Significance
The plant genome encodes resistance (R) genes that are one of the major genomic resources to enhance disease resistance in various crops. R gene products, R proteins, serve as intracellular receptors for pathogen effectors, leading to activation of effector-triggered immunity. Due to the importance of R proteins, elucidation of their signaling pathways is an important research goal. We revealed that OsSPK1, a GDP/GTP exchange factor for the small GTPase OsRac1, is a direct binding protein of the rice R protein Pit, which is a resistance protein to rice blast fungus. OsSPK1 is a key signaling molecule in Pit signaling. Our results provide a critical new insight into molecular mechanisms underlying R protein activation and new knowledge for crop improvement.
Keywords: GEF, R protein, rice, small GTPase, effector-triggered immunity
Abstract
Resistance (R) genes encode intracellular nucleotide-binding/leucine-rich repeat-containing (NLR) family proteins that serve as critical plant immune receptors to induce effector-triggered immunity (ETI). NLR proteins possess a tripartite domain architecture consisting of an N-terminal variable region, a central nucleotide-binding domain, and a C-terminal leucine-rich repeat. N-terminal coiled-coil (CC) or Toll-interleukin 1 receptor (TIR) domains of R proteins appear to serve as platforms to trigger immune responses, because overexpression of the CC or TIR domain of some R proteins is sufficient to induce an immune response. Because direct downstream signaling molecules of R proteins remain obscure, the molecular mechanisms by which R proteins regulate downstream signaling are largely unknown. We reported previously that a rice R protein named Pit triggers ETI through a small GTPase, OsRac1, although how Pit activates OsRac1 is unclear. Here, we identified OsSPK1, a DOCK family guanine nucleotide exchange factor, as an interactor of Pit and activator for OsRac1. OsSPK1 contributes to signaling by two disease-resistance genes, Pit and Pia, against the rice blast fungus Magnaporthe oryzae and facilitates OsRac1 activation in vitro and in vivo. The CC domain of Pit is required for its binding to OsSPK1, OsRac1 activation, and the induction of cell death. Overall, we conclude that OsSPK1 is a direct and key signaling target of Pit-mediated immunity. Our results shed light on how R proteins trigger ETI through direct downstream molecules.
Plants have evolved a two-layered innate immune system to avoid infections by pathogens (1, 2). The first layer is governed by pattern recognition receptors that recognize microbe-associated molecular patterns (MAMPs), resulting in the initiation of early immune responses (3, 4). This process is called MAMP-triggered immunity (MTI) and is the basal immune response of plants against pathogen invasion. Pathogens can secrete effectors [also called avirulence (Avr) proteins] that enter plant cells to perturb various aspects of host cell physiology, including MTI signaling. Plants have also developed the second layer of defense against pathogens. In this branch of the immune system, plants employ resistance (R) proteins that act as intracellular receptors to perceive pathogenic effectors (5–7). This defense system is termed effector-triggered immunity (ETI), and triggers the hypersensitive response and the production of reactive oxygen species (ROS) (1, 2).
Most of the R genes encode nucleotide-binding (NB) and leucine-rich repeat (LRR) domain-containing (NLR) family proteins (5–7). NLR proteins possess a tripartite domain architecture consisting of an N-terminal region, a central NB-ARC (NB and APAF-1, certain R gene products, and CED-4) domain, and C-terminal LRRs. Based on their N-terminus structures, NLR proteins fall into two major subclasses: NLRs with a coiled-coil (CC) domain (CNLs) or with a Toll-interleukin 1 receptor (TIR) domain (TNLs) (5). CC and TIR domains appear to act as platforms to induce immune responses, because overexpression of the CC domain of barley MLA10, maize Rp-1D, and tobacco NRG1, or of the TIR domain of flax L6 and Arabidopsis RPS4, is sufficient to trigger cell death in Nicotiana benthamiana (8–12). To date, several binding partners that are associated with the N-terminal CC domain of NLR proteins—such as RIN4, PBS1, BSL1, Pto, ZED1, and RanGAP2—have been identified, but they mainly contribute to monitoring/perception of pathogen effectors rather than signal transduction of R proteins (13–19). The molecular mechanisms of immune responses induced by NLR proteins are thus largely unknown, because direct downstream signaling molecules of R proteins remain obscure.
Like other small GTPases, Rac/Rop family small GTPases function as molecular switches by cycling between GDP-bound inactive and GTP-bound active forms (20, 21). Rac/Rop family small GTPases serve as regulators of various cellular events, including pollen tube growth, development, and immunity (20, 22–26). Guanine nucleotide exchange factors (GEFs) control the transition from inactive (GDP-bound) to active (GTP-bound) forms of small GTPases. In plants, GEFs are categorized into three subclasses according to their catalytic GEF domain: a plant-specific Rop nucleotide exchanger (PRONE), a diffuse B-cell lymphoma homology-pleckstrin homology (DH-PH), and a conserved DOCK homology region 2 (DHR2) domain (27–30).
We have previously revealed that the small GTPase OsRac1 serves as a key regulator in both MTI and ETI (20, 25) and that it contributes to MTI induced by fungal elicitors, such as chitin and sphingolipids (31, 32). Two chitin coreceptors, namely the RLP OsCEBiP and the RLK OsCERK1, work together to sense chitin and directly phosphorylate OsRacGEF1, thereby resulting in the activation of OsRacGEF1 (33) and subsequently in OsRac1 activation and MTI (33). The OsRac1 complexes comprise 15 components, including NADPH oxidase and OsMPK6, which play key roles in MTI (20, 25, 34–36). A DH-PH–type GEF, SWAP70, regulates chitin-induced ROS production and defense gene expression, probably through OsRac1 (28). We have also explored OsRac1-binding proteins and identified Pit, which is an R protein for rice blast fungus, and revealed that OsRac1 functions as a downstream molecular switch of Pit to control ROS production and cell death (37). We further found that anchoring of Pit to the plasma membrane through palmitoylation, a lipid modification, is required for Pit-induced OsRac1 activation on the plasma membrane (38). However, Pit does not have a GEF domain; we do not know how it activates OsRac1 in planta.
In this study, we identified a Pit interactor, OsSPK1, which is a DOCK family GEF protein. Pit associated with OsSPK1 through its CC domain, and regulated OsRac1 activation. Our results provide mechanistic clues to how CNL proteins trigger the activation of downstream molecules.
Results
OsSPK1 Is an Interactor of the R Protein Pit.
We have previously revealed that the R protein Pit regulates ETI through the activation of the small GTPase OsRac1 (37). However, the identity of direct activators of OsRac1 in Pit signaling remains unclear. It is known that GEFs that facilitate the release of GDP from small GTPases serve as activators of small GTPases. We and another group have reported two different types of GEFs for OsRac1, a PRONE-type GEF (OsRacGEF1) and a DH-PH–type GEF (OsSWAP70), that contribute to OsRac1-dependent MTI (28, 33). To examine the possibility that OsRacGEF1 and OsSWAP70 function in Pit-mediated resistance, we first tested interactions between Pit and these two GEFs using yeast two-hybrid assays. However, we could not detect any interaction between Pit and either GEF under our conditions (SI Appendix, Fig. S1 A–C). Next, we employed a different type of GEF, OsSPK1, which belongs to the DOCK family of GEFs and shows 76% identity with Arabidopsis SPK1 (SI Appendix, Fig. S1D), to test the interaction (29). OsSPK1 has three conserved domains: DHR1 (amino acids 466–637); DHR2 (amino acids 1384–1833), which functions as the GEF domain; and DHR3 (amino acids 150–294) (Fig. 1A). The N-terminal CC domain of Pit associated with the C-terminal fragment of OsSPK1 (amino acids 1002–1835) containing the DHR2 domain in a yeast two-hybrid assay, but we could not detect any interaction in the other domain combinations (Fig. 1B). To further confirm this interaction, we performed coimmunoprecipitation (co-IP) assays by transiently expressing Pit and OsSPK1 in N. benthamiana leaves. When green fluorescent protein (GFP)-tagged Pit was precipitated by anti-GFP antibody, OsSPK1-Myc coprecipitated with Pit-GFP (Fig. 1C). Consistent with this, a direct interaction between the CC domain of Pit and OsSPK1 (amino acids 1334–1835) was observed in an in vitro pull-down assay (Fig. 1D) and a yeast two-hybrid assay (SI Appendix, Fig. S2A).
Fig. 1.
OsSPK1 interacts with the R protein Pit in rice. (A) Schematic representation of Pit and OsSPK1 deletion mutants used in this study. (B) Interaction between Pit and OsSPK1 in a yeast two-hybrid assay. Yeast growth on selective plates without histidine [His (−)] indicates a positive interaction. (C) In vivo interaction between Pit and OsSPK1. Pit-GFP and OsSPK1-Myc were transiently coexpressed in N. benthamiana leaves. Co-IP was carried out with anti-GFP antibody, and the proteins were detected by Western blot with anti-GFP and anti-Myc antibodies. (D) Direct interaction between Pit and OsSPK1 in an in vitro binding assay. Purified MBP or MBP-tagged OsSPK1 (amino acids 1334–1835) immobilized to Sepharose was incubated with His-SUMO–tagged Pit CC. After washing, the bound proteins were eluted by addition of the sample buffer for immunoblotting. (E and F) Localization of OsSPK1 in N. benthamiana leaves. (E) The leaves were injected with Agrobacterium carrying YFP-OsSPK1 (green), and stained with FM4-64 (red; plasma membrane marker). Open arrowheads represent PM and closed arrowheads show the position of YFP-OsSPK1, which does not merge with PM. (Scale bars, 25 μm.) (F) YFP-OsSPK1 (green) and mRFP-HDEL (red; ER marker) were cotransformed into tobacco leaves. Insets are enlargements (3×) relative to white dashed boxes. (Scale bars, 25 μm.) (G) Subcellular distribution of native OsSPK1 in rice cells. Western blots of rice suspension cell fractions separated using a commercial kit. Each fraction was blotted with antibodies against proteins that localize to specific subcellular compartments. UGPase, BiP, and H+ATPase were used as markers for cytoplasm, ER, and plasma membrane, respectively. Cyt, cytosol fraction; EM, endomembrane fraction; PM, plasma membrane fraction; TM, total membrane fraction; Total, total protein extract. (H) BiFC assay of Pit and OsSPK1 in N. benthamiana leaves. Expression of these genes was driven by the CaMV 35S promoter. Empty vector served as a negative control. FM4-64 was used as a plasma membrane marker. (Scale bars, 25 μm.)
Next, we looked at the subcellular distribution of YFP-OsSPK1 in N. benthamiana under a fluorescence microscope. Before observing its localization, we successfully detected intact YFP-OsSPK1 protein in N. benthamiana leaves by immunoblotting (SI Appendix, Fig. S2B). The fluorescent dye FM4-64 was used as a PM marker. YFP-OsSPK1 showed a punctate distribution (Fig. 1E). We carefully observed the localization of YFP-OsSPK1 and found that it displayed a transvacuolar strand-like distribution and merged only rarely with FM4-64 (Fig. 1E, arrowheads, Inset). It has been reported that AtSPK1 is a peripheral membrane protein that accumulates at the endoplasmic reticulum (ER), and promotes the formation of a specialized domain termed the ER exit site (39). Therefore, we compared the localization of YFP-OsSPK1 with an ER marker monomer RFP (mRFP)-HDEL and found that YFP-OsSPK1 colocalized partially with mRFP-HDEL (Fig. 1F). The cytoplasm and ER are packed tightly against the plasma membrane in tobacco cells and it is technically difficult to determine conclusively the localization of OsSPK1. Therefore, we undertook biochemical fractionation to clarify its localization. We roughly fractionated rice suspension cells into four compartments: total membrane (plasma membrane and endomembrane), plasma membrane, endomembrane (ER, Golgi, mitochondria, plastids, and so forth), and cytosol. We found that OsSPK1 was localized mainly in the endomembrane fraction (Fig. 1G). Consistently, YFP-OsSPK1 was also detected mainly in the membrane fraction in N. benthamiana (SI Appendix, Fig. S2D). Taking these data together, we find that SPK1 might be associated only with subdomains of the ER or other endomembranes.
We attempted to assess the interaction between OsSPK1 and Pit in planta using a bimolecular fluorescence complementation (BiFC) assay. We confirmed that our BiFC proteins were intact by immunoblotting (SI Appendix, Fig. S2F). YFP signal of BiFC between Pit-nYFP and OsSPK1-cYFP was well merged with the plasma membrane marker FM4-64 (Fig. 1H) and was different from the punctate localization of YFP-OsSPK1 (Fig. 1E) but we were unable to detect fluorescence in the pair with a negative control, indicating that the OsSPK1-Pit complex is localized mainly in the plasma membrane and the recruitment of OsSPK1 to the plasma membrane occurs through its interaction with Pit (Fig. 1H and SI Appendix, Fig. S2E). We also monitored the localization of YFP-OsSPK1 coexpressed with Pit but we could not detect any change in YFP-OsSPK1 localization. This may be due to a low-affinity or transient interaction between Pit and OsSPK1 (SI Appendix, Fig. S5 A and B, a–c).
OsSPK1 Contributes to Pit-Mediated Disease Resistance.
To test whether OsSPK1 functions in Pit-mediated resistance, like OsRac1 (37), we chose rice cultivar K59, which carries a functional Pit gene, and the avirulent blast fungus Magnaporthe oryzae race 007.0 (40). When we used a punch method, all three independent lines of OsSPK1 RNAi plants developed larger lesions than WT plants after infection with the avirulent race of rice blast fungus (Fig. 2 A–C and SI Appendix, Fig. S3A). To precisely measure fungal growth in OsSPK1 RNAi plants, we conducted DNA-based real-time PCR to quantify the amount of DNA of M. oryzae using two sets of primers specific for M. oryzae Pot2 and rice Ubiquitin. The qPCR analysis revealed that the infection ratio of MoPot2/OsUbq in OsSPK1 RNAi plants was much higher than that in WT plants at 7 d postinoculation (dpi) (Fig. 2D). We also performed a spray inoculation assay using the SPK1 RNAi lines. Pit triggered a unique resistance lesion induced by inoculation of the avirulent M. oryzae strain, with a brown ring (halo) around the fungal penetration site (SI Appendix, Fig. S3B, arrowheads) (41). This halo is a typical symptom of Pit-mediated resistance and was observed in K59 WT. In contrast, OsSPK1 RNAi lines did not show halo rings, and exhibited susceptible symptoms and enhanced fungal growth (SI Appendix, Fig. S3 B–D), demonstrating that OsSPK1 RNAi compromises Pit-mediated disease resistance to avirulent rice blast fungus. We examined the possibility that OsRacGEF1 and OsSWAP70 also participate in Pit-mediated disease resistance using OsRacGEF1 and OsSWAP70 RNAi plants. The lesion induced by rice blast fungus in OsRacGEF1 RNAi plants was comparable to that in WT plants under conditions in which we clearly observed an effect of OsSPK1 RNAi, implying that OsRacGEF1 is not involved in the Pit signaling pathway (Fig. 2 E and F). However, we were unable to obtain OsSWAP70 RNAi suspension cells or plants, suggesting that OsSWAP70 is an essential gene for development in rice cultivar K59.
Fig. 2.
OsSPK1 contributes to Pit-mediated resistance in rice immunity. (A) Transcript levels of OsSPK1 were measured by qPCR in OsSPK1 RNAi plants. Numbers 1, 17, and 32 indicate independent RNAi transgenic lines. OsUbq was used as an internal control. Bars are SE (**P < 0.01; n = 3). (B–D) Infection assays of OsSPK1 RNAi plants with the incompatible M. oryzae race 007.0. (B) Photographs show typical phenotypes of OsSPK1 RNAi plants after infection at 7 dpi. (Scale bar, 5 mm.) (C) Quantitative analysis of lesions induced by the blast fungus was performed at 7 dpi. Relative lesion length (WT = 1) is shown. Error bars indicate SE (*P < 0.05; n ≥ 48). (D) Growth of the incompatible M. oryzae race in OsSPK1 RNAi plants was measured by qPCR. Relative infection ratio (WT = 1) is shown. Error bars indicate SE (**P < 0.01; n ≥ 8). (E and F) Infection assays of OsRacGEF1 RNAi plants with the incompatible M. oryzae race 007.0. (E) Quantitative analysis of lesions induced by the blast fungus was performed at 7 dpi. Relative lesion length (WT = 1) is shown. Error bars indicate SE (**P < 0.01; n ≥ 48). (F) Growth of the incompatible M. oryzae race in OsSPK1 and OsRacGEF1 RNAi plants was measured by qPCR and normalized with endogenous OsUbq. Relative infection ratio (WT = 1) is shown. Error bars indicate SE (**P < 0.01; n ≥ 8). (G) Transcript levels of OsSPK1 were measured by qPCR in OsSPK1 RNAi suspension cells and normalized with endogenous OsUbq expression. Numbers 17 and 32 indicate independent RNAi transgenic lines. Bars are SE (**P < 0.01; n = 3). (H) Cell death activity induced by Pit in OsSPK1 RNAi rice protoplasts. Relative luciferase activity (GUS = 100) is shown. Bars are SE (**P < 0.01, n = 6).
To further confirm roles of OsSPK1 in rice immunity, we used a luciferase reporter system to monitor the effect of OsSPK1 knockdown on Pit-induced cell death in rice protoplasts. In this system, we transformed protoplasts with Pit and luciferase vectors, and measured the viability of suspension cells based on luminescence. In the WT K59 background, the luciferase activity in cells expressing Pit was much lower than that in cells expressing control GUS, meaning that Pit induces cell death in rice protoplasts (Fig. 2 G and H); this Pit-induced cell death was suppressed in two independent lines of K59 OsSPK1 RNAi suspension cells, indicating that OsSPK1 is required for Pit-induced cell death. Taken together, these results demonstrate that OsSPK1 contributes to the Pit-mediated immune response to rice blast fungus.
OsSPK1 Acts as a GEF Toward OsRac1.
OsSPK1 possesses the typical GEF domain DHR2, which is known to activate small GTPases (29). To examine whether OsSPK1 has GEF activity toward OsRac1, we first ascertained the interaction between OsRac1 and OsSPK1 by a yeast two-hybrid assay, and found that only the C-terminal fragment of OsSPK1 (amino acids 1002–1835) containing the DHR2 domain associated with OsRac1 WT (Fig. 3A). Next, to examine relationships between OsRac1 activation states and the binding activities of OsRac1 to OsSPK1, we generated three OsRac1 mutants: a dominant-negative form of OsRac1 (DN-OsRac1), a constitutively active form (CA-OsRac1), and OsRac1 D125N, which is analogous to a Ras mutant (D119N) having lower nucleotide affinity and higher GEF affinity. We then tested the interactions between OsSPK1 and these mutants (27, 37). The binding activity of all three OsRac1 mutants to OsSPK1 was comparable to that of OsRac1 WT (Fig. 3B). We verified these interactions by in vivo co-IP experiments, using tobacco leaves transiently coexpressing OsSPK1 and OsRac1 (Fig. 3C). As with the result of the yeast two-hybrid assay, all OsRac1 mutants interacted with OsSPK1 in a similar fashion to OsRac1 WT, and these findings are consistent with a previous report on an Arabidopsis PRONE GEF, RopGEF14, and its interaction with the small GTPase Rop1 (42).
Fig. 3.
OsSPK1 interacts with the small GTPase OsRac1. (A) Identification of the OsRac1-binding region in OsSPK1 by a yeast two-hybrid assay. (B) Yeast two-hybrid assays of OsSPK1 and OsRac1 mutants. (A and B) Yeast growth on selective plates without histidine [His (−)] and with 10 mM 3-AT indicates a positive interaction. (C) GFP-OsRac1 mutants and OsSPK1-Myc were transiently coexpressed in N. benthamiana leaves. Co-IP was carried out with anti-GFP antibody, and the proteins were detected by Western blot with anti-GFP and anti-Myc antibodies. (D) BiFC assay of OsRac1 mutants and OsSPK1 in N. benthamiana leaves. Expression of these genes was driven by the CaMV 35S promoter. Empty vector served as a negative control. FM4-64 was used as a plasma membrane marker. Enlarged images of the boxed areas are shown, Right. (Scale bars, 25 μm.)
We also examined the interaction between OsSPK1 and OsRac1 in planta using a BiFC assay. YFP fluorescence from BiFC between nYFP-OsRac1 WT and OsSPK1-cYFP was observed at the plasma membrane (Fig. 3D and SI Appendix, Fig. S4), and there was no obvious change in this interaction when we used the CA and DN forms of nYFP-OsRac1. To further validate this interaction, we coexpressed nontag versions of OsRac1 with YFP-OsSPK1. When we expressed YFP-OsSPK1 alone, YFP-OsSPK1 exhibited the punctate distribution and was hardly distributed in the plasma membrane (SI Appendix, Fig. S5 A and B, a and b). However, the coexpression of OsRac1 WT, CA, and DN promoted plasma membrane localization of YFP-OsSPK1, resulting in the disappearance of punctate distribution and the enhancement of continuous distribution of YFP-OsSPK1 in the plasma membrane, like OsRac1 (43) (SI Appendix, Fig. S5 A and B, d–f), indicating that OsSPK1 interacts with OsRac1 at the plasma membrane in an OsRac1 activation-independent manner.
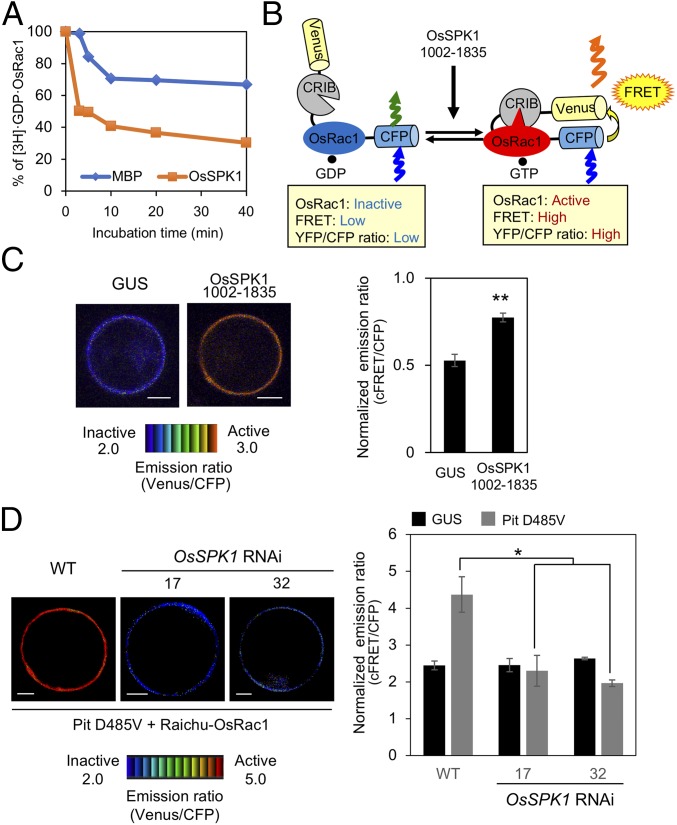
Next, we tried to measure in vitro GEF activity of OsSPK1 for OsRac1 using a recombinant OsSPK1 (amino acids 1334–1835) fused with maltose-binding protein (MBP). If OsSPK1 exhibits GEF activity toward OsRac1, it should enhance the release of tritium-labeled GDP from OsRac1. Accordingly, we monitored the change of the radioactivity of 3H-labeled GDP bound to OsRac1 to test whether OsSPK1 shows GEF activity toward OsRac1 (44). The time-dependent dissociation of GDP from OsRac1 was enhanced in the presence of OsSPK1 compared with a negative control MBP (Fig. 4A), indicating that OsSPK1 possesses GEF activity for OsRac1 in vitro. To monitor in vivo activation of OsRac1 by OsSPK1, we employed a FRET sensor called Ras and interacting protein chimeric unit (Raichu)-OsRac1 (37, 45). In this sensor, intramolecular binding of the active GTP-OsRac1 to the Cdc42/Rac interactive binding domain brings CFP closer to Venus, enabling FRET from CFP to Venus when OsRac1 is activated (Fig. 4B). The resulting Venus fluorescence represents the activation state of OsRac1 in vivo: low and high ratios of Venus/CFP fluorescence correspond to low and high levels of OsRac1 activation, respectively. The ratio of Venus/CFP fluorescence of Raichu-OsRac1 in rice protoplasts expressing OsSPK1 (amino acids 1002–1835) was much higher than that in protoplasts expressing a negative control GUS (Fig. 4C). Thus, we conclude that OsSPK1 displays GEF activity toward OsRac1 and activates OsRac1 both in vitro and in vivo.
Fig. 4.
OsSPK1 functions as a GEF protein toward OsRac1. (A) In vitro GEF assay of OsSPK1. In vitro GEF activity of OsSPK1 was detected by the dissociation of [3H]-labeled GDP from OsRac1. MBP was a negative control. (B) Schematic overview of the Raichu-OsRac1 sensor used to monitor OsRac1 activation in vivo. (C) Detection of in vivo OsRac1 activation by OsSPK1 using Raichu-OsRac1 FRET. Emission ratio images (Left) of confocal laser-scanning micrographs of rice protoplasts coexpressing Raichu-OsRac1 and OsSPK1 (amino acids 1002–1835) or control GUS. The color scale from blue to red represents low to high levels of OsRac1 activation. (Scale bars, 5 μm.) Statistical analysis (Right) of OsRac1 activation using Raichu-OsRac1 with normalized emission ratios of Venus to CFP. Bars are SE (**P < 0.01, n = 60). (D) Effect of OsSPK1 RNAi on Pit D485V-induced OsRac1 activation. Emission ratio images (Left) of confocal laser-scanning micrographs of OsSPK1 RNAi and WT rice protoplasts coexpressing Raichu-OsRac1 and Pit D485V or control GUS. The color scale from blue to red represents low to high levels of OsRac1 activation. (Scale bars, 5 μm.) Statistical analysis (Right) of OsRac1 activation using Raichu-OsRac1 with normalized emission ratios of Venus to CFP. Bars are SE (*P < 0.05, n = 60).
To examine whether Pit leads to OsRac1 activation through OsSPK1, we monitored the OsRac1 activation level in OsSPK1 RNAi lines and found that Pit D485V-triggered OsRac1 activation in OsSPK1 RNAi was much lower than that in WT, indicating that Pit induces OsRac1 activation through OsSPK1 (Fig. 4D).
The CC Domain of Pit Is Critical for Binding to OsSPK1.
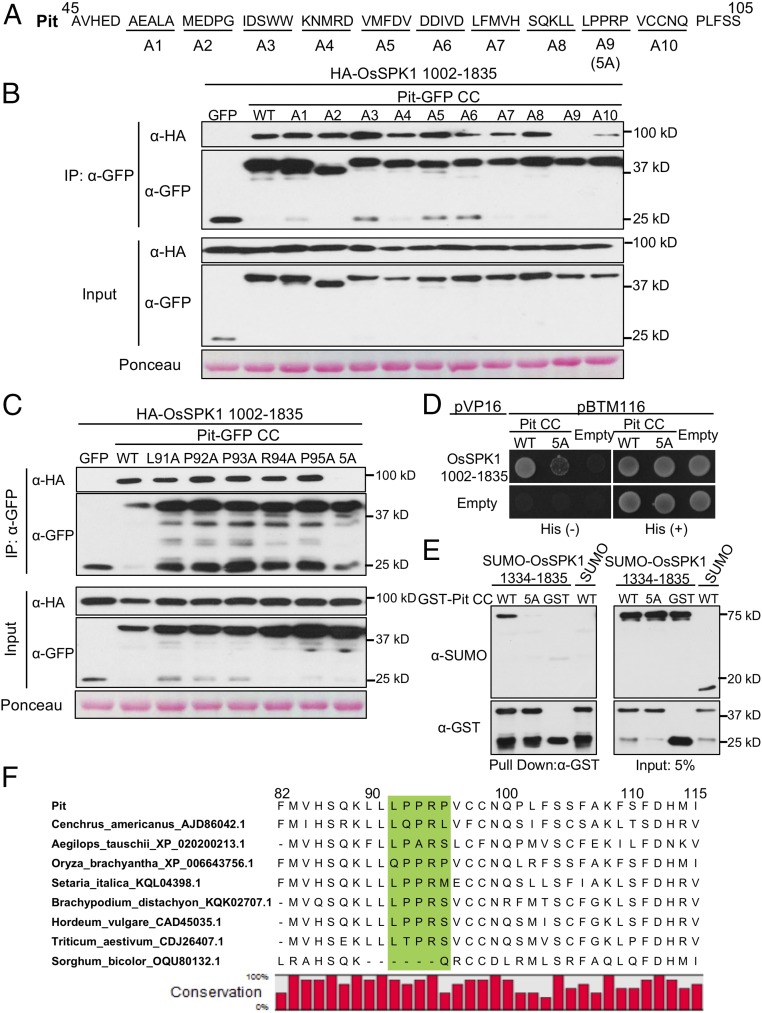
Accumulating evidence indicates that the CC domain of R proteins orchestrates a variety of functions involved in immunity, such as induction of cell death, perception of pathogen, and binding to cofactors (6). To reveal the mechanism of how Pit regulates and transduces signals to the downstream molecule OsSPK1, we focused further on the CC domain of Pit. To identify important residues of Pit that bind to OsSPK1, we systematically added a series of five consecutive alanine substitutions from residues 51–100 of the CC domain of Pit (Fig. 5A), and tested their interaction with OsSPK1 in a co-IP assay using N. benthamiana leaves (Fig. 5B). Among the tested mutants, the interaction of Pit with OsSPK1 was completely abolished in the Pit A9 mutant (having alanine substitutions of 91LPPRP95). Interestingly, residues 91–95 in Pit orthologs contain a consensus proline-rich motif, PxxP (where “x” is any amino acid) (Fig. 5F). Next, we further generated five single alanine mutants within Pit 91LPPRP95 but found no clearly reduced binding of these mutants to OsSPK1 (Fig. 5C), indicating that these five residues participate additively in binding to OsSPK1. Thus, we redesignated Pit A9 as Pit 5A and employed this mutant for further analyses. To confirm the result of the co-IP assay, we also performed a yeast-two hybrid assay (Fig. 5D and SI Appendix, Fig. S6) and an in vitro binding assay (Fig. 5E) and found that both results corroborate the idea that residues 91–95 in Pit CC domain are crucial determinants for its binding to OsSPK1.
Fig. 5.
The CC domain of Pit is critical for binding to OsSPK1. (A) Schematic representation of Pit mutants. Groups of five consecutive residues of Pit from residues 51–100 as shown in the underlines were mutated by alanine substitutions. Pit A9 is the same as Pit 5A and corresponds to Pit 91AAAAA95. (B) OsSPK1-binding properties of alanine substitutions of A1 to A10. (C) Analysis of the OsSPK1-binding activity of single mutations within Pit 91LPPRP95. Amino acids were individually mutated to alanine. (B and C) Binding activity of the Pit mutants to OsSPK1 was evaluated by coexpressing the indicated Pit CC-GFP mutants with HA-OsSPK1 (amino acids 1002–1835) in N. benthamiana leaves followed by co-IP. Total protein extract was immunoprecipitated with anti-GFP antibody, and Western blot was then carried out with anti-GFP and anti-HA antibodies. Proteins on the transferred membrane were stained by Ponceau. (D) Yeast two-hybrid assay of Pit 5A mutant with OsSPK1. Yeast growth on selective plates without histidine [His (−)] indicates a positive interaction. (E) In vitro pull-down assay of OsSPK1 with Pit WT or 5A mutant. Purified GST or GST-tagged Pit CC immobilized on Glutathione Sepharose 4B beads was incubated with His-SUMO-OsSPK1 (amino acids 1334–1835). Western blot was then carried out with anti-GST and anti-SUMO antibodies. (F) Multiple alignment of Pit homologs in various species. The residues (91LPPRP95) of Pit highlighted with a green background are critical for binding to OsSPK1.
The CC Domain of Pit Is Indispensable for OsRac1 Activation and the Induction of Immunity.
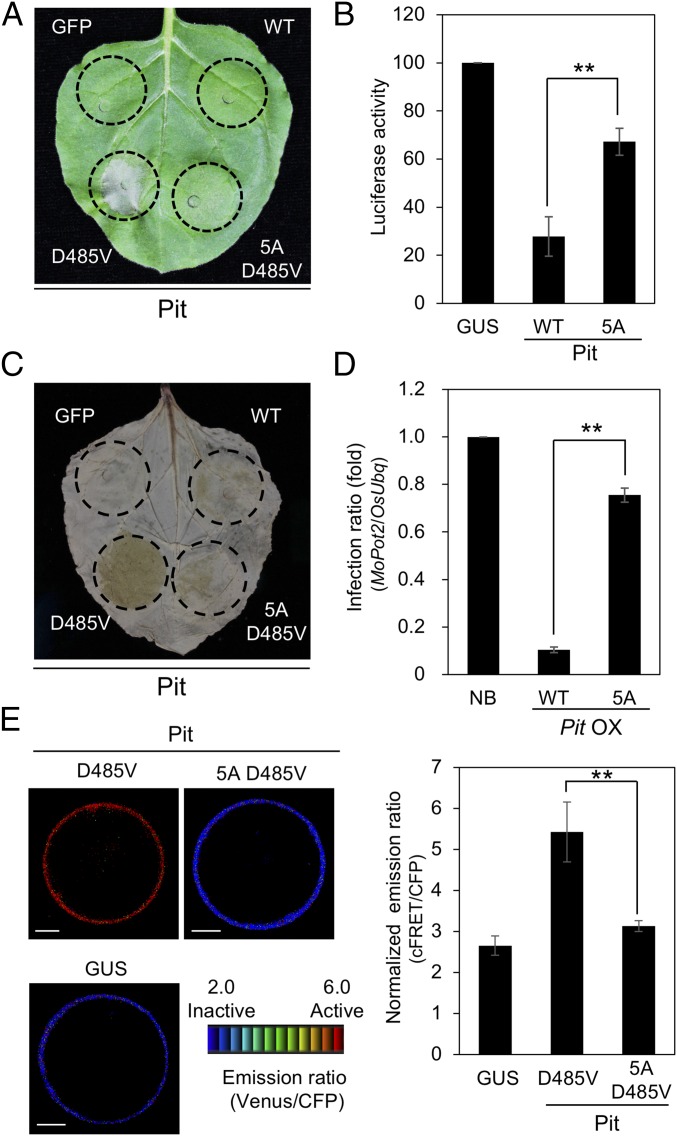
To assess the importance of the CC domain of Pit in immunity, we employed a transient expression system using N. benthamiana leaves to test the effects of Pit 5A on cell death and ROS production. We have previously generated Pit D485V, which works as a constitutively active mutant and induces cell death without pathogen infection in tobacco (37). At 2 dpi, strong cell death was observed in Pit D485V, while the cell death and ROS production induced by Pit 5A D485V were much weaker (Fig. 6 A and C and SI Appendix, Fig. S7A). In N. benthamiana, the expression of Pit 5A D485V was much higher than that of Pit D485V, implying that the reduction of cell death and ROS production by the addition of 5A mutation is not simply due to the decrement of Pit protein stability (SI Appendix, Fig. S7B). Pit WT clearly induced cell death in rice protoplasts, but this was significantly impaired in Pit 5A (Fig. 6B and SI Appendix, Fig. S7C). Similarly, plants expressing Pit 5A were more susceptible to avirulent rice blast fungus than those expressing Pit WT (Fig. 6D and SI Appendix, Fig. S7 D–F). However, Pit 5A-GFP retains proper plasma membrane localization, which is critical for Pit function, as reported previously (SI Appendix, Fig. S7G) (38). Taking these data together, we conclude that the CC domain of Pit plays important roles in immunity through its binding to OsSPK1.
Fig. 6.
The CC domain of Pit is indispensable for induction of immunity. (A) Effect of 91LPPRP95 on Pit D485V-induced cell death in N. benthamiana leaves, photographed at 2 dpi. (B) Cell death activity of Pit 91LPPRP95 and control GUS in rice protoplasts. Pit 5A and corresponds to Pit 91AAAAA95. Relative luciferase activity (GUS = 100) is shown. Error bars indicate SE (**P < 0.01, n = 3). (C) Effect of 91LPPRP95 on Pit D485V-induced ROS production in N. benthamiana. ROS production was detected by DAB staining at 2 dpi. (D) Responses of Nipponbare WT and Pit WT- or 5A-overexpressing plants to infection with the incompatible M. oryzae race 007.0. Growth of blast fungus in Nipponbare plants was measured by qPCR. Relative infection ratio (NB = 1) is shown. Error bars indicate SE (**P < 0.01; n ≥ 8). (E) In vivo OsRac1 activation by Pit mutants. Emission ratio images (Left) of confocal laser-scanning micrographs of rice protoplasts coexpressing Raichu-OsRac1 and either the Pit mutants or GUS. (Scale bars, 5 μm.) Normalized emission ratios (Right) of Venus to CFP. Bars are SE (**P < 0.01, n = 60).
We have reported, using a Raichu-OsRac1 system, that Pit D485V induces OsRac1 activation in rice protoplasts (37, 38). To examine the effects of Pit amino acids 91–95 on Pit D485V-induced OsRac1 activation, we monitored the in vivo activation level of OsRac1 in rice protoplasts expressing Pit 5A D485V. OsRac1 activation was high in protoplasts expressing Pit D485V but was compromised in protoplasts expressing Pit 5A D485V (Fig. 6E), implying that the binding of Pit to OsSPK1 is required for OsRac1 activation in vivo. These results indicate that Pit forms a complex with OsSPK1 through its CC domain and activates OsRac1 at the plasma membrane, resulting in the induction of Pit-mediated immunity (see, for example, Fig. 8).
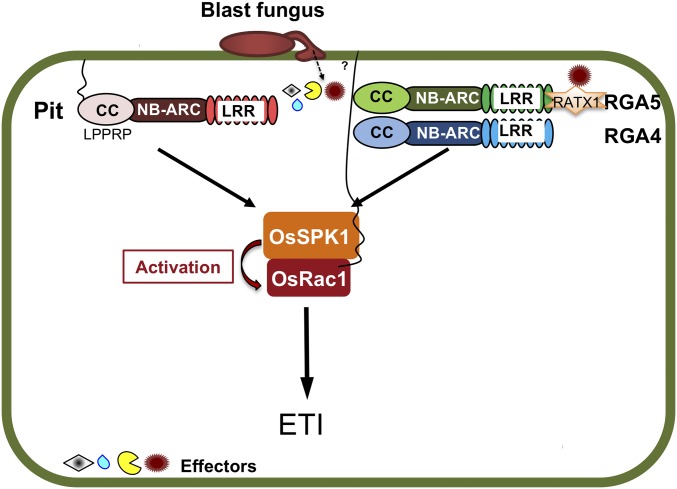
Fig. 8.
R protein-OsSPK1-OsRac1 modules in rice immunity. Model of the R protein-OsSPK1-OsRac1 modules in rice immunity. R protein Pit and a pair of R proteins RGA4 and RGA5 perceive effectors from rice blast fungus and then transduce their signals to the downstream molecule OsSPK1 by direct binding. Active OsSPK1 leads to OsRac1 activation at the plasma membrane, resulting in the induction of ETI.
OsSPK1 Participates in RGA4-Mediated Resistance.
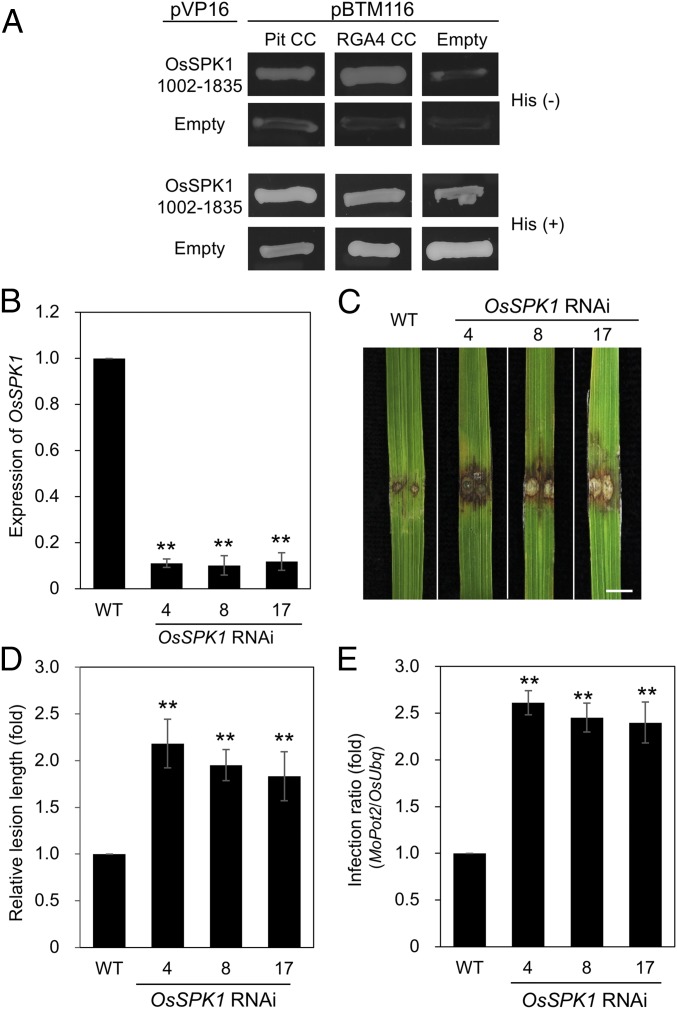
We have previously found that OsRac1 RNAi or overexpression of DN OsRac1 compromises an R protein RGA4-mediated resistance (31, 43, 46). Here, we found that OsSPK1 is a direct activator for OsRac1 and plays a key role in Pit-mediated disease resistance to rice blast fungus (Figs. 1–4). These results raised the possibility that OsSPK1 also functions in RGA4-mediated signaling. We therefore tested the interaction between OsSPK1 and RGA4 and found that OsSPK1 also interacted with RGA4 in a yeast two-hybrid assay (Fig. 7A). Moreover, the knockdown of OsSPK1 attenuated resistance and enhanced fungal growth compared with Kinmaze WT (Fig. 7 B–E), indicating that OsSPK1 also functions downstream of RGA4.
Fig. 7.
OsSPK1 Is Involved in Pia-mediated Immunity. (A) Yeast two-hybrid assay of Pit and RGA4 with OsSPK1. Yeast growth on selective plates without histidine [His (−)] indicates a positive interaction. (B–E) Infection assays of OsSPK1 RNAi plants with the incompatible M. oryzae race P131. Numbers 4, 8, and 17 indicate independent RNAi transgenic lines. (B) Transcript levels of OsSPK1 were measured by qPCR in OsSPK1 RNAi Kinmaze plants and normalized with endogenous OsUbq expression. Bars are SE (**P < 0.01; n = 3). (C) Photograph shows typical phenotypes of OsSPK1 RNAi plants at 7 dpi. (Scale bar, 5 mm.) (D) Quantitative analysis of lesions induced by the blast fungus was performed at 7 dpi. Relative lesion length (WT = 1) is shown. Error bars indicate SE (**P < 0.01; n ≥ 48). (E) Growth of the incompatible M. oryzae race P131 in OsSPK1 RNAi plants was measured by qPCR. Relative infection ratio (WT = 1) is shown. Error bars indicate SE (**P < 0.01; n ≥ 10).
Discussion
Pit Forms an Immune Complex with OsSPK1 and OsRac1.
In this study, we identified an interactor of the rice R protein Pit, named OsSPK1, which is an activator protein for the small GTPase OsRac1, and found that it interacted with the CC domain of Pit. Results from the knockdown of OsSPK1, and from a Pit mutant that displays reduced binding to OsSPK1, showed that OsSPK1 plays key roles in Pit-mediated disease resistance to rice blast fungus (Fig. 8). We found that the GEF protein OsSPK1 interacts with and activates OsRac1 both in vitro and in vivo (Fig. 4); this SPK1-Rac/Rop pathway is conserved from plants to animals (30). Arabidopsis SPK1 controls two heteromeric complexes, termed WAVE and actin-related protein (ARP) 2/3, to organize the actin cytoskeleton, probably through small GTPase AtRops, in pavement cells (29, 47). Similarly, human DOCK3, a homolog of SPK1, activates Rac1 and regulates the actin cytoskeleton through the WAVE complex to control tumor cell movement through the actin cytoskeleton (48). Therefore, it is possible that OsSPK1 also regulates the actin cytoskeleton and enhances ETI through OsRac1, because actin dynamics play a role in the activation of ETI in Arabidopsis (49).
Our present and previous studies revealed that Pit interacts with OsSPK1 through its CC domain (Figs. 1 and 5) but binds to OsRac1 via its NB-ARC domain at the plasma membrane (37), implying that Pit forms a ternary complex. OsSPK1 is likely endomembrane associated and partially cytosolic (Fig. 1 E and G and SI Appendix, Fig. S2C). However, no clear association with the ER could be evidenced (Fig. 1F). Therefore, SPK1 might be associated only with subdomains of the ER (39) or other endomembranes. Our data further suggest the recruitment of OsSPK1 to the plasma membrane during its interaction with Pit and OsRac1 (Figs. 1 and 3). This ternary complex may function as a module for OsRac1 activation at the plasma membrane. Localization to the 2D plasma membrane may effectively enhance the local concentration of Pit, OsSPK1, and OsRac1 relative to their concentration in the 3D cytosol to efficiently trigger OsRac1 activation to induce ETI. Further studies are necessary to clarify more fully the relationship between Pit and OsSPK1.
OsSPK1 and OsRac1 Play Vital Roles in R Protein-Mediated Disease Resistance.
We and another group have shown that OsRac1 and its homologs are involved in signaling by two more R proteins in addition to Pit. Pia-mediated resistance to rice blast fungus is controlled by a pair of R genes, RGA4 and RGA5 (46, 50). RGA4 and RGA5 are CNL proteins that directly interact with each other, and act as a coimmune receptor (46). RGA4 serves as an executor of immune induction because it triggers cell death without pathogen effector. We have previously revealed that OsRac1 RNAi or overexpression of DN-OsRac1 compromises RGA4-mediated resistance, indicating that OsRac1 participates in RGA4 and RGA5-mediated resistance (31, 43). Here, we found that RGA4 interacted with OsSPK1 in a yeast two-hybrid assay, and that OsSPK1 RNAi suppressed RGA4-mediated resistance (Fig. 7). These results raise the possibility that the OsSPK1-OsRac1 module additionally contributes to the RGA4-mediated pathway. Moreover, overexpression of DN-OsRac1 in tobacco leaves suppresses the synchronous production of hypersensitive response and ROS that is triggered by the tobacco N gene, indicating that a tobacco OsRac1 homolog contributes to N gene-mediated disease resistance (51). N protein is a TNL protein but it requires the helper R protein NRG1, a CNL protein, to confer resistance against the tobacco mosaic virus (52). We have previously used affinity-column chromatography to isolate OsRac1-binding proteins and identified five NLR proteins in the elution fraction of the active GTP-bound form of OsRac1 (53). Additionally, OsRac1 interacts with a known rice NLR-type R protein, Pib, in vitro (37). Taking these observations together, it is possible that OsRac1 and its homologs are common molecular switches in CNL-mediated signaling, and that OsSPK1 also participates in these pathways.
The CC Domain Is Important for the Binding of Pit to OsSPK1.
In this study, we found that 91LPPRP95 in Pit are crucial residues for binding to the DHR2 domain of OsSPK1; they contain the consensus sequence of the proline-rich motif PxxP, which is known to preferentially interact with Src homology 3 (SH3) domains. Interactions between proline-rich motifs and SH3 domains contribute to the assembly and targeting of larger protein complexes involved in key cellular processes, such as cytoskeletal rearrangements and cell growth in animals (54). Homologs of rice Pit were found only in Poaceae and Oryza clade of the Pit homologs maintain the proline-rich motif. However, the Pit homolog of Sorghum bicolor completely lacks this proline-rich motif and there are some variations at the position of P95 of Pit in the other species (Fig. 5F).
The first and best-characterized example of the proline-rich motif/SH3 interaction is that between the adaptor protein Grb2 and a GEF protein, SOS, for the small GTPase Ras in animals (54). Upon growth factor treatment, the SH3 domain of Grb2 binds to the proline-rich domain of SOS, leading to activation of the GEF activity of SOS and resulting in Ras activation; these observations provided the first indication that interactions between proline-rich motifs and SH3 domains play an important role in small GTPase activation. We cannot exclude that the 5A mutation disturbs overall structural integrity of the CC domain of Pit. However, the protein of Pit 5A mutant was stably expressed in our experiments, and hence data additionally support the general importance of the CC domain of Pit in its interaction with OsSPK1. Because the PxxP motif is conserved in predicted Pit orthologs but absent from RGA4, the structural details for CNLs–SPK1 interaction need further investigation.
Activities of GEF proteins are regulated by three factors: intermolecular interactions with other proteins, intramolecular interactions between GEF domains and other domains, and posttranslational modifications, such as phosphorylation (55, 56). As far as we know, there is only one report that a DHR2-binding protein regulates the GEF activity of a DOCK family protein (57). The protein phosphatase PP2A interacts with the DHR2 domain of DOCK6 and dephosphorylates it at S1194, and this dephosphorylation may affect the GEF activity of DOCK6. Here, we identified a second DHR2-binding protein, which manipulates OsSPK1 activity, and future study should throw light on new regulation mechanisms of GEF proteins.
Experimental Procedures
Plasmid Construction.
For all assays except a pull-down assay involving Pit fragments, the ORFs of each gene and deletion mutant were amplified, cloned into the pENTR d-TOPO vector (Invitrogen), and then transferred by LR reaction into various destination vectors, depending on the experiment. These destination vectors included pGWBs, pBTM116, pVP16, p2k, p2k-pANDA, Ubq-GW, Ubq-GW-3×Flag, pCold, and pMal (New England Biolabs). For recombinant protein expression in Escherichia coli for a pull-down assay, Pit deletion mutants were directly cloned into the 6×His-SUMO vector. To generate Pit mutants, mutagenesis was performed by PCR with specific primers (SI Appendix, Table S1).
Yeast Two-Hybrid Assay.
To test protein-protein interactions in yeast cells, baits containing various genes were cloned into pBTM116 and preys were produced by cloning genes into pVP16. This two-hybrid system detects protein–protein interactions in nuclei. The C-terminal cysteine of OsRac1, which is required for plasma membrane localization, apparently inhibits the transfer of OsRac1 into nuclei and was therefore replaced by serine. Yeast strain L40 cells were transformed with the pBTM116 and pVP16 constructs.
Agroinfiltration of N. benthamiana Leaves.
Agroinfiltration of N. benthamiana was performed as described previously (37). Agrobacterium tumefaciens strain GV3101, harboring the helper plasmid pSoup and binary plasmids carrying the cDNAs of OsRac1, Pit, OsSPK1, or GUS, was used to infiltrate leaves of 4-wk-old N. benthamiana plants. In all experiments, we also used the p19 silencing suppressor to enhance gene expression. For coexpression of multiple proteins, Agrobacterium carrying the appropriate constructs and p19 helper plasmid was mixed and used for infiltration. Agrobacterium transformants were grown overnight to an optical density at 600 nm (OD600) of around 0.8. The cultures were collected and resuspended in 10 mM MgCl2, 10 mM Mes-NaOH (pH 5.6), and 150 µM acetosyringone, adjusted to OD600 = 0.4, and incubated at 23 °C for 2–3 h before infiltration. The uppermost three or four leaves were selected for injection and plants were kept in a growth room at 26 °C for 2 d.
Cell Death Assay in N. benthamiana.
A. tumefaciens strains carrying constructs expressing Pit mutant proteins were infiltrated into N. benthamiana leaves according to the method described above. Each strain was infiltrated in a circle of 1-cm diameter on each of 15 leaves for three independent experiments. After 2–3 d, cell death symptoms were observed.
qRT-PCR.
Total RNA extraction and cDNA synthesis were performed according to TRIzol reagent (Invitrogen) and cDNA synthesis kit (Vazyme) protocols, respectively. The cDNA was used for quantitative analysis using SYBR Green Supermix (Bio-Rad) on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). OsUbiquitin was used as an internal control for normalization. Sequences of qRT-PCR and RT-PCR primers are shown in SI Appendix, Table S1.
Raichu-OsRac1 FRET Analysis.
To analyze the activation of OsRac1 by OsSPK1 in vivo, we employed the Raichu intramolecular FRET system, as described previously (37, 45). Rice protoplasts were transfected with Raichu-OsRac1 and OsSPK1 (amino acids 1002–1835) or GUS vectors by the PEG method. For Fig. 4C, 10–12 h after transfection, the cells were imaged using an Olympus IX-81 inverted microscope with a Yokogawa CSU22 confocal scanner, equipped with an EM-CCD C9100-02 cooled charge-coupled device camera (Hamamatsu Photonics). Raichu-OsRac1 was excited using a 440-nm diode laser (iFLEX 2000; Point Source). The CFP and Venus filters were 480 ± 15 nm and 535 ± 20 nm, respectively. For Figs. 4D and 6E, the transformed cells were imaged using a Leica SMD FLCS microscope. Raichu-OsRac1 was excited using a 440-nm solid-state laser. The CFP and Venus filters were 470 ± 20 nm and 550 ± 25 nm, respectively.
Rice Cultivation and Infection with Rice Blast Fungus.
The japonica rice cultivars K59, Kinmaze, and Nipponbare and the M. oryzae strains Ina86-137 (Race 007.0) and P131 were used (40, 58). Blast fungus growth and punch infection of leaf blades were measured and performed as described previously (31, 37). Lesion length was measured 7 dpi with blast fungus. Photographs of disease lesions were taken at 7 dpi. For spray infection, 2-wk-old rice plants were sprayed with blast fungus (5 × 105 spores per milliliter; 0.05% Tween 20) and then kept at high humidity in an infection assay box for 20–24 h at 25 °C in the dark. After that, plants were transferred to a growth chamber (day: 14 h, 28 °C; night: 10 h, 25 °C; humidity: 60–70%). Five- to 7-d after inoculation, leaves that were fully developed at the time of inoculation were photographed and used for DNA analyses.
Protein Expression and Purification.
The GST/His-fused protein Pit CC and His/MBP-fused protein OsSPK1 (amino acids 1334–1835) were expressed in E. coli strain BL21(DE3) Codon Plus, which was grown at 37 °C to OD600 = 0.8. After induction with a final concentration of 0.3 mM isfopropyl-β-d-thiogalactopyranoside (IPTG) for 15 h at 16 °C, the cells were collected by centrifugation, lysed by sonicating in a homogenization buffer [20 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, 1 mM DTT], and then centrifuged at 15,500 × g for 1 h. The supernatants were purified by affinity chromatography using Ni-NTA agarose resin, Glutathione Sepharose 4B and amylose resin, respectively, and further purified on a HiLoad 16/600 Superdex 200 column (GE Healthcare).
In Vitro Binding Assay.
Equal amounts of MBP/His-SUMO fusion protein OsSPK1 (amino acids 1334–1835) and GST/His-SUMO-fused Pit CC were mixed with pull-down buffer [50 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 10% glycerol, 0.1% Triton X-100, 1 mM EDTA, and 1 mM DTT] to 200 μL for each reaction and then rotated at 4 °C for 30 min. The mixture was applied to 30 µL Amylose resin (New England Biolabs) or Glutathione Sepharose 4B (GE Healthcare) and incubated at 4 °C for 2–3 h. The beads were then washed five times for 5 min each time with pull-down buffer, and the bound proteins were eluted with 100 µl 2× SDS loading buffer and subjected to immunoblot assay with anti-SUMO (A01693; GenScript), anti-GST (sc-138; Santa Cruz Biotechnology), and anti-MBP (E8032S; New England Biolabs) antibodies.
Subcellular Localization.
A. tumefaciens strain GV3101 carrying constructs expressing YFP-OsSPK1 and Pit-GFP mutants were infiltrated into N. benthamiana leaves according to the method described above. After 2 d, the infiltrated areas were examined under a Leica TCS-SP8 microscope. For samples stained with FM4-64 (F34653; Invitrogen), infiltrated leaves were injected with FM4-64 solution before observation. Fluorescence signals from FM4-64 or mRFP and YFP or GFP were captured by sequential excitations with 561- and 514- or 488-nm lasers, respectively. For chloroplast signals, the autofluorescence of chloroplasts was collected at the emission wavelengths from 650 nm to 720 nm. Peak intensity was analyzed using LAS X software (Application for Leica TCS-SP8 microscope).
Detection and Quantitative Analysis of ROS.
A. tumefaciens strains carrying constructs expressing mutant Pit proteins were infiltrated into N. benthamiana leaves according to the method described above. Each strain was infiltrated in a circle of 1-cm diameter on each of 15 leaves for three independent experiments. To visualize ROS in situ, the agroinfiltrated leaves were detached at 36 h postinfection, before cell death symptoms occurred, and incubated in 1 mg/mL DAB solution for 5 h at room temperature. The leaves were then decolorized by boiling several times with absolute ethanol in a microwave oven until the chlorophyll was removed completely. The DAB-stained leaves were scanned, and the pixel intensities of the agroinfiltrated regions were quantified using ImageJ software (National Institutes of Health). The mean pixel intensity from three spots outside the infiltrated regions on each leaf was used for background subtraction. Relative DAB staining intensity was calculated based on the pixel intensity of the Pit D485V-agroinfiltrated region (control) on each leaf, to facilitate comparisons between different leaves.
Luciferase Activity Assay in Rice Protoplasts.
Protoplasts were prepared as described previously from rice suspension cells (37). The firefly luciferase gene (LUC) expressed under the control of the maize Ubiquitin promoter was used as a reporter to monitor protoplast viability. Pit WT or Pit 5A vector was cotransfected together with 2 µg of LUC plasmid into rice protoplasts (5 × 106 cells/mL) by the PEG method. Protein from protoplast samples was harvested 40 h after transfection at 30 °C using a 1× lysis buffer provided in the Luciferase Assay Report Kit (Promega) and centrifuged at 21,500 × g at 4 °C for 1 min. A 20-µL aliquot of the supernatant was then mixed with 100 µL luciferase substrate from the kit, in a 96-well microtiter plate (Thermo Fisher Scientific) specific for a microplate reader machine. Luciferase activity was measured with the appropriate program. In each experiment, we prepared three replicate samples and used mean values to display the result. The reduction in luminescence was compared and normalized with protoplasts expressing control GUS. The experiment was repeated three independent times.
In Vitro GEF Assay.
Effects of OsSPK1 on the dissociation of [3H] GDP from Rho family proteins were assayed as described previously (44). The [3H] GDP-bound form of OsRac1 was obtained by incubating 20 pmol of OsRac1 protein with 1 μM [3H] GDP (1,000–2,000 cpm/pmol) for 20 min at 30 °C in buffer I [20 mM Tris⋅HCl (pH 7.5), 1 mM DTT, 5 mM MgCl2, and 10 mM EDTA]. To prevent the dissociation of [3H] GDP from OsRac1, MgCl2 was added to a final concentration of 20 mM, and the mixtures were immediately cooled on ice. The dissociation of [3H] GDP was performed at 25 °C by adding a 200-fold excess of unlabeled GTP and the indicated amount of MBP-OsSPK1 to buffer II [50 mM Tris⋅HCl (pH 8.0), 1 mM DTT, 10 mM MgCl2, and 2.9 mM EDTA]. The reaction was stopped at the indicated time by adding 2 mL of ice-cold buffer III [20 mM Tris⋅HCl (pH 7.5), 20 mM MgCl2, and 100 mM NaCl]. The diluted mixtures were filtered through nitrocellulose filters, which were then washed several times with the same solution. The radioactivity trapped on the filters was counted.
Co-IP Assays.
A. tumefaciens GV3101 carrying constructs expressing Pit, OsSPK1, and OsRac1 mutants were mixed based on the experimental designs and infiltrated into N. benthamiana leaves according to the method described above. Proteins were isolated by the addition of IP buffer [20 mM Tris⋅HCl (pH 7.5), 150 mM NaCl, 10% glycerol, 0.2% Nonidet P-40, 1 mM EGTA (pH 7.5), 5 mM DTT, and EDTA-free protease inhibitor (Roche)] and the extracts were cleared by centrifugation at 21,500 × g for 15 min at 4 °C. Fifty microliters of each supernatant was saved as input samples for later immunoblot analysis and the remainder was incubated with 20 µL anti-GFP agarose resin (GFP-Trap A, gta-20; Chromotek) for 2 h at 4 °C. After washing five times with IP buffer the immunocomplexes were eluted by boiling in 100 µL of 2× SDS loading buffer. The IP samples were washed by centrifugation and then subjected to immunoblot analysis with the indicated antibodies together with input samples.
BiFC.
A. tumefaciens strain GV3101 carrying constructs expressing OsSPK1-cYFP, nYFP-OsRac1 variants, and Pit-nYFP mutants was infiltrated into N. benthamiana leaves according to the method described above. After 2 d, the infiltrated areas were examined using a Leica TCS-SP8 microscope.
Cell Fractionation.
Liquid nitrogen-frozen rice suspension cells (about 300 mg) were ground to fine powder with a pestle and mortar, and different cell fractions were then extracted according to the manual of a plasma membrane extraction kit (SM-005-P; Invent Biotechnologies) (Fig. 1G). Tobacco leaf extracts expressing YFP-OsSPK1 were separated into membrane and soluble protein fractions, as described previously (SI Appendix, Fig. S2D) (59). Anti-UGPase (AS06 180: cytosol; Agrisera), BiP (AS09 481: ER; Agrisera), cAPX (AS06 180: cytosol; Agrisera) and anti-H+ATPase (AS07 260: plasma membrane; Agrisera) antibodies were used.
Statistical Analysis.
Means were compared using a t test (two-tailed; type 2; significant, P < 0.05). Analysis of t tests was carried out using Microsoft Excel v2013. SEs and SDs were calculated.
Supplementary Material
Acknowledgments
We thank Dr. Jianming Li and Dr. Rosa Lozano-Duran (Shanghai Center for Plant Stress Biology) for the endoplasmic reticulum marker HDEL; the Core Facility of Transformation at the Shanghai Center for Plant Stress Biology for excellent technical assistance; members of the Laboratory of Plant Molecular Genetics at the Nara Institute of Science and Technology and the Laboratory of Signal Transduction and Immunity at the Shanghai Center for Plant Stress Biology for valuable support and discussions. Y.K. was supported by the National Natural Science Foundation in China (Grants 31572073 and 31772246); Chinese Academy of Sciences Hundred Talents Program (Grant 173176001000162114); Strategic Priority Research Program of the Chinese Academy of Sciences (B) (Grant XDB27040202); Chinese Academy of Sciences, Shanghai Institutes for Biological Sciences; Shanghai Center for Plant Stress Biology, Chinese Academy of Sciences Center of Excellence for Molecular Plant Sciences; Japan Society for the Promotion of Science KAKENHI; and the Takeda Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813058115/-/DCSupplemental.
References
- 1.Chisholm ST, Coaker G, Day B, Staskawicz BJ. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell. 2006;124:803–814. doi: 10.1016/j.cell.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Dodds PN, Rathjen JP. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11:539–548. doi: 10.1038/nrg2812. [DOI] [PubMed] [Google Scholar]
- 3.Tang D, Wang G, Zhou JM. Receptor kinases in plant-pathogen interactions: More than pattern recognition. Plant Cell. 2017;29:618–637. doi: 10.1105/tpc.16.00891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couto D, Zipfel C. Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol. 2016;16:537–552. doi: 10.1038/nri.2016.77. [DOI] [PubMed] [Google Scholar]
- 5.Cui H, Tsuda K, Parker JE. Effector-triggered immunity: From pathogen perception to robust defense. Annu Rev Plant Biol. 2015;66:487–511. doi: 10.1146/annurev-arplant-050213-040012. [DOI] [PubMed] [Google Scholar]
- 6.Jones JD, Vance RE, Dangl JL. Intracellular innate immune surveillance devices in plants and animals. Science. 2016;354:aaf6395. doi: 10.1126/science.aaf6395. [DOI] [PubMed] [Google Scholar]
- 7.Maekawa T, Kufer TA, Schulze-Lefert P. NLR functions in plant and animal immune systems: So far and yet so close. Nat Immunol. 2011;12:817–826. doi: 10.1038/ni.2083. [DOI] [PubMed] [Google Scholar]
- 8.Collier SM, Hamel LP, Moffett P. Cell death mediated by the N-terminal domains of a unique and highly conserved class of NB-LRR protein. Mol Plant Microbe Interact. 2011;24:918–931. doi: 10.1094/MPMI-03-11-0050. [DOI] [PubMed] [Google Scholar]
- 9.Bernoux M, et al. Structural and functional analysis of a plant resistance protein TIR domain reveals interfaces for self-association, signaling, and autoregulation. Cell Host Microbe. 2011;9:200–211. doi: 10.1016/j.chom.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swiderski MR, Birker D, Jones JD. The TIR domain of TIR-NB-LRR resistance proteins is a signaling domain involved in cell death induction. Mol Plant Microbe Interact. 2009;22:157–165. doi: 10.1094/MPMI-22-2-0157. [DOI] [PubMed] [Google Scholar]
- 11.Wang GF, et al. Molecular and functional analyses of a maize autoactive NB-LRR protein identify precise structural requirements for activity. PLoS Pathog. 2015;11:e1004674. doi: 10.1371/journal.ppat.1004674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maekawa T, et al. Coiled-coil domain-dependent homodimerization of intracellular barley immune receptors defines a minimal functional module for triggering cell death. Cell Host Microbe. 2011;9:187–199. doi: 10.1016/j.chom.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Ade J, DeYoung BJ, Golstein C, Innes RW. Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc Natl Acad Sci USA. 2007;104:2531–2536. doi: 10.1073/pnas.0608779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mackey D, Holt BF, 3rd, Wiig A, Dangl JL. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell. 2002;108:743–754. doi: 10.1016/s0092-8674(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 15.Mucyn TS, et al. The tomato NBARC-LRR protein Prf interacts with Pto kinase in vivo to regulate specific plant immunity. Plant Cell. 2006;18:2792–2806. doi: 10.1105/tpc.106.044016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacco MA, Mansoor S, Moffett P. A RanGAP protein physically interacts with the NB-LRR protein Rx, and is required for Rx-mediated viral resistance. Plant J. 2007;52:82–93. doi: 10.1111/j.1365-313X.2007.03213.x. [DOI] [PubMed] [Google Scholar]
- 17.Tameling WI, Baulcombe DC. Physical association of the NB-LRR resistance protein Rx with a Ran GTPase-activating protein is required for extreme resistance to Potato virus X. Plant Cell. 2007;19:1682–1694. doi: 10.1105/tpc.107.050880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders DG, et al. Host protein BSL1 associates with Phytophthora infestans RXLR effector AVR2 and the Solanum demissum immune receptor R2 to mediate disease resistance. Plant Cell. 2012;24:3420–3434. doi: 10.1105/tpc.112.099861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis JD, et al. The Arabidopsis ZED1 pseudokinase is required for ZAR1-mediated immunity induced by the Pseudomonas syringae type III effector HopZ1a. Proc Natl Acad Sci USA. 2013;110:18722–18727. doi: 10.1073/pnas.1315520110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawano Y, Kaneko-Kawano T, Shimamoto K. Rho family GTPase-dependent immunity in plants and animals. Front Plant Sci. 2014;5:522. doi: 10.3389/fpls.2014.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agrawal GK, Iwahashi H, Rakwal R. Small GTPase ‘Rop’: Molecular switch for plant defense responses. FEBS Lett. 2003;546:173–180. doi: 10.1016/s0014-5793(03)00646-x. [DOI] [PubMed] [Google Scholar]
- 22.Szymanski DB. Breaking the WAVE complex: The point of Arabidopsis trichomes. Curr Opin Plant Biol. 2005;8:103–112. doi: 10.1016/j.pbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Yalovsky S. Protein lipid modifications and the regulation of ROP GTPase function. J Exp Bot. 2015;66:1617–1624. doi: 10.1093/jxb/erv057. [DOI] [PubMed] [Google Scholar]
- 24.Craddock C, Lavagi I, Yang Z. New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol. 2012;22:492–501. doi: 10.1016/j.tcb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawano Y, Shimamoto K. Early signaling network in rice PRR-mediated and R-mediated immunity. Curr Opin Plant Biol. 2013;16:496–504. doi: 10.1016/j.pbi.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Feiguelman G, Fu Y, Yalovsky S. ROP GTPases structure-function and signaling pathways. Plant Physiol. 2018;176:57–79. doi: 10.1104/pp.17.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berken A, Thomas C, Wittinghofer A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature. 2005;436:1176–1180. doi: 10.1038/nature03883. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi K, et al. SWAP70 functions as a Rac/Rop guanine nucleotide-exchange factor in rice. Plant J. 2012;70:389–397. doi: 10.1111/j.1365-313X.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 29.Basu D, Le J, Zakharova T, Mallery EL, Szymanski DB. A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proc Natl Acad Sci USA. 2008;105:4044–4049. doi: 10.1073/pnas.0710294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meller N, Merlot S, Guda C. CZH proteins: A new family of Rho-GEFs. J Cell Sci. 2005;118:4937–4946. doi: 10.1242/jcs.02671. [DOI] [PubMed] [Google Scholar]
- 31.Ono E, et al. Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA. 2001;98:759–764. doi: 10.1073/pnas.021273498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suharsono U, et al. The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA. 2002;99:13307–13312. doi: 10.1073/pnas.192244099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akamatsu A, et al. An OsCEBiP/OsCERK1-OsRacGEF1-OsRac1 module is an essential early component of chitin-induced rice immunity. Cell Host Microbe. 2013;13:465–476. doi: 10.1016/j.chom.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Thao NP, et al. RAR1 and HSP90 form a complex with Rac/Rop GTPase and function in innate-immune responses in rice. Plant Cell. 2007;19:4035–4045. doi: 10.1105/tpc.107.055517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakashima K, et al. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007;51:617–630. doi: 10.1111/j.1365-313X.2007.03168.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, et al. The Hop/Sti1-Hsp90 chaperone complex facilitates the maturation and transport of a PAMP receptor in rice innate immunity. Cell Host Microbe. 2010;7:185–196. doi: 10.1016/j.chom.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Kawano Y, et al. Activation of a Rac GTPase by the NLR family disease resistance protein pit plays a critical role in rice innate immunity. Cell Host Microbe. 2010;7:362–375. doi: 10.1016/j.chom.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 38.Kawano Y, et al. Palmitoylation-dependent membrane localization of the rice resistance protein pit is critical for the activation of the small GTPase OsRac1. J Biol Chem. 2014;289:19079–19088. doi: 10.1074/jbc.M114.569756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Kotchoni SO, Samuels AL, Szymanski DB. SPIKE1 signals originate from and assemble specialized domains of the endoplasmic reticulum. Curr Biol. 2010;20:2144–2149. doi: 10.1016/j.cub.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi K, Yoshida H. Refunctionalization of the ancient rice blast disease resistance gene Pit by the recruitment of a retrotransposon as a promoter. Plant J. 2009;57:413–425. doi: 10.1111/j.1365-313X.2008.03694.x. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi K, et al. Serotonin attenuates biotic stress and leads to lesion browning caused by a hypersensitive response to Magnaporthe oryzae penetration in rice. Plant J. 2016;85:46–56. doi: 10.1111/tpj.13083. [DOI] [PubMed] [Google Scholar]
- 42.Gu Y, Li S, Lord EM, Yang Z. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell. 2006;18:366–381. doi: 10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen L, et al. Analysis of the Rac/Rop small GTPase family in rice: Expression, subcellular localization and role in disease resistance. Plant Cell Physiol. 2010;51:585–595. doi: 10.1093/pcp/pcq024. [DOI] [PubMed] [Google Scholar]
- 44.Taya S, et al. Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF. J Cell Biol. 2001;155:809–820. doi: 10.1083/jcb.200106139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong HL, et al. In vivo monitoring of plant small GTPase activation using a Förster resonance energy transfer biosensor. Plant Methods. 2018;14:56. doi: 10.1186/s13007-018-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Césari S, et al. The NB-LRR proteins RGA4 and RGA5 interact functionally and physically to confer disease resistance. EMBO J. 2014;33:1941–1959. doi: 10.15252/embj.201487923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uhrig JF, et al. The role of Arabidopsis SCAR genes in ARP2-ARP3-dependent cell morphogenesis. Development. 2007;134:967–977. doi: 10.1242/dev.02792. [DOI] [PubMed] [Google Scholar]
- 48.Sanz-Moreno V, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 49.Tian M, et al. Arabidopsis actin-depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB. Plant Physiol. 2009;150:815–824. doi: 10.1104/pp.109.137604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okuyama Y, et al. A multifaceted genomics approach allows the isolation of the rice Pia-blast resistance gene consisting of two adjacent NBS-LRR protein genes. Plant J. 2011;66:467–479. doi: 10.1111/j.1365-313X.2011.04502.x. [DOI] [PubMed] [Google Scholar]
- 51.Moeder W, Yoshioka K, Klessig DF. Involvement of the small GTPase Rac in the defense responses of tobacco to pathogens. Mol Plant Microbe Interact. 2005;18:116–124. doi: 10.1094/MPMI-18-0116. [DOI] [PubMed] [Google Scholar]
- 52.Peart JR, Mestre P, Lu R, Malcuit I, Baulcombe DC. NRG1, a CC-NB-LRR protein, together with N, a TIR-NB-LRR protein, mediates resistance against tobacco mosaic virus. Curr Biol. 2005;15:968–973. doi: 10.1016/j.cub.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 53.Nakashima A, et al. RACK1 functions in rice innate immunity by interacting with the Rac1 immune complex. Plant Cell. 2008;20:2265–2279. doi: 10.1105/tpc.107.054395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarrinpar A, Bhattacharyya RP, Lim WA. The structure and function of proline recognition domains. Sci STKE. 2003;2003:RE8. doi: 10.1126/stke.2003.179.re8. [DOI] [PubMed] [Google Scholar]
- 55.Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17:496–510. doi: 10.1038/nrm.2016.67. [DOI] [PubMed] [Google Scholar]
- 56.Côté JF, Vuori K. GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 2007;17:383–393. doi: 10.1016/j.tcb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyamoto Y, et al. Akt and PP2A reciprocally regulate the guanine nucleotide exchange factor Dock6 to control axon growth of sensory neurons. Sci Signal. 2013;6:ra15. doi: 10.1126/scisignal.2003661. [DOI] [PubMed] [Google Scholar]
- 58.Xue M, et al. Comparative analysis of the genomes of two field isolates of the rice blast fungus Magnaporthe oryzae. PLoS Genet. 2012;8:e1002869. doi: 10.1371/journal.pgen.1002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El Kasmi F, et al. Signaling from the plasma-membrane localized plant immune receptor RPM1 requires self-association of the full-length protein. Proc Natl Acad Sci USA. 2017;114:E7385–E7394. doi: 10.1073/pnas.1708288114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.