Abstract
Background
Laterally spreading tumours are separated in subclasses: granular, homogenous or nodular mixed; and non-granular, flat or pseudodepressed. For every subtype, a proper risk of submucosal invasive cancer has been described in Asian series.
Objective
The aim of the study was to determine the rate of cancer and submucosal invasive cancer in a Western series of endoscopic-resected laterally spreading tumours and their endoscopic predictive factors.
Methods
A total of 374 laterally spreading tumours ≥20 mm were resected by endoscopy in our single centre between 2012–2016. We analysed endoscopic and pathological data from our prospective database, determining the rates of cancer and submucosal invasive cancer according to the subtype of laterally spreading tumour.
Results
The rates of submucosal invasive cancer for granular homogenous, granular nodular mixed, non-granular flat, non-granular pseudodepressed laterally spreading tumours were 4.9%, 15.9%, 3.0% and 19.4%, respectively. Endoscopic mucosal resection was used in 58.0% and endoscopic submucosal dissection in 42.0%. Endoscopic submucosal dissection was associated with a higher rate of en-bloc resection (87.3% vs 26.3%; p < 0.0001), and a lower risk of recurrence (7.6% vs 15.2%; p = 0.026). Adverse event rates were not statistically different (9.5% vs 6.4%, p = 0.26). Predictive endoscopic factors of submucosal invasive cancer were: invasive pit pattern (hazard ratio = 33 (8.81–143.3)), non-granular pseudodepressed laterally spreading tumours (hazard ratio = 11.9 (0.89–146.2)), and granular nodular mixed laterally spreading tumours (hazard ratio = 3.42 (0.99–13.0)).
Conclusions
The risk of submucosal invasive cancer varies according to the laterally spreading tumour subtype. Three factors were associated with submucosal invasion and should justify an endoscopic submucosal dissection: non-granular pseudodepressed laterally spreading tumours, granular nodular mixed laterally spreading tumours subtypes and invasive pit pattern.
Keywords: Laterally spreading tumour, endoscopic submucosal dissection, endoscopic mucosal resection, invasive cancer
Key summary
Laterally spreading tumours (LSTs) are divided in four subtypes based on their morphological features: granular homogenous (LST-G-H) or nodular mixed (LST-G-N), and non-granular flat (LST-NG-F) or pseudodepressed (LST-NG-PD).
Every subtype has been correlated with a risk of invasive cancer in Asian series.
This Western series shows the same cancer risks regarding LST subtypes.
Predictive factors for invasive cancer were: LST-NG-PD, LST-G-N and invasive pit pattern, and should justify endoscopic submucosal dissection (ESD).
Introduction
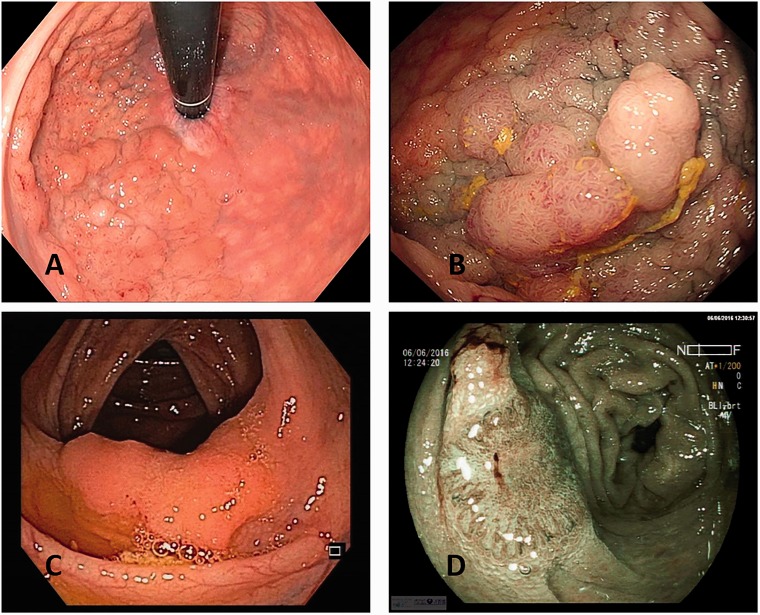
Laterally spreading tumours (LSTs) represent the most common nonpolypoid colorectal neoplasm found during colonoscopy, described for the first time by Kudo et al.,1 and may represent around 15% of colonic neoplasms detected in colonoscopy.2,3 According to the definition accepted in all countries, these lesions are defined as 10 mm or greater in diameter with a low vertical axis, extending laterally along the luminal wall. LSTs are classified according to the morphological aspect: the granular type (LST-G), and the non-granular type (LST-NG) with a smooth surface. In the LST-G group, the homogenous type (LST-G-H) is differentiated from the nodular mixed type (LST-G-N) (Figure 1(a) and (b)); in the LST-NG group, flat (LST-NG-F) and pseudodepressed (LST-NG-PD) lesions are also separated (Figure 1(c) and (d)). This classification has been correlated to a various risk of neoplasia and submucosal invasion (SMI).4–6 For LST-G-Hs, the risk of SMI was around 2%, while it reached between 28–40 % for LST-NG-PDs.4,6 Considering that the type of LST was correlated with the risk of cancer, the ability of the endoscopist to characterise the LST is crucial, leading to the choice of the therapeutic endoscopic approach.7–9 Indeed, endoscopic submucosal dissection (ESD) should be required when the risk of SMI is high, ensuring en bloc resection, whereas endoscopic mucosal resection (EMR) is often sufficient for the low-risk lesions.10,11
Figure 1.
(a) Endoscopic image of granular homogenous laterally spreading tumour (LST-G-H) with white light; (b) granular nodular mixed laterally spreading tumour (LST-G-N) with white light; (c) non-granular flat laterally spreading tumour (LST-NG-F) with white light; (d) image of non-granular pseudodepressed laterally spreading tumour (LST-NG-PD) with blue light imaging (BLI).
Data about LSTs are mainly provided from Asian series and the literature on the Western population is scarce. Thus, it is not clear if LST characteristics are similar while this knowledge is essential before practising therapeutic endoscopy. The aim of our study was to determine the rates of cancer and SMI for LSTs in a large series of French patients, and the predictive factors of cancer in those lesions.
Materials and methods
Patients
We conducted a retrospective analysis of prospectively collected data from our single centre database on colorectal LSTs≥20 mm that were removed by endoscopic procedures, between January 2012–December 2016. All lesions were histologically diagnosed as adenoma, adenocarcinoma or sessile serrated lesions (SSA/P). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee. The local ethics committee of the Cochin Teaching Hospital, Paris, France, approved the study in January 2017. French legislation on studies of anonymised retrospective data do not require informed consent.
Endoscopic examination
All endoscopic procedures were performed by three experienced operators (SC, FP, SL). Colonoscopies were performed using magnifying colonoscopes (EC-600WR, EC-760RV/M, 760ZP-VM, Fujinon, Tokyo, Japan–CF-H180AI, Olympus Medical System, Tokyo, Japan) following bowel preparation in a split-dose regimen (polyethylene glycol ± sennoside B). Laterally spreading tumours were prospectively described in our database and subclassified according to the standard classification after a precise examination comprising conventional observation ± magnification, and virtual chromoendoscopy (Fuji Intelligent Chromo Endoscopy (FICE) or blue light imaging (BLI) system for Fujifilm scopes, Narrow Band Imaging (NBI) system for Olympus scopes). For LST-G-Ns, the size of the nodule was estimated and macronodule was defined above a 10-mm diameter. For LST-NG-PDs, depression was defined as a well-circumscribed circular deep depression. For each lesion, the following characteristics were also described in the database: size, location, invasive pit pattern or not. The size of the lesion was evaluated by the endoscopist referring to the size of the snare and other material that have been used for the resection. It was then approached within a range of 5 mm. In the case of monobloc ESD, size was also measured on the resected specimen. Invasive pit pattern was defined as a NICE 3 (NBI international colorectal endoscopy classification) or Sano 3 or Kudo V lesion,12–14 after virtual colouration, even if these classifications are not validated for the BLI system. The choice of the classification was left to the operator’s discretion. For missing data, pictures of the lesions were retrospectively analysed when possible. Treatment’s characteristics were also reported: techniques (monobloc or piece-meal mucosectomy (EMR), classical submucosal dissection (ESD) or hybrid ESD),15 and their adverse events (perforation and bleeding). The choice of resection technique was left to the operator’s choice, considering the risk of cancer or SMI. Hybrid ESD was mainly used as a rescue therapy during a difficult colorectal ESD.
Histopathological evaluation
All removed tumours were sent for histological examination and sectioned at 2–3 mm intervals. The histological classification was made according to the European Society of Gastrointestinal Endoscopy (ESGE) guidelines,16 based on the pathologist reports. Maximal histology was subclassified as adenoma with low-grade or high-grade dysplasia, intramucosal carcinoma, submucosal invasive carcinoma (SMIC) or sessile serrated adenomas/polyps with or without dysplasia.17 For every SMI lesion, the depth of submucosal invasion was measured, from the lower muscularis mucosa to the deepest area of cancer.18 If the muscularis mucosa was not identified, depth was measured from the surface of the tumour. Budding, differentiation and lymphovascular invasion were also reported.
R0 resection was defined as in the ESGE guidelines,16 and curative resection was considered for R0 resection of low-grade or high-grade adenomas and intramucosal adenocarcinomas. For submucosal adenocarcinomas, depth < 1 mm in submucosa, well differentiation, absence of budding and lymphovascular invasion were also required.16
Statistical analysis
Statistical analyses were calculated using R version 3.1.3. Data are given as mean values and standard deviations, or percentages. The characteristics of each subtype of LST were compared using the chi-squared or Fisher’s exact test for binary data, and t test for continuous data. Both 95% confidence intervals (CIs) for odds ratios (ORs) that did not include 1.0 and p values < 0.05 were considered to be statistically significant. The multivariate logistic regression model included the significant factors from univariate analysis.
Results
Characteristics of LSTs
A total of 374 consecutive LSTs were eligible in our study from 322 different patients. Clinicopathological characteristics and endoscopic procedures are summarised in Table 1. The indication of the colonoscopy leading to the diagnosis of LST was a personal history of polyp or cancer in 47.4%, a familial history of cancer in 4.8%, digestive symptoms in 24.4%, a positive screening test in 11.5% and other reasons in 11.9%. Lesions were localised in the right colon, transverse colon, left colon and rectum in 44.0%, 9.7%, 13.9% and 32.4%, respectively. The mean size of LSTs was 40.6 mm ± 21.2 (20–140). According to the LST classification, we observed 28.6% of LST-G-N, 27.3% of LST-G-H, 35.8% of LST-NG-F and 8.3% of LST-NG-PD.
Table 1.
Clinico-pathological features of laterally spreading tumours (LSTs).
| Patients’ characteristics | Patients: n = 374 | |
| Mean age (years) | 67.7 ± 12.4 (15–93) | |
| Sex ratio (male/female) | 194/180 | |
| Mean size, mm (range) | 40.6 ± 21.2 (20–140) | |
|
n
|
% |
|
| Location | ||
| Right colon | 166 | 44.4 |
| Rectum | 123 | 32.9 |
| Left colon | 52 | 13.9 |
| Transverse colon | 33 | 8.8 |
| LST classification | ||
| LST-G homogenous | 102 | 27.3 |
| LST-G nodular mixed | 107 | 28.6 |
| LST-NG flat | 134 | 35.8 |
| LST-NG pseudodepressed | 31 | 8.3 |
| Histology | ||
| Tubulovillous adenoma | 205 | 54.8 |
| Villous adenoma | 83 | 22.2 |
| Tubular adenoma | 28 | 7.5 |
| SSA/P | 58 | 15.5 |
| Histology | ||
| No neoplasia | 41 | 11.0 |
| Low-grade neoplasia | 120 | 32.1 |
| High-grade neoplasia | 143 | 38.2 |
| Carcinomas | 70 | 18.7 |
| Intra-mucosal carcinomas | 38 | 10.2 |
| Submucosal carcinoma | 32 | 8.6 |
| T1sm > 1000 µm | 18 | 4.8 |
LST-G: granular laterally spreading tumour; LST-NG: non-granular laterally spreading tumour; SSA/P: sessile serrated adenoma.
Overall, we found adenomas with low-grade dysplasia in 32.1%, adenomas with high-grade dysplasia in 38.2%, intramucosal adenocarcinoma (Tis) in 10.2%, and adenocarcinoma with SMI in 8.6% (32 lesions). Among SMIC lesions, 18 LSTs had submucosal invasion deeper than 1000 µm, six had budding, nine had lymphovascular invasion and none were poorly differentiated.
Results regarding resection technique
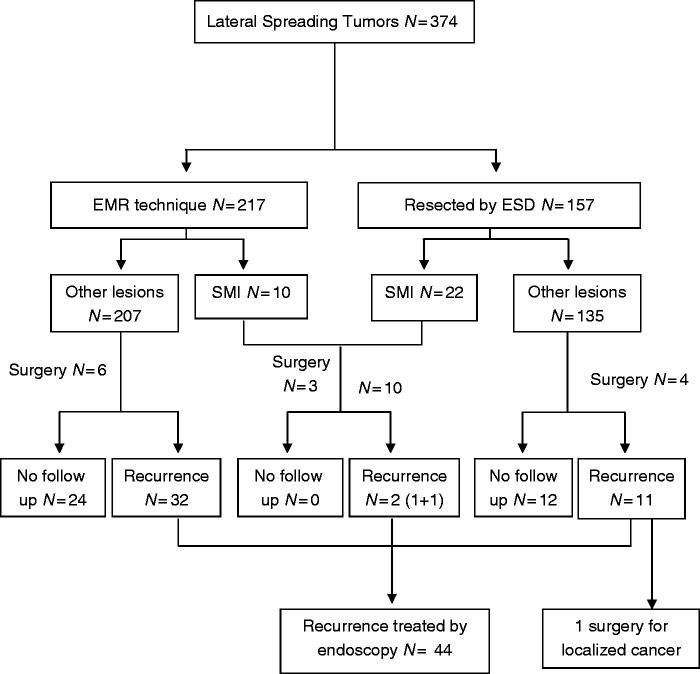
In our series, EMR was used for 217 lesions (57 with monobloc technique, and 160 with piece-meal EMR), and ESD for 157 lesions (103 ‘classic’ ESD, and 54 ESD with hybrid technique) (see Figure 2). The mean size of the lesions was significantly higher in the ESD group (Table 2) (50.0 mm versus 34.3 mm; p < 0.0001). The procedure duration was longer for ESD technique (172.1 min versus 89.3 min; p < 0.0001), as well as the hospitalization duration (2.2 days versus 0.97 days; p < 0.0001).
Figure 2.
Flow chart of the laterally spreading tumours (LSTs) of the study.
Table 2.
Comparison of endoscopic resection techniques.
| EMR | ESD | p | |
|---|---|---|---|
| Number (n/%) | 217/58.0 | 157/42.0 | |
| Lesion size (mm) | 34.3 ± 15.8 (20–130) | 50.0 ± 24.7 (20–140) | <0.00001 |
| Procedure duration (min) | 89.4 ± 42.1 (31–291) | 172.1 ± 89.8 (35– 665) | <0.00001 |
| Hospitalization (days) | 0.97 ± 1.32 (0–10) | 2.20 ± 1.79 (0–10) | <0.00001 |
| Cancer (n/%) | 25/11.5 | 44/28.0 | 0.00005 |
| T1sm (n/%) | 10/4.7 | 22/14.0 | 0.00133 |
| En bloc resection (n/%) | 57/26.3 | 137/87.3 | <0.00001 |
| R0 | 209/96.3 | 148/94.3 | 0.36 |
| R1 | 8/3.7 | 9/5.7 | |
| Complication | |||
| Bleeding | 9/4.1 | 6/3.8 | NS |
| Perforation | 5/2.3 | 9/5.7 | 0.08 |
| Recurrence | 33/15.2 | 12/7.6 | 0.026 |
EMR endoscopic mucosal resection; ESD: endoscopic submucosal dissection.
En bloc resection was more frequent with ESD rather than with EMR (87.3% versus 26.3%; p < 0.0001), with a higher R0 resection rate (83.4% versus 26.3%; p < 0.0001).
Adverse event rates were not statistically different between ESD and EMR (9.5% versus 6.4%, p = 0.26). Perforation was the most frequent adverse event after ESD (5.7% versus 2.3%; p = 0.08), and was mostly medically managed. Three patients underwent surgery because of perforation, two in the EMR group, and one in the ESD group. Bleeding was also not different between the two techniques (3.8% versus 4.1%)
The median time for follow-up was 15.0 ± 11.7 months (3–58 months) and 36 patients were lost to follow-up (9.6%). Local recurrence rate within one year was 7.6% with ESD (12 patients), and 15.2% with EMR (33 patients; p = 0.026). Comparing ESD and hybrid EDS also showed a different recurrence rate (respectively 4.9% against 13.0%), but not statistically significant (p = 0.07). Histology before the relapse was adenocarcinoma for six patients, high-grade dysplasia for 21, low-grade dysplasia for 16, and SSA/P for two patients. In the EMR group, 29 patients out of the 33 patients had a piecemeal resection. Concerning the ESD group, six had a R0 resection, five had a piecemeal resection, with non interpretable margins (Rx resection), and one had invasion on the deep margin.
Almost all recurrent lesions were endoscopically resected for low- or high-grade dysplasia. Only one patient needed surgery for an invasive recurrence with a localised cancer classified pT1sm2N0M0. This recurrence occured two years after a first resection by hybrid ESD of a SSA/P lesion with high grade dysplasia.
Mucosal and submucosal invasion according to the LST subtype
In the LST-G group, there were 49/209 (23.4%) adenocarcinomas of which 22 (10.5%) were with SMI. Cancer and cancer with SMI were found in LST-G-H in 11.8% and 4.9%, and in LST-G-N in 34.5% and 15.9% (p < 0.0001).
In the LST-NG group, there were 21/168 (12.5%) adenocarcinomas and 10 (6.0%) with SMI. Cancer and cancer with SMI were found in LST-NG-PD in 32.3% and 19.4%, and in LST-NG-F in 8.2% and 3.0% (p < 0.0001).
SMIC was statistically more frequent in LST-G-N and LST-NG-PD (15.9% and 19.4%) compared to LST-G-H and LST-NG-F (p < 0.0001) (Table 3).
Table 3.
Rate of adenocarcinoma, and submucosal invasion in every subtype of laterally spreading tumour (LST).
| LST-G-H | LST-G-N | LST-NG-F | LST-NG-PD | |
|---|---|---|---|---|
| Number (n/%) | 102/27.3 | 107/28.6 | 134/35.8 | 31/8.3 |
| EMR (n/%) | 66/64.7 | 38/35.5 | 99/73.9 | 14/45.2 |
| ESD (n/%) | 36/35.3 | 69/64.5 | 35/26.1 | 17/54.8 |
| Cancer (n/%) | 12/11.8 | 37/34.5 | 11/8.2 | 10/32.3 |
| SMIC (n/%) | 5/4.9 | 17/15.9 | 4/3.0 | 6/19.4 |
| Complication | 9/8.8 | 10/9.3 | 8/6.0 | 2/6.5 |
| Recurrence | 10/9.8 | 18/16.8 | 14/10.4 | 3/9.7 |
EMR endoscopic mucosal resection; ESD: endoscopic submucosal dissection; LST-G-H: granular homogenous LST; LST-G-N: granular nodular mixed LST; LST-NG-F: non-granular flat LST; LST-NG-PD: non-granular pseudodepressed LST; SMIC: submucosal invasive carcinoma.
Characteristics of the lesions regarding location of the lesions are summarised in Table 4. Proximal lesions (including right and transverse colon) were associated with less cancer and less SMI compared to distal lesions including left colon and rectum (respectively 11.9% vs 26.0%, p = 0.0005; and 5.0% vs 12.7%, p = 0.008). Lesion size was significantly smaller for proximal lesions (34.0 mm vs 48.8 mm; p < 0.0001). EMR technique was most frequently used for proximal lesions (35.3% vs 22.4%; p = 0.006), and also recurrence rate was significantly higher for proximal lesions (15.4% vs 8.1%; p = 0.03). The adverse event rate was not significantly different regarding the location of the lesion.
Table 4.
Characteristics of laterally spreading tumours (LSTs) regarding location of the lesion.
| Proximal colon | Distal colon | p Value | |
|---|---|---|---|
| Number | 201/ 53.7 | 173/46.3 | |
| EMR | 156/77.6 | 112/64.7 | 0.006 |
| ESD | 45/22.4 | 61/35.3 | 0.006 |
| Size | 34.0 ± 13.6 (20–100) | 48.8 ± 25.8 (20–140) | <0.00001 |
| Cancer | 24/11.9 | 45/26.0 | 0.00047 |
| T1sm | 10/5.0 | 22/12.7 | 0.0076 |
| Complication | 12/6.0 | 17/9.8 | 0.16 |
| Perforation | 6 | 8 | |
| Bleeding | 6 | 9 | |
| Recurrence | 31/15.4 | 14/8.1 | 0.030 |
EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection.
Endoscopic predictors of deep submucosal invasion
For all lesions, univariate and multivariate analysis showed several factors statistically associated with SMI (Table 5). In the multivariate analysis, invasive pit pattern (hazard ratio (HR) = 33.0 (95% CI: 8.81–143.3); p < 0.001), depression into the lesion (HR = 11.9 (95% CI: 0.89–146.2); p = 0.049), and the size of the lesion (HR = 1.03 (95% CI: 1.01–1.05); p = 0.007) were significantly associated with adenocarcinomas with SMI. The presence of a large nodule and the non-lifting sign were not statistically significant.
Table 5.
Predictor of submucosal invasion: univariate and multivariate analysis.
| Univariate analysis (HR; p) | Multivariate analysis (HR; p) | |
|---|---|---|
| Invasive pit pattern | 18.6 (7.6–46.5); p = <0.00001 | 33.0 (8.81–143.3) ; p = <0.00001 |
| LST-NG IIa + IIc | 15.0 (3.16–79.0); p = 0.025 | 11.9 (0.89–146.2) ; p = 0.049 |
| LST-G nodular mixed | 3.45 (1.66–7.14); p = 0.0008 | 3.42 (0.99–13.0) ; p = 0.056 |
| Size | 1.03 (1.00–1.03); p = 0.009 | 1.03 (1.01–1.05); p = 0.007 |
| Good lifting | 0.41 (0.19–0.92); p = 0.025 | 0.85 (0.26–3.02) ; p = 0.79 |
| Spontaneous bleeding | 25.7 (7.6–101.8); p = <0.00001 | 2.12 (0.32–13.4); p = 0.42 |
HR: hazard ratio; LST-G: granular laterally spreading tumour; LST-NG: non-granular laterally spreading tumour.
Size was associated with SMIC, with a low HR of 1.03. Different sizes of the tumour were evaluated to define the most discriminating threshold, and 40 mm was the most relevant. LSTs greater than 40 mm had a significantly higher rate of SMIC (5.7% versus 13.5%; p = 0.009).
Finally, the ability of the operators to predict SMIC was evaluated. Sensitivity was 57% (95% CI: 0.44–0.67), and specificity reached 90% (95% CI: 0.87–0.93). There was no significant difference between the three operators, respectively p = 0.10 and p = 0.95. Virtual colouration techniques were also compared. Sensitivity and specificity were respectively 0.56 (95% CI: 0.34–0.75) and 0.88 (95% CI: 0.77–0.93) with the BLI system from Fujifilm scopes and 0.63 (95% CI: 0.46–0.78) and 0.91 (95% CI: 0.84–0.95) with the NBI system from Olympus scopes. There was no significant difference between these two systems, respectively p = 0.59 and p = 0.29.
Discussion
This study reports a large series of colorectal LSTs from a Western population and demonstrates the similarity of the cancer and SMI risk according to the subtype of LSTs, with Asian series suggesting that this classification is useful in daily practice to appreciate the potential risk of carcinoma before endoscopic resection. Thus, LST-NG-PD and LST-G-N were associated with a higher risk of cancer, respectively 32.3% and 34.5%.
Cong et al. have reported a series of 177 LSTs treated by ESD: the LST-G-N type was correlated with higher rate of high-grade intraepithelial neoplasia (32.2% versus 10.8%, p = 0.003) and submucosal carcinomas (13.6% versus 1.5%; p = 0.01) compared to LST-G-H, that was very similar to our series (34.5% versus 11.8%, p < 0.0001, and 15.9% versus 4.9%, p = 0.009).19 The LST-NG-PD exhibited the highest rate of submucosally invasive cancer (16.7%) that is close to our results (19.4%). In a Japanese retrospective series of 822 LSTs, LST-NG was associated with SMIC in 39%, with an invasion site under a depression in 45%.9 For LST-G, SMIC was detected in 19% of cases, mainly under a nodule (56%) and a depression (28%). Recently, a meta-analysis was published reporting the results of 48 studies about LSTs and the SMI risk. Overall, 8.5% of LSTs contained SMIC, varying according to the subtype: 31.6% in LST-NG-PD, 10.5% in LST-G-N, 4.9% in LST-NG-F and 0.5% in LST-G-H.20 Most patients in this meta-analysis were provided from Asian series.
Finally, two large Western studies were recently published.20 Probst et al. have reported results of ESD for early rectal neoplasia: the SMI was very high, reaching 71.4% for LST-NG and 17.9% for LST-G-N. Of note, 25 out of the 30 lesions with SMI underwent a non-curative endoscopic resection because of a deeper invasion exceeding 1000 µm: inclusion of these patients in this endoscopic series could probably explain the very high rate of SMI for LST-NG that would have rather needed a surgical resection. Another large multicentre cohort has reported the SMI rate of 2277 lesions in the Western population, from 4.7% in LST-G to 12.5% in LST-NG that was consistent with our results.20
Endoscopic diagnosis of depth of invasion is crucial in deciding which lesions are suitable for endoscopic treatment or surgery, and if piecemeal EMR rather than ESD could be performed. Several classifications have been developed to predict the submucosal cancer from advanced colonic mucosal neoplasia. NICE classification evaluates the lesion’s colour, and surface pattern, and has been shown to be a valid tool for predicting deep submucosal carcinomas. 12The Kudo classification uses more precise description of the pit pattern. It is the most commonly used classification in Asian literature and is helpful to separate early from deep SMI.14,21
In our study, the most important factors in multivariate analysis predicting SMI were an invasive pit pattern, a depressed lesion and the presence of a large nodule. This results confirm results of other studies.4,9,22 In the study of Yamada et al.,9 a large nodule (>10 mm), a depression and an invasive pattern according to the Kudo classification were statistically significant for LST-G lesions. In LST-NG, submucosal mass-like elevation, depression and invasive pit pattern were statistically significant predictors. Data from the Western population were similar, showing that Kudo Pit pattern V, a depressed component, non-granular surface morphology and increasing size, rectosigmoid location and 0-Is or 0-IIa + Is Paris classification were also significant.20
Size was significantly associated with an increase of the SMI rate with a cut-off of 40 mm. Other studies showed this impact but the threshold is different between these studies and is also different according to the subtype of LST.9,22 In a recent meta-analysis, the rates of SMIC for LSTs were 4.6% in 10–19 mm lesions, 9.2% in 20–29 mm, and 16.5% for lesions larger than 30 mm.20 Thus, the size should probably be considered for invasiveness prediction, and the therapeutic decision.
Considering the redundancy of these three predictive factors in the larges series of LST, we believe that large nodule, depression and invasive/non-invasive pit pattern have to be described for LSTs in each report. Moreover, the presence of one of these factors should lead to an ESD rather than EMR, due to the high risk of submucosal lesion for which an en bloc resection is required. Tumour size may also be considered, but its relevance, independently of the other predictive factors, deserves to be evaluated. Finally, this finding concerns almost half of LST lesions since it systematically includes LST-G-N, LST-NG-PD and every lesion with an invasive pattern.
A limitation of our results is the monocentric study, as well as the retrospective design, but we underlined that data was provided from a prospective database and most of the pit pattern diagnoses and characterization of lesions were made by the operator during the procedure. The lack of long-term outcomes is another potential limitation to correctly evaluate the local recurrence rate.
Finally, we showed that the Western experience of ESD is close to the Asian experience. The perforation rate was 5.6% which is similar to Saito et al.’s series (4.9% to 11.8%), such as the en-bloc resection rates of 87.3% (85.0–89.0% in Saito et al.’s series).23 The R0 resection rate was lower in the current series, but this could be explained by the fact that we mixed ESD and hybrid ESD.19,24 Our EMR comparison group also showed the same data as those published previously.10,24,25
In conclusion, this monocentric Western series confirms the available data about LSTs from Asian countries and recent Western series. LST-NG-PD and LST-G-N subtypes are associated with a high risk of SMI, justifying an en bloc resection with ESD. Piecemeal resection could be proposed for other subtypes with caution: a critical prerequisite is the ability to predict an invasive pattern.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
Informed consent
French legislation on studies of anonymised retrospective data do not require informed consent.
References
- 1.Kudo S, Kashida H, Tamura T, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg 2000; 24: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 2.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA 2008; 299: 1027–1035. [DOI] [PubMed] [Google Scholar]
- 3.Kaku E, Oda Y, Murakami Y, et al. Proportion of flat- and depressed-type and laterally spreading tumor among advanced colorectal neoplasia. Clin Gastroenterol Hepatol 2011; 9: 503–508. [DOI] [PubMed] [Google Scholar]
- 4.Kudo S, Lambert R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc 2008; 68: S3–S47. [DOI] [PubMed] [Google Scholar]
- 5.Lambert R, Tanaka S. Laterally spreading tumors in the colon and rectum. Eur J Gastroenterol Hepatol 2012; 24: 1123–1134. [DOI] [PubMed] [Google Scholar]
- 6.Facciorusso A. Non-polypoid colorectal neoplasms: Classification, therapy and follow-up. World J Gastroenterol 2015; 21: 5149–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uraoka T, Saito Y, Matsuda T, et al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut 2006; 55: 1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oka S, Tanaka S, Kanao H, et al. Therapeutic strategy for colorectal laterally spreading tumor. Dig Endosc 2009; 21: S43–S46. [DOI] [PubMed] [Google Scholar]
- 9.Yamada M, Saito Y, Sakamoto T, et al. Endoscopic predictors of deep submucosal invasion in colorectal laterally spreading tumors. Endoscopy 2016; 48: 456–464. [DOI] [PubMed] [Google Scholar]
- 10.Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011; 140: 1909–1918. [DOI] [PubMed] [Google Scholar]
- 11.Nakajima T, Saito Y, Tanaka S, et al. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc 2013; 27: 3262–3270. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: Validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc 2013; 78: 625–632. [DOI] [PubMed] [Google Scholar]
- 13.Uraoka T, Saito Y, Ikematsu H, et al. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc Off J Jpn Gastroenterol Endosc Soc 2011; 23: S112–S115. [DOI] [PubMed] [Google Scholar]
- 14.Liu H-H, Kudo S-E, Juch J-P. Pit pattern analysis by magnifying chromoendoscopy for the diagnosis of colorectal polyps. J Formos Med Assoc Taiwan Yi Zhi 2003; 102: 178–182. [PubMed] [Google Scholar]
- 15.Bae JH, Yang D-H, Lee S, et al. Optimized hybrid endoscopic submucosal dissection for colorectal tumors: A randomized controlled trial. Gastrointest Endosc 2016; 83: 584–592. [DOI] [PubMed] [Google Scholar]
- 16.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015; 47: 829–854. [DOI] [PubMed] [Google Scholar]
- 17.Schlemper R, Riddell R, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol 2012; 17: 1–29. [DOI] [PubMed] [Google Scholar]
- 19.Cong Z-J, Hu L-H, Ji J-T, et al. A long-term follow-up study on the prognosis of endoscopic submucosal dissection for colorectal laterally spreading tumors. Gastrointest Endosc 2016; 83: 800–807. [DOI] [PubMed] [Google Scholar]
- 20.Probst A, Ebigbo A, Märkl B, et al. Endoscopic submucosal dissection for early rectal neoplasia: Experience from a European center. Endoscopy 2016; 49: 222–232. [DOI] [PubMed] [Google Scholar]
- 21.Hurlstone DP, Cross SS, Adam I, et al. Endoscopic morphological anticipation of submucosal invasion in flat and depressed colorectal lesions: Clinical implications and subtype analysis of the Kudo type V pit pattern using high-magnification-chromoscopic colonoscopy. Colorectal Dis Off J Assoc Coloproctology G B Irel 2004; 6: 369–375. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Zhan Q, Xiang L, et al. Clinicopathological characteristics of laterally spreading colorectal tumor. PLoS One 2014; 9: e94552–e94552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 24.Fujiya M, Tanaka K, Dokoshi T, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: A meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc 2015; 81: 583–595. [DOI] [PubMed] [Google Scholar]
- 25.Wang J. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: A meta-analysis. World J Gastroenterol 2014; 20: 8282–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]