Abstract
State prescription drug monitoring programs (PDMPs) aim to reduce risky controlled-substance prescribing, but early programs had limited impact. Several states implemented robust features in 2012–2013, such as mandates that prescribers register with the PDMP and regularly check it; some states allow prescribers to fulfill the latter requirement by designating delegates to check the registry. The effects of robust PDMP features have not been fully assessed. We used commercial claims data to examine effects of robust PDMPs in four states on overall and high-risk opioid prescribing, comparing those results to trends in similar states without robust PDMPs. By the end of 2014, the mean morphine-equivalent dosages that providers dispensed declined by 77, 57, 38, and 6 mg per person per quarter in Kentucky, New Mexico, Tennessee, and New York, respectively, relative to comparison states. Only in Kentucky did the absolute percentage of enrollees filling opioid prescriptions decline versus its comparator state, namely by 2% by the end of 2014. Robust PDMPs may be able to significantly reduce opioid dosages dispensed, percentage of patients receiving opioids, and measures of high-risk prescribing.
Introduction
Opioid misuse and associated harms have grown to epidemic proportions. From 1999–2015, opioid prescriptions in the U.S. tripled,1 paralleled by rates of opioid-related overdose deaths, emergency department visits, and substance use treatment admissions.2,3 In 2015, prescription opioids were involved in 15,000 deaths.4 Most people using opioid analgesics non-medically obtained their drugs from a prescription written either for them or for an acquaintance.5 Thus, efforts to reduce opioid-related harm often focus on moderating prescribing.
State prescription drug monitoring programs (PDMPs) electronically store information about prescriptions filled by patients for drugs with misuse potential, including opioids. Because they offer a wealth of information to prescribers, the Centers for Disease Control and Prevention describe PDMPs as “among the most promising state-level interventions to improve painkiller prescribing, inform clinical practice, and protect patients at risk,” and recommend regular PDMP queries in their opioid prescribing guidelines for chronic pain.6,7
PDMP implementation has proliferated since 1990, and now all states except Missouri have operational programs. Many early programs had technical deficiencies, lacked comprehensive data,8,9 and were little-used. Their effectiveness in reducing opioid prescriptions and opioid-related overdoses was limited.10–18 More recently, states have attempted to increase PDMP participation and improve the clinical utility of PDMP data. Today’s most robust PDMPs require prescribers to register (“registration mandate”) and to query a database prior to prescribing opioids (“use mandate”), and allow prescribers’ delegates to check the PDMP.19,20 Online Appendix A details the features of robust PDMP policies.21
Recent literature finds that PDMPs—particularly those with use mandates or that are implemented in combination with pain clinic laws that regulate the ownership, operation, and management of facilities primarily engaged in the treatment of pain—are associated with decreases in opioid prescribing, doctor shopping, and overdoses.22–26 However, one study found that registration mandates, not use mandates, reduce opioid prescribing among Medicaid patients.27 As well, previous research does not evaluate effects of a package of robust PDMP features on opioid prescribing26 or on PDMP effects among adults with commercial insurance, who account for over half of opioid prescriptions in many states.28
Using a rigorous longitudinal design, we compare prescribing outcomes in states with “robust” PDMPs (defined below) to outcomes in states with weak or no PDMPs. We hypothesized that states introducing robust PDMPs would experience reductions in several population-level measures of opioid prescribing, including the proportion of patients filling opioid prescriptions and the total dosage of opioids prescribed per patient. We also hypothesized that enrollees receiving opioids in states with robust PDMPs would experience reductions, relative to comparison states and to a pre-implementation period, in high-dose opioid prescribing and receipt from multiple prescribers and pharmacies.
Methods
Our quasi-experimental analyses of individual-level health insurance claims data (2010–2014) for commercially-insured adults compared states implementing robust PDMPs to matched states without robust PDMPs.
States with robust PDMPs and comparison states.
Based on careful review of state PDMP laws, we devised a comprehensive, novel coding scheme to identify states that implemented robust PDMPs before January 1, 2014. Robust PDMPs exhibited by the end of 2013 at least 8 of 10 features that facilitate prescriber access to comprehensive, timely data and/or have been established in prior PDMP evaluation literature as important to increasing prescriber use and utility of the data. These were prescriber access to the PDMP; a use mandate of any kind (including those that require prescribers to check the database only if they suspect misuse or diversion, rather than based on objective prescribing criteria); a comprehensive use mandate that requires prescribers to check the PDMP regularly (including for any initial prescription of Schedule II-III drugs to a patient); operation by a health agency; at least weekly dispensing data updates; monitoring of all controlled substances on the federal schedules II-IV; a registration mandate (or automatic registration); delegate access; proactive reporting of suspicious prescribing or dispensing; and no prescriber immunity for failure to check the PDMP.11,19–20,22–27 Online Appendix A and Appendix Exhibits A1-A2 describe if and when robust PDMP features were implemented across all 50 states.21
We identified Kentucky, New Mexico, Tennessee, and New York as “intervention states” with robust PDMPs. Each state implemented a robust PDMP as of the quarter in which it exceeded the 8-feature threshold by unveiling a set of reforms on top of baseline program features. Kentucky implemented 4 new features (a use mandate, a comprehensive use mandate, a registration mandate, and delegate access) and New Mexico implemented 3 new features (a use mandate, a comprehensive use mandate, and a registration mandate) in the third quarter of 2012 to qualify as having a robust PDMP, thereby providing 9 post-implementation quarters for observation in each state. Tennessee implemented 3 new features (a comprehensive use mandate, a registration mandate, and weekly data updates) in the first two quarters of 2013 to qualify, thereby providing 6 post-implementation quarters for observation. New York implemented 6 new features (a use mandate, a comprehensive use mandate, proactive reporting, no prescriber immunity, at least weekly data updates, and delegate access) in the third quarter of 2013 to qualify, thereby providing 5 post-implementation quarters for observation. Importantly, no other relevant program feature changes closely preceded robust PDMP implementation in any intervention state.21
We selected neighboring comparison states without robust PDMPs that had similar primary outcome trends during the pre-implementation period and 5 or fewer of the 10 PDMP features. We compared Missouri to Kentucky, Texas to New Mexico, Georgia to Tennessee, and New Jersey to New York, as shown on a map in online Appendix Exhibit A3.21
Opioid prescribing.
We used deidentified Optum data (OptumInsight, Eden Prairie, MN) to quantify prescribed opioids dispensed to enrollees in plans offered by a large national health insurer.29,30 From the study states, we included prescription claims for adults aged 18 to 64 years enrolled between January 1, 2010 and December 31, 2014. The study start date provided adequate time to evaluate baseline trends, including effects of two national interventions associated with decreases in opioid-related overdoses and prescribing that occurred in the fourth quarter of 2010: reformulation of OxyContin to a tamper-resistant extended-release form and withdrawal of propoxyphene from the market.29 Because rates in our outcomes of interest declined similarly among comparator states following these national interventions and well before our state PDMP implementation periods, we included 2010 data in our analysis (Exhibits 1–2).
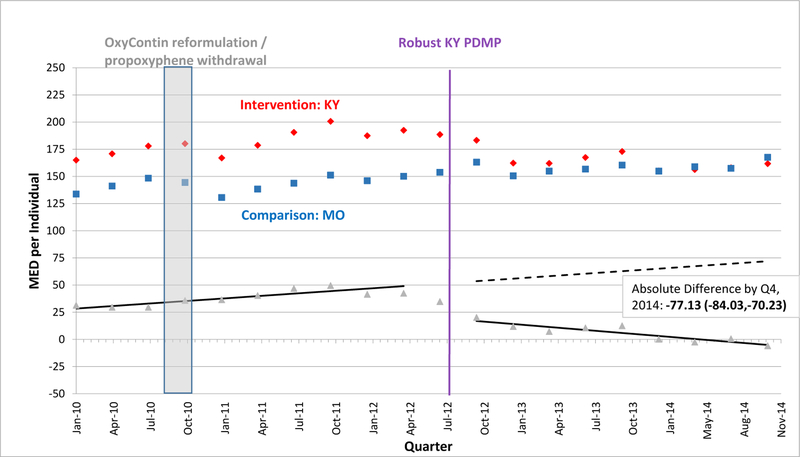
EXHIBIT 1.
Morphine Equivalent Dosage Dispensed per Individual per Quarter in Kentucky versus Missouri, 2010–2014
SOURCE: Authors’ analysis of Optum data (OptumInsight, Eden Prairie, MN), 2010–2014.
NOTES: Abbreviations: PDMP, prescription drug monitoring program; MED, morphine equivalent dosage in milligrams; Q, quarter. A fitted regression line shows the difference between adjusted intervention state (red) and comparison state (blue) quarterly values in the baseline period, and continues as a predicted regression line in the follow-up period, after robust PDMP implementation in the intervention state. We calculated regression lines using population-level interrupted time series linear models, after adjusting for individual age, gender, race/ethnicity, education-level, and poverty-level at each quarter using the STATA margins command. We provide the absolute difference between intervention and comparison state levels by the fourth quarter of 2014 with a 95% confidence interval as an estimate of policy effect.
A vertical bar shows when two national interventions associated with decreases in opioid-related overdoses and prescribing occurred during the fourth quarter of 2010: reformulation of OxyContin to a tamper-resistant extended-release form and withdrawal of propoxyphene from the market.
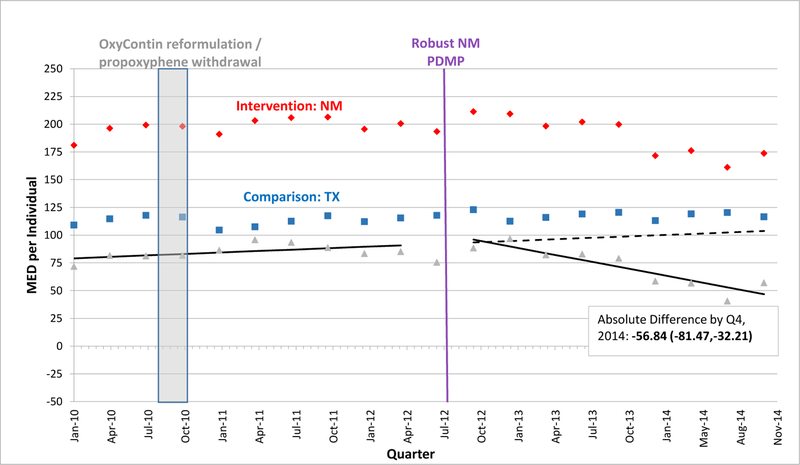
EXHIBIT 2.
Morphine Equivalent Dosage Dispensed per Individual per Quarter in New Mexico versus Texas, 2010–2014
SOURCE: Authors’ analysis of Optum data (OptumInsight, Eden Prairie, MN), 2010–2014.
NOTES: Abbreviations: PDMP, prescription drug monitoring program; MED, morphine equivalent dosage in milligrams; Q, quarter. A fitted regression line shows the difference between adjusted intervention state (red) and comparison state (blue) quarterly values in the baseline period, and continues as a predicted regression line in the follow-up period, after robust PDMP implementation in the intervention state. We calculated regression lines using population-level interrupted time series linear models, after adjusting for individual age, gender, race/ethnicity, education-level, and poverty-level at each quarter using the STATA margins command. We provide the absolute difference between intervention and comparison state levels by the fourth quarter of 2014 with a 95% confidence interval as an estimate of policy effect.
A vertical bar shows when two national interventions associated with decreases in opioid-related overdoses and prescribing occurred during the fourth quarter of 2010: reformulation of OxyContin to a tamper-resistant extended-release form and withdrawal of propoxyphene from the market.
Statistical analyses.
We used two quasi-experimental designs to assess robust PDMP effects independent of other changes to opioid prescribing: interrupted time series with comparison series and difference-in-difference analysis.
The first design, population-level comparative interrupted time series analyses, captured quarterly event rates contemporaneously in 4 intervention/comparison states pairs. The primary outcomes modeled were percentage of enrollees filling opioid prescriptions and mean morphine equivalent dosage (MED) dispensed per enrollee, a measure that standardizes opioid dosages prescribed. We adjusted quarterly primary outcomes for changes in this open cohort’s characteristics during the study period and between state comparator sets.31 We then used aggregate-level segmented linear regression32 to model the differenced outcomes between intervention and comparison states for each of the 4 pairs separately. We detail the interrupted time series statistical analyses in online Appendix A.21
To ensure that patients entering or leaving the study population were not biasing results and to generate interpretable relative change estimates, we conducted sensitivity difference-in-differences analyses on cohorts of adults with any opioid receipt (i.e., at least 1 opioid prescription fill during the study period) and adults with chronic non-cancer-related opioid receipt who were continuously enrolled from 1 year before to 1 year after the robust PDMP implementation quarter. We defined adults with “chronic non-cancer-related opioid receipt” as those with opioid fills in each quarter of the year prior to the PDMP implementation quarter(s) for reasons not related to a non-benign cancer diagnosis. Adjusting for individual covariates, we modeled mean MED dispensed, the number of opioid fills, and the following “high-risk opioid prescribing measures”: average daily MED of ≥ 100 mg, and mean number of quarters in which enrollees used ≥ 3 doctors or ≥ 3 pharmacies to fill opioid prescriptions. We repeated analyses using an alternative comparison state for each intervention state. We detail sensitivity difference-in-differences analyses in online Appendix B.21
We performed analyses using SAS 9.3 (Cary, NC) and Stata 12 (College Station, Texas).
Study limitations.
We used administrative data and therefore cannot observe opioid dispensing not billed to insurance (e.g., cash purchases). Nevertheless, our data represent a substantial market share; approximately two-thirds of controlled substances are paid for by commercial insurance.28,33 Second, our cohort was limited to commercially-insured adults ages 18 to 64—a population with high opioid analgesic use34—and our results may not be generalizable to other populations. Third, we focused primarily on opioid prescribing outcomes, rather than opioid-related injuries. These intermediate outcomes are more proximal to PDMP implementation, but do not directly measure the health impact of PDMPs. Fourth, by defining one cohort for difference-in-difference analyses as persons with opioid fills at any time during the study period, we may have introduced bias if those receiving opioids when robust PDMPs were and were not in place differ. To mitigate this risk, we required this cohort to be continuously enrolled and controlled for individual characteristics.
Fifth, in our comparator pairs, baseline levels were different and baseline trends for some outcomes were not parallel between the states compared in each set.32 However, effect estimates from comparative interrupted time series designs are robust even with such differences, particularly when effects observed at the time of an intervention are immediate and dramatic. Finally, our analysis did not account for other state policy interventions that may have occurred at the same time, although we researched opioid policies in the study states and identified none that could have explained our findings except perhaps for Kentucky’s pain clinic law.35
Results
All 4 states with robust PDMPs exhibited reductions in the opioid dosages prescribed to commercially-insured individuals versus their comparisons, and only Kentucky also exhibited a sustained reduction in the percentage of persons filling opioid prescriptions. The number of opioid prescription fills per person receiving opioids in a year also declined following robust PDMP implementation in all 4 states examined versus comparison states. High-risk opioid prescribing measures only declined among individuals receiving opioids in Kentucky relative to Missouri.
Sample characteristics.
In the interrupted time series analyses of an open cohort of all adult enrollees in commercial insurance plans, enrollment and demographic composition within each state remained quite consistent over time. Sample sizes ranged from 36,100–867,500 enrollees in the month before robust PDMP implementation. Average age was 39–42, half were male, and mean enrollment was 34–52 months. Most of the sample was white, with substantial proportions of persons classified as Hispanic and African American in some states. Online Appendix A and Exhibits A4-A7 detail open cohort characteristics within each state comparator set.21
Changes in population-level opioid prescribing.
By the end of 2014, opioid dosages prescribed declined significantly and in clinically meaningful quantities in all states with robust PDMPs relative to their comparisons. Also, the proportion of enrollees filling opioid prescriptions declined in Kentucky versus its comparison state.
Pre-implementation trends in the percentage of enrollees filling opioid prescriptions per quarter were parallel among each state comparator set. Among 2 of the 4 state comparator sets, pre-implementation trends in mean MED dispensed per enrollee were parallel (Exhibits 1–4). Pre-implementation levels were generally higher in intervention than comparison states, albeit still usually parallel. Online Appendix Exhibits A8 and A9 display the trends in percentage of enrollees filling opioid prescriptions per quarter and quantify the differences in pre- and post-robust PDMP implementation levels and trends between state comparator sets, respectively.21
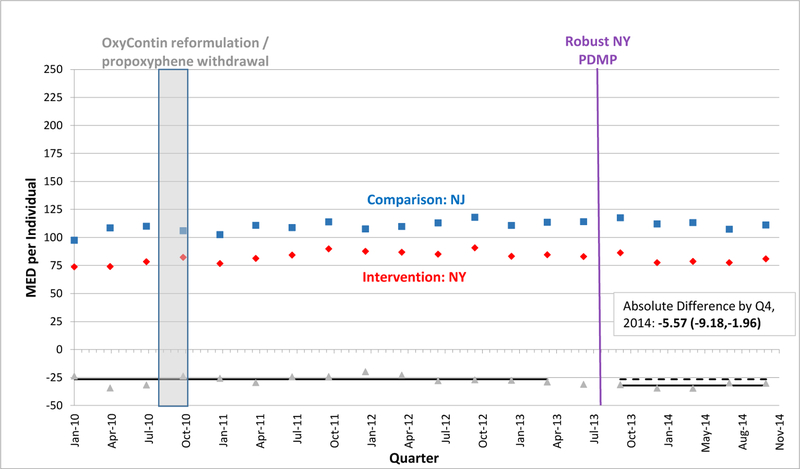
EXHIBIT 4.
Morphine Equivalent Dosage Dispensed per Individual per Quarter in New York versus New Jersey, 2010–2014
SOURCE: Authors’ analysis of Optum data (OptumInsight, Eden Prairie, MN), 2010–2014.
NOTES: Abbreviations: PDMP, prescription drug monitoring program; MED, morphine equivalent dosage in milligrams; Q, quarter. A fitted regression line shows the difference between adjusted intervention state (red) and comparison state (blue) quarterly values in the baseline period, and continues as a predicted regression line in the follow-up period, after robust PDMP implementation in the intervention state. We calculated regression lines using population-level interrupted time series linear models, after adjusting for individual age, gender, race/ethnicity, education-level, and poverty-level at each quarter using the STATA margins command. We provide the absolute difference between intervention and comparison state levels by the fourth quarter of 2014 with a 95% confidence interval as an estimate of policy effect.
A vertical bar shows when two national interventions associated with decreases in opioid-related overdoses and prescribing occurred during the fourth quarter of 2010: reformulation of OxyContin to a tamper-resistant extended-release form and withdrawal of propoxyphene from the market.
In the quarter after robust PDMP implementation, the percentage of sampled persons filling opioid prescriptions declined significantly in 3 states. In Kentucky, where the rate of opioid fills was approximately 10.2% in the quarter before robust PDMP implementation, the absolute percentage fell by 1.3% compared to Missouri in the quarter immediately after PDMP implementation. Tennessee exhibited similar reductions to Kentucky along this outcome, while New York’s reductions were smaller in magnitude. However, these initial reductions were not sustained in Tennessee and New York over time. By the end of the study period, or the fourth quarter of 2014, Kentucky exhibited the most sustained decline, with 1.6% fewer persons filling opioid prescriptions compared to Missouri.
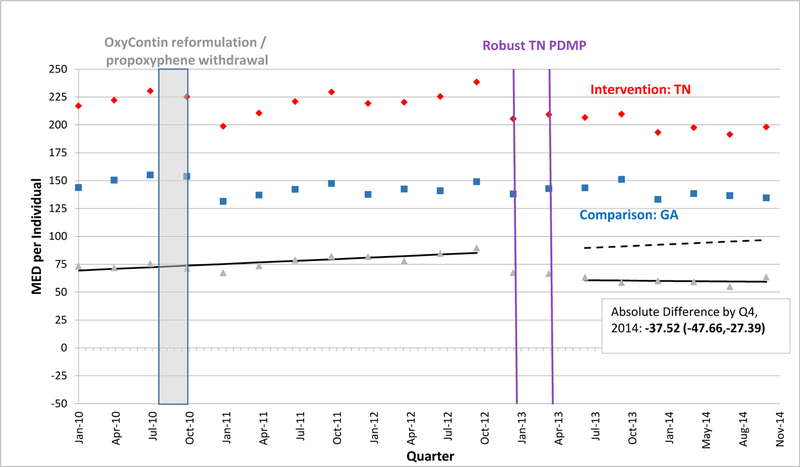
In the quarter after robust PDMP implementation, the mean MED level dispensed per person declined significantly in 3 states. In Kentucky, where mean MED was approximately 192.4 mg per quarter pre-implementation, the absolute level fell by 9.2 mg within Kentucky and by 31.6 mg relative to Missouri (Exhibit 1). Just after PDMP implementation, mean MED also fell in Tennessee and New York relative to their comparators, but by smaller amounts than in Kentucky (Exhibits 3, 4). Enrollees in Kentucky and New Mexico also experienced downward post-implementation trends versus comparisons (Exhibits 1, 2). By the fourth quarter of 2014, Kentucky exhibited the greatest decline in mean MED dispensed per enrollee, an absolute reduction of 77.1 mg relative to Missouri. However, absolute reductions were also significant by the end of the study period – albeit in smaller magnitudes – for the other 3 states relative to their comparators (Exhibits 2–4). In Kentucky, New Mexico, and Tennessee, these reductions approximately equate to each commercially-insured person receiving 10, 8, and 5 fewer 5-mg oxycodone tablets in a quarter, respectively.
EXHIBIT 3.
Morphine Equivalent Dosage Dispensed per Individual per Quarter in Tennessee versus Georgia, 2010–2014
SOURCE: Authors’ analysis of Optum data (OptumInsight, Eden Prairie, MN), 2010–2014.
NOTES: Abbreviations: PDMP, prescription drug monitoring program; MED, morphine equivalent dosage in milligrams; Q, quarter. A fitted regression line shows the difference between adjusted intervention state (red) and comparison state (blue) quarterly values in the baseline period, and continues as a predicted regression line in the follow-up period, after robust PDMP implementation in the intervention state. We calculated regression lines using population-level interrupted time series linear models, after adjusting for individual age, gender, race/ethnicity, education-level, and poverty-level at each quarter using the STATA margins command. We provide the absolute difference between intervention and comparison state levels by the fourth quarter of 2014 with a 95% confidence interval as an estimate of policy effect.
A vertical bar shows when two national interventions associated with decreases in opioid-related overdoses and prescribing occurred during the fourth quarter of 2010: reformulation of OxyContin to a tamper-resistant extended-release form and withdrawal of propoxyphene from the market.
Changes in opioid prescribing among persons who received opioids.
Difference-in-difference analyses among continuously enrolled individuals who received any opioids were consistent with the population-level analyses of all adult enrollees, which revealed reductions in opioid dosages dispensed to individuals after robust PDMP implementation. Also among this continuous cohort who received opioids, a robust PDMP was associated with significant relative reductions in the number of opioid prescriptions filled across all states studied. In Kentucky, the robust PDMP was also associated with significant reductions in high-risk opioid prescribing measures, such as those receiving opioids in high dosages and from multiple prescribers or pharmacies.
Among individuals with any opioid receipt in Kentucky, average opioid prescription fills were 2.4 and 2.1 in the years pre- and post-robust PDMP implementation, respectively, as compared to 2.1 and 2.3 in Missouri. Enrollees in Kentucky received mean MED of 4177.1 mg and 4024.6 mg pre- and post-robust PDMP implementation, as compared to 3922.4 mg and 4627.6 mg in Missouri. Online Appendix A10 provides estimates for pre- and post-robust PDMP implementation outcome levels among cohorts with opioid receipt.21
According to the difference-in-difference analyses, the mean MED dispensed in the year after robust PDMP implementation declined in Kentucky by 857.6 (relative: −18.3%) compared to Missouri. Relative reductions in this measure were also significant, albeit smaller in magnitude than in Kentucky, in New Mexico, Tennessee, and New York (Exhibit 5). We also detected significant reductions in the number of opioid fills in Kentucky of 0.4 (relative: −16.2%), and, to a lesser degree, in the other 3 states in the year after implementation relative to their comparators (Exhibit 5).
EXHIBIT 5.
Effect of Robust PDMPs on Opioid Prescribing Outcomes among Continuous Enrollees who Received Opioidsa
| Mean Change Baseline to
Follow-Up, Intervention vs. Comparison |
||||
|---|---|---|---|---|
| Absolute | Relative, % | |||
| Est | (95% CI) | Est | (95% CI) | |
| a) Kentucky vs. Missouri (n=55,654) | ||||
| Mean No. Opioid Fills/Enrollee | −0.39 | (−0.46, −0.32)*** | −16.15 | (−18.71, −13.60)*** |
| Mean MED Dispensed/Enrollee | −857.61 | (−1143.93,−571.28)*** | −18.33 | (−23.53,−13.13)*** |
| Percent of Enrollees with Daily MED ≥ 100 |
−0.20 | (−0.32, −0.07)* | −20.42 | (−32.03, −8.80)** |
| Mean Q Opioid Rx Filled with ≥ 3
Doctors/Enrollee |
−0.02 | (−0.02,−0.01)*** | −40.44 | (−50.36, −30.54)*** |
| Mean Q Opioid Rx Filled with ≥ 3
Pharmacies/Enrollee |
−0.01 | (−0.01,−0.00)*** | −38.06 | (−52.72, −23.39)*** |
| b) New Mexico vs. Texas (n=173,860) | ||||
| Mean No. Opioid Fills/Enrollee | −0.14 | (−0.22, −0.05)*** | −6.79 | (−10.16, −3.42)*** |
| Mean MED Dispensed/Enrollee | −270.49 | (−860.69,319.71) | −10.72 | (−17.83,−3.62)** |
| Percent of Enrollees with Daily MED ≥ 100 |
−0.04 | (−0.24, 0.16) | −8.82 | (−23.78, 6.13) |
| Mean Q Opioid Rx Filled with ≥ 3
Doctors/Enrollee |
−0.00 | (−0.01, 0.01) | −6.46 | (−22.92, 10.00) |
| Mean Q Opioid Rx Filled with ≥ 3
Pharmacies/Enrollee |
0.00 | (−0.00, 0.01) | 13.05 | (−10.12, 36.30) |
| c) Tennessee vs. Georgia (n=65,623) | ||||
| Mean No. Opioid Fills/Enrollee | −0.11 | (−0.17, −0.06)*** | −5.23 | (−7.81, −2.79)*** |
| Mean MED Dispensed/Enrollee | −446.60 | (−850.68,−42.53)* | −10.43 | (−16.93,−3.93)** |
| Percent of Enrollees with Daily MED ≥ 100 |
−0.07 | (−0.18, 0.04) | −8.76 | (−19.92, 2.40) |
| Mean Q Opioid Rx Filled with ≥ 3
Doctors/Enrollee |
−0.00 | (−0.01, 0.00) | −2.85 | (−14.53, 8.83) |
| Mean Q Opioid Rx Filled with ≥ 3 Pharmacies/Enrollee | 0.00 | (−0.00, 0.01) | 8.72 | (−10.45, 27.90) |
| d) New York vs. New Jersey (n=50,358) | ||||
| Mean No. Opioid Fills/Enrollee | −0.04 | (−0.09, 0.01)† | −2.93 | (−6.00, 0.14)† |
| Mean MED Dispensed/Enrollee | −232.35 | (−406.63,−58.07)* | −10.54 | (−18.42,−2.67)* |
| Percent of Enrollees with Daily MED ≥ 100 |
−0.03 | (−0.14, 0.09) | −1.43 | (−15.95, 13.09) |
| Mean Q Opioid Rx Filled with ≥ 3
Doctors/Enrollee |
−0.00 | (−0.01, 0.00) | −8.56 | (−23.03, 5.91) |
| Mean Q Opioid Rx Filled with ≥ 3
Pharmacies/Enrollee |
0.00 | (−0.00, 0.01) | 14.81 | (−10.06, 39.68) |
SOURCE: Authors’ analysis of Optum data (OptumInsight, Eden Prairie, MN), 2011–2014.
NOTES: Abbreviations: PDMP, prescription drug monitoring program; MED, morphine equivalent dosage in milligrams; Q, Quarters; Rx, Prescriptions.
All rates/changes estimated using the Stata margins and/or nlcom commands, adjusted for age, gender, race/ethnicity, education-level, poverty-level, and Adjusted Clinical Group score. Mean change baseline to follow up is defined as the difference between the year after and the year before quarter(s) of robust PDMP implementation in the intervention versus comparison state.
Intervention states with robust PDMPs include Kentucky, New Mexico, Tennessee, and New York.
Comparison states include Missouri, Texas, Georgia, and New Jersey.
p<0.1
p<0.05
p<0.01
p<0.001
In analyses of potentially high-risk opioid prescribing among persons who received any opioids, only Kentucky experienced significant post-implementation reductions in relation to its comparator, Missouri. The percentage of persons in Kentucky with daily MED ≥ 100 mg declined by 0.2% (relative: −20.4%). Also in Kentucky, the number of quarters when opioid prescriptions were filled with ≥ 3 doctors per enrollee or at ≥ 3 pharmacies per enrollee fell by 0.02 (relative: −40.4%) and 0.01 (relative: −38.1%), respectively (Exhibit 5).
Our findings were generally consistent with results generated when examining opioid prescribing outcomes among continuous enrollees with baseline chronic non-cancer-related opioid receipt. The magnitude of pre-and post-measurements along all outcomes was higher for this chronic cohort than for the cohort with any opioid receipt. For example, enrollees in Kentucky were filling 13.5 and 11.0 opioid prescriptions for non-cancer-related conditions on average pre- and post-robust PDMP implementation, respectively, as compared to 14.1 and 12.8 in Missouri.
As compared to the cohort with any opioid receipt, difference-in-difference changes for the chronic non-cancer-related opioid receipt cohort were larger in absolute terms, smaller in relative terms, and somewhat less significant. As in the main analyses, the most dramatic and consistent reductions along all outcomes among this chronic cohort were observed in Kentucky relative to Missouri. For example, post-implementation absolute and relative reductions in the following outcomes were observed in Kentucky as compared to Missouri: enrollees with daily MED ≥ 100 mg declined by 1.43% (relative: −13.3%); quarters when opioid prescriptions were filled with ≥ 3 doctors per enrollee fell by 0.06 (relative: −37.8%); and quarters when opioid prescriptions were filled at ≥ 3 pharmacies per enrollee dropped by 0.04 (relative: −32.7%). Online Appendix B and Exhibits A10-A13 present complete difference-in-differences results for the chronic non-cancer-related opioid receipt cohort.21
Our findings were also generally consistent with results generated when using alternative comparison states. Notably, our Kentucky findings relative to Missouri, a state with no PDMP, were qualitatively similar to those relative to Indiana, a state with a PDMP only lacking in a comprehensive PDMP mandate and registration mandate during the study period. Online Appendix B and Exhibits A10-A13 provide complete difference-in-differences results for alternative comparator state sets.21
Discussion
We evaluated changes in opioid prescribing after robust PDMP implementation in 4 states. In all states studied, robust PDMP implementation was associated with sustained declines in the total opioid dosage prescribed and number of opioid fills. Implementation was less consistently associated with reduced percentages of patients prescribed opioids. The magnitude and statistical significance of effect varied across intervention states, with Kentucky exhibiting the most dramatic and consistent decreases along all outcomes, followed by Tennessee and New Mexico. We also found that Kentucky’s robust PDMP was associated with reductions in potentially high-risk opioid prescribing measures (i.e., high-dose prescriptions and individuals receiving prescriptions from multiple providers).
Our results are generally consistent with recent studies in other populations that have found reductions in opioid prescribing following implementation of PDMP mandates.22–24,27 Specifically, other analyses have found that registration mandates, rather than use mandates, reduce the number of Schedule II opioid prescriptions among Medicaid enrollees;27 use mandates are associated with reductions in quantity prescribed, as well as doctor and pharmacy shopping in the Medicare population;22,24 and use mandates paired with pain clinic laws are associated with lower opioid dosages prescribed.23
Our study benefits from a more detailed legal and policy analysis and classification of PDMP robustness than previous studies. We also employed a longitudinal, controlled design and compared multiple states along many opioid prescribing outcomes. We assessed PDMP effects among commercially-insured adults, a population not previously investigated but among whom opioid prescribing is highly prevalent.28,34 We also focused on prescribing outcomes more proximal to PDMP use, rather than more distal outcomes like overdoses.25,26 We found differential effects even among states with robust PDMPs, which may explain mixed or conflicting findings in other studies.10–18,22–24,27
We observed the smallest effects in New York, where rates of opioid prescribing were lower at baseline, so PDMP checks might have been less likely to yield salient information and influence prescribers’ decisions. Additionally, New York’s PDMP had fewer robust features than the other intervention states. Most notably, it lacked a registration mandate.
Kentucky’s program might serve as a model for other jurisdictions given the consistent and dramatic effects following robust PDMP implementation that we detected. In July 2012, Kentucky implemented PDMP use and registration mandates and allowed delegates to use the registry. Delegate PDMP checks have helped prescribers to satisfy the use mandate requirement.20 Kentucky’s PDMP also benefited from increased administrative staffing to support its operations, more frequent (daily) updates to the data, and input from prescribers that made the PDMP user-friendly.20,35 From 2011 to 2014, PDMP queries increased over 500%, and from 2012 to 2013, PDMP registrants increased by almost 70%. By July 2013, 95% of in-state practitioners with the authority to prescribe controlled substances were PDMP-registered.20,35–36
Of note, Kentucky implemented a pain clinic law concurrent with PDMP upgrades in 2012, which led to closure of many pain clinics and perhaps may have contributed to some effects observed.24,35Our data do not reflect changes in prescription opioids paid for with cash, a common practice in pain clinics.37 However, to the extent our data do capture some prescription opioids dispensed at pain clinics, any of the prescribing outcomes measured in Kentucky could be affected by the pain clinic law in addition to the robust PDMP.
Both Tennessee and New Mexico, which have PDMPs with features similar to Kentucky’s and also benefited from implementation support and increased PDMP usership, experienced significant effects after implementation. Concurrent with robust PDMP implementation, Tennessee upgraded hardware configuration to handle increased query volume and educated prescribers about the PDMP.38 From 2012–2013, Tennessee PDMP registrants increased 56% while PDMP queries more than doubled from 1.86 to 4.50 million.38 Although New Mexico does not make available pre-implementation registration and use rates, a gradual monthly increase in PDMP queries after robust implementation from 34,000 queries in January 2013 to 100,000 queries in December 2014 suggests that PDMP participation increased.39 Because these states, like Kentucky, allowed delegates to use PDMPS in addition to implementing comprehensive use and registration mandates for prescribers, this trio of policy features may be important to include in a PDMP for significant and clinically meaningful reductions in opioid prescribing.
Unintended consequences of increased PDMP use, such as reduced prescribing when medically indicated or patient substitution to illicit opioid sources, should be monitored closely, although a qualitative assessment of Kentucky’s robust PDMP implementation did not detect adverse unintended consequences.35 Newer features not studied here also deserve future attention, including automated interstate sharing of data and interoperability of PDMP data and medical records.20 Despite these areas of uncertainty, the evidence is compelling that robust PDMPs can make a significant difference in curbing opioid prescribing.
Conclusions
Our findings indicate that PDMPs that incorporate robust design features can significantly reduce the proportion of commercially-insured adults who receive opioid prescriptions as well as the strength of those prescriptions. States interested in refining their PDMPs to reduce opioid prescribing should consider implementing the following features that were adopted by Kentucky, New Mexico, and Tennessee: a comprehensive use mandate, a registration mandate, delegate access, increased program administration capacity, frequent (ideally at least daily) data updates, and priority given to the user-friendliness of the system. The majority of states still lack many of these features.20
Supplementary Material
Contributor Information
Rebecca L. Haffajee, Department of Health Management and Policy, University of Michigan School of Public Health
Michelle M. Mello, Stanford Law School and the Department of Health Research and Policy, Stanford University School of Medicine, Stanford, California
Fang Zhang, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts
Alan M. Zaslavsky, Department of Health Care Policy, Harvard Medical School, Boston, Massachusetts
Marc R. Larochelle, Boston University School of Medicine and Boston Medical Center, Boston, Massachusetts
J. Frank Wharam, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts
References
- 1.Centers for Disease Control & Prevention. Vital signs: changes in opioid prescribing in the United States, 2006–2015. Morbid & Mortality Wkly Rep 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths. Morbid & Mortality Wkly Rep 2016;64(50):1378–82. [DOI] [PubMed] [Google Scholar]
- 3.Office of National Drug Control Policy, Executive Office of the President. 2010 national survey on drug use and health: highlights September 2011. https://www.whitehouse.gov/sites/default/files/ondcp/Fact_Sheets/nsduh_fact_sheet_9-7-11_0.pdf. Accessed May 17, 2016.
- 4.Centers for Disease Control & Prevention. Prescription opioid overdose data Dec. 16, 2016. https://www.cdc.gov/drugoverdose/data/overdose.html. Accessed April 10, 2017.
- 5.Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: summary of national findings, NSDUH Series H-48, HHS Publication No. (SMA) 14–4863 Rockville, MD: Substance Abuse and Mental Health Services Administration; (2014). [Google Scholar]
- 6.Centers for Disease Control & Prevention. Prescription drug monitoring programs (PDMPs) March 23, 2016. http://www.cdc.gov/drugoverdose/pdmp/index.html. Accessed May 18, 2016.
- 7.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. JAMA 2016;315(15):1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyo RA, Irvine JM, Millet LM, et al. Measures such as interstate cooperation would improve the efficacy of programs to track controlled drug prescriptions. Health Aff (Millwood) 2013;32(3):603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutkow L, Turner L, Lucas E, Hwang C, Alexander GC. Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access. Health Aff (Millwood) 2015;34(3):484–92. [DOI] [PubMed] [Google Scholar]
- 10.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med 2011;12(5):747–54. [DOI] [PubMed] [Google Scholar]
- 11.Reifler LM, Droz D, Bailey JE, et al. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med 2012;13(3):434–42. [DOI] [PubMed] [Google Scholar]
- 12.Reisman RM, Shenoy PJ, Atherly AJ, Flowers CR. Prescription opioid usage and abuse relationships: an evaluation of state prescription drug monitoring program efficacy. Subst Abuse 2009;3:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rutkow L, Chang HY, Daubresse M, Webster DW, Stuart EA, Alexander GC. Effect of Florida’s prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Intern Med 2015; 175(10):1642–49. [DOI] [PubMed] [Google Scholar]
- 14.Brady JE, Wunsch H, DiMaggio C, Lang BH, Giglio J, Li G. Prescription drug monitoring and dispensing of prescription opioids. Pub Health Rep 2014;129(2):139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baehren DF, Marco CA, Droz DE, Sinha S, Callan EM, Akpunonu P. A statewide prescription drug monitoring program affects emergency department prescribing behavior. Ann Intern Med 2010;56(1):19–23. [DOI] [PubMed] [Google Scholar]
- 16.Bao Y, Pan Y, Taylor A, et al. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff (Millwood) 2016;35(6):1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yarbrough CR. Prescription drug monitoring programs produce a limited impact on painkiller prescribing in Medicare Part D [published online January 18, 2017]. Health Serv Res doi: 10.1111/1475-6773.12652. [DOI] [PMC free article] [PubMed]
- 18.Moyo P, Simoni-Wastila L, Griffin BA, et al. Impact of prescription drug monitoring programs (PDMPs) on opioid utilization among Medicare beneficiaries in 10 U.S. States. Addiction 2017; 112(10):1784–1796. [DOI] [PubMed] [Google Scholar]
- 19.Haffajee RL, Jena AB, Weiner SG. Mandatory use of prescription drug monitoring programs. JAMA 2015;313(9):891–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The PEW Charitable Trusts. Prescription drug monitoring programs: evidence-based practice to optimize prescriber use December 2016. http://www.pewtrusts.org/en/research-and-analysis/reports/2016/12/prescription-drug-monitoring-programs/. Accessed April 10, 2017.
- 21.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 22.Rasubala L, Pernapati L, Velasquez X, Burk J, Ren YF. Impact of mandatory prescription drug monitoring program on prescription of opioid analgesics by dentists. PLoS ONE 2015:10(8):e0135957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowell D, Zhang K, Noonan RK, Hockenberry JM. State level mandatory provider review of prescription drug monitoring program data combined with pain clinic laws reduces opioid prescribing and opioid overdose death rates. Health Aff (Millwood) 2016;35(10):1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchmueller T, Carey C. The effect of prescription drug monitoring programs on opioid utilization in Medicare. NBER Working Paper Series http://www.nber.org/papers/w23148. Accessed March 30, 2017.
- 25.Patrick SW, Fry CE, Jones TF, Buntin MB. Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff (Millwood) 2016;35(7):1324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pardo B Do more robust prescription drug monitoring programs reduce prescription opioid overdose. Addiction 2017;112(10):1773–1783. [DOI] [PubMed] [Google Scholar]
- 27.Wen H, Schackman BR, Aden B, Bao Y. States with prescription drug monitoring mandates saw a reduction in opioids prescribed to Medicaid Enrollees. Health Aff (Millwood) 2017;36(4):733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM. Controlled substance prescribing patterns—prescription behavior surveillance system, eight states, 2013. Morbid & Mortality Wkly Rep 2015;6(SS09):1–14. [DOI] [PubMed] [Google Scholar]
- 29.Larochelle MR, Zhang F, Ross-Degnan D, Wharam JF. Rates of opioid dispensing and overdose after introduction of abuse-deterrent extended-release oxycodone and withdrawal of propoxyphene. JAMA Intern Med 2015;175(6):978–87. [DOI] [PubMed] [Google Scholar]
- 30.Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med 2016;164(1):1–9. [DOI] [PubMed] [Google Scholar]
- 31.Williams R Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata J 2012;12:308–31. [Google Scholar]
- 32.Wagner A, Soumerai S, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm & Therapeutics 2002; 27:299–309. [DOI] [PubMed] [Google Scholar]
- 33.Dasgupta N, Kramer ED, Zalman MA, et al. Association between non-medical and prescriptive usage of opioids. Drug Alcohol Depend 2006;82(2):135–42. [DOI] [PubMed] [Google Scholar]
- 34.Blue Cross Blue Shield. America’s opioid epidemic and its effect on the nation’s commercially-insured population June 2017. https://www.bcbs.com/the-health-of-america/reports/americas-opioid-epidemic-and-its-effect-on-the-nations-commercially-insured. Accessed July 11, 2017.
- 35.Freeman PR, Goodin A, Troske S, Talbert J. Kentucky house bill 1 impact evaluation March 2015. Lexington, KY: CHFS; http://www.chfs.ky.gov/NR/rdonlyres/8D6EBE65-D16A-448E-80FF-30BED11EBDEA/0/KentuckyHB1ImpactStudyReport03262015.pdf. Accessed June 5, 2016. [Google Scholar]
- 36.Kentucky Cabinet for Health and Family Services. KASPER trend report Q4 2015 Frankfort, KY: CHFS; http://www.chfs.ky.gov/NR/rdonlyres/12F90847-46BB-4AD5-9ABB-19FA10C2AF79/0/KASPERQuarterlyTrendReportQ42012.pdf. Accessed May 18, 2016. [Google Scholar]
- 37.Rigg KK, March SJ, Inciardi JA. Prescription drug abuse & diversion: the role of the pain clinic. J Drug Issues 2010;40(3):681–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NASCIO Data, Information and Knowledge Management Initiative, The State of Tennessee. Tennessee controlled substance monitoring database: phase I & phase II start September 1, 2012 – completion December 1, 2013. http://www.nascio.org/portals/0/awards/nominations2014/2014/2014TN2-Controlled%20Substance%20Monitoring%20Database2.pdf. Accessed June 5, 2016.
- 39.New Mexico Board of Pharmacy. Prescription monitoring program: PMP statistics Albuquerque, NM: NM Board of Pharmacy; http://www.nmpmp.org/Stats/Default.aspx. Accessed June 5, 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.