Abstract
Background
The association between celiac disease (CD) and the development of lymphoid and gastrointestinal (GI) malignancies have been reported. However, data are scarce yet needed to develop evidence-based follow-up programs.
Objective
The objective of this article is to assess relative (RR) and absolute risks of lymphoma and GI carcinoma for newly diagnosed patients.
Methods
A case-control design to determine RR was performed with cases (lymphoma or GI carcinoma) and controls (melanoma or basal cell carcinoma) diagnosed 1994–2014, retrieved from the Dutch nationwide population-based pathology database (PALGA). Within this population, individuals with histologically proven CD before or simultaneously diagnosed with the malignancy were identified.
Results
A total of 349/301,425 cases (0.1%) and 282/576,971 (0.05%) controls were diagnosed with CD. Risk of T-cell lymphoma, predominantly enteropathy-associated T-cell lymphoma (EATL), was strongly associated with CD diagnosis (RR = 35.8 (95% CI 27.1–47.4)). Although most often synchronously diagnosed, T-cell lymphoma RR ≥ 1 year after CD diagnosis was still elevated (RR = 12.7 (95% CI 7.6–21.3)). Other CD-associated malignancies were small bowel adenocarcinoma (RR = 11.9 (95% CI 8.2–17.2)) and esophageal squamous cell carcinoma (RR = 3.5 (95% CI 2.1–5.8)). Absolute risks were relatively low. Other types of lymphomas and GI carcinomas were not associated with CD.
Conclusion
Increased risk for specific malignancies in CD should alert physicians for EATL (both intestinal and extraintestinal) and small bowel adenocarcinoma in patients with CD diagnosed at age ≥ 50 years.
Keywords: Celiac disease, enteropathy-associated T-cell lymphoma, small bowel adenocarcinoma, squamous cell carcinoma, follow-up
Key points
What is the current knowledge?
- Although celiac disease usually runs a benign course, it is associated with certain malignancies.
- Enteropathy-associated T-cell lymphoma and small bowel adenocarcinoma are the best-known associated malignancies.
- Data regarding relative and absolute risks for malignancies over time after celiac disease diagnosis are insufficiently known but necessary for evidence-based follow-up guidelines.
What is new here?
- After diagnosis, celiac disease patients have an elevated risk of being diagnosed with T-cell lymphoma, small bowel adenocarcinoma and esophageal squamous cell carcinoma.
- Absolute celiac disease-associated risks of malignancies were relatively low with a highest absolute risk of 4.3% for T-cell lymphoma in males between the age of 50 and 80 years when CD is diagnosed at age 50 years.
- In patients with enteropathy-associated T-cell lymphoma, lymphoma and celiac disease were often synchronously diagnosed.
Introduction
Celiac disease (CD) is an autoimmune-mediated enteropathy caused by ingestion of gluten with a prevalence of 0.5%–1% in the Western population.1 Symptoms resolve in most patients once a gluten-free diet (GFD) is started. Although the disease runs a benign course in the majority of patients, associations between CD and the development of various malignancies have been reported. Of these, the associations with enteropathy-associated T-cell lymphoma (EATL) and small bowel adenocarcinoma are best supported.2,3 However, the relative (RR) and absolute risks for these serious complications are still insufficiently known and risk estimates for other types of malignancies, such as other lymphoma types and gastrointestinal (GI) carcinomas, are inconsistent.4–10 Systematic data on the evolution of risk over time after CD diagnosis are sparse. Although some studies suggested an association, it is largely unclear whether the risk of developing malignant diseases is influenced by a strict GFD.11,12 As the most important consequence of these uncertainties, no evidence-based guidelines for counseling and screening of newly diagnosed CD patients can be given.
In this population-based, nationwide study, we aim to assess the RR and absolute risks of being diagnosed with a carcinoma in the GI tract and malignant lymphoma after a histologically confirmed diagnosis of CD and to describe the characteristics of this unselected, population-based group of patients to support counseling of newly diagnosed patients.
Patients and methods
Design and study population: case-control study
To estimate RR and absolute risks of developing GI carcinomas and different types of malignant lymphomas in newly diagnosed CD patients, we performed a population-based, case-control study comparing the prevalence of a history of CD in patients with malignant lymphoma or GI carcinoma (cases) to controls. Patients with basal cell carcinoma (BCC) or melanoma were selected as controls since a causal relationship with CD has never been suggested for these malignancies and since these controls could be selected from the same database as the cases. Identification was based on data from the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA), which has included nationwide coverage of all academic and nonacademic centers since 1991.13 Standardized coding of diagnoses and pseudonymization of patients allows for anonymized comprehensive searches and population-based epidemiological studies.
We conducted a case-control study rather than a cohort study of all individuals registered with CD in PALGA because we aimed to restrict our study to patients with a histologically confirmed CD diagnosis using very strict criteria. Fifty percent of putative CD patients did not fulfill these strict criteria. It would have been too labor intensive to perform this confirmation step for all putative CD diagnoses in the entire PALGA database (1994–2014, > 32,000 all ages, > 12,000 above 50 years). Therefore, a case-control study is a more efficient approach than a cohort study when it is necessary to obtain more detailed verification of “the exposure” (CD).
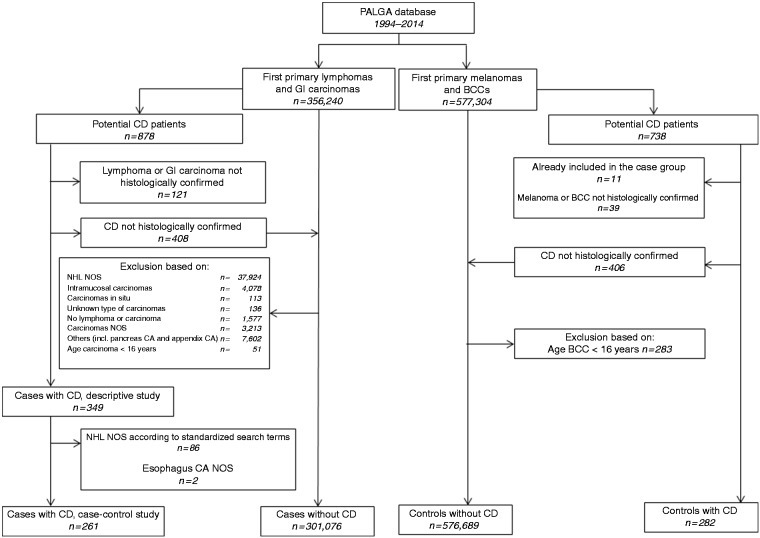
The selection strategy for the case and control groups is depicted in Figure 1. For detailed descriptions and selection criteria, see Supplementary File 1. In brief, all patients with a first primary diagnosis of any class of malignant lymphoma or GI carcinoma diagnosed between January 1994 and December 2014 in the Netherlands were selected. In this study population we identified all patients with a histologically supported diagnosis of CD, diagnosed between January 1994 and December 2014, prior or up to three months after the diagnosis of lymphoma or carcinoma. All CD diagnoses in the cases and controls were checked based on the original pathology report conclusion texts. The strict criteria for histologically confirmed CD involved a pathology diagnosis stating: (1) in accordance with CD diagnosis, (2) Marsh IIIA, IIIB or IIIC, with Marsh IIIA included only if at least two out of three criteria (intraepithelial lymphocytosis, crypt hyperplasia and villous atrophy14) were mentioned, or (3) (sub)total villous atrophy together with intraepithelial lymphocytes and/or the presence of CD antibodies, reported in the conclusion of the histological report.
Figure 1.
Selection process of case and control patients.
BCC: basal cell carcinoma; CA: carcinoma, CD: celiac disease; GI: gastrointestinal; NHL: non-Hodgkin lymphoma; NOS: not otherwise specified; PALGA: Dutch nationwide population-based pathology database.
For the control population we identified all patients with a first primary diagnosis of BCC or melanoma between January 1994 and December 2014 in the PALGA database. Patients with both lymphoma or GI carcinoma and BCC or melanoma were considered as cases. Subsequently, for the selected control patients, an identical strategy to identify CD patients was applied as for the case patients
Design and study population: descriptive study of characteristics of lymphoma and GI carcinoma in CD patients
We examined the characteristics of lymphoma and GI carcinoma in CD patients, based on the original pathology report conclusion texts from PALGA.
Diagnoses of lymphomas in patients with a history of CD were confirmed by an expert pathologist (DDJ) based on the original pathology report conclusion text and reclassified according to the 2008 World Health Organization classification if possible.15
Statistical analysis
For each lymphoma and GI carcinoma subgroup, odds ratios (ORs) including 95% confidence intervals (95% CI) associated with CD history were calculated with adjustment for age at time of case or control diagnosis (continuous variable) and gender. Since the OR approximates the RR, we denote ORs as RRs. Effect modification by gender or age at CD diagnosis was evaluated by fitting separate CD effects for levels of the effect modifier. These analyses included a main effect for the effect modifier. Interaction effects between CD and continuous effect modifiers were used as trend test. To analyze whether time between CD diagnosis and malignancy ( < 1 year vs ≥ 1 year) affected the RRs, analyses were restricted to case/control diagnoses between 2004 and 2014 and CD diagnoses between 1994 and 2014 to perform an analysis in which all patients had at least a minimum potential time between CD and malignancy of 10 years. Interval between CD and malignancy of patients with case/control diagnosis before CD (maximum of three months) were in this analysis coded as simultaneous CD and case/control diagnosis.
A sensitivity analysis was performed excluding all patients with CD diagnosis three months before until three months after case/control diagnosis, to analyze the effect of a potential diagnostic bias since patients with BCC or melanoma are far less likely to undergo upper endoscopic examination than patients with GI carcinoma, and thereby less likely to be diagnosed with CD.
For calculation of the absolute risk among CD patients, we multiplied the general population incidence rates by the CD-associated RR (including 95% CI). Data from the general population were obtained from the Netherlands Cancer Registry (2005–2009).16 We calculated the risk of malignancy until age 80 years, the average life expectancy of the Dutch population,17 for individuals alive and free of malignancy at age “x” years (life table risk), “x” ranging between 50 and 75, taking competing causes of death into account. The range 50–75 was chosen based on the distribution of age in the CD group in this study. We assumed that the RR of cancer associated with CD is homogeneous across age groups and that all-cause mortality among CD patients is similar to the general population.
Statistical analyses were performed using SPSS version 22.0 software (SPSS Inc, Chicago, IL, USA). A p value of < 0.05 was considered as statistically significant.
Ethics
This study was approved by the Scientific Board of PALGA complying with the regulations for anonymized (epidemiological) studies and considered to remain outside the restrictions of the Medical Research Involving Human Subjects Act (WMO) by the Medical Ethics Review Committee of VU University Medical Center on April 6, 2016 (METC-VUMC 2016.133). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Results
From the nationwide population-based PALGA database, we identified 301,425 cases with lymphoma or GI carcinoma. Among these patients, a confirmed diagnosis of CD was listed in 349 (0.1%). Of 576,971 control patients with BCC or melanoma, 282 had a history of histologically confirmed CD (0.05%).
Risks for lymphoma and GI carcinoma in CD patients
With correction for age at time of case or control diagnosis and gender, we found significantly elevated RRs associated with a CD history for all T-cell lymphoma (RR = 35.8 (95% CI 27.1–47.4)), for duodenal adenocarcinoma (RR = 10.2 (95% CI 6.2–17.0)), distal small bowel adenocarcinoma (RR = 14.4 (95% CI 8.5–24.2)) and squamous cell carcinoma (SCC) of the esophagus (RR = 3.5 (95% CI 2.1–5.8)) (Table 1). The CD-associated risk increase for esophageal SCC was restricted to female patients (RR = 5.9 (95% CI 3.3–10.3)) (Table 2). Other classes of malignant lymphoma or GI carcinoma were not associated with CD history. In T-cell lymphomas and GI carcinomas associated with CD, a nonstatistically significantly increased risk was found in patients diagnosed with CD at an older age (Table 2).
Table 1.
Celiac disease-related relative risks for lymphoma and gastrointestinal carcinoma.
| Group | Total number | Celiac disease n (%) | No celiac disease n (%) | Adjusteda RR (95% CI) |
|---|---|---|---|---|
| Control group | ||||
| BCC/melanoma | 576,971 | 282 (0.05%) | 576,689 (99.95%) | 1.0 (reference) |
| Case group | ||||
| T-cell NHL | 4046 | 63 (1.6%) | 3983 (98.4%) | 35.8 (27.1–47.4)b |
| B-cell NHL | 25,183 | 17 (0.07%) | 25,166 (99.93%) | 1.4 (0.9–2.3) |
| Hodgkin lymphoma | 8076 | 3 (0.04%) | 8073 (99.96%) | 1.0 (0.3–3.3) |
| Adenocarcinoma esophagus | 18,322 | 12 (0.07%) | 18,310 (99.93%) | 1.5 (0.8–2.6) |
| Squamous cell carcinoma esophagus | 9776 | 16 (0.2%) | 9760 (99.8%) | 3.5 (2.1–5.8)b |
| Adenocarcinoma stomach | 32,281 | 12 (0.04%) | 32,269 (99.96%) | 0.8 (0.4–1.4) |
| Adenocarcinoma duodenum | 3237 | 16 (0.5%) | 3221 (99.5%) | 10.2 (6.2–17.0)b |
| Adenocarcinoma jejunum/ileum | 2129 | 15 (0.7%) | 2114 (99.3%) | 14.4 (8.5–24.2)b |
| Adenocarcinoma colorectal | 195,244 | 105 (0.05%) | 195,139 (99.95%) | 1.1 (0.9–1.4) |
| Squamous cell carcinoma anus | 3043 | 2 (0.07%) | 3041 (99.93%) | 1.4 (0.3–5.5) |
BCC: basal cell carcinoma; CI: confidence interval; n: number; NHL: non-Hodgkin lymphoma; RR: relative risk; SCC: squamous cell carcinoma.
Adjusted for gender and age at case or control diagnosis, based on unconditional logistic regression.
Statistically significant.
Table 2.
CD-associated relative risks for lymphoma and gastrointestinal carcinoma: Stratified analysis by gender and age at CD diagnosis.
| T-cell lymphoma |
Small bowel adenocarcinomaa |
Esophageal SCC |
Colorectal carcinoma |
|
|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| No CD | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| CD | 35.8 (27.1–47.4) | 11.9 (8.2–17.2) | 3.5 (2.1–5.8) | 1.1 (0.9–1.4) |
| Gender | ||||
| Male | 35.9 (24.1–53.4) | 12.4 (7.1–21.7) | 1.2 (0.4–3.9) | 1.1 (0.8–1.5) |
| Female | 35.7 (24.1–53.0) | 11.4 (6.9–18.9) | 5.9 (3.3–10.3) | 1.2 (0.9–1.6) |
| p heterogeneity | NS | NS | 0.02 | NS |
| Age at CD diagnosis | ||||
| <60 years | 27.4 (18.1–41.5) | 8.1 (4.0–16.6) | 1.7 (0.5–5.2) | 1.0 (0.7–1.5) |
| ≥60 years | 46.1 (31.7–67.0) | 14.2 (9.2–22.1) | 4.7 (2.6–8.2) | 1.2 (0.9–1.6) |
| p heterogeneity | NS | NS | NS | NS |
CD: celiac disease; CI: confidence interval; NS: not significant; RR: relative risk; SCC: squamous cell carcinoma.
Duodenum, jejunum and ileum.
Subgroup analysis according to time between CD and malignancy showed that the association between CD and T-cell lymphoma decreased from an RR of 157.6 (95% CI 92.2–269.6) within one year after CD diagnosis to 12.7 (95% CI 7.6–21.3) after ≥ 1 year (p value for trend = 0.001). The association between small bowel adenocarcinoma also decreased ≥ 1 year after CD diagnosis, from an RR of 38.0 (95% CI 17.8–81.0) to 6.4 (3.7–11.2), although the p value for trend was not significant. This decrease over time was not noted for esophageal SCC (Table 3). Analysis of the risk of colorectal carcinoma over time showed that the risk was elevated during the first year after CD diagnosis (RR = 5.1 (95% CI 3.1–8.3)), whereas it was decreased after that period (RR = 0.7 (95% CI 0.5–0.9)), p value for trend < 0.001.
Table 3.
CD-related relative risks for lymphoma and gastrointestinal carcinoma, by time since CD diagnosis.
| T-cell lymphoma | Small bowel adenocarcinomaa | Esophageal SCC | Colorectal carcinoma | ||
|---|---|---|---|---|---|
| RRb (95% CI) | RRb (95% CI) | RRb (95% CI) | RRb (95% CI) | ||
| No CD | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | |
| CD | 30.4 (21.9–42.2) | 9.7 (6.3–15.1) | 3.9 (2.2–6.6) | 1.2 (0.9–1.5) | |
| Time since CD diagnosis | |||||
| <1 year n (cases with CD) | 157.7 (92.2–269.6) n = 28 | 38.0 (17.8–81.0) n = 9 | 4.9 (1.2–20.6) n = 2 | 5.1 (3.1–8.3) n = 43 | |
| ≥ 1 year n (cases with CD) | 12.7 (7.6–21.3) n = 16 | 6.4 (3.7–11.2) n = 13 | 3.7 (2.1–6.7) n = 12 | 0.7 (0.5–0.9) n = 50 | |
| p heterogeneity | <0.001c | <0.001d | NS | <0.001c | |
CD: celiac disease; CI: confidence interval; NS: not significant; RR: relative risk; SCC: squamous cell carcinoma.
Duodenum, jejunum and ileum.
Adjusted for gender and age at case or control diagnosis, based on unconditional logistic regression.
p value trend < 0.001.
p value trend = 0.10.
Sensitivity analyses were performed including only patients whose case or control diagnosis was established > 3 months after CD diagnosis. Results showed that T-cell lymphoma (RR = 17.6 (95% CI 11.9–26.0)), duodenal and distal small bowel adenocarcinoma (RR = 6.2 (95% CI 3.2–12.1) and RR = 9.3 (95% CI 4.8–18.1), respectively) and SCC of the esophagus (RR = 3.1 (95% CI 1.7–5.4)) were still associated with CD, although the associations for T-cell lymphoma and small bowel adenocarcinoma were weaker than in the primary analysis. Sensitivity analyses showed decreased risk for colorectal carcinoma after CD diagnosis (RR = 0.7 (95% CI 0.5–0.9), p = 0.02), whereas no association was found in the primary analysis.
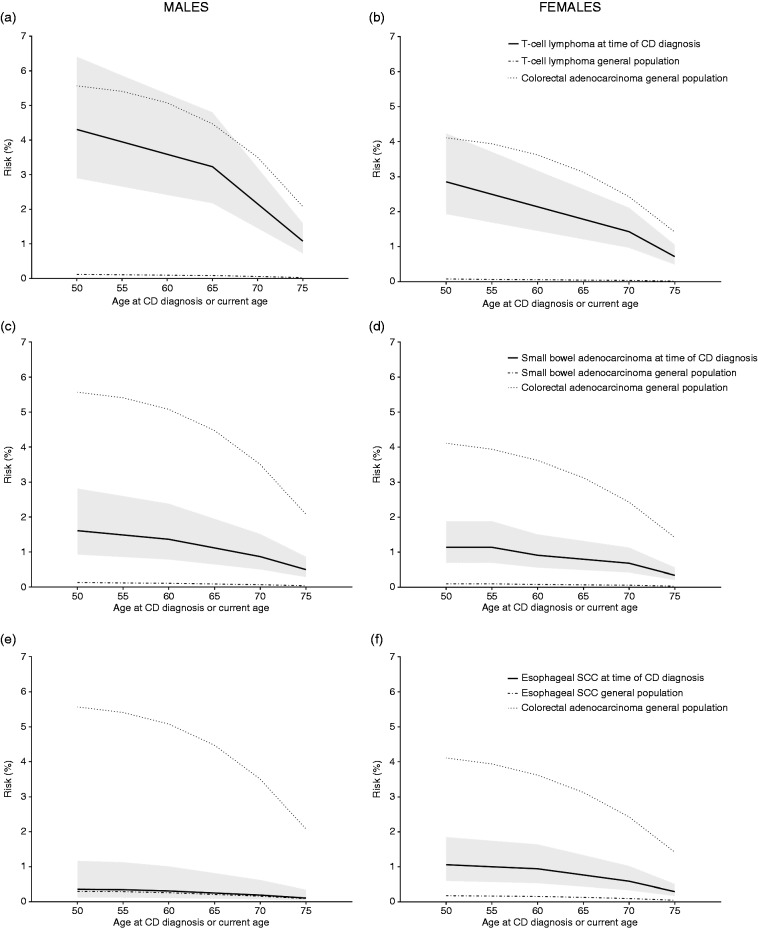
Absolute cumulative risk estimates were calculated based on data from the Netherlands Cancer Registry. Based on the distribution of age at CD diagnosis (Table 4) in our study, we chose to provide risk estimates from the age of 50 years since our data would not be sufficiently representative for younger patients. This resulted in risks as depicted in Figure 2 with a highest absolute risk of 4.3% for T-cell lymphoma in males between the ages 50 and 80 years when CD is diagnosed at age 50 years.
Table 4.
Characteristics of lymphomas and GI carcinomas in CD patients.
| Adenocarcinoma |
Squamous cell carcinoma |
Large T-cell lymphoma (EATL/ALCL/PTCL) |
B-cell lymphoma |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Esophagus | Stomach | Duodenum | Jejunum/ Ileum | Colorectal | Esophagus | GI localization | Non-GI localization | DLBCL | |
| Number | 12 | 12 | 16 | 15 | 105 | 16 | 95 | 33 | 20 |
| Gender (M/F) (%) | 83%/17% | 42%/58% | 31%/69% | 60%/40% | 42%/58% | 19%/81% | 62%/38% | 42%/58% | 60%/40% |
| Median age at malignancy diagnosis (years) (IQR) | 69 (66–75) | 61 (49–72) | 68 (64–76) | 69 (61–73) | 70 (62–74) | 70 (64–73) | 63 (57–69) | 66 (56–70) | 75 (59–77) |
| Median age at CD diagnosis (years) (IQR) | 69 (65–73) | 60 (47–64) | 67 (59–72) | 63 (59–69) | 65 (57–73) | 63 (60–70) | 61 (56–67) | 62 (51–67) | 67 (58–74) |
| Median time between CD and malignancy (months) (IQR) | 13 (0–59) | 20 (0–87) | 38 (0–90) | 12 (1–79) | 11 (0–66) | 40 (10–108) | 0 (0–18) | 25 (1–81) | 23 (4–56) |
ALCL: anaplastic large cell lymphoma; CD: celiac disease; DLBCL: diffuse large B-cell lymphoma; EATL: enteropathy-associated T-cell lymphoma; F: female; GI: gastrointestinal; IQR: interquartile range; M: male; PTCL: peripheral T-cell lymphoma.
Figure 2.
Absolute risk of malignancies until the age of 80 years by current age or age at time of celiac disease (CD) diagnosis. The risks present the risk for developing the malignancy of interest, i.e. T-cell lymphoma ((a) and (b)), small bowel adenocarcinoma ((c) and (d)) and esophageal squamous cell carcinoma (SCC) (2(e) and (f)), by age 80 years for patients cancer free and diagnosed with CD at age 50, 55, etc. years. As reference, we added the risk for colorectal carcinoma at current age in the general population. Example: The risk of being diagnosed with a T-cell lymphoma from CD diagnosis until the age of 80 years (including synchronous diagnosis) for a male when CD is diagnosed at the age of 60 is 3.6% (95% confidence interval 2.4%–5.3%). The risk for a male without CD developing a T-cell lymphoma between ages 60 and 80 years is 0.1%. The risk for a male from the general population developing colorectal adenocarcinoma between the age of 60 to 80 years is 5.1%.
Descriptive characteristics of lymphoma and GI carcinoma in CD patients
An overview of the characteristics of the most frequent lymphoma classes and GI carcinomas in CD patients are summarized in Table 4 and an overview of all lymphomas and GI carcinomas is depicted in Supplementary File 2. A total of 169 patients were diagnosed with various classes of lymphomas and 180 with a carcinoma in the GI tract.
Malignant lymphoma
For this descriptive study, all lymphomas, including the lymphomas encoded as non-Hodgkin lymphoma not otherwise specified in the PALGA database, were subdivided when possible. Signs of CD (i.e. by serological and/or histological supportive features in nonlymphoma-involved mucosa) are a defining parameter for classification of large T-cell lymphoma as EATL. Outside this context, identical morphological and immunophenotypical tumor cell features as in EATL may be seen in other classes of large T-cell lymphoma, which are then classified as peripheral T-cell lymphoma (PTCL) or anaplastic large cell lymphoma (ALCL), anaplastic lymphoma kinase negative (ALK–).15 Therefore, because in the context of this study, classification as EATL, PTCL or ALCL in the presence of CD may be considered largely arbitrary, these classes were combined.
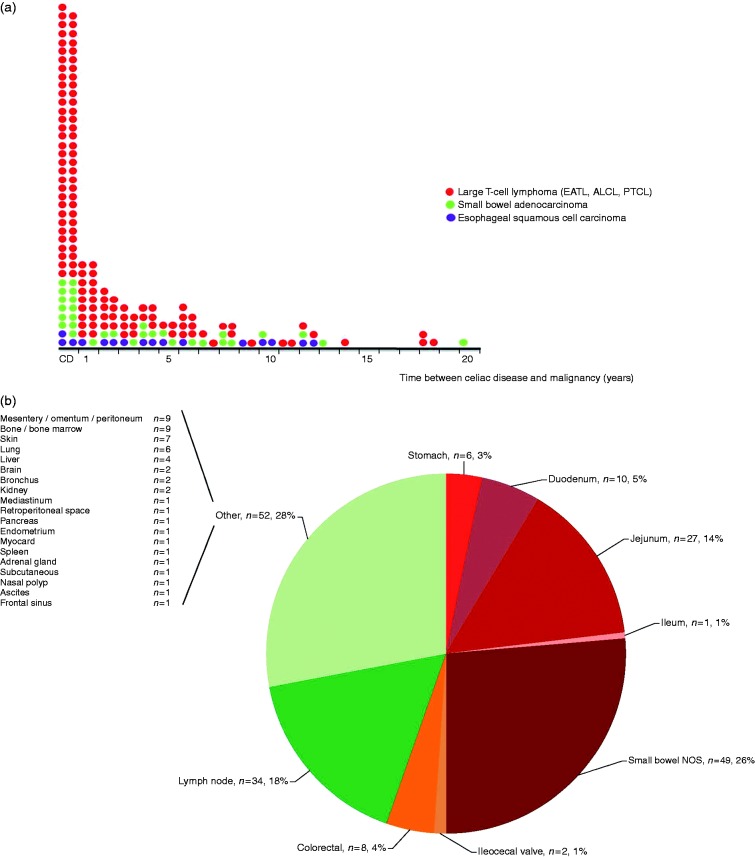
As expected, the EATL group was most prevalent among malignant lymphoma classes (128/169, 76%) in CD patients. Age at time of CD diagnosis ranged between 28 and 78 years (median 62), with only 13% diagnosed before the age of 50. In half of the patients, CD and EATL were simultaneously diagnosed, defined as within three months before to three months after diagnosis of CD (64/128, 50%) (Figure 3(a)). Seventeen percent were diagnosed >5 years after CD diagnosis and 6% after >10 years. The most prevalent location involved the GI tract (103/189 localizations in 128 patients, 54% of all sites involved) with a predominance of small bowel involvement (87/189, 46%), but highly diverse extranodal sites (both primary and secondary) such as skin, lung and brain were noted (Figure 3(b), 52/189) as well as primary and secondary nodal involvement (34/189 localizations). This underpins the importance of also considering EATL in biopsies at non-GI sites in CD patients. GI predominance was not seen in any other classes of lymphoma, including diffuse large B-cell lymphoma.
Figure 3.
Descriptive characteristics of malignancies associated with celiac disease (CD). (a) Time between celiac disease and various malignancies associated with CD. Each dot represents an individual event. ALCL: anaplastic large cell lymphoma; CD: malignancy diagnosed three months before until three months after celiac CD diagnosis; EATL: enteropathy-associated T-cell lymphoma; PTCL: peripheral T-cell lymphoma. (b) Sites of enteropathy-associated T-cell lymphoma involvement. Total n (EATL) = 128.
NOS: not otherwise specified.
Small bowel adenocarcinoma
In this series, 31 CD patients with small bowel adenocarcinoma were identified. Age at time of CD diagnosis ranged between 46 and 81 years (median 65) with only 13% diagnosed before the age of 50. A synchronous diagnosis of malignancy and CD occurred in 13/31 patients (42%), 32% were diagnosed >5 years after CD diagnosis and 7% after >10 years. Locations involved were the duodenum (16/31, 52%), jejunum (9/31, 29%), ileum (1/31, 3%) and small bowel not otherwise specified (5/31, 16%).
Esophageal SCC
Sixteen CD patients with esophageal SCC were identified, of whom 13 were female (81%). Age at time of CD diagnosis in these patients ranged between 57 and 77 years (median 63). In contrast to EATL and small bowel adenocarcinoma, only a very few patients were diagnosed simultaneously with CD (3/16, 19%); 31% were diagnosed >5 years after CD diagnosis and 6% >10 years after CD diagnosis.
Discussion
With a strictly kept GFD, CD runs a benign course in most patients. In a minority, however, severe complications including malignant diseases may develop that may require tailored screening and follow-up programs in newly diagnosed CD patients. To propose evidence-based guidelines, information on RR and absolute risks of specific complications as well as risk profiles over time after CD diagnosis are needed but are currently sparse. In this population-based, nationwide case-control study we assessed the risk for malignant lymphomas and carcinomas in the GI tract in newly diagnosed patients with a histologically confirmed diagnosis of CD. Our study provides several lines of information important for clinical care.
First, if present, EATL, and to a lesser extent small bowel carcinoma, is most often synchronously diagnosed with CD. It can be inferred from these findings that in the large majority of cases, patients had silent CD, or, intuitively less likely, that CD developed concomitantly with the development of the malignancy. Whether timely diagnosis and treatment of CD would have prevented these complications is unknown. The decline in risk of EATL diagnosed ≥1 year after CD diagnosis may support the hypothesis that recovery of small bowel architecture and reduction of immune activity after introducing a GFD could be (partly) protective for developing these malignancies. However, risk for T-cell lymphoma, small bowel adenocarcinoma and esophageal SCC remain elevated ≥1 year after CD diagnosis. Whether these patients had been on a strict GFD is unknown and a subject for further research. Our findings of an elevated risk for T-cell lymphomas and small bowel adenocarcinomas fit the results of previous studies, although risk estimates vary widely with standardized incidence ratios of 19–51 for T-cell lymphomas and 0–34 for small bowel adenocarcinomas.4,5,18 Although reports of esophageal SCC after CD have been published,19,20 risk estimates are lacking in the literature.
A second important finding is that extraintestinal presentation of EATL is common. In fact, 26% of the EATLs had no intestinal involvement. Extraintestinal involvement of EATL was especially seen in patients with secondary EATL, i.e. diagnosed after CD diagnosis.
Thirdly, it is important that our large study does not show increased risks for developing other classes of non-Hodgkin lymphoma, including B-cell lymphoma and Hodgkin lymphoma, as well as anal SCC and for adenocarcinoma at other GI sites than the small bowel.
This study is the first to provide absolute risks of developing CD-associated malignancies and demonstrates that the absolute risk of being diagnosed with a T-cell lymphoma before age 80 years for patients diagnosed with CD at age 50 years (4.3% for males and 2.9% for females) is lower than the risk for colorectal adenocarcinoma in the general population (5.6% for males and 4.1% for females). For a CD patient diagnosed at age 50, the absolute risks to be diagnosed with a small bowel adenocarcinoma (1.6% in males and 1.1% in females) or esophageal SCC (0.4% in males, 1.1% in females) before the age of 80 years were not as high as the risk for T-cell lymphoma in the same population.
This study has some limitations. First, this study is based on the 21-year period in which the PALGA database can provide complete nationwide data, and both the CD diagnosis and the malignancy diagnosis are restricted to this period. A limitation of this approach is that potential follow-up is limited to a maximum of 21 years, which is not long enough to assess risk of GI tract cancer and lymphoma in patients with a CD diagnosis during childhood and adolescence. Therefore, our conclusions relate to adult patients with a primary CD diagnosis.
Secondly, it cannot be entirely excluded that biopsies on which the diagnosis of CD was based in this study were not index biopsies since we could include histopathological reports only since January 1994. This may theoretically have influenced the analysis stratified by time since CD. It is not common practice to take follow-up biopsies in most CD patients, however, and the effect is therefore likely small.
For the primary analysis we also included patients with simultaneous CD and case or control diagnosis. This may have resulted in a higher detection rate of CD in the case group compared to the control group, since patients with a GI tumor are more likely to have undergone upper endoscopy during work-up than patients diagnosed with a melanoma or BCC, for which this is not commonly performed. This may have been a source of bias since approximately 80% of CD patients are undiagnosed.21 However, we wanted to include these synchronously diagnosed patients in the primary analysis from an epidemiological and biological perspective to provide the actual risk of malignancies in CD patients. To exclude this source of bias and to calculate clinical relevant risks, however, we performed a sensitivity analysis including only patients with a lymphoma or GI carcinoma diagnosis ≥3 months after CD diagnosis. This analysis showed that RRs associated with CD were still elevated for esophageal SCC, small bowel adenocarcinoma and T-cell lymphoma, albeit not as strong as in the original analysis. Based on these (gender-specific) RRs from the sensitivity analysis, we calculated absolute risks for malignancies ≥3 months after CD diagnosis until the age of 80 for a CD patient diagnosed between ages 50 and 75 years. Supplementary File 3 represents these risks useful for daily clinical practice. These absolute risks were lower than the absolute risks calculated from an epidemiological and biological point of view (these are shown in Figure 2) with a highest absolute risk of 1.8% (vs 4.3% in the original absolute risk analysis) of being diagnosed with a T-cell lymphoma from ≥3 months after CD diagnosis until the age of 80 years once CD is diagnosed in a male at age 50 years. This lower absolute risk is due to the large amount of synchronic presentations of CD and T-cell lymphoma, and to a lesser amount, CD and small bowel adenocarcinoma.
In conclusion, newly diagnosed CD patients have an increased risk of EATL, of which are a significant part without GI involvement, small bowel adenocarcinoma and, in females, esophageal SCC. This is particularly seen in patients diagnosed with CD above the age of 50 years. Despite the fact that the RRs for EATL and small bowel adenocarcinoma decrease one year after CD diagnosis, these risks remain increased compared to non-CD patients. Yet the absolute risk of being diagnosed with EATL or small bowel carcinoma during follow-up remains small. While in many cases, diagnosis of EATL will unveil CD diagnosis, risk of developing EATL remains elevated >1 year after CD diagnosis.
Based on the results of this study, we feel that the low risk of being diagnosed with these malignancies during CD follow-up does not warrant standardized screening programs in CD patients. We recommend being suspicious for an underlying malignancy in CD patients, especially those diagnosed at ages older than 50 years who present with severe symptoms, do not clinically improve after starting a strict GFD or deteriorate during follow-up.
Supplemental Material
Supplemental material, REVISION Supplementary File 1 for Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up by Tom van Gils, Petula Nijeboer, Lucy IH Overbeek, Michael Hauptmann, Daan AR Castelijn, Gerd Bouma, Chris JJ Mulder, Flora E van Leeuwen and Daphne de Jong in United European Gastroenterology Journal
Supplemental Material
Supplemental material, Supplementary File 2 for Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up by Tom van Gils, Petula Nijeboer, Lucy IH Overbeek, Michael Hauptmann, Daan AR Castelijn, Gerd Bouma, Chris JJ Mulder, Flora E van Leeuwen and Daphne de Jong in United European Gastroenterology Journal
Supplemental Material
Supplemental material, Supplementary File 3 for Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up by Tom van Gils, Petula Nijeboer, Lucy IH Overbeek, Michael Hauptmann, Daan AR Castelijn, Gerd Bouma, Chris JJ Mulder, Flora E van Leeuwen and Daphne de Jong in United European Gastroenterology Journal
Supplemental Material
Supplemental material for Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up by Tom van Gils, Petula Nijeboer, Lucy IH Overbeek, Michael Hauptmann, Daan AR Castelijn, Gerd Bouma, Chris JJ Mulder, Flora E van Leeuwen and Daphne de Jong in United European Gastroenterology Journal
Acknowledgements
Author contributions includes the following: study concept and design: T.G., G.B., C.J.J.M., F.E.L. and D.D.J.; acquisition of data: T.G., P.N., D.A.R.C., L.I.H.O. and D.D.J.; analysis and interpretation of data: T.G., M.H., F.E.L. and D.D.J.; drafting of the manuscript: T.G., F.L. and D.D.J.; critical revision of the manuscript for important intellectual content: P.N., L.I.H.O., D.A.R.C., M.H., G.B. and C.J.J.M.; statistical analysis: T.G. and M.H.; and study supervision: D.D.J. and F.E.L.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Scientific Board of PALGA complying with the regulations for anonymized (epidemiological) studies and considered to remain outside the restrictions of the Medical Research Involving Human Subjects Act (WMO) by the Medical Ethics Review Committee of VU University Medical Center on April 6, 2016 (METC-VUMC 2016.133). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Informed consent
Informed consent is not applicable, please see ethics approval
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med 2007; 357: 1731–1743. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis 2014; 46: 97–104. [DOI] [PubMed] [Google Scholar]
- 3.Verbeek WH, Van De Water JM, Al-Toma A, et al. Incidence of enteropathy-associated T-cell lymphoma: A nation-wide study of a population-based registry in The Netherlands. Scand J Gastroenterol 2008; 43: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 4.Ilus T, Kaukinen K, Virta LJ, et al. Incidence of malignancies in diagnosed celiac patients: A population-based estimate. Am J Gastroenterol 2014; 109: 1471–1477. [DOI] [PubMed] [Google Scholar]
- 5.Smedby KE, Akerman M, Hildebrand H, et al. Malignant lymphomas in coeliac disease: Evidence of increased risks for lymphoma types other than enteropathy-type T cell lymphoma. Gut 2005; 54: 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oruç N, Ozütemız O, Tekın F, et al. Celiac disease associated with B-cell lymphoma. Turk J Gastroenterol 2010; 21: 168–171. [DOI] [PubMed] [Google Scholar]
- 7.Elli L, Contiero P, Tagliabue G, et al. Risk of intestinal lymphoma in undiagnosed coeliac disease: Results from a registered population with different coeliac disease prevalence. Dig Liver Dis 2012; 44: 743–747. [DOI] [PubMed] [Google Scholar]
- 8.Mahmud B, Mohammadkazem A, Hasan S, et al. Esophagus adenocarcinoma in a young patient with celiac disease; is celiac disease a predisposing factor for esophagus adenocarcinoma as well as squamous cell carcinoma? Clin J Gastroenterol 2014; 7: 324–327. [DOI] [PubMed] [Google Scholar]
- 9.Grainge MJ, West J, Solaymani-Dodaran M, et al. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: A cohort study. Aliment Pharmacol Ther 2012; 35: 730–739. [DOI] [PubMed] [Google Scholar]
- 10.Green PH, Fleischauer AT, Bhagat G, et al. Risk of malignancy in patients with celiac disease. Am J Med 2003; 115: 191–195. [DOI] [PubMed] [Google Scholar]
- 11.West J, Logan RF, Smith CJ, et al. Malignancy and mortality in people with coeliac disease: Population based cohort study. BMJ 2004; 329: 716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Askling J, Linet M, Gridley G, et al. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002; 123: 1428–1435. [DOI] [PubMed] [Google Scholar]
- 13.Casparie M, Tiebosch AT, Burger G, et al. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 2007; 29: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rostami K, Kerckhaert J, Tiemessen R, et al. Sensitivity of antiendomysium and antigliadin antibodies in untreated celiac disease: Disappointing in clinical practice. Am J Gastroenterol 1999; 94: 888–894. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of tumours of haematopoietic and lymphoid tissues, 4th ed Lyon, France: IARC Press, 2008. [Google Scholar]
- 16.The Netherlands Cancer Registry, http://www.cijfersoverkanker.nl/ (2017).
- 17.World Health Organization. World Health Statistics 2016: Monitoring health for the SDGs Annex B: Tables of health statistics by country, WHO region and globally 2016. http://www.who.int/gho/publications/world_health_statistics/2016/EN_WHS2016_AnnexB.pdf?ua=1.
- 18.Catassi C, Fabiani E, Corrao G, et al. Risk of non-Hodgkin lymphoma in celiac disease. JAMA 2002; 287: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 19.Holmes GK, Stokes PL, Sorahan TM, et al. Coeliac disease, gluten-free diet, and malignancy. Gut 1976; 17: 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selby WS, Gallagher ND. Malignancy in a 19-year experience of adult celiac disease. Dig Dis Sci 1979; 24: 684–688. [DOI] [PubMed] [Google Scholar]
- 21.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol 2012; 107: 1538–1544. quiz 1537, 1545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, REVISION Supplementary File 1 for Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up by Tom van Gils, Petula Nijeboer, Lucy IH Overbeek, Michael Hauptmann, Daan AR Castelijn, Gerd Bouma, Chris JJ Mulder, Flora E van Leeuwen and Daphne de Jong in United European Gastroenterology Journal
Supplemental material, Supplementary File 2 for Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up by Tom van Gils, Petula Nijeboer, Lucy IH Overbeek, Michael Hauptmann, Daan AR Castelijn, Gerd Bouma, Chris JJ Mulder, Flora E van Leeuwen and Daphne de Jong in United European Gastroenterology Journal
Supplemental material, Supplementary File 3 for Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up by Tom van Gils, Petula Nijeboer, Lucy IH Overbeek, Michael Hauptmann, Daan AR Castelijn, Gerd Bouma, Chris JJ Mulder, Flora E van Leeuwen and Daphne de Jong in United European Gastroenterology Journal
Supplemental material for Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up by Tom van Gils, Petula Nijeboer, Lucy IH Overbeek, Michael Hauptmann, Daan AR Castelijn, Gerd Bouma, Chris JJ Mulder, Flora E van Leeuwen and Daphne de Jong in United European Gastroenterology Journal