific biomarker for the early detection of nephrotoxicant-induced proximal tubular injury.
Cystatin C (CysC) is a small molecule of 13.3 kDa, 122 amino acid non-glycosylated basic cysteine protease (lysosomal proteinases) inhibitor, preventing breakdown of certain intracellular and extracellular proteins within the body, constitutively expressed by all nucleated cells, and is produced and released to the plasma at a steady rate [40]. The current study result of significantly increased urinary Cystatin C in Gentamicin at 100 mg/kg on Day 4 and Day 8 which is in accordance with report from Fuchs et al. [53]. The suggested mechanism of reduced reabsorptive capability caused by proximal tubular cell breakdown associated with increased urine Cystatin C correlates with findings in this study. This suggests that it is a sensitive and specific biomarker for the early detection of nephrotoxicant-induced renal injury.
NGAL is a 25 KDa glycoprotein that is expressed at low levels in several human tissues, including epithelial cells located in the proximal tubule and neutrophils [43,44]. NGAL has been reported to be increased within the thick ascending limb of the loop of Henle, distal tubule and collecting duct with nephrotoxic injury in rodents and humans. EMA and US FDA encouraged the conduct of nonclinical and exploratory clinical analyses to evaluate the translational relevance of changes in urinary NGAL for clinical trial authorization [48]. In the current study, significant increase in urinary NGAL were seen in Gentamicin at 100 mg/kg on Day 4 and 8. There was a trend in increase in NGAL in other treated group (30 mg/kg) on Day 4 and 8 as well. This seems to correlate well with minimal histological changes seen in distal tubule or collecting ducts with Gentamicin on Day 4 but moderate to severe changes seen in Day 8. The fold change in NGAL is comparable to serum BUN or creatinine on Day 4 with Gentamicin treated animals. However, on day 8 the NGAL was changed by 2.2 and 9.1 times with Gentamicin at 30 and 100 mg/kg/day, respectively. The fold change in serum creatinine and BUN on Day 8 at 30 mg/kg/day of Gentamicin was 1.0 (both serum creatinine and BUN), and 3.4 and 5.6 times at 100 mg/kg of Gentamicin respectively.
On Day 4 and 8, kidneys were pale, discolored and enlarged in animals treated with Gentamicin at 30 and 100 mg/kg. Additionally, perirenal edema in animals treated with Gentamicin at 100 mg/kg on Day 8. These findings were correlated with organ weight increase and tubular epithelial cell degeneration/necrosis and tubular epithelial cell regeneration/basophilia in the cortex and the medulla on Day 8. Pale discoloration of kidneys were reported previously by Gautier et al. [54] and Alarifi et al. [56]. No treatment related changes in kidney weights seen in any dose levels of Gentamicin treated groups on Day 4. On Day 8, the increase in kidney weight relative to body weight was seen in both doses Gentamicin which is in accordance with Gautier et al. [54]. This increase in relative kidney weights correlated with the histopathological changes seen in these groups.
The extensive dose-dependent severity of lesions noticed on Day 8 with Gentamicin treated animals were in accordance with several fold increases in the appearance of urinary biomarkers primarily Clusterin and Kim-1 in both doses, followed by increase in Cystatin C and NGAL at 100 mg/kg of Gentamicin. The magnitude of changes seen were 7.8 and 9.3; 3.2 and 14.6; 2.6 and 31.9 and 2.2 and 9.1 with Clusterin, Kim-1, Cystatin C and NGAL at 30 and 100 mg/kg of Gentamicin. There were no increase in serum BUN and creatinine at 30 mg/kg and marginal extent of 3.4 and 5.6 fold increase in serum creatinine and BUN at 100 mg/kg of Gentamicin indicating better sensitivity and specificity of urinary biomarkers over these conventional markers. The Day 4 findings seen in this study with Gentamicin is correlated with report from Gautier et al. [54]. The Day 8 histological findings showed that the cortex of the kidney was more affected than the medulla with Gentamicin administration. This indicate that a relatively higher concentration of gentamicin reaching the cortex via the bloodstream than that entering the medulla. The findings seen in this study with Gentamicin is correlated with the reported findings from [42,51,54,[57], [58], [59], [60], [61]].
Further similar work should be conducted in other species like mouse, hamster and dogs as well which will help in improving the percentage of prediction of nephrotoxicity of drugs and thus reduce attrition of novel drugs during advanced development stage. Standardization and validation of these novel biomarkers in dogs would also help whenever rodents are not the pharmacologically active species for novel drugs and thus to evaluate nephrotoxicity of novel drugs early during drug development.
5. Conclusion
This experimental result suggests Clusterin as highly sensitive urinary biomarker for Gentamicin induced nephrotoxicity in male Sprague Dawley rats. The temporal changes in increase in urinary Clusterin, Kim-1, Cystatin C and NGAL were noticed in Gentamicin treated animals. These urinary biomarkers showed several fold change compared to serum creatinine and BUN level. It is noteworthy that all these renal biomarkers had earlier-onset changes than either serum BUN or creatinine that demonstrates sensitivity of using these noninvasive biomarkers in evaluating drug induced nephrotoxicity. This experiment in male Sprague Dawley rats demonstrated once again that traditional markers fail to detect minimal to moderate kidney injury and minimal use in Clinical practice to monitor the patients. Hence, it is recommended to use these urinary biomarkers both in preclinical studies and Clinical studies or patients for monitoring of nephrotoxicity.
Conflict of interest statement
We, hereby declare that we have no conflict of interest related to this manuscript or research conducted and any organization or financial institution.
Acknowledgements
Authors thank Glenmark Pharmaceuticals Limited, Mumbai for providing facility for this work. No external grant has been used for this work.
References
- 1.Garrett M.D., Workman P. Discovering novel chemotherapeutic drugs for the third millennium. Eur. J. Cancer. 1999;35:2010–2030. doi: 10.1016/s0959-8049(99)00280-4. [DOI] [PubMed] [Google Scholar]
- 2.Lesko L.J., Atkinson A.J., Jr. Use of biomarkers and surrogate endpoints in drug development and regulatory decision-making: criteria, validation, strategies. Annu. Rev. Pharmacol. Toxicol. 2001;41:347–366. doi: 10.1146/annurev.pharmtox.41.1.347. [DOI] [PubMed] [Google Scholar]
- 3.Kola, Landis Can the pharmaceutical industry reduce attrition rates. Nat. Rev. Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller, Gstraunthaler G. Nephrotoxicity testing in vitro-what we know and what we need to know. Environ. Health Perspect. 1998;106:559–569. doi: 10.1289/ehp.98106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Naim A.B., Abdel-Wahab M.H., Attia F.F. Protective effects of vitamin E and probucol against gentamicin-induced nephrotoxicity in rats. Pharmacol. Res. 1999;40(2):183–187. doi: 10.1006/phrs.1999.0494. [DOI] [PubMed] [Google Scholar]
- 6.Hansen M., Christrup L.L., Jarlov J.O. Gentamicin dosing in critically ill patients. Acta Anaesthesiol. Scand. 2001;45:734–740. doi: 10.1034/j.1399-6576.2001.045006734.x. [DOI] [PubMed] [Google Scholar]
- 7.Nagai J., Saito M., Adachi Y., Yumoto R. Inhibition of gentamicin binding to rat renal brush-border membrane by megalin ligands and basic peptides. J. Control. Release. 2006;112:43–50. doi: 10.1016/j.jconrel.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Ali B.H., Al–Salam S., Al-Husseinia I., Nemmar A. Comparative protective effect of N-acetyl cysteine and tetramethylpyrazine in rats with gentamicin nephrotoxicity. J. Appl. Toxicol. 2008;29:302–307. doi: 10.1002/jat.1409. [DOI] [PubMed] [Google Scholar]
- 9.Priyamvada S., Priyadarshini M., Arivarasu N.A. The protective effect of dietary fish oil on gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Prostaglandins Leukot. Essent. Fatty Acids. 2008;78:369–381. doi: 10.1016/j.plefa.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Ali B.H., Al Zaabi M., Blunden G., Nemmar A. Experimental gentamicin nephrotoxicity and agents that modify it: a minireview of recent research. Mini review. Basic Clin. Pharmacol. Toxicol. 2011;9:225–232. doi: 10.1111/j.1742-7843.2011.00728.x. [DOI] [PubMed] [Google Scholar]
- 11.Sandhu J.S., Sehgal A., Gupta O., Singh A. Aminoglycoside nephrotoxicity revisited. JIACM. 2007;8(4):331–333. 2007. [Google Scholar]
- 12.Gonzalez L.S., Spencer J.P. Aminoglycosides: a practical review. Am. Fam. Physician. 1998;58:1811–1820. [PubMed] [Google Scholar]
- 13.Houghton Donald C., English J., Bennett W.M. Chronic tubulointerstitial nephritis and renal insufficiency associated with long-term sub-therapeutic gentamicin. J. Lab. Clin. Med. 1988;112:694–703. [PubMed] [Google Scholar]
- 14.Abdel-Daim M.M., Abuzead S.M., Halawa S.M. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8:e72991. doi: 10.1371/journal.pone.0072991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Daim M.M., Abd Eldaim M.A., Mahmoud M.M. Trigonella foenum-graecum protection against deltamethrin-induced toxic effects on haematological, biochemical, and oxidative stress parameters in rats. Can. J. Physiol. Pharmacol. 2014;92:679–685. doi: 10.1139/cjpp-2014-0144. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sayed E., Abdel-Daim M.M. Protective role of Cupressuflavone from Cupressus macrocarpa against carbon tetrachloride-induced hepato- and nephrotoxicity in mice. Planta Med. 2014;80:1665–1671. doi: 10.1055/s-0034-1383211. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Daim M.M., El-Ghoneimy A. Synergistic protective effects of ceftriaxone and ascorbic acid against subacute deltamethrin-induced nephrotoxicity in rats. Ren. Fail. 2015;37:297–304. doi: 10.3109/0886022X.2014.983017. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Daim M.M., Taha R., Ghazy E.W., El-Sayed Y.S. Synergistic ameliorative effects of sesame oil and alpha-lipoic acid against subacute diazinon toxicity in rats: hematological, biochemical, and antioxidant studies. Can. J. Physiol. Pharmacol. 2016;94:81–88. doi: 10.1139/cjpp-2015-0131. [DOI] [PubMed] [Google Scholar]
- 19.Al-Sayed E., Abdel-Daim M.M., Kilany O.E., Karonen M., Sinkkonen J. Protective role of polyphenols from Bauhinia hookeri against carbon tetrachloride-induced hepato- and nephrotoxicity in mice. Ren. Fail. 2015;37:1198–1207. doi: 10.3109/0886022X.2015.1061886. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Daim M.M. Synergistic protective role of ceftriaxone and ascorbic acid against subacute diazinon-induced nephrotoxicity in rats. Cytotechnology. 2016;68:279–289. doi: 10.1007/s10616-014-9779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fahmy N.M., Al-Sayed E., Abdel-Daim M.M., Karonen M., Singab A.N. Protective effect of Terminalia muelleri against carbon tetrachloride-induced hepato and nephro-toxicity in mice and characterization of its bioactive constituents. Pharm. Biol. 2016;54:303–313. doi: 10.3109/13880209.2015.1035794. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Daim M.M., Abdeen A. Protective effects of rosuvastatin and vitamin E against fipronil-mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem. Toxicol.: Br. Ind. Biol. Res. Assoc. 2018;114:69–77. doi: 10.1016/j.fct.2018.01.055. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Daim M.M., El-Sayed Y.S., Eldaim M.A., Ibrahim A. Nephroprotective efficacy of ceftriaxone against cisplatin-induced subchronic renal fibrosis in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2017;390:301–309. doi: 10.1007/s00210-016-1332-5. [DOI] [PubMed] [Google Scholar]
- 24.Jones S.E., Jomary C. Clusterin. Int. J. Biochem. Cell Biol. 2002;34:427–431. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 25.Kujiraoka T., Takano M., Hattori H. Apolipoprotein j (apo j) Nippon Rinsho. 2004;62:117–120. [PubMed] [Google Scholar]
- 26.Schepeler Troels, Mansilla Francisco, Christensen Lise L. Clusterin expression can be modulated by changes in TCF1 mediated Wnt signaling. J. Mol. Signal. 2007;2:6–18. doi: 10.1186/1750-2187-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg M.E., Silkensen J. Clusterin: physiologic and pathophysiologic considerations. Int. J. Biochem. Cell Biol. 1995;27:633–645. doi: 10.1016/1357-2725(95)00027-m. [DOI] [PubMed] [Google Scholar]
- 28.Amin R.P., Vickers A.E., Sistare F., Thompson K.L., Roman R.J., Lawton M., Kramer J., Hamadeh H.K., Collins J., Grissom S., Bennett L., Tucker C.J., Wild S., Kind C., Oreffo V., Davis J.W., 2nd, Curtiss S., Naciff J.M., Cunningham M., Tennant R., Stevens J., Car B., Bertram T.A., Afshari C.A. Identification of putative gene based markers of renal toxicity. Environ. Health Perspect. 2004;112:465–479. doi: 10.1289/ehp.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witzgall R., Brown D., Schwarz C., Bonventre J.V. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J. Clin. Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hidaka S., Kränzlin B., Gretz N., Witzgall R. Urinary clusterin levels in the rat correlate with the severity of tubular damage and may help to differentiate between glomerular and tubular injuries. Cell Tissue Res. 2002;310:289–296. doi: 10.1007/s00441-002-0629-5. [DOI] [PubMed] [Google Scholar]
- 31.Girton R.A., Sundin D.P., Rosenberg M.E. Clusterin protects renal tubular epithelial cells from gentamicin-mediated cytotoxicity. Am. J. Physiol. Renal Physiol. 2002;282:F703–F709. doi: 10.1152/ajprenal.00060.2001. [DOI] [PubMed] [Google Scholar]
- 32.Aulitzky W.K., Schlegel P.N., Wu D.F., Cheng C.Y., Chen C.L., Goldstein M., Reidenberg M., Bardin C.W. Measurement of urinary clusterin as an index of nephrotoxicity. Proc. Soc. Exp. Biol. Med. 1992;199:93–96. doi: 10.3181/00379727-199-43335. [DOI] [PubMed] [Google Scholar]
- 33.Ichimura T., Bonventre J.V., Bailly V., Wei H., Hession C.A., Cate R.L., Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 34.Ichimura T., Hung C.C., Yang S.A., Stevens J.L., Bonventre J.V. Kidney injury molecule-1; a tissue and urinary biomarker for nephrotoxicant induced renal injury. Am. J. Physiol. Ren. Physiol. 2004;286:F552–563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 35.Vaidya V.S., Ramirez V., Ichimura T., Bobadilla N.A., Bonventre J.V. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am. J. Physiol. Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 36.Tonomura Y., Tsuchiya N., Torii M., Uehara T. Evaluation of the usefulness of urinary biomarkers for nephrotoxicity in rats. Toxicology. 2010;273:53–59. doi: 10.1016/j.tox.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 37.Vaidya V.S., Ozer J.S., Dieterle F., Collings F.B., Ramirez V., Troth S., Muniappa N., Thudium D., Gerhold D., Holder D.J., Bobadilla N.A., Marrer E., Perentes E., Cordier A., Vonderscher J., Maurer G., Goering P.L., Sistare F.D., Bonventre J.V. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Togashi Y., Sakaguchi Y., Miyamoto M., Miyamoto Y. Urinary cystatin C as a biomarker for acute kidney injury and its immunohistochemical localization in kidney in the CDDP treated rats. Exp. Toxicol. Pathol. 2012;64:797–805. doi: 10.1016/j.etp.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Ichimura T., Asseldonk E.J., Humphreys B.D., Gunaratnam L., Duffield J.S., Bonventre J.V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin. Nephrol. 1992;38(Suppl. 1):S20–S27. [PubMed] [Google Scholar]
- 41.Dieterle Frank, Perentes Elias, Cordier André, Roth Daniel R., Verdes Pablo, Grenet Olivier, Pantano Serafino, Moulin Pierre, Wahl Daniel, Mahl Andreas, End Peter, Staedtler Frank, Legay François, Carl Kevin, Laurie David, Chibout Salah-Dine, Vonderscher Jacky, Maurer G.érard. Urinary clusterin, cystatin C, β2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat. Biotechnol. 2010;28:463–472. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 42.Ozer JosefS., Dieterle Frank, Troth Sean, Perentes Elias, Cordier André, Verdes Pablo, Staedtler Frank, Mahl Andreas, Grenet Olivier, Roth DanielR., Wahl Daniel, Legay François, Holder Daniel, Erdos Zoltan, Vlasakova Katerina, Jin Hong, Yu Yan, Muniappa Nagaraja, Forest Tom, Clouse HollyK., Reynolds Spencer, Bailey Wendy J., Thudium DouglasT., Topper Michael J., Skopek Thomas R., Sina JosephF., Glaab WarrenE., Vonderscher Jacky, Maurer G.érard, Chibout Salah-Dine, Sistare Frank D., Gerhold David L. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat. Biotechnol. 2010;28:486–496. doi: 10.1038/nbt.1627. [DOI] [PubMed] [Google Scholar]
- 43.Le Cabec V., Calafat J., Borregaard N. Sorting of the specific granule protein, NGAL, during granulocytic maturation of HL-60 cells. Blood. 1997;89:2113–2121. [PubMed] [Google Scholar]
- 44.Flower D.R., North A.C., Sansom C.E. The lipocalin protein family: structural and sequence overview. Biochim. Biophys. Acta. 2000;1482:9–24. doi: 10.1016/s0167-4838(00)00148-5. [DOI] [PubMed] [Google Scholar]
- 45.Mishra J., Ma Q., Prada A., Mitsnefes M., Zahedi K., Yang J., Barasch J., Devarajan P. Identification of neutrophil gelatinase associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 46.Mishra J., Mori K., Ma Q., Kelly C., Barasch J., Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am. J. Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 47.Bolignano D., Donato V., Coppolino G., Campo S., Buemi A., Lacquaniti A., Buemi M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008;52:595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 48.EMA/651243/2014. Letter of Support for PSTC translational Drug-Induced Kidney Injury (DIKI) biomarkers.
- 49.Haase M., Bellomo R., Devarajan P., Schlattmann P., Haase-Fielitz A. NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am. J. Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann D., Fuchs T.C., Henzler T., Matheis K.A. Evaluation of a urinary kidney biomarker panel in rat models of acute and subchronic nephrotoxicity. Toxicology. 2010;277:49–58. doi: 10.1016/j.tox.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Zhou Y., Vaidya V.S., Brown R.P. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury and chromium. Toxicol. Sci. 2008;101:159–170. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rouse Rodney L., Zhang Jun, Stewart Sharron R. Comparative profile of commercially available urinary biomarkers in preclinical drug induced kidney injury and recovery in rats. Kidney Int. 2011;79:1186–1197. doi: 10.1038/ki.2010.463. [DOI] [PubMed] [Google Scholar]
- 53.Fuchs Tobias Christian, Frick Katharina, Emde Barbara. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol. Pathol. 2012;40:1031–1048. doi: 10.1177/0192623312444618. [DOI] [PubMed] [Google Scholar]
- 54.Gautier J.C., Gury T., Guffroy M. Comparison between male and female Sprague-Dawley rats in the response of urinary biomarkers to injury induced by gentamicin. Toxicol. Pathol. 2014;42:1105–1116. doi: 10.1177/0192623314524489. [DOI] [PubMed] [Google Scholar]
- 55.Bailly V., Zhang Z., Meier W. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J. Biol. Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 56.Alarifi Saud, Al-Doaiss Amin, Alkahtani Saad, Al Farraj S.A. Blood chemical changes and renal histological alterations induced by gentamicin in rats. Saudi J. Biol. Sci. 2012;19:103–110. doi: 10.1016/j.sjbs.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bounous D.I., Westcott D.M., Dambach H. Presented at Society of Toxicology, 2007EMA/651243/2014. Letter of Support for PSTC Translational Drug-Induced Kidney Injury (DIKI) Biomarkers. 2007. Evaluation of novel biomarkers of nephrotoxicity in rats treated with gentamicin. [Google Scholar]
- 58.Zhang Jun, Brown Ronald P., Shaw Martin. Immunolocalization of Kim-1, RPA-1, and RPA-2 in kidney of gentamicin-, mercury-, or chromium-treated rats: relationship to renal distributions of iNOS and nitrotyrosine. Toxicol. Pathol. 2008;36:397–409. doi: 10.1177/0192623308315832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozbek E., Cekmen M., Ilbey Y.O. Atorvastatin prevents gentamicin induced renal damage in rats through the inhibition of p38-MAPK and NF-kappaB pathways. Ren. Fail. 2009;31:382–392. doi: 10.1080/08860220902835863. [DOI] [PubMed] [Google Scholar]
- 60.Balakumar P., Rohilla A., Thangathirupathi A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol. Res. 2010;62:179–186. doi: 10.1016/j.phrs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki Daisuke, Yamada Atsushi, Umeno Hitomi, Kurihara Hiroshi, Nakatsuji Shunji, Fujihira Shiro, Tsubota Kenjiro, Ono Mihoko, Moriguchi Akira, Watanabe Kouji, Seki Jiro. Comparison of the course of biomarker changes and kidney injury in a rat model of drug-induced acute kidney injury. Biomarkers. 2011;16:553–566. doi: 10.3109/1354750X.2011.613123. [DOI] [PubMed] [Google Scholar]