Abstract
Summary
Gene expression changes over the lifespan and varies among different tissues or cell types. Gene co-expression also changes by sex, age, different tissues or cell types. However, gene expression under the normal state and gene co-expression in the human brain has not been fully defined and quantified. Here we present a database named Brain EXPression Database (BrainEXP) which provides spatiotemporal expression of individual genes and co-expression in normal human brains. BrainEXP consists of 4567 samples from 2863 healthy individuals gathered from existing public databases and our own data, in either microarray or RNA-Seq library types. We mainly provide two analysis results based on the large dataset: (i) basic gene expression across specific brain regions, age ranges and sexes; (ii) co-expression analysis from different platforms.
Availability and implementation
Supplementary information
Supplementary data are available at Bioinformatics online.
1 Introduction
Comprehensive knowledge about the pattern of gene expression in normal human brain (‘normal’ means no neuropsychiatric disorders) is fundamental to understand the molecular function and physiology of human brain. Spatiotemporal analyses of human brain have identified many genes differentially expressed among brain regions (Johnson et al., 2009; Kang et al., 2011; Miller et al., 2014), age stages (Johnson et al., 2009; Kang et al., 2011; Miller et al., 2014) and sexes (Kang, et al., 2011). Quantification of the spatiotemporal expression in human brain is essential for understanding the neurodevelopment, gender differences and neuropsychiatric disorders.
Gene co-expression, which may reflect regulatory relationships among genes, was also implicated in neurodevelopment (Bakken et al., 2016; Johnson et al., 2009; Miller et al., 2014) and the etiology of multiple brain disorders (Bakken et al., 2016; Tebbenkamp et al., 2014). Weighted gene co-expression network analysis (WGCNA) (Langfelder and Horvath, 2008) is a popular method to construct the gene co-expression network for identifying sets of genes with correlated patterns among samples. Genes with high topological overlap matrix named modules can be defined by this method through the dynamic tree cut algorithm. Modules of coordinated expression of genes and co-regulation relationships can be identified.
Here, we developed a novel database called Brain EXPression (BrainEXP), which provides the normal brain expression levels and co-expression analysis results for reference. Compared with other databases of the same type (Colantuoni et al., 2011; Higgs et al., 2006; Miller et al., 2014), BrainEXP provides a more in-depth analysis based on the largest sample-sizes. Combined data from several published databases and our own data were strictly analyzed by consistent workflow. Thus, large sample-sizes and abundant brain regions or age stages are included in BrainEXP. BrainEXP is the first database that supplies gene co-expression information based on WGCNA in the human brain data.
2 Materials and methods
Currently, BrainEXP contains 4567 normal human brain samples of 2863 normal individuals from both our own data and existing public databases, including the Gene Expression Omnibus database (Barrett et al., 2013), ArrayExpress (Kolesnikov et al., 2015), Genotype-Tissue Expression project (Consortium, 2013), Brain Cloud (Colantuoni et al., 2011), Brainspan (Miller et al., 2014), Stanley Medical Research Institute online genomics database (SMRIDB) (Higgs et al., 2006) and BrainGVEx (Psych et al., 2015). Datasets with fewer than 15 samples were not included. The samples’ ages range from embryonic stages to late adulthood, involving 56 brain regions. The raw expression data were measured by eight platforms, two RNA-Seq and six microarray platforms. Data from different platforms were analyzed separately with stringent quality control and a consistent pre-processing pipeline (see Supplementary Material). ComBat was used to correct the batch effect within and among datasets (Johnson et al., 2007; Leek et al., 2012).
The database was designed using relational tables and was implemented by Microsoft’s SQL Server Management Studio. Its website was built using C# on Windows Server.
3 Results
BrainEXP consists of two major parts: spatiotemporal expression variations and WGCNA co-expression networks described as follows (also see Fig. 1):
Fig. 1.
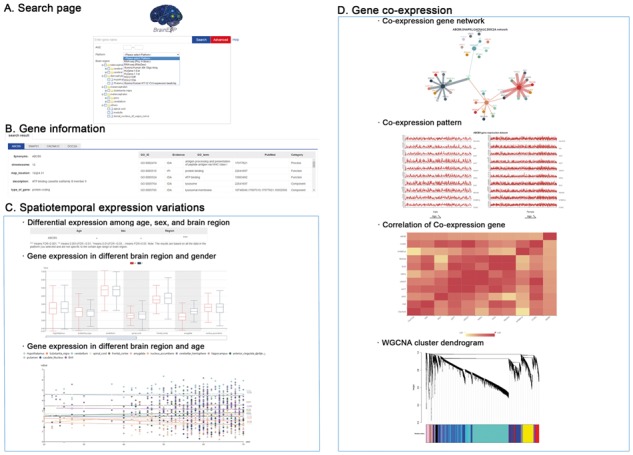
The query and search results page. (A) The Search page. (B) The gene information interface. (C) The Spatiotemporal expression variations interface. (D) The Gene co-expression results interface
3.1 Spatiotemporal expression variations
BrainEXP provides three types of charts: The Differential expression analysis, the Gene expression in different brain regions and sexes shown by boxplot and the Gene expression in different brain regions and ages demonstrated by scatterplot.
3.2 WGCNA co-expression networks
We applied WGCNA to get the matrix of gene co-expression values. The results are displayed through four different modes. The first is the Co-expression gene network. Gene nodes connect if the corresponding genes significantly co-expressed across samples at each platform. We choose top 10 co-expressed genes ordered by the adjacency (the higher the absolute adjacency value, the stronger the connection will be) (see Supplementary Material). Second, the Co-expression pattern shows the other gene expression levels with the similar pattern. Next, the Correlation of co-expression genes gives the correlation coefficients of these 11 genes (top 10 genes plus the query) displayed by the heat map. Finally, the WGCNA cluster dendrogram will tell you in which module the query gene is detected and how many genes are in the same module.
3.3 The advantages of combined datasets
The increase of sample size can improve the statistical power, overcome the influence of outliers and represent population better. We test whether a larger dataset can produce more information in the differential expression analysis and co-expression analysis. In differential expression analysis, since different datasets have different age ranges and brain regions, which may affect the results, we analyzed sex-related genes only. The test dataset chosen from our database has the same brain region (Hippocampus), platform (HG-U133P) and age range (22∼68 years old). This combined dataset contains 30 (12 and 18) samples. The results showed that 21 sex-related genes were detected in 30-sample dataset, while zero was detected in either 12- or 18-sample datasets. So, the combined dataset identified more sex-related genes.
In the WGCNA analysis, larger sample data resulted in more robust and refined results (Langfelder and Horvath, 2008). In summary, the combined data are proven valuable in detecting differential expression and co-expression.
3.4 Future update
The data will be updated as new human brain expression datasets are available. The datasets involving different cell types will be included in the future. New functions such as the gene co-expression in particular brain regions, age stages and sex will be added.
Supplementary Material
Acknowledgements
We sincerely thank Chicago Biomedical Consortium for its supports (to C. Liu). All the data contributors are sincerely appreciated for data submitted in the GEO and other databases. We are grateful to Qingres | Journal of Psychiatry and Brain Science for hosting our webserver.
Funding
This work was supported by National Natural Science Foundation of China grants 81401114, 31571312, the National Key Plan for Scientific Research and Development of China (2016YFC1306000), Innovation-Driven Project of Central South University (No. 2015CXS034, 2018CX033) (to C. Chen) and NIH grants 1 U01 MH103340-01, 1R01ES024988 (to C. Liu).
Conflict of Interest: none declared.
References
- Bakken T.E. et al. (2016) A comprehensive transcriptional map of primate brain development. Nature, 535, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T. et al. (2013) NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res., 41, D991–D995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantuoni C. et al. (2011) Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature, 478, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium G.T. (2013) The genotype-tissue expression (GTEx) project. Nat Genet., 45, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs B.W. et al. (2006) An online database for brain disease research. BMC Genomics, 7, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.B. et al. (2009) Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron, 62, 494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W.E. et al. (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics, 8, 118–127. [DOI] [PubMed] [Google Scholar]
- Kang H.J. et al. (2011) Spatio-temporal transcriptome of the human brain. Nature, 478, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov N. et al. (2015) ArrayExpress update–simplifying data submissions. Nucleic Acids Res., 43, D1113–D1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics, 9, 559.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek J.T. et al. (2012) The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics, 28, 882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.A. et al. (2014) Transcriptional landscape of the prenatal human brain. Nature, 508, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psych E.C. et al. (2015) The PsychENCODE project. Nat Neurosci, 18, 1707–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebbenkamp A.T. et al. (2014) The developmental transcriptome of the human brain: implications for neurodevelopmental disorders. Curr. Opin. Neurol., 27, 149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


