Significance
Neurodegenerative diseases are a leading cause of morbidity and mortality among older adults, the fastest-growing segment of the US population. Neurodegenerative disease risk is reduced by high dietary intake of the omega-3 fatty acid, docosahexaenoic acid (DHA), a highly abundant lipid in the brain. Yet the fundamental mechanisms regulating brain DHA enrichment remain unknown. Here, we have made a key discovery that Acyl-CoA synthetase 6 (Acsl6) is required to specifically enrich DHA in the brain. Of importance, mice lacking Acsl6 have impaired motor function and increased astrogliosis, demonstrating the critical need for Acsl6-mediated lipid metabolism in neurological health. This work provides critical insight into longstanding mysteries surrounding brain DHA metabolism and has broad-reaching health implications.
Keywords: fatty acid metabolism, neurometabolism, docosahexaenoic acid, acyl-CoA synthetase, brain lipids
Abstract
Docosahexaenoic acid (DHA) is an omega-3 fatty acid that is highly abundant in the brain and confers protection against numerous neurological diseases, yet the fundamental mechanisms regulating the enrichment of DHA in the brain remain unknown. Here, we have discovered that a member of the long-chain acyl-CoA synthetase family, Acsl6, is required for the enrichment of DHA in the brain by generating an Acsl6-deficient mouse (Acsl6−/−). Acsl6 is highly enriched in the brain and lipid profiling of Acsl6−/− tissues reveals consistent reductions in DHA-containing lipids in tissues highly abundant with Acsl6. Acsl6−/− mice demonstrate motor impairments, altered glutamate metabolism, and increased astrogliosis and microglia activation. In response to a neuroinflammatory lipopolysaccharide injection, Acsl6−/− brains show similar increases in molecular and pathological indices of astrogliosis compared with controls. These data demonstrate that Acsl6 is a key mediator of neuroprotective DHA enrichment in the brain.
The omega-3 docosahexaenoic acid (DHA) and omega-6 arachidonic acid (AA) are the most abundant polyunsaturated fatty acids in the brain as the healthy brain contains ∼15% DHA, three to four times higher than the amount of DHA in any other tissue, and ∼13% AA (1–4). Importantly, low dietary intake of DHA, afflicting a majority of the US population (5), increases the risk of neurodegenerative diseases and related molecular events in rodents and humans (6–8). The neuroprotective properties of DHA are attributed to its ability to act as an antioxidant, increase membrane fluidity, and serve as the precursor for specialized proresolving mediators that attenuate inflammation and oxidative stress (9–12). Thus, low brain DHA results in neurodegenerative symptomology, suggesting a role for brain DHA metabolism in the onset and progression of neurodegeneration. However, little is known about the fundamental regulatory mechanisms controlling brain fatty acid metabolism and incorporation into phospholipids.
Cellular fatty acid metabolism is initiated by the activation of free fatty acids to form acyl-CoA to trap fatty acids within cells and provide the substrate for nearly all fatty acid metabolic processes, including membrane phospholipid biosynthesis. The generation of acyl-CoAs is mediated by the Acyl-CoA synthetase (ACS) family of enzymes with diverse substrate preferences, regulatory mechanisms, binding partners, expression patterns across tissues, and subcellular localization (13–16). We and others have shown that these distinct properties allow the ACS family of enzymes to channel specific fatty acids toward directed metabolic fates (17–19). Of these ACS enzymes, ACSL6 is nearly exclusively expressed in the brain according to mRNA abundance, suggesting that it may play an important and unique role in regulating brain lipid metabolism (15, 20, 21). However, the role of Acsl6 in regulating brain lipid metabolism in vivo has remained unknown. We have generated an Acsl6-deficient mouse (Acsl6−/−) and show that Acsl6-deficient mice exhibit reduced abundance of brain DHA, suggesting that Acsl6 is required for the incorporation and enrichment of the omega-3 fatty acid DHA in the brain. In agreement with the neuroprotective effects of DHA, Acsl6−/− mice exhibit motor dysfunction and increased astrogliosis. These data demonstrate that Acsl6 is critical for DHA metabolism in the central nervous system and that Acsl6-mediated lipid metabolism is critical for normal brain function and neuroprotection.
Results
Acsl6 Is Highly Enriched in the Central Nervous System.
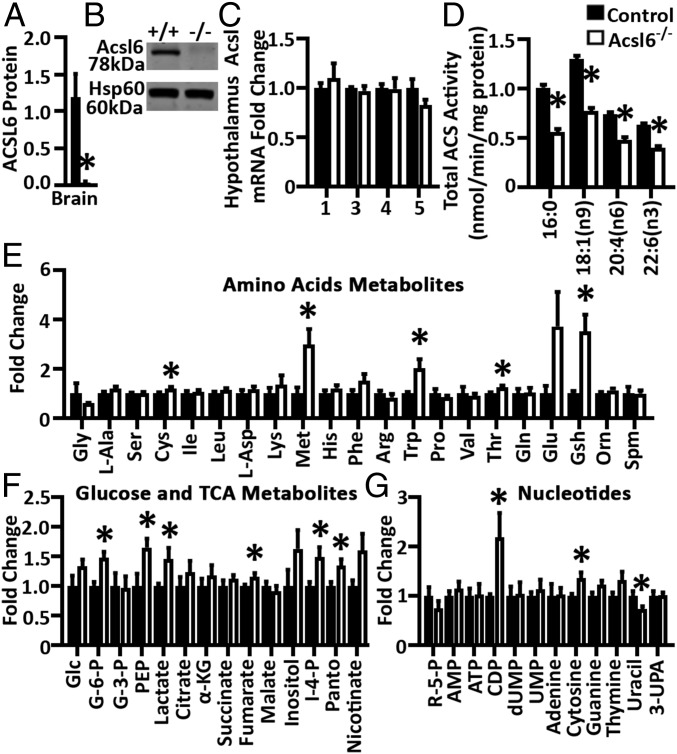
To confirm that Acsl6 protein, like its mRNA, is enriched in the central nervous system, an antibody was generated and used to demonstrate enrichment of ACSL6 protein in mouse brain, with minor expression detected in the spine, eye, and testis (Fig. 1A). Expression of Acsl6 was barely detected in all other tissues assayed (Fig. 1A). Acsl6 protein was abundant across brain regions but compared with hypothalamus, hippocampus, and cortex, Acsl6 was most abundant in the midbrain, medulla/pons, and cerebellum (Fig. 1B). Along the course of development, Acsl6 mRNA and protein was detectable at low levels in embryonic and early postnatal brain but increased at ∼7 d of age and linearly up to day 28, remaining abundant up to 1 y of age, data that represents a combination of male and female mice (Fig. 1 C and D). Thus, brain Acsl6 protein is induced 25-fold from fetus to adult brain, later in development than several DHA-metabolizing enzymes (FABP5 and Mfsd2a) (22–24). These data suggest that mouse Acsl6 is a developmental-induced central nervous system and testes-specific protein.
Fig. 1.
Acsl6 is highly enriched in the central nervous system. Quantification of Western blot for Acsl6 normalized to HSP60 in mouse tissues (A), brain regions (B), and mRNA (C) and protein (D) in brain across development, n = 3. BAT, brown adipose tissue; Cere, cerebellum; Epi, epididymal white adipose tissue; Gastro, gastrocnemius; Hipp, hippocampus; Hyp, hypothalamus; Med/Pons, medulla oblongata and pons; Mid, midbrain. Data represent averages ± SEM.
Generation of Acsl6 Knockout Mice.
To determine the role and importance of Acsl6-mediated fatty acid metabolism and function, we generated an Acsl6 conditionally deficient mouse strain (Acsl6flox/flox) and bred these mice to CMV-Cre expressing mice to generate a total body germ-line deletion of Acsl6 (Acsl6−/−) (SI Appendix, Fig. S1A). Acsl6−/− mice were viable and born at expected Mendelian ratios (SI Appendix, Fig. S1B). The loss of Acsl6 protein in Acsl6−/− was confirmed by Western blotting (Fig. 2 A and B). The mRNA of other Acsl isoforms were not induced to compensate for the loss of Acsl6 (Fig. 2C). Initial rates of ACS activity were measured in total membrane fractions using radiolabeled fatty acid substrates, encompassing potential activity from all 25 ACS enzymes and assay conditions optimized using oleate (OA) as the substrate (SI Appendix, Table S1). The loss of Acsl6 reduced total ACS activity for palmitate (PA) (C16:0) by 44%, OA (C18:1n9) by 41%, AA (20:4n6) by 36%, and DHA (22:6n3) by 37% in the midbrain (Fig. 2D), and similarly in the cortex (SI Appendix, Fig. S1C). Thus, the total ACS activity was reduced ∼40% in Acsl6−/− brain. The loss of Acsl6 did not alter body length, weight, or food intake compared with littermate controls (SI Appendix, Fig. S1 D–F), nor did it alter brain weight, length, and width (SI Appendix, Fig. S1 G and H) or cerebellar ultrastructure (SI Appendix, Fig. S1I) at 2 mo of age. Together, these data show that the loss of Acsl6 does not impact viability and that Acsl6 contributes to ∼40% of total ACS activity in the brain.
Fig. 2.
Generation of Acsl6 knockout mice. Quantification (A) and Western blot (B) image of Acsl6 protein from control and Acsl6−/−, n = 3. (C) mRNA abundance of Acsl isoforms 1, 3, 4, and 5 from control and Acsl6−/− hypothalamus, n = 5. (D) Initial rate of ACS activity for [14C]16:0, [14C]18:1n9, [14C]20:4n-6, and [14C]22:6n-3 from control and Acsl6−/− midbrain, n = 5–6. Amino acid (E), glucose and TCA (F), and nucleotide (G) metabolites in 2-mo-old female Acsl6−/− hippocampus relative to control, n = 8. Data represent averages ± SEM; *P ≤ 0.05 by Student’s t test.
To gain insight into how Acsl6 affects neurometabolism more broadly, an unbiased metabolomics analysis was performed on control and Acsl6−/− hippocampus from 2-mo-old mice following an overnight fast. Acsl6−/− mice exhibit alterations in hippocampal tryptophan, glutathione, methionine, and cysteine that have potential implications for disruptions in antioxidant and neurotransmitter homeostasis (Fig. 2E). Several metabolites related to glucose and energy metabolism were altered in the Acsl6−/− hippocampus including, lactate, glucose-6-phosphate, phosphoenolpyruvate, fumarate, and pantothenate (CoA synthesis) (Fig. 2F). Acsl6−/− hippocampal nucleotides, cytosine and uracil, and the nucleotide synthesis intermediate, CDP, were altered (Fig. 2G), which may reflect altered nucleic acid synthase or transcriptional activity.
Acsl6 Deficiency Decreases Brain Omega-3 Docosahexaenoate-Containing and Increases Omega-6 Arachidonate-Containing Lipids.
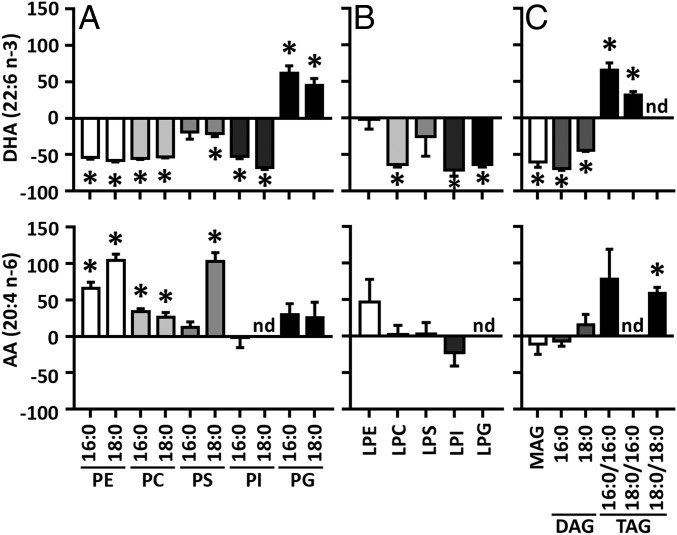
To determine how the loss of Acsl6 affects brain lipid composition, global unbiased lipidomic profiling of the cerebellum from 2-mo-old control and Acsl6−/− female mice was performed. The fatty acid profile of Acsl6−/− cerebellum, compared with controls, was marked by 22–71% reductions in phospholipids, lysophospholipids, monoacylglycerol, and diacylglycerol containing DHA and 25–61% increases in nearly every lipid species containing AA (Fig. 3). The pattern of reduced DHA and increased AA in Acsl6−/− cerebellum was also reflected in the free fatty acid and in the ether-linked and plasmalogen lipids (SI Appendix, Table S2). This level of reduced DHA, and increased AA, is similar to that seen after two generations of breeding rodents on an omega-3–deficient diet, suggesting that Acsl6 is a major contributor to brain DHA enrichment (25). Phospholipids containing the monounsaturated fatty acid OA remained generally unchanged between genotypes. To determine if overnight fasting altered lipid composition in this genetic model, lipidomics was performed on cerebellum of control and Acsl6−/− mice challenged with overnight fasting to reveal similar genotypic alterations compared with the fed state (SI Appendix, Table S2). Together, these data show that loss of Acsl6 reduced brain DHA content with a concomitant increase in the omega-6 fatty acid AA, suggesting that Acsl6 is critical for the incorporation of DHA into brain lipids.
Fig. 3.
Acsl6 deficiency decreases brain omega-3 docosahexaenoate-containing and increases omega-6 arachidonate-containing lipids. Lipid profile of phospholipids (A), lysophospholipids (B), MAG, DAG, and TAG (C) in 2-mo-old female cerebellum expressed as % change in the Acsl6−/− brains relative to the control samples, n = 5–6. Data represent averages ± SEM; *P ≤ 0.05 by Student’s t test.
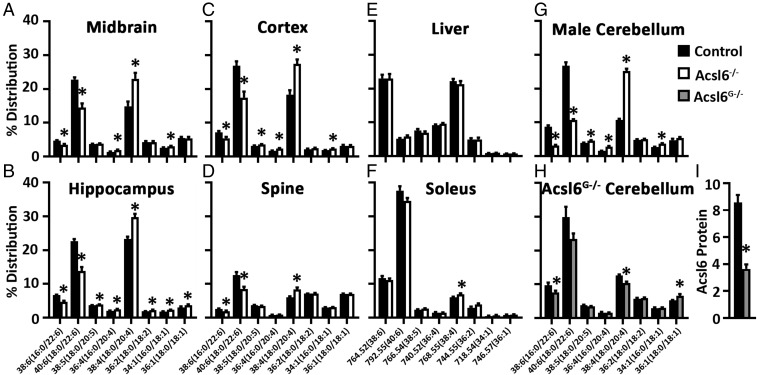
To confirm Acsl6-mediated DHA deficiency across brain regions and spine, lipidomics was performed in midbrain, hippocampus, cortex, and spine of 2-mo-old female control and Acsl6−/− mice. All Acsl6−/− brain regions and spine had consistent 24–40% reductions in predicted DHA-containing and 26–54% increases in AA-containing phospholipids (Fig. 4 A–D), similar to the cerebellum (Fig. 3). To determine if Acsl6 deficiency altered peripheral lipid homeostasis, lipidomics was performed on liver (Fig. 4E) and soleus muscle (Fig. 4F) in control and Acsl6−/− mice to reveal no genotype effect on lipid content in these tissues. These data suggest that tissues expressing Acsl6 require it for DHA enrichment and that loss of Acsl6 does not impact whole-body lipid homeostasis.
Fig. 4.
Acsl6 deficiency decreases omega-3 docosahexaenoate across the central nervous system. Phosphatidylethanolamine profile in 2-mo-old female midbrain (A), hippocampus (B), cortex (C), spine (D), liver (E), soleus (F), 6-mo-old male cerebellum (G), and 4- to 7-mo-old male Acsl6G/− cerebellum (H) expressed as % ion intensity distribution in Acsl6−/− or Acsl6G−/− relative to control, x axis represents carbon:unsaturated bonds, predicted fatty acid composition, or m/z, n = 5–6. (I) Western blot quantification of Acsl6 from control and Acsl6G−/− cerebellum normalized to β-tubulin, n = 5. Data represent averages ± SEM; *P ≤ 0.05 by Student’s t test.
To determine if Acsl6 deficiency-mediated impact on brain lipid metabolism was similar across sexes and with aging, lipidomics was performed on 6-mo-old male cerebellum (Fig. 4G) to demonstrate similar fatty acid profile compared with 2-mo-old female mice (Fig. 3). To determine the cell type-specific contribution of Acsl6-mediated lipid metabolic control, an astrocyte-specific Acsl6 knockout mouse (Acsl6G−/−) was generated resulting in a 57% reduction in Acsl6 protein in cerebellum (Fig. 4I). Acsl6G−/− cerebellum lipidomic analysis revealed similar reductions in DHA compared with Acsl6−/− mice; however, AA was decreased, rather than increased, in Acsl6G−/− (Fig. 4 G and H). These data suggest that approximately half of brain Acsl6 is expressed in astrocytes and that Acsl6 is critical for DHA enrichment independent of cell type.
Loss of Acsl6 Disrupts Motor Function.
Because dietary DHA deficiency perturbs motor function in rodents (26) and Acsl6 loss results in brain and spine DHA deficiency, we assessed motor and neurosensory function in Acsl6−/− mice. Acsl6−/− performed poorly during a wire hang test compared with controls (Fig. 5A). Poor performance during a wire hang test could be due to reduced strength; however, the control and Acsl6−/− mice performed similarly in the neuromuscular grip strength assessment, suggesting no disruption in upper body strength (SI Appendix, Fig. S2A). Rotarod tests were performed to assess motor coordination and showed a trend toward reduced performance by Acsl6−/− mice at 12 mo of age (Fig. 5B). Open field data showed no alterations in time spent in center versus periphery and no differences in locomotor activity, suggesting that Acsl6−/− mice do not have altered anxiety in the open field test (SI Appendix, Fig. S2B). Sensorimotor assessment by adhesive removal test showed similar time spent attempting to remove the sticker between Acsl6−/− and control, but the Acsl6−/− made more failed attempts to remove the adhesive compared with controls (Fig. 5C). The startle response of Acsl6−/− mice trended toward a reduction in response to an electrical impulse to the footpad and was significantly reduced in response to acoustic stimuli (Fig. 5 D and E). However, fear potentiated startle and prepulse inhibition were not different between genotypes (SI Appendix, Fig. S2 C and D). Together these data show impaired neurosensory and motor function in Acsl6−/− mice.
Fig. 5.
Loss of Acsl6 disrupts motor control. (A) Control and Acsl6−/− 6-mo-old mice average latency to fall in the wire hang test normalized to body weight, n = 14–15. (B) Rotarod performance for 12-mo-old male control and Acsl6−/− mice during three consecutive days, n = 15–16. (C) Adhesive removal test removal time (Left) and number of failed attempts (Right) by 12-mo-old control and Acsl6−/− mice. (D) Average startle response during the fear conditioning session (FC) to a foot shock and during the fear testing session (FT) to a 100-dB tone (noise = N) and to noise + light stimulus in 6-mo-old control and Acsl6−/− mice, n = 28–30. (E) Average startle response to acoustic stimuli in 6-mo-old control and Acsl6−/− mice. Data represent averages ± SEM; *P ≤ 0.05 by Student’s t test.
Acsl6−/− Mice Exhibit Potentiated Astrogliosis and Microglia Activity.
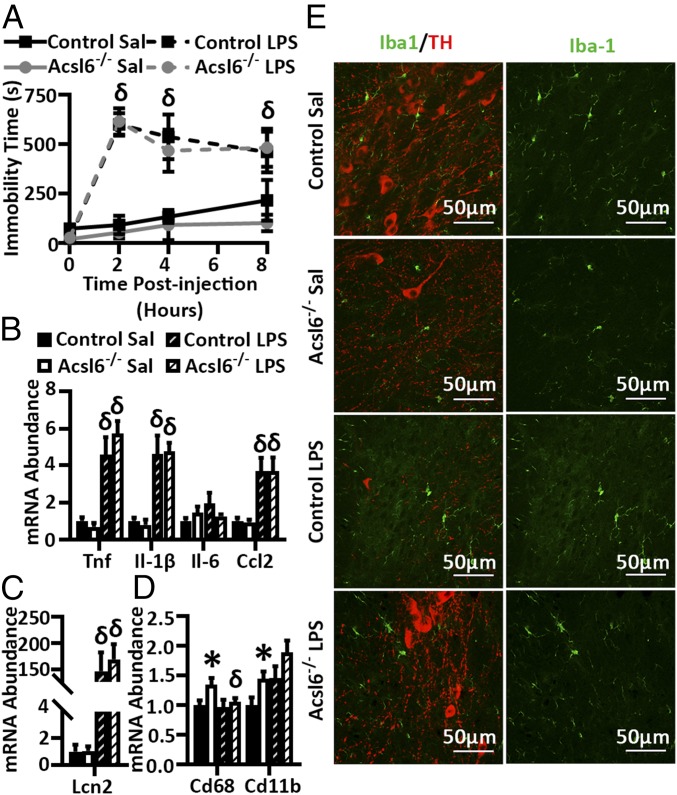
DHA has been shown to attenuate neuroinflammation in response to lipopolysaccharide (LPS) exposure in some, but not all, reports (27–31). To determine if Acsl6-mediated DHA deficiency altered neuroinflammation in the brain and in response to LPS, control and Acsl6−/− male mice were given a single i.p. injection of LPS. No genotype effect was observed for immobility or hippocampal mRNA abundance of the inflammatory genes with or without LPS at 2 and 6 mo of age (Fig. 6 A–C and SI Appendix, Fig. S2E). However, the mRNA abundance of the microglia markers, CD68 and CD11b, was increased in Acsl6−/− hippocampus, compared with saline-injected controls (Fig. 6D), and immunohistochemical staining of microglia by Iba1 in the substantia nigra showed morphology consistent with microglia activation, as demonstrated by the retracted processes and increased cell body size, in Acsl6−/− mice (Fig. 6E) (32). Motor-controlling dopaminergic neurons originate in the substantia nigra and extend to the striatum. Quantification of Acsl6−/− striatal terminal density of tyrosine hydroxylase-positive dopaminergic neurons increased, compared with controls (SI Appendix, Fig. S2F). Together these data suggest increased microglia activation and/or activity in Acsl6−/− mice.
Fig. 6.
Acsl6 deficiency increases microglia activity. Percent immobility and hippocampal mRNA abundance (A) of inflammatory (B and C) and microglial genes (D) in control and Acsl6−/− saline (Sal) or LPS treated 6-mo-old male mice, n = 7–8. (E) Representative images of Iba1 and tyrosine hydroxylase (TH) stained substantia nigra of control and Acsl6−/− mice injected with saline or LPS, n = 5. (Scale bars: 50 μm.) Data represent averages ± SEM; * by genotype, δ by treatment, P ≤ 0.05 by Student’s t test.
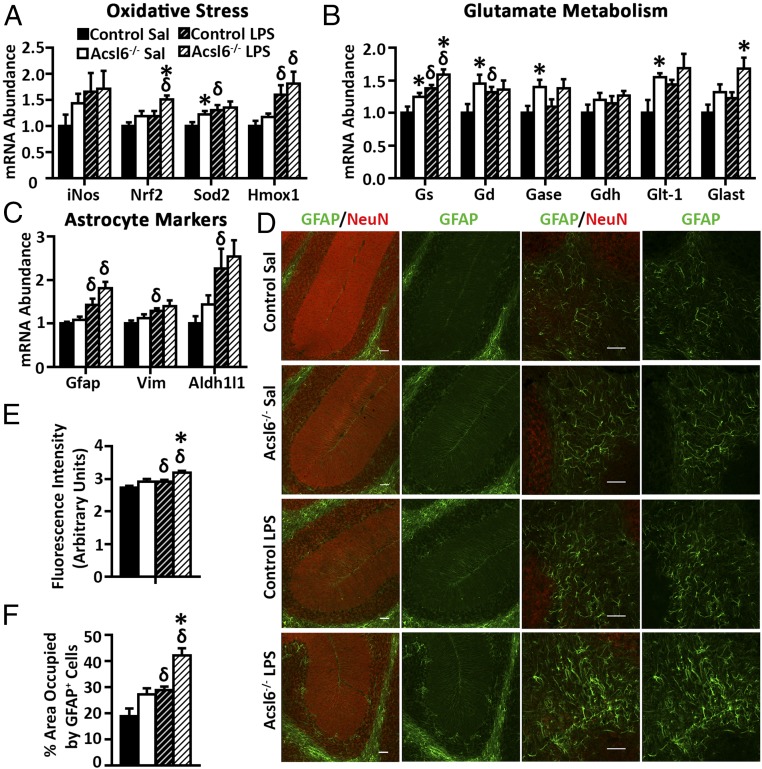
LPS-induced inflammatory response includes altered glutamate homeostasis, oxidative stress, and astrocyte activation (33–35). The mRNA abundance of genes related to glutamate metabolism, oxidative stress, and astrocyte activation in hippocampus were increased in Acsl6−/− compared with controls (Fig. 7 A–C). In agreement, immunohistochemistry in the cerebellum of Acsl6−/− mice compared with controls showed increased GFAP signal (Fig. 7D). Specifically, in cerebellar gray matter, GFAP immunoreactivity in the protoplasmic astrocytes surrounding Purkinje cells was increased in Acsl6−/−, compared with controls, with and without LPS (Fig. 7D). In cerebellar white matter where fibrous astrocytes reside, GFAP immunoreactivity was increased 7% and 9% in Acsl6−/− mice compared with controls with or without LPS, respectively, suggesting increased reactivity of the Acsl6-deficient astrocytes (Fig. 7 D and E). Furthermore, the area occupied by GFAP+ cells in white matter was increased 45% in Acsl6−/− mice, compared with controls, suggesting increased number of astrocytes (Fig. 7F). Together, these data show that loss of Acsl6-elevated glutamate and oxidative stress-related gene markers, and increased astrogliosis, an effect that remains elevated after a proinflammatory LPS challenge.
Fig. 7.
Increased astrogliosis in Acsl6−/− mice. mRNA abundance of oxidative stress (A), glutamate metabolism (B), and astrocyte markers (C) in hippocampus of control and Acsl6−/− saline (Sal) or LPS treated 6-mo-old male mice, n = 7–8. Representative (D) and quantification (E and F) of GFAP (green) and NeuN (red) stained in the cerebella of control and Acsl6−/− mice injected with saline or LPS, n = 5. (Scale bars: 50 μm.) Data represent mean ± SEM; * by genotype, δ by treatment, P ≤ 0.05 by Student’s t test.
Discussion
While DHA is the most abundant polyunsaturated fatty acid in the brain, models to study DHA in the brain are limited. The most predominant model is to modify dietary omega-3 fatty acid intake for multiple generations, a model confounded by whole-body and transgenerational effects. A model of DHA deficiency was reported in mice lacking major facilitator superfamily domain-containing 2A (Mfsd2a). These mice have a ∼50% reduction of DHA in brain due to impaired uptake of DHA-containing lysophospholipids through the blood–brain barrier (36). While this work provides evidence for a mechanism of DHA uptake into the brain, the contribution of lysophospholipids to brain DHA pool is minimal (37). Here, we report the loss of Acsl6, resulting in brain DHA deficiency. The requirement of Acsl6 to ligate DHA to the glycerol backbone argues a mechanism for Acsl6-mediated DHA enrichment that is independent of Mfsd2a. To date, the metabolic handling of blood-derived lipids once inside brain parenchyma has remained unclear. Here, we provide critical insight into Acsl6 as a major mediator of brain parenchyma DHA metabolism.
The existence of Acsl6 splice variants in the brain and their reported alternative substrate preferences, as well as Acsl6 cell type-specific expression, have complicated the implications of Acsl6 in lipid biology. Here, we were surprised to find nearly equivalent reductions in ACS activity for saturated, monounsaturated, omega-3 and omega-6 fatty acids in the Acsl6−/− brains relative to controls. We used the monounsaturated fatty acid oleate to optimize the enzyme assay conditions, thus optimization of the assay conditions using each substrate individually may be warranted to more accurately depict substrate-dependent enzyme activity. However, our lipidomic profiling reveal consistent and significant reductions in DHA-containing lipids, strongly suggesting that, in vivo, Acsl6 is critical for DHA incorporation into brain lipids. Acsl6 deficiency induced reduction in brain DHA is likely due to compounding mechanisms that control substrate (i.e., DHA) accessibility via shuttling-, transport-, or phospholipid remodeling-related processes in a cell type-dependent manner, an area of research that requires further investigation.
DHA has received recent attention for its antiinflammatory properties (9–11, 38–40). However, not all reports are consistent with reduced neuroinflammation by DHA (27–31, 41–43). Because dietary DHA manipulation involves whole-body metabolism of DHA, the neuroprotective effects could be elicited by responses outside the central nervous system. Recently, the i.c.v. injection of LPS to mice with increased DHA content, due to dietary fish oil supplementation or fat-1 transgene expression, revealed little to no protective role for DHA in the neuroinflammatory response to LPS (30). In agreement with these results, our data show that the neuroinflammatory response to LPS was not augmented in male Acsl6-mediated DHA deficiency. However, at baseline, the loss of Acsl6 did increase microglia activation and astrogliosis. These data suggest that brain neuroinflammatory responses are increased by Acsl6 deficiency, potentially due to the loss of DHA and increase in AA; however, LPS-mediated inflammation is not dependent on Acsl6-mediated lipid metabolism.
Several human genome mapping studies have linked the ACSL6 loci to schizophrenia (44–47). Here, we demonstrate motor symptoms, alterations to glutamate and dopaminergic homeostasis, all of which are consistent with characteristics of schizophrenia observed in patient and animal models (6, 48, 49). Several of these characteristics are also indicative of parkinsonism, such as alterations in the motor function, dopaminergic, glutamate, and microglia homeostasis consistent with symptoms and pathology of Parkinson’s disease patients and animal models (31, 50, 51). Thus, future investigation into the effects of Acsl6 on age-related neurodegenerative and psychiatric diseases are warranted.
In summary, we show that the loss of Acsl6 reduces DHA content in tissues in which it is highly expressed. The loss of Acsl6 disrupted brain metabolism, impaired motor function, and induced microglia activity and astrogliosis, indicative of neurological stress.
Experimental Procedures
Acsl6 knockout mice were created using a vector designed by the NIH-sponsored knockout mouse program to target Acsl6 exon 2 and injected into C57BL/6 embryonic stem cells to generate Acsl6 conditional mice (Acsl6flox/flox) by Ingenious Targeting, Inc. Acsl6flox/flox mice were bred to CMV-Cre (Jackson Laboratories stock no. 006054) or GFAP-Cre (Jackson Laboratories stock no. 024098) transgenic mice to produce germ-line global (Acsl6−/−) or GFAP-driven astrocyte-specific (Acsl6G−/−) knockout mice. Mice were maintained on chow diet with soy oil as lipid source (Teklad Global 18% Protein Rodent Diet; Envigo) and 12-h light-dark cycles. All experiments were approved by Purdue Animal Care and Use Committee. For the LPS challenge, 6-mo-old male mice were injected intraperitoneally with sterile saline or Escherichia coli lipopolysaccharide (0.33 mg/kg; 396,000 EU/kg; serotype 0127:B8; Sigma) and tissues harvested 8 h after injection (52, 53).
Supplementary Material
Acknowledgments
We thank Natalie Mudd for assistance with mouse behavior assessment, Kolapo Ajuwon for use of the oxymax apparatus, and Shihuan Kuang for use of the grip strength apparatus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.G.M. is a guest editor invited by the Editorial Board.
See Commentary on page 12343.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807958115/-/DCSupplemental.
References
- 1.Crawford MA, Casperd NM, Sinclair AJ. The long chain metabolites of linoleic avid linolenic acids in liver and brain in herbivores and carnivores. Comp Biochem Physiol B. 1976;54:395–401. doi: 10.1016/0305-0491(76)90264-9. [DOI] [PubMed] [Google Scholar]
- 2.Crawford MA, et al. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins Leukot Essent Fatty Acids. 2013;88:5–13. doi: 10.1016/j.plefa.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Diau GY, et al. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 2005;3:11. doi: 10.1186/1741-7015-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joffre C, et al. Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot Essent Fatty Acids. 2016;114:1–10. doi: 10.1016/j.plefa.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Papanikolaou Y, Brooks J, Reider C, Fulgoni VL., 3rd U.S. Adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from NHANES 2003-2008. Nutr J. 2014;13:31. doi: 10.1186/1475-2891-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardoso C, Afonso C, Bandarra NM. Dietary DHA and health: Cognitive function ageing. Nutr Res Rev. 2016;29:281–294. doi: 10.1017/S0954422416000184. [DOI] [PubMed] [Google Scholar]
- 7.Lukiw WJ, Bazan NG. Docosahexaenoic acid and the aging brain. J Nutr. 2008;138:2510–2514. doi: 10.3945/jn.108.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yashodhara BM, et al. Omega-3 fatty acids: A comprehensive review of their role in health and disease. Postgrad Med J. 2009;85:84–90. doi: 10.1136/pgmj.2008.073338. [DOI] [PubMed] [Google Scholar]
- 9.Basil MC, Levy BD. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15:511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 13.Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J Lipid Res. 2007;48:2736–2750. doi: 10.1194/jlr.M700378-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Mashek DG, et al. Revised nomenclature for the mammalian long-chain acyl-CoA synthetase gene family. J Lipid Res. 2004;45:1958–1961. doi: 10.1194/jlr.E400002-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Ellis JM, Bowman CE, Wolfgang MJ. Metabolic and tissue-specific regulation of acyl-CoA metabolism. PLoS One. 2015;10:e0116587. doi: 10.1371/journal.pone.0116587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol. 2010;21:212–217. doi: 10.1097/mol.0b013e32833884bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis JM, et al. Adipose acyl-CoA synthetase-1 directs fatty acids toward β-oxidation and is required for cold thermogenesis. Cell Metab. 2010;12:53–64. doi: 10.1016/j.cmet.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis JM, et al. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol Cell Biol. 2011;31:1252–1262. doi: 10.1128/MCB.01085-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowman TA, et al. Acyl CoA synthetase 5 (ACSL5) ablation in mice increases energy expenditure and insulin sensitivity and delays fat absorption. Mol Metab. 2016;5:210–220. doi: 10.1016/j.molmet.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhotra KT, Malhotra K, Lubin BH, Kuypers FA. Identification and molecular characterization of acyl-CoA synthetase in human erythrocytes and erythroid precursors. Biochem J. 1999;344:135–143. [PMC free article] [PubMed] [Google Scholar]
- 21.Fujino T, Yamamoto T. Cloning and functional expression of a novel long-chain acyl-CoA synthetase expressed in brain. J Biochem. 1992;111:197–203. doi: 10.1093/oxfordjournals.jbchem.a123737. [DOI] [PubMed] [Google Scholar]
- 22.Pélerin H, et al. Gene expression of fatty acid transport and binding proteins in the blood–brain barrier and the cerebral cortex of the rat: Differences across development and with different DHA brain status. Prostaglandins Leukot Essent Fatty Acids. 2014;91:213–220. doi: 10.1016/j.plefa.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Berger JH, Charron MJ, Silver DL. Major facilitator superfamily domain-containing protein 2a (MFSD2A) has roles in body growth, motor function, and lipid metabolism. PLoS One. 2012;7:e50629. doi: 10.1371/journal.pone.0050629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Y, et al. Fatty acid-binding protein 5 facilitates the blood-brain barrier transport of docosahexaenoic acid. Mol Pharm. 2015;12:4375–4385. doi: 10.1021/acs.molpharmaceut.5b00580. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Y, Huang Y, Chen Z-Y. Distribution, depletion and recovery of docosahexaenoic acid are region-specific in rat brain. Br J Nutr. 2005;94:544–550. doi: 10.1079/bjn20051539. [DOI] [PubMed] [Google Scholar]
- 26.Levant B, Ozias MK, Carlson SE. Sex-specific effects of brain LC-PUFA composition on locomotor activity in rats. Physiol Behav. 2006;89:196–204. doi: 10.1016/j.physbeh.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Wong SW, et al. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J Biol Chem. 2009;284:27384–27392. doi: 10.1074/jbc.M109.044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matt SM, Lawson MA, Johnson RW. Aging and peripheral lipopolysaccharide can modulate epigenetic regulators and decrease IL-1β promoter DNA methylation in microglia. Neurobiol Aging. 2016;47:1–9. doi: 10.1016/j.neurobiolaging.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi Z, et al. Fish oil prevents lipopolysaccharide-induced depressive-like behavior by inhibiting neuroinflammation. Mol Neurobiol. 2017;54:7327–7334. doi: 10.1007/s12035-016-0212-9. [DOI] [PubMed] [Google Scholar]
- 30.Trépanier MO, Hopperton KE, Giuliano V, Masoodi M, Bazinet RP. Increased brain docosahexaenoic acid has no effect on the resolution of neuroinflammation following intracerebroventricular lipopolysaccharide injection. Neurochem Int. 2018;118:115–126. doi: 10.1016/j.neuint.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Trépanier MO, Hopperton KE, Orr SK, Bazinet RP. N-3 polyunsaturated fatty acids in animal models with neuroinflammation: An update. Eur J Pharmacol. 2016;785:187–206. doi: 10.1016/j.ejphar.2015.05.045. [DOI] [PubMed] [Google Scholar]
- 32.Switzer RC, Butt MT. Fundamental Neuropathology for Pathologists and Toxicologists: Principles and Techniques. Wiley; Hoboken, NJ: 2011. Histological markers of neurotoxicity; pp. 181–190. [Google Scholar]
- 33.Stigger F, et al. Inflammatory response and oxidative stress in developing rat brain and its consequences on motor behavior following maternal administration of LPS and perinatal anoxia. Int J Dev Neurosci. 2013;31:820–827. doi: 10.1016/j.ijdevneu.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Qin L, Liu Y, Hong J-S, Crews FT. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61:855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takaki J, et al. L-glutamate released from activated microglia downregulates astrocytic L-glutamate transporter expression in neuroinflammation: The ‘collusion’ hypothesis for increased extracellular L-glutamate concentration in neuroinflammation. J Neuroinflammation. 2012;9:275. doi: 10.1186/1742-2094-9-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen LN, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–506. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- 37.Chen CT, et al. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci Rep. 2015;5:15791. doi: 10.1038/srep15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. Br J Nutr. 2012;107(Suppl 2):S171–S184. doi: 10.1017/S0007114512001560. [DOI] [PubMed] [Google Scholar]
- 39.Serhan CN. Treating inflammation and infection in the 21st century: New hints from decoding resolution mediators and mechanisms. FASEB J. 2017;31:1273–1288. doi: 10.1096/fj.201601222R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Layé S, Nadjar A, Joffre C, Bazinet RP. Anti-inflammatory effects of omega-3 fatty acids in the brain: Physiological mechanisms and relevance to pharmacology. Pharmacol Rev. 2018;70:12–38. doi: 10.1124/pr.117.014092. [DOI] [PubMed] [Google Scholar]
- 41.Park T, Chen H, Kevala K, Lee J-W, Kim H-Y. N-docosahexaenoylethanolamine ameliorates LPS-induced neuroinflammation via cAMP/PKA-dependent signaling. J Neuroinflammation. 2016;13:284. doi: 10.1186/s12974-016-0751-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rey C, et al. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav Immun. 2016;55:249–259. doi: 10.1016/j.bbi.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 43.Choi JY, et al. Antarctic krill oil diet protects against lipopolysaccharide-induced oxidative stress, neuroinflammation and cognitive impairment. Int J Mol Sci. 2017;18:2554. doi: 10.3390/ijms18122554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo XJ, et al. Association of haplotypes spanning PDZ-GEF2, LOC728637 and ACSL6 with schizophrenia in Han Chinese. J Med Genet. 2008;45:818–826. doi: 10.1136/jmg.2008.060657. [DOI] [PubMed] [Google Scholar]
- 45.Chowdari KV, et al. DNA pooling: A comprehensive, multi-stage association analysis of ACSL6 and SIRT5 polymorphisms in schizophrenia. Genes Brain Behav. 2007;6:229–239. doi: 10.1111/j.1601-183X.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, et al. Haplotypes spanning SPEC2, PDZ-GEF2 and ACSL6 genes are associated with schizophrenia. Hum Mol Genet. 2006;15:3329–3342. doi: 10.1093/hmg/ddl409. [DOI] [PubMed] [Google Scholar]
- 47.Kurotaki N, et al. Identification of novel schizophrenia loci by homozygosity mapping using DNA microarray analysis. PLoS One. 2011;6:e20589. doi: 10.1371/journal.pone.0020589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das UN. Polyunsaturated fatty acids and their metabolites in the pathobiology of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:122–134. doi: 10.1016/j.pnpbp.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res. 2004;152:49–57. doi: 10.1016/j.bbr.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 50.Lee HJ, et al. Docosahexaenoic acid prevents paraquat-induced reactive oxygen species production in dopaminergic neurons via enhancement of glutathione homeostasis. Biochem Biophys Res Commun. 2015;457:95–100. doi: 10.1016/j.bbrc.2014.12.085. [DOI] [PubMed] [Google Scholar]
- 51.Jagmag SA, Tripathi N, Shukla SD, Maiti S, Khurana S. Evaluation of models of Parkinson’s disease. Front Neurosci. 2016;9:503. doi: 10.3389/fnins.2015.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Godbout JP, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 53.Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300–316. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.