Significance
Using strawberry fruit as a model system, we uncover the mechanistic interactions between auxin, gibberellic acid (GA), and abscisic acid (ABA) that regulate the entire process of fruit development. Interlinked regulatory loops control ABA levels during fruit development. During the early stages, auxin/GA turns on a feedback loop to activate the removal of ABA via FveCYP707A4a-dependent catabolism needed for fruit growth. Down-regulation of auxin/GA results in the suppression of the feedback loop and the activation of the ABA biosynthesis-dependent feedforward loop, leading to a steep ABA accumulation for fruit ripening. The interlinked regulatory loops provide a conceptual framework that underlies the connection between the regulation of fruit growth and that of ripening as well as a molecular basis for manipulation of fruit sizes and ripening times.
Keywords: fruit development, auxin, gibberellic acid, hormone interaction
Abstract
Fruit growth and ripening are controlled by multiple phytohormones. How these hormones coordinate and interact with each other to control these processes at the molecular level is unclear. We found in the early stages of Fragaria vesca (woodland strawberry) fruit development, auxin increases both widths and lengths of fruits, while gibberellin [gibberellic acid (GA)] mainly promotes their longitudinal elongation. Auxin promoted GA biosynthesis and signaling by activating GA biosynthetic and signaling genes, suggesting auxin function is partially dependent on GA function. To prevent the repressive effect of abscisic acid (ABA) on fruit growth, auxin and GA suppressed ABA accumulation during early fruit development by activating the expression of FveCYP707A4a encoding cytochrome P450 monooxygenase that catalyzes ABA catabolism. At the onset of fruit ripening, both auxin and GA levels decreased, leading to a steep increase in the endogenous level of ABA that drives fruit ripening. ABA repressed the expression of FveCYP707A4a but promoted that of FveNCED, a rate-limiting step in ABA biosynthesis. Accordingly, altering FveCYP707A4a expression changed the endogenous ABA levels and affected FveNCED expression. Hence, ABA catabolism and biosynthesis are tightly linked by feedback and feedforward loops to limit ABA contents for fruit growth and to quickly increase ABA contents for the onset of fruit ripening. These results indicate that FveCYP707A4a not only regulates ABA accumulation but also provides a hub to coordinate fruit size and ripening times by relaying auxin, GA, and ABA signals.
The control of fruit size and shape and ripening is of great interest to biologists at large and to plant scientists and breeders in particular. Fruit development is divided into the early phase when fruits start growing, and the ripening phase when fruits undergo dramatic developmental changes such as softening, color changes, and production of aromatic and flavor compounds. How these phases are developmentally coordinated and what determines the transition between the two phases remain unknown. Auxin and gibberellic acid (GA) promote fruit growth in the early phase in many plant species (1–8). In strawberry, auxin synthesized in achenes, in combination with GA, plays central roles in fruit development in the early phase (7–9). In general, the strawberry fruit refers to the receptacle and achenes (10, 11). Receptacles with achenes removed fail to grow, but this growth defect is rescued by auxin and/or GA (8, 9). In contrast, applications of auxin and/or GA inhibit fruit ripening in various plant species (12–14). Moreover, the endogenous auxin and GA levels in strawberry fruits greatly decreased in the late stages, when abscisic acid (ABA) level increases dramatically (8, 15, 16). In nonclimacteric strawberry fruits, ABA, but not ethylene, which is the key ripening hormone in climacteric fruits such as peach, apple, banana, and tomato, is believed to be the main regulator of ripening processes such as softening of fruits and accumulation of anthocyanin (17–25). Generally, the fluctuation of endogenous ABA contents contributes to the progression of some developmental sequences in response to internal and external environmental cues, such as the sequential seed maturation, dormancy, and germination (26, 27). ABA and GA often counteract each other in the regulation of these processes (28–31). In strawberry fruits, the growth promoters auxin/GA also seem to inhibit fruit ripening by opposing ABA’s function (14). However, how these plant hormones interact to modulate fruit growth and ripening are largely unknown at the molecular level.
The major ABA biosynthesis pathway is regulated by the rate-limiting enzyme 9-cis-epoxycarotenoid dioxygenase (NCED) (32–35). On the other hand, ABA’s 8′-hydroxylation catalyzed by the CYP707A cytochrome P450 monooxygenase family is responsible for rapid deactivation of ABA (36–39). Both of the cultivated octoploid strawberry, Fragaria ananasa and the diploid strawberry, Fragaria vesca, have at least three members of NCED genes and five members of CYP707A genes (7, 40, 41). In the ripening stage of strawberry fruit, NCED gene expression increases sharply (25, 40, 41). Furthermore, virus-induced gene silencing (VIGS) of FaNCED1 gene caused significant decrease in ABA levels and delayed ripening in fruit (25). These observations raised many important questions about the regulation of fruit growth and ripening by plant hormones, for example, what regulates ABA biosynthesis and catabolism, how the balance of ABA biosynthesis and catabolism is regulated during fruit development, and how ABA fluctuation contributes to plant hormone cross talk and coordination among different stages of fruit development. Most of the previous molecular studies of ABA function in strawberry fruits focused on only ripening processes (23–25). However, like other fruits, the development of strawberry fruits involves several well-coordinated sequential stages. In addition to ripening, ABA is well known as a stress-inhibiting hormone and as a growth-inhibiting hormone (28–31). Thus, detailed studies on the regulation of ABA levels and its interaction with other hormones during the whole fruit developmental process is sorely needed.
By categorizing early fruit developmental stages and analyzing their gene expression profiles in Fragaria vesca, Kang et al. (7) convincingly showed the great potential of the diploid woodland strawberry of Fragaria vesca as a model plant to study the hormonal regulation of early fruit development. To understand how early fruit development is coordinated with fruit ripening, here we extended the detailed analysis of the whole fruit developmental process from flowers to ripened fruit, and showed that the fluctuation of ABA levels (low in early development and sharply increasing during ripening) during fruit development is mainly controlled by FveNCED5 and FveCYP707A4a genes, which control the bottleneck steps of ABA biosynthesis and inactivation, respectively. Importantly, we demonstrate that FveCYP707A4a is a central regulatory point that coordinates the hormonal relay between the early growth-promoting auxin and GA and the late ripening-promoting ABA in strawberry fruit development.
Results
Detailed Description of Fruit Development.
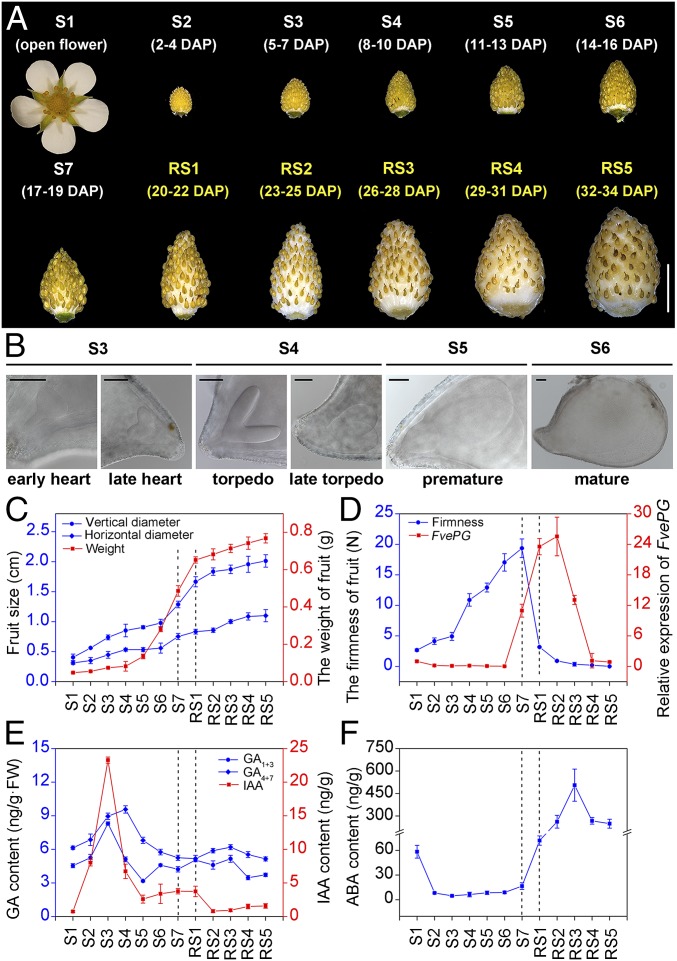
Early fruit development starting from anthesis was divided into five stages (S1–S5) in the diploid strawberry Fragaria vesca in previous reports (7, 42). To dissect hormonal control of fruit developmental transition from early growth to ripening stages, we extended the characterization beyond S5 in Fragaria vesca Yellow Wonder 5AF7. We divided the entire fruit developmental processes into 12 stages, including S1–S7 and ripening stage 1 (RS1) to RS5 (Fig. 1A). S6 is characterized by mature embryo with two large cotyledons filling up the entire seed (Fig. 1B). We observed a steep rise in the weight of fruits between S5 and RS1 and a sudden drop of the firmness of fruits between S7 and RS1 (Fig. 1 C and D). Thus, the sharp increase in fruit weight and the initiation of ripening coincided at S7 (Fig. 1 C and D). The increase in the weight of fruits gradually declines after RS1 (Fig. 1C). Polygalacturonase (PG) gene activation is typically used as a marker for fruit softening (43, 44). The PG mRNA expression rapidly increases at S7, rising to the peak at RS1 and RS2, which coincides with decrease in the firmness of fruits (Fig. 1D). These observations suggest that fruits make the transition to ripening at S7. In line with these observations, we propose that the 12 stages of fruit development can be categorized into three phases, the early fruit developmental phase (S1–S6), the transitional phase (S7 and RS1), and the ripening phase (RS2–RS5).
Fig. 1.
A refined description of Fragaria vesca fruit developmental stages and their relationship with changes in the content of auxin, gibberellin, and ABA. (A) A detailed description of different fruit developmental stages. Fruit development includes early growth and late ripening phases. The early phase, which is further divided into seven stages, S1–S7, is characterized by gradual increase in fruit (receptacle and achene) size, weight, and firmness. S1–S5 were described previously (7). The ripening phase, divided into RS1–RS5. For each stage, the corresponding days after pollination (DAP) are indicated. (Scale bar: 1 cm.) (B) Embryos inside cleared seeds of different developmental stages imaged with DIC optics. (Scale bar: 100 μM.) (C) Quantification of fruit dimension (length and width) and weight in different stages. Error bars represent SD of 20 fruits. (D) Changes in fruit firmness and the expression of PG gene in fruit (receptacle and achene) in different developmental stages. FveACTIN was used as the internal control. Error bars represent SD of three independent replicates (20 fruits were used for each replicate). (E and F) Changes in IAA, GAs (E), and ABA (F) contents of fruit (receptacle and achene) in different developmental stages. Error bars represent SD of three independent replicates (10 fruits were used for each replicate).
We investigated the endogenous level of auxin, GA, and ABA in entire fruit (achene and receptacle) for each stage (S1–RS5). After S1, indole-3-acetic acid (IAA) and GA levels increased coincidentally (Fig. 1E). The endogenous level of IAA and GA1,3 had a similar pattern in the early stages, while a slight increase in GA4,7 was observed through S1 to S4, and then gradually decreased (Fig. 1E). Interestingly, the change in the ABA level showed an inverse pattern to that of IAA and GA during fruit development. The ABA level was relatively high at S1, but decreased when GA and IAA levels started to increase and stayed at extremely low throughout S2–S7 (Fig. 1 E and F). A sudden increase in the ABA level occurred at the ripening onset (RS1) (Fig. 1F). Together, our results reveal coordinated changes in the levels of auxin, GAs, and ABA during different stages of fruit development, which hint at a synergism between IAA and GA and an antagonism between IAA/GA and ABA.
Auxin and GA Differentially Regulate Fruit Growth and Shape During Early Fruit Development.
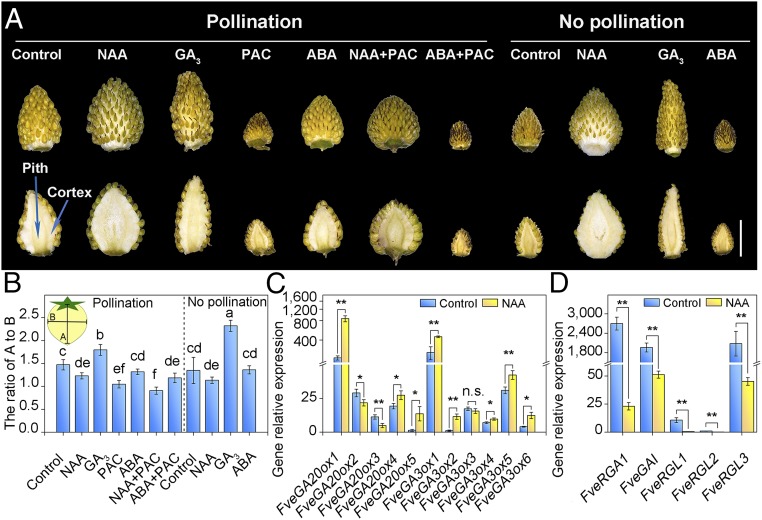
Auxin and GA promote fruit growth in various species including cultivated Fragaria annanasa and Fragaria vesca (4, 8, 9, 45, 46). Kang et al. (7) showed that double treatment of auxin and GA promoted fruit growth efficiently compared with auxin treatment. To further investigate the effect of these hormones on Fragaria vesca fruit development, we exogenously applied GA and NAA in developing fruits. Hormone treatments were started at S1 (Methods). NAA promoted fruit growth in both width and length, but GA primarily promoted fruit elongation in Fragaria vesca (Fig. 2 A and B and SI Appendix, Table S1). The ratio of length to width in fruits treated with GA was significantly higher in no pollination fruits (without fertilization) compared with pollination fruit (with fertilization) (Fig. 2B).
Fig. 2.
Fruit growth is promoted by auxin through GA-dependent and -independent pathways, but is inhibited by ABA. (A) The shape and size of fruits treated with indicated hormones (long-term treatment; Methods). (Upper) Images of whole fruits treated with different hormones. (Lower) Treated fruits were cut in half to show the pith and cortex of receptacles. Photos were taken at 14 d after the first hormone application. (Scale bar: 5 mm.) (B) The ratios of the length to width of receptacle. Letter in figure indicates significant differences between groups [P < 0.05, one-way ANOVA, Tukey’s honest significant difference (HSD) post hoc test]. Error bars represent SD of three independent replicates (20 fruits were used for each replicate). (C and D) qRT-PCR analysis of transcript levels for GA biosynthesis (C) and GA signaling genes (D) in NAA-treated pollination fruits (receptacle and achene). Total RNAs were isolated at 14 d after the first hormone application. FveACTIN was used as the internal control. Error bars represent SD of three independent replicates (20 fruits were used for each replicate). Student’s t test, **P < 0.01 and *P< 0.05. P value for each t test was adjusted by Bonferroni procedure. n.s., not significant.
To study the functional relationship between auxin and GA, we cotreated early fruit with 1-naphthaleneacetic acid (NAA) and GA biosynthesis inhibitor paclobutrazol (PAC) and found that PAC reduced the length of the fruits compared with treatment with NAA alone (Fig. 2 A and B, and SI Appendix, Table S1), suggesting that NAA may promote fruit elongation via GA. However, the size of fruit cotreated with NAA and PAC was significantly larger than that of the fruit treated with PAC alone, indicating that NAA promotes fruit size through both GA biosynthesis-dependent and -independent pathways (Fig. 2A). Auxin also promotes GA biosynthesis in fruits of other plant species (3, 47, 48). In agreement with the regulation of GA biosynthesis by auxin, we found that in pollinated fruits NAA treatments increased transcript levels for three—GA20ox-1, -4, and 5—and five—GA3ox-1, -2, -4, -5, and -6—genes encoding GA-biosynthesis enzymes compared with mock treatments (Fig. 2C). In many plant species, the negative regulators of GA signaling, DELLA proteins, act downstream of GA receptor (49–52). Interestingly, the expression of FveGAI, FveRGA1, and FveRGL3 (encoding DELLA and highly expressed in fruits) was clearly suppressed in auxin-treated pollinated fruits (Fig. 2D). Similar effects of NAA on the expression of these genes were also observed in no-pollination fruits (SI Appendix, Fig. S1 A and B). Therefore, we propose that auxin activates not only GA biosynthesis but also GA signaling in the fruits (Fig. 2 C and D).
High Levels of ABA Inhibit the Growth of Fruits in the Early Stages.
Since the pattern of ABA accumulation is opposite to that of GA and auxin in the early phase (Fig. 1 E and F), we sought to understand the function of ABA in fruit development. We found that application of ABA inhibited fruit growth distinctly in the early stages (Fig. 2A). The inhibition of fruit growth by ABA was confirmed by down- or up-regulation of endogenous ABA (see details below; see Figs. 4 C and H and 5 C and F). ABA often acts antagonistically with GA (28–31). Indeed, the inhibition of fruit growth by ABA was enhanced by PAC treatment (Fig. 2A). Thus, we propose that the rise in the GA level in the early stage antagonizes the inhibitory effect of ABA, while the rapid drop of ABA level in S2 ensures GA function to promote fruit growth in the early fruit development.
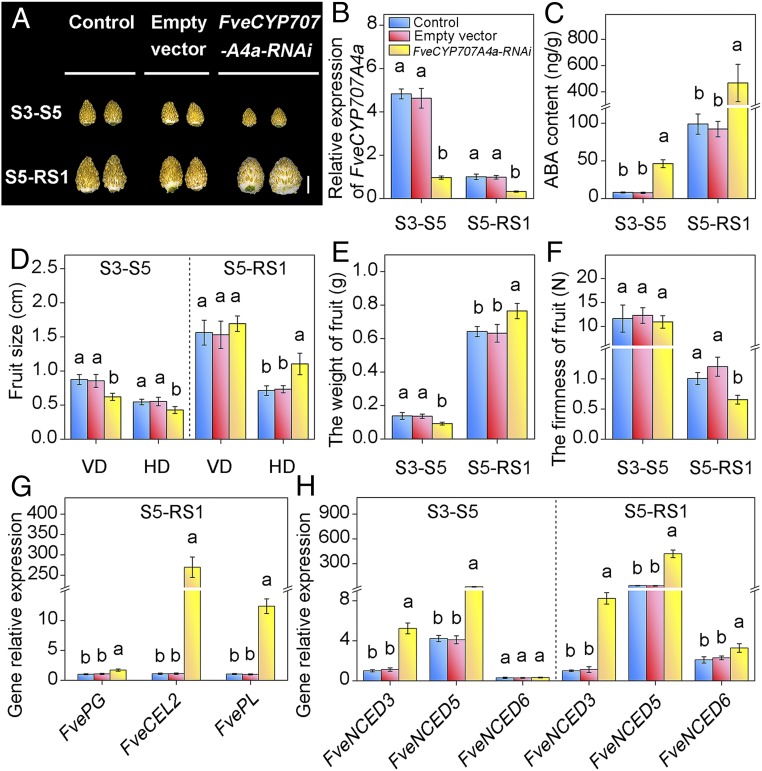
Fig. 4.
Transient silencing of FveCYP707A4a reveals dual function of ABA catabolism in fruit growth and ripening. (A) FveCYP707A4aRNAi S3–S5 inhibited fruit growth, while the FveCYP707A4aRNAi S5–RS1 promoted fruit ripening. FveCYP707A4aRNAi construct and the empty vector were injected into the fruits at 5 DAP (for FveCYP707A4aRNAi S3–S5) and 13 DAP (for FveCYP707A4aRNAi S5–RS1), respectively. Photos were taken 7 d after injection. (Scale bar: 5 mm.) (B) qRT-PCR analysis of transcript levels for FveCYP707A4a in FveCYP707A4aRNAi S3–S5 and FveCYP707A4aRNAi S5-RS1 fruits. Error bars represent SD of three independent replicates (15–20 fruits were used for each replicate). (C) FveCYP707A4a on ABA contents. Error bars represent SD of three independent replicates (10 fruits were used for each replicate). (D–F) The effect of transient silencing of Fve CYP707A4a on fruit (receptacle and achene) size (D), fresh weight (E), and firmness (F). Error bars represent SD of three independent replicates (15–20 fruits were used for each replicate). (G and H) qRT-PCR analysis of transcript levels for ripening-related genes (G) and FveNCED genes (H) in FveCYP707A4aRNAi S3–S5 and FveCYP707A4aRNAi S5–RS1 fruits. Error bars represent SD of three independent replicates. FveACTIN was used as the internal control for qRT-PCR analysis. Letter in figure indicates significant differences between groups (P < 0.05, one-way ANOVA, Tukey’s HSD post hoc test).
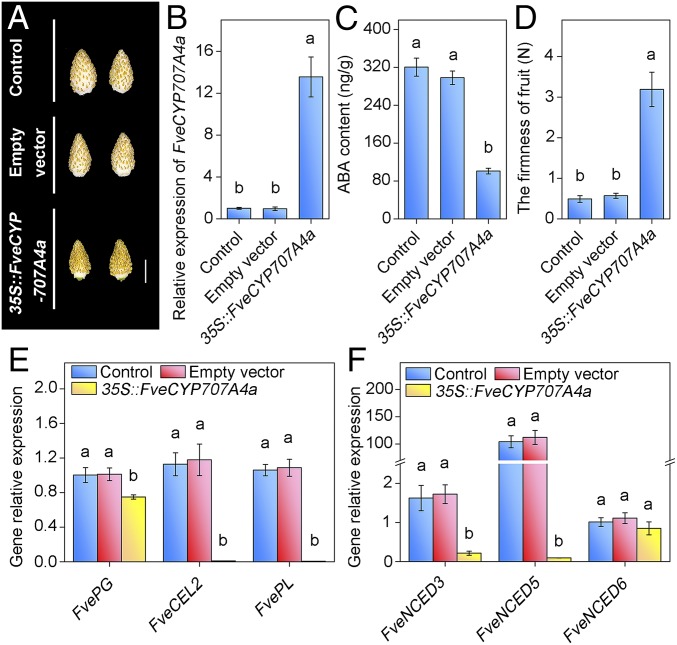
Fig. 5.
Transient overexpression of FveCYP707A4a delayed fruit ripening. FveCYP707A4a was transiently overexpressed using an Agrobacterium infiltration method (65). Infiltration was performed at S7, and all analyses were conducted at 7 d after Agrobacterium infiltration. (A) Fruits overexpressing FveCYP707A4a remained unripe, while control fruits became ripe. (Scale bar: 1 cm.) (B) qRT-PCR analysis showed dramatic increase in FveCYP707A4a transcript levels in 35S::FveCYP707A4a fruits. Error bars represent SD of three independent replicates (15–20 fruits were used for each replicate). (C) ABA content in 35S::FveCYP707A4a fruits. Error bars represent SD of three independent replicates (10 fruits were used for each replicate). (D–F) The effect of transient expression of 35S::FveCYP707A4a on fruit firmness (D), transcript levels for three ripening-related genes (E), and NCED genes (F). Error bars represent SD of three independent replicates (15–20 fruits were used for each replicate). Letter in figure indicates significant differences between groups (P < 0.05, one-way ANOVA, Tukey’s HSD post hoc test).
ABA Catabolism and Biosynthesis in Fruit Development.
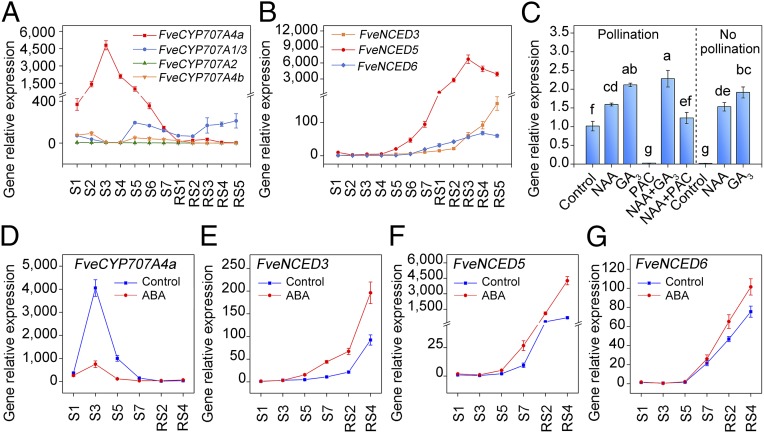
To understand the molecular basis for the fluctuation of ABA levels during fruit development, we examined the expression of NCED genes, which encode carotenoid dioxygenases, the rate-limiting enzyme for ABA biosynthesis in various plant species (7, 25, 40, 41). All three FveNCED genes (SI Appendix, Fig. S2 B and D) exhibited low expression in the early stages but were highly expressed in the ripening stages of fruit development (SI Appendix, Fig. S3B). In particular, FveNCED5 expression was highly expressed in receptacle (SI Appendix, Fig. S3B), started to increase after S4, and reached the peak at RS3 (Fig. 3B). FveNCED3 and FveNCED6 gene expression started to increase after S7 but was lower than FveNCED5 by nearly two orders of magnitudes, indicating that FveNCED5 is the predominant FveNCED gene regulating the ABA level during fruit development (Fig. 3B).
Fig. 3.
Temporal pattern and hormonal regulation of FveCYP707A4a and FveNCED5 expression during fruit development. (A–G) qRT-PCR analysis of transcript levels in fruit (receptacle and achene). The expression level of FveCYP707As (A) and FveNCEDs (B) in fruits of different developmental stages. Error bar represent SD of three independent replicates (15–20 fruits were used for each replicate). (C) Effects of auxin and GA on the expression of FveCYP707A4a. RNAs were isolated at 14 d after first hormone application. FveACTIN was used as internal control. Letter in figure indicates significant differences between groups (P < 0.05, one-way ANOVA, Tukey’s HSD post hoc test). Error bar represent SD of three independent replicates (15–20 fruits were used for each replicate). (D–G) qRT-PCR analysis of transcript levels in fruit (receptacle and achene). The expression level of FveCYP707A4a (D), FveNCED3 (E), FveNCED5 (F), and FveNCED6 (G) in different developmental stages with or without ABA treatment during fruit development; FveACTIN was used as the internal control. Error bars represent SD of three independent replicates (15–20 fruits were used for each replicate).
Interestingly, we noticed a time lag between the FveNCED5 expression and the rise in the ABA level, as an increase in FveNCED5 expression started at S4, but endogenous ABA contents remained low until S7 (Figs. 1F and 3B). In addition, all of the FveNCED genes were very weakly expressed in S1, but ABA level in S1 was relatively high (Figs. 1F and 3B). These results suggested the presence of other factor(s) to control the ABA level in the early stages. We found that FveBG genes, encoding β-glucosidase, which catalyzes the hydrolysis of glucose-conjugated ABA into active ABA (53, 54), were expressed in S1 (SI Appendix, Fig. S4 A–C), suggesting that FveBG expression may contribute to ABA accumulation in S1. However, this expression pattern is not sufficient to explain the low ABA level in the early stage of fruit development from S2 to S7. To identify other factor(s) that control ABA level during fruit development, we conducted a BLAST search of the Fragaria vesca genome for the CYP707A members of the p450 superfamily, which catabolize ABA via its dehydroxylation (36–39). Five potential CYP707A genes were identified (SI Appendix, Fig. S2 A and C). Most of FveCYP707A genes were highly expressed in achene; however, only FveCYP707A4a was highly expressed in the early stages of fruit development and was mainly expressed in receptacles (SI Appendix, Fig. S3A). Interestingly, a steep rise in FveCYP707A4a expression was observed at S3, indicating active down-regulation of ABA level during the early stages of fruit development (Fig. 3A). The FveCYP707A4a transcript level decreased dramatically to a very low level after S7 (the ripening initiation phase described above), concomitant with the initial point of the rapid rise in the ABA level (Fig. 1F). Furthermore, the Arabidopsis stable transgenic line overexpressing FveCYP707A4a clearly exhibited reduced ABA accumulation and accelerated seed germination and cotyledon greening (SI Appendix, Fig. S5 A–C). Taken together, these results suggest that FveCYP707A4a is involved in ABA catabolism during Fragaria vesca fruit development.
The FveCYP707A4a Is a Key Hormonal Cross-Talk Point in Early Fruit Development.
We next sought to understand the mechanism for maintaining FveCYP707A4a expression to the high level at the early stages of fruit development. We speculated that auxin and/or GA may control the expression, because auxin and GA are the main plant hormones in the early stages and because of our results implying the antagonism between GA and ABA function in the early-stage fruits (Fig. 2 A and B). Furthermore, the highest peaks of these hormones coincide with that of FveCYP707A4a expression at S3 (Figs. 1E and 3A). Indeed, GA application in pollinated and nonpollinated fruits promoted the expression of FveCYP707A4a at S5 but not other FveCYP707A genes (Fig. 3C and SI Appendix, Fig. S6 A–C). The increase was particularly pronounced in no pollination (Fig. 3C). Importantly, we found that FveCYP707A4a expression was induced after short-time GA treatment in fruit at S1 (SI Appendix, Fig. S7A). Further experiments suggest that GA is the main hormone that promotes the FveCYP707A4a expression. First, GA alone and cotreatment with GA and NAA induced FveCYP707A4a expression to a similar level (Fig. 3C). Second, GA biosynthesis inhibitor PAC greatly reduced FveCYP707A4a expression (Fig. 3C). Finally, the GUS expression in fruits injected with proFveCYP707A4a::GUS was greatly induced by GA treatment (SI Appendix, Fig. S8 A–C). Altogether, these results strongly suggest that GA maintains low endogenous ABA levels through the up-regulation of the FveCYP707A4a gene during early fruit development.
FveCYP707A4a Is Essential for the Maintenance of Low ABA Levels During Early Fruit Development.
To further analyze the function of FveCYP707A4a, we conducted transient silencing of FveCYP707A4a at the S3 (the highest peak of the FveCYP707A4a gene expression) by using a VIGS system (55). FveCYP707A4a expression was greatly suppressed in 7 d (at S5, FveCYP707A4aRNAi S3–S5) after injecting the TRV vector at S3 (Fig. 4B and SI Appendix, Fig. S9). In agreement with the FveCYP707A4a expression, greatly higher levels of ABA in FveCYP707A4aRNAi S3–S5 fruits were observed, compared with mock control (Fig. 4C). These results indicate that the ABA hydroxylation level was down-regulated in FveCYP707A4aRNAi fruits. Interestingly, the size and weight of FveCYP707A4aRNAi S3–S5 fruits were smaller (Fig. 4 A and D) and lighter (Fig. 4E), respectively, than controls, confirming the inhibition of early fruit growth by elevated ABA content as suggested by ABA application (Fig. 2A).
ABA Regulates FveNCED and FveCYP707A4a Expression to Interlink ABA Biosynthesis and Catabolism.
We next investigated the mechanism by which the ABA level rises sharply during the transition from the early phase to the late phase. As shown in Fig. 4H, down-regulation of FveCYP707A4a expression at the early stages induced a fivefold increase in the expression of both FveNCED5 and FveNCED3 in FveCYP707A4aRNAi S3–S5, suggesting a connection between ABA catabolism and biosynthesis. Furthermore, we observed a dramatic increase in FveNCED5 mRNA expression during the transitional phase (S7–RS1) (Fig. 3B). These results hint at the existence of an ABA-mediated feedforward regulation of FveNCED5 expression. Both long-term and short-term ABA treatments greatly increased the expression of FveNCED5 in fruits in the late stages (Fig. 3 E–G and SI Appendix, Fig. S7C), further supporting the feedforward loop between ABA and FveNCED5 during fruit development. The feedforward loop can explain the sharp up-regulation of FveNCED5 immediately after S7 stage (Fig. 3B).
For a feedforward loop of ABA-FveNCED5 to work, we anticipate that the suppression of FveCYP707A4a expression is necessary. Interestingly, the expression of FveCYP707A4a, but not other FveCYP707A genes, was greatly inhibited by both short- and long-term ABA treatment (Fig. 3D and SI Appendix, Figs. S7B and S10 A–C), suggesting a feedback loop between ABA level and FveCYP707A4a expression during fruit development.
Transient Silencing of FveCYP707A4a at S5 Caused Flying Start of Fruit Ripening.
To further test the hypothesis about the interconnected regulatory loops of ABA biosynthesis and catabolism, we transiently down-regulated FveCYP707A4a by using the VIGS system at S5 and observed ABA levels and fruit phenotype at RS1 (FveCYP707A4aRNAi S5–RS1) (SI Appendix, Fig. S9). As expected, we observed significant suppression of FveCYP707A4a expression and dramatically higher level of ABA in 7 d after injecting the TRV vectors at RS1 (Fig. 4 B and C). Furthermore, FveNCED expression was extremely high in FveCYP707A4aRNAi S5–RS1 fruits (Fig. 4H). These results confirm the connection between ABA biosynthesis and catabolism, highlighting the importance of the interconnected regulation loops for the steep increase of FveNCED5 gene expression and ABA level at the late stage.
Consistent with the dramatic increase in ABA levels and FveNCED5 gene expression, FveCYP707A4aRNAi S5–RS1 fruits exhibited accelerated ripening (Fig. 4 A and H). The firmness of FveCYP707A4aRNAi S5-RS1 fruit was significantly lower than that of control (Fig. 4F). Furthermore, strong expression of marker genes FveCEL2 (endo-β-1,4-glucanase) and FvePL (pectatelyase) (56, 57) for ripening was observed in FveCYP707A4aRNAi S5–RS1fruits (Fig. 4G). In agreement with the accelerated ripening, FveCYP707A4aRNAi S5–RS1 fruits became wider and heavier than that of controls 7 d after the injection of TRV vector (at RS1) (Fig. 4 A, D, and E). Interestingly, the fruit enlargement phenotype in FveCYP707A4aRNAi S5–RS1 fruits is in contrast to the reduction in fruit sizes in FveCYP707A4aRNAi S3–S5 fruits (Fig. 4A).
Overexpression of FveCYP707A4a Reduced FveNCED Expression and ABA Accumulation, Delaying Ripening.
To further investigate the importance of regulating FveCYP707A4a expression in the modulation of fruit development, we transiently overexpressed the FveCYP707A4a gene by using the agrobacterium infiltration into S7 fruits. The FveCYP707A4a expression was 13.5 times higher in the fruits with overexpression of FveCYP707A4a gene (35S::FveCYP707A4a) compared with that of control (Fig. 5B). Interestingly, we observed a significant decrease in the expression of FveNCED genes in the fruits expressing 35S::FveCYP707A4a (Fig. 5F) and greatly reduced ABA levels compared with control fruits (Fig. 5C). These results further support the feedforward regulation loop between ABA and FveNCED expression proposed above and is consistent with the low FveNCED expression in the early stages. In agreement with these results, we observed a clear delay in ripening in 35S::FveCYP707A4a fruits (Fig. 5 A and D). The fruits of 35S::FveCYP707A4a still remained unripe in 7 d after infiltration, and the significant increase in the firmness of receptacles was observed in 35S::FveCYP707A4a (Fig. 5 A and D). Furthermore, the expression of ripening marker genes FvePL and FveCEL2 was significantly lower in the fruits of 35S::FveCYP707A4a compared with that of control (Fig. 5E). Taken together, our results indicate that FveCYP707A4a is a central regulator of fruit development by relaying different hormones and controlling the tightly linked ABA catabolism and biosynthesis.
Discussion
It has been known that auxin and GA promote fruit growth and expansion but delay ripening in various species, while ABA promotes nonclimacteric fruit ripening (1, 2, 12–14). However, the molecular basis of their coordination during fruit development remains largely unknown. In the current study, using the strawberry fruit as a model, we have constructed a molecular framework for the coordination and relay between auxin, GA, and ABA throughout all of the fruit developmental stages. Our findings provide evidence that auxin, acting through GA, promotes the expression of FveCYP707A4a gene that promotes ABA catabolism, which is required for fruit growth in the early stages of fruit development (Figs. 2 C and D, 3C, and 6A). Furthermore, we have demonstrated that ABA catabolism and biosynthesis are interconnected by FveCYP707A4a-based feedback and FveNCED-mediated feedforward loops to control a rapid rise in the ABA level required for fruit ripening. Our results suggest that FveCYP707A4a is a key cross-talk point of plant hormones, auxin, GA, and ABA in fruit development and is a central regulator of the transition from the early growth phase to the ripening phase.
Fig. 6.
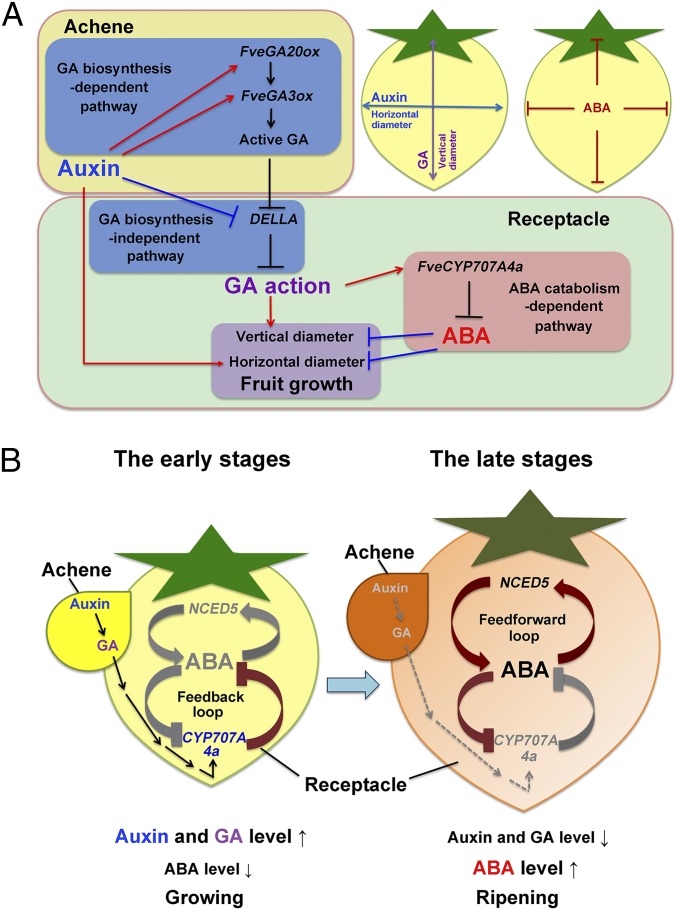
Auxin and GA promote fruit growth by activating ABA catabolism in the early stage and are important for the regulation of interlinked regulatory loops of ABA catabolism and biosynthesis during fruit development. (A) In the early stages, auxin promotes fruit growth in both width and length in GA-independent and -dependent pathways, respectively. Auxin promotes GA biosynthesis and signaling, which promotes fruit elongation only. In contrast, ABA inhibits fruit growth both in width and length, while auxin and GA activate ABA catabolism to ensure efficient fruit growth. (B) The interconnected ABA catabolism-mediated double–negative-feedback loop and ABA biosynthesis-mediated feedforward loops in fruit development. Auxin and GA from achene in early stages promote the expression of the FveCYP707A4a gene to ensure the endogenous ABA level is extremely low. At the initiation of ripening stage, auxin and GA levels decrease to a threshold, and so does the FveCYP707A4a expression, leading to the activation of the feedforward loop for the steep increase of ABA level to trigger transition from “growing” to “ripening.” The high level of ABA in the late stages further inhibits the expression of FveCYP707A4a to enhance the activation of the feedforward loop.
In the early phase of fruit development, cells divide and expand in a specific spatial pattern, which plays a crucial role in determining the size and shape of fruits. In most species, if not all, this early phase is controlled by both auxin and GA (1, 2). These hormones are likely to promote cell division and/or cell expansion, but the precise mode of their action is unclear. Furthermore, how auxin and GA coordinate to regulate fruit growth remains largely enigmatic. Our results here suggest that auxin promotes both GA biosynthesis and signaling in the strawberry fruits. Apart from the GA-dependent auxin action as discussed above, auxin also modulates fruit growth and shape in a GA-independent manner (horizontal diameter in Figs. 2 A and B and 6A and SI Appendix, Table S1). Specifically, our data suggest that auxin promotes the width of fruit independent of GA (Fig. 2A and SI Appendix, Table S1). In agreement with our observation, Kang et al. (7) also observed the results that double treatment of GA and auxin showed synergy effects for fertilization-independent fruit enlargement, suggesting auxin and GA have common as well as unique roles in fruit growth. Exogenously applied auxin and GA control cell elongation and expansion through altering microtubule array organization (58–62). Detailed transcriptomic analysis and a genetic approach need to be employed to investigate this specific role of auxin in fruit growth.
Apart from their direct roles in regulating fruit size and shape, auxin and GA also need to suppress hormones that inhibit growth such as ABA. We showed that GA promotes ABA catabolism gene FveCYP707A4a expression to prevent ABA accumulation in fruits. In deepwater rice, GA promotes expression of one of the CYP707As, suggesting that a common regulation mechanism exist in different plant species (63).
Several sets of our results show strong evidence that the FveCYP707A4a-based feedback loop and FveNCED-mediated feedforward loop are tightly interconnected with each other. For example, the steep increase of FveNCED5 expression coincides with the time when FveCYP707A4a expression declines to the lowest level (Fig. 3 A and B). Transiently up-regulating and knocking down FveCYP707A4a expression caused dramatic down- and up-regulation of FveNCED5 expression, respectively (Figs. 4H and 5F). Furthermore, the feedback down-regulation of FveCYP707A4a by ABA ensures the steep increase in ABA level in the ripening stage (Fig. 6B). Intriguingly, these interconnected regulation loops are regulated by auxin and GA at the key cross-talk point, the FveCYP707A4a expression (Fig. 6B). In the presence GA, the feedback loop-mediated ABA catabolism prevents the accumulation of ABA in fruits (Fig. 6B). However, after auxin and GA levels go down before the initiation of fruit ripening, FveCYP707A4a expression also declines. This down-regulation of FveCYP707A4a expression seemed to be a trigger for the activation of FveNCED5-mediated feedforward loop in the late stage of fruit development (Fig. 6B). The high level of ABA in the late stage further inhibits FveCYP707A4a expression (Fig. 6B).
Importantly, the interconnected regulation loops we propose here can explain how auxin and GA inhibit fruit ripening on one hand, and how the steep increase of FveNCED gene expression and ABA contents can be initiated at the transition from early growth phase to the ripening phase of fruit development (Fig. 6B). We suspect the endogenous and exogenous environmental conditions control the speed of the fruit growth and advance of developmental program through regulation of the ABA-linked interconnected regulation loops. The high-level expression of FveCYP707A4a might be important to give fruits enough time to have ripening competent in the early stage. Interestingly, we observed mature embryo in achene just before transition stage (at S6). Once embryos become mature, FveCYP707A4a expression reaches a significantly low level and the NCED-linked feedforward loop can be activated to contribute to the steep increase of ABA level that realizes survival strategies such as quick receptacle fruit ripening and consequent efficient dispersing seeds at appropriate timing.
In conclusion, we have established a molecular framework for the sequential and coordinate action of auxin, GA, and ABA in their regulation of strawberry fruit development (Fig. 6 A and B). Our framework explains how these hormones coordinately fluctuate, relay, interact, and coordinate to achieve their roles in the regulation of fruit size, shape, and ripening. The promotion of fruit growth by auxin and GA and their inhibition of fruit ripening are highly conserved in various plant species. Rapid fruit ripening either activated by ABA or ethylene is also quite common (1, 2). The speedy transition to ripening is likely to commonly require the activation of the double-feedback loops for the instant rise of ripening hormone as we have shown here for Fragaria vesca. Therefore, our findings have established a conceptual framework of fruit development, which provides not only an important basis for further mechanistic studies of fruit development in the Fragaria vesca model system but also an exciting paradigm for the broader understanding of the mechanisms for fruit development in many other plant species.
Methods
Plant Materials and Growth Conditions.
Diploid strawberry plants (Fragaria vesca), Yellow Wonder 5AF7 (YW5AF7) (64), planted in pots (90 mm × 90 mm × 90 mm) were used in this study. The seedlings were grown and maintained in a growth room with the following conditions: 22 °C, 60% humidity, and a 16-h photoperiod. Hand pollination was performed by using downy water bird feather to obtain pollinated fruit. For Arabidopsis transformation, Arabidopsis thaliana (ecotype Columbia) was used.
Hormone Treatments.
Long-term plant hormone treatments were performed every 2 d after hand pollination (pollinated fruit) or after emasculation (no-pollination fruit) as described previously (7). The first hormone treatments for long-term treatment were performed for pollinated fruit at 1 d after pollination (DAP). For short-term hormone treatment, plant hormone was applied only one time at 1 DAP. The PAC treatment was performed as well as the hormone treatment. The concentrations for each plant hormone were 500 µM for NAA, and GA3, 100 µM for PAC and ABA.
Visualizing Embryo Development.
Individual achenes were removed from fertilized flowers at the indicated stages. The seeds were manually dissected out of the ovary under a dissecting microscope, fixed for 10 min in 1:1 acetic acid/ethanol, and then transferred to Hoyer’s solution (42) to clear for 6–12 h. Hoyer’s solution consists of 50 mL of distilled H2O, 30 g of gum arabic, 200 g of chloral hydrate, and 20 g of glycerin. After cleared, samples were examined with a Nikon ECL1PSE E600 W microscope using bright Weld and differential interference contrast (DIC) optics and then photographed. About 15 seeds from three fruits at each stage were examined.
Firmness Analysis.
The firmness of fresh fruit was measured with a texture analyzer (GY-4; Handpi) fitted with a cylindrical plunger of 6 mm in diameter. Each fruit was penetrated 10 mm during the test was recorded. Each fruit was measured three times in the equatorial zone for each fruit.
RNA Isolation and qRT-PCR.
Total RNA was extracted using the polysaccharide and polyphenolics-rich RNAprep Pure Kit (Tiangen); all tissues from at least five plants were combined to form one biological replicate. cDNA was synthesized from total RNA using the PrimeScript RT reagent Kit (Perfect Real Time) (Takara). Real-time quantitative PCR was performed in the ABI 7500 Real-Time PCR System (Applied Biosystems) using SYBR Premix Ex Taq II (Takara). In Arabidopsis and strawberry, AtACTIN2 and FveACTIN were used as internal controls, respectively. Primer sequences used for qRT-PCR in this study are shown in SI Appendix, Table S2.
Construction of Plasmid DNA.
For FveCYP707A4aRNAi, the pTRV1 and pTRV2 VIGS vectors (55) were used in this study. A 326-bp cDNA fragment (from span of base pairs 305–630) of FveCYP707A4a was amplified and inserted into pTRV2. To generate the FveCYP707A4a overexpression vector, full-length FveCYP707A4a cDNA (1,383 bp) was amplified and inserted into the pCAMBIA1305 vector (pCAMBIA1305-FveCYP707A4a/35S::FveCYP707A4a). To generate the FveCYP707A4a promoter::GUS vector, the promoter region was amplified and inserted into the PBI121 vector containing the GUS reporter gene. Primer sequences used for vector construction are shown in SI Appendix, Table S3. The accession numbers are shown in SI Appendix, Table S4.
Transient Overexpression and RNAi in Fruit.
Agrobacterium tumefaciens strain GV3101 containing pTRV1, pTRV2, and the pTRV2 derivative pTRV2-FveCYP707A4a was used for RNAi, and containing pCAMBIA1305-FveCYP707A4a/35S::FveCYP707A4a, was used for transient overexpression. Agrobacterium infiltration was performed as described previously (65). To examine the effect of FveCYP707A4a-RNAi on strawberry fruit development and ripening, fruits in S3 and S5 stages were selected. To examine the effect of overexpression of FveCYP707A4a on strawberry fruit ripening, fruits in S7 stage were selected. Fifteen to 20 fruits from 10 independent plants selected for inoculation for each experiment. The fruits were evaluated 7 d after injection.
Hormone Measurements.
The entire fruit (achene and receptacle) was used for all hormone measurements. All samples were ground to powder under liquid nitrogen. After extraction, 900 μL of 70% (vol/vol) MeOH (methanol), 100 μL of 500 ng/mL [2H6]-ABA (OlChemIm), and 50 ng/mL [13C6]-IAA (OlChemIm) isotope internal standard were added to each sample (200 mg). After full oscillation, samples were kept at −20 °C for overnight. The samples then were vortexed and sonicated for 2 min, and centrifuged in 4 °C for 30 min at 36,000 × g (first extraction). The supernatant was transferred to a 2-mL centrifugal tube and kept at −20 °C. A volume of 0.5 mL of 70% (vol/vol) MeOH was added to the deposit, vortexed, and sonicated for 5 min, and centrifuged at 4 °C for 5 min at 36,000 × g (second extraction); the supernatant was transferred to the supernatant from the first extraction. A volume of 700 μL of 2% (vol/vol) NH4OH (ammonium hydroxide) was added to the supernatant after the supernatant was evaporated to 300 μL at room temperature, and then centrifuged at 4 °C for 3 min at 36,000 × g. A volume of 2 mL of MeOH, 2 mL of 2% (vol/vol) NH4OH, 2 mL of 2% (vol/vol) NH4OH, and samples were added to CNW Poly-Sery MAX (60 mg/3 mL) cartridges (ANPEL) in turn. A volume of 2 mL of 2% (vol/vol) NH4OH and 2 mL MeOH were added again in turn, and the fraction was first collected. A volume of 2 mL of MeOH and 2 mL of 1% (vol/vol) FA (dissolved in MeOH) were added in turn, and then fraction was collected again. The two pieces of fraction were centrifuged to dry. A volume of 200 μL of 70% MeOH was added and vortexed for 1 min. A certain amount of sample was put in the sample bottle and then was analyzed. The GA contents were measured by an ELISA as described previously (66).
GUS Histochemical Staining and Quantitative Assays.
The fruits injected in S5 stage for 3 d with or without GA3 treatment were collected for GUS staining. For GA3 treatment, fruits were sprayed with 100 μM GA3 for 3 h. For histochemical staining of GUS, fresh fruits were incubated in X-Gluc solution at 37 °C for 12 h. After bleaching with 75% ethanol, the stained samples were observed with an OLYMPUS SZX16-DP72 stereo fluorescence microscope. Quantification of GUS activity was performed as described previously (67).
Supplementary Material
Acknowledgments
We thank Dr. Yan Xiong (Fujian Agriculture and Forestry University) for his careful and critical comments on this manuscript. Fragaria vesca, Yellow Wonder 5AF7 was kindly provided by Dr. J. P. Slovin (US Department of Agriculture). The work was supported by National Natural Science Foundation of China Grant 31670187 (to C.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. Z.-Y.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812575115/-/DCSupplemental.
References
- 1.Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C. Fruit development and ripening. Annu Rev Plant Biol. 2013;64:219–241. doi: 10.1146/annurev-arplant-050312-120057. [DOI] [PubMed] [Google Scholar]
- 2.McAtee P, Karim S, Schaffer R, David K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci. 2013;4:79. doi: 10.3389/fpls.2013.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serrani JC, Ruiz-Rivero O, Fos M, García-Martínez JL. Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J. 2008;56:922–934. doi: 10.1111/j.1365-313X.2008.03654.x. [DOI] [PubMed] [Google Scholar]
- 4.de Jong M, Mariani C, Vriezen WH. The role of auxin and gibberellin in tomato fruit set. J Exp Bot. 2009;60:1523–1532. doi: 10.1093/jxb/erp094. [DOI] [PubMed] [Google Scholar]
- 5.Csukasi F, et al. Gibberellin biosynthesis and signalling during development of the strawberry receptacle. New Phytol. 2011;191:376–390. doi: 10.1111/j.1469-8137.2011.03700.x. [DOI] [PubMed] [Google Scholar]
- 6.Devoghalaere F, et al. A genomics approach to understanding the role of auxin in apple (Malus x domestica) fruit size control. BMC Plant Biol. 2012;12:7. doi: 10.1186/1471-2229-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang C, et al. Genome-scale transcriptomic insights into early-stage fruit development in woodland strawberry Fragaria vesca. Plant Cell. 2013;25:1960–1978. doi: 10.1105/tpc.113.111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nitsch JP. Growth and morphogenesis of the strawberry as related to auxin. Am J Bot. 1950;37:211–215. [Google Scholar]
- 9.Archbold D, Dennis FJ. Quantification of free ABA and free and conjugated IAA in strawberry achene and receptacle tissue during fruit development. J Am Soc Hortic Sci. 1984;109:330–335. [Google Scholar]
- 10.Darrow GM. The Strawberry: History, Breeding, and Physiology. Holt, Rinehart and Winston; New York: 1966. [Google Scholar]
- 11.Hancock JF. Strawberries. CABI Publishing; Wallingford, UK: 1999. [Google Scholar]
- 12.Dostal HC, Leopold AC. Gibberellin delays ripening of tomatoes. Science. 1967;158:1579–1580. doi: 10.1126/science.158.3808.1579. [DOI] [PubMed] [Google Scholar]
- 13.Given NK, Venis MA, Gierson D. Hormonal regulation of ripening in the strawberry, a non-climacteric fruit. Planta. 1988;174:402–406. doi: 10.1007/BF00959527. [DOI] [PubMed] [Google Scholar]
- 14.Martfnez GA, Chaves AR, Añón MC. Effect of gibberellic acid on ripening of strawberry fruits (Fragaria annanassa Duch.) J Plant Growth Regul. 1994;13:87–91. [Google Scholar]
- 15.Nitsch JP. Free auxins and free tryptophane in the strawberry. Plant Physiol. 1955;30:33–39. doi: 10.1104/pp.30.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Symons GM, et al. Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot. 2012;63:4741–4750. doi: 10.1093/jxb/ers147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams-Phillips L, Barry C, Giovannoni J. Signal transduction systems regulating fruit ripening. Trends Plant Sci. 2004;9:331–338. doi: 10.1016/j.tplants.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Zaharah SS, Singh Z, Symons GM, Reid JB. Role of brassinosteroids, ethylene, abscisic acid, and indole-3-acetic acid in mango fruit ripening. J Plant Growth Regul. 2011;31:363–372. [Google Scholar]
- 19.Kondo S, Inoue K. Abscisic acid (ABA) and 1-aminocyclopropane-l-carboxylic acid (ACC) content during growth of “Satohnishiki” cherry fruit, and the effect of ABA and ethephon application on fruit quality. J Hortic Sci. 1997;72:221–227. [Google Scholar]
- 20.Trainotti L, Pavanello A, Casadoro G. Different ethylene receptors show an increased expression during the ripening of strawberries: Does such an increment imply a role for ethylene in the ripening of these non-climacteric fruits? J Exp Bot. 2005;56:2037–2046. doi: 10.1093/jxb/eri202. [DOI] [PubMed] [Google Scholar]
- 21.Deytieux C, et al. Proteome analysis of grape skins during ripening. J Exp Bot. 2007;58:1851–1862. doi: 10.1093/jxb/erm049. [DOI] [PubMed] [Google Scholar]
- 22.Koyama K, Sadamatsu K, Goto-Yamamoto N. Abscisic acid stimulated ripening and gene expression in berry skins of the Cabernet Sauvignon grape. Funct Integr Genomics. 2010;10:367–381. doi: 10.1007/s10142-009-0145-8. [DOI] [PubMed] [Google Scholar]
- 23.Kano Y, Asahira T. Role of cytokinin and abscisic acid in the maturing of strawberry fruits. J Jpn Soc Hortic Sci. 1981;50:31–36. [Google Scholar]
- 24.Jiang YM, Joyce DC. ABA effects on ethylene production, PAL activity, anthocyanin and phenolic contents of strawberry fruit. Plant Growth Regul. 2003;39:171–174. [Google Scholar]
- 25.Jia HF, et al. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011;157:188–199. doi: 10.1104/pp.111.177311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi M, et al. Fluctuation of endogenous gibberellin and abscisic acid levels in germinating seeds of barley. Biosci Biotechnol Biochem. 2014;59:1969–1970. [Google Scholar]
- 27.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 28.Lovegrove A, Hooley R. Gibberellin and abscisic acid signalling in aleurone. Trends Plant Sci. 2000;5:102–110. doi: 10.1016/s1360-1385(00)01571-5. [DOI] [PubMed] [Google Scholar]
- 29.Razem FA, Baron K, Hill RD. Turning on gibberellin and abscisic acid signaling. Curr Opin Plant Biol. 2006;9:454–459. doi: 10.1016/j.pbi.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Shu K, Zhou W, Yang W. APETALA 2-domain-containing transcription factors: Focusing on abscisic acid and gibberellins antagonism. New Phytol. 2018;217:977–983. doi: 10.1111/nph.14880. [DOI] [PubMed] [Google Scholar]
- 31.Shu K, et al. ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels. Plant J. 2016;85:348–361. doi: 10.1111/tpj.13109. [DOI] [PubMed] [Google Scholar]
- 32.Qin X, Zeevaart JA. The 9-cis-epoxycarotenoid cleavage reaction is the key regulatory step of abscisic acid biosynthesis in water-stressed bean. Proc Natl Acad Sci USA. 1999;96:15354–15361. doi: 10.1073/pnas.96.26.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iuchi S, et al. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001;27:325–333. doi: 10.1046/j.1365-313x.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 34.Tan BC, et al. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003;35:44–56. doi: 10.1046/j.1365-313x.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 35.Lefebvre V, et al. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006;45:309–319. doi: 10.1111/j.1365-313X.2005.02622.x. [DOI] [PubMed] [Google Scholar]
- 36.Gillard DF, Walton DC. Abscisic acid metabolism by a cell-free preparation from Echinocystis lobata liquid endoserum. Plant Physiol. 1976;58:790–795. doi: 10.1104/pp.58.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krochko JE, Abrams GD, Loewen MK, Abrams SR, Cutler AJ. (+)-Abscisic acid 8′-hydroxylase is a cytochrome P450 monooxygenase. Plant Physiol. 1998;118:849–860. doi: 10.1104/pp.118.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kushiro T, et al. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′-hydroxylases: Key enzymes in ABA catabolism. EMBO J. 2004;23:1647–1656. doi: 10.1038/sj.emboj.7600121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millar AA, et al. Seed dormancy and ABA metabolism in Arabidopsis and barley: The role of ABA 8′-hydroxylase. Plant J. 2006;45:942–954. doi: 10.1111/j.1365-313X.2006.02659.x. [DOI] [PubMed] [Google Scholar]
- 40.Ji K, et al. Non-climacteric ripening in strawberry fruit is linked to ABA, FaNCED2 and FaCYP707A1. Funct Plant Biol. 2012;39:351–357. doi: 10.1071/FP11293. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Ding G, Gu T, Ding J, Li Y. Bioinformatic and expression analyses on carotenoid dioxygenase genes in fruit development and abiotic stress responses in Fragaria vesca. Mol Genet Genomics. 2017;292:895–907. doi: 10.1007/s00438-017-1321-5. [DOI] [PubMed] [Google Scholar]
- 42.Hollender CA, Geretz AC, Slovin JP, Liu Z. Flower and early fruit development in a diploid strawberry, Fragaria vesca. Planta. 2012;235:1123–1139. doi: 10.1007/s00425-011-1562-1. [DOI] [PubMed] [Google Scholar]
- 43.Redondo-Nevado J, Moyano E, Medina-Escobar N, Caballero JL, Muñoz-Blanco J. A fruit-specific and developmentally regulated endopolygalacturonase gene from strawberry (Fragaria x ananassa cv. Chandler) J Exp Bot. 2001;52:1941–1945. doi: 10.1093/jexbot/52.362.1941. [DOI] [PubMed] [Google Scholar]
- 44.Villarreal NM, Rosli HG, Martínez GA, Civello PM. Polygalacturonase activity and expression of related genes during ripening of strawberry cultivars with contrasting fruit firmness. Postharvest Biol Technol. 2008;47:141–150. [Google Scholar]
- 45.Serrani JC, Fos M, Atarés A, García-Martínez JL. Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv Micro-Tom of tomato. J Plant Growth Regul. 2007;26:211–221. [Google Scholar]
- 46.Serrani JC, et al. Inhibition of auxin transport from the ovary or from the apical shoot induces parthenocarpic fruit-set in tomato mediated by gibberellins. Plant Physiol. 2010;153:851–862. doi: 10.1104/pp.110.155424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dorcey E, Urbez C, Blázquez MA, Carbonell J, Perez-Amador MA. Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J. 2009;58:318–332. doi: 10.1111/j.1365-313X.2008.03781.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, et al. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell. 2009;21:1428–1452. doi: 10.1105/tpc.108.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itoh H, Ueguchi-Tanaka M, Matsuoka M. Molecular biology of gibberellins signaling in higher plants. Int Rev Cell Mol Biol. 2008;268:191–221. doi: 10.1016/S1937-6448(08)00806-X. [DOI] [PubMed] [Google Scholar]
- 50.Xu H, Liu Q, Yao T, Fu X. Shedding light on integrative GA signaling. Curr Opin Plant Biol. 2014;21:89–95. doi: 10.1016/j.pbi.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 51.Li W, et al. FveRGA1, encoding a DELLA protein, negatively regulates runner production in Fragaria vesca. Planta. 2018;247:941–951. doi: 10.1007/s00425-017-2839-9. [DOI] [PubMed] [Google Scholar]
- 52.Caruana JC, Sittmann JW, Wang W, Liu Z. Suppressor of Runnerless encodes a DELLA protein that controls runner formation for asexual reproduction in strawberry. Mol Plant. 2018;11:230–233. doi: 10.1016/j.molp.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Sauter A, Dietz KJ, Hartung W. A possible stress physiological role of abscisic acid conjugates in root-to-shoot signalling. Plant Cell Environ. 2002;25:223–228. doi: 10.1046/j.1365-3040.2002.00747.x. [DOI] [PubMed] [Google Scholar]
- 54.Li Q, et al. The role of FaBG3 in fruit ripening and B. cinerea fungal infection of strawberry. Plant J. 2013;76:24–35. doi: 10.1111/tpj.12272. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Schiff M, Marathe R, Dinesh-Kumar SP. Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N-mediated resistance to tobacco mosaic virus. Plant J. 2002;30:415–429. doi: 10.1046/j.1365-313x.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 56.Llop-Tous I, Domínguez-Puigjaner E, Palomer X, Vendrell M. Characterization of two divergent endo-β-1,4-glucanase cDNA clones highly expressed in the nonclimacteric strawberry fruit. Plant Physiol. 1999;119:1415–1422. doi: 10.1104/pp.119.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Figueroa CR, et al. Softening rate of the Chilean strawberry (Fragaria chiloensis) fruit reflects the expression of polygalacturonase and pectate lyase genes. Postharvest Biol Technol. 2008;49:210–220. [Google Scholar]
- 58.Shibaoka H. Involvement of wall microtubules in gibberellin promotion and kinetin inhibition of stem elongation. Plant Cell Physiol. 1974;15:255–263. [Google Scholar]
- 59.Shibaoka H. Regulation of gibberellins of the orientation of cortical micro tubules in plant cells. Aust J Plant Physiol. 1993;20:461–470. [Google Scholar]
- 60.Ishida K, Katsumi M. Immunofluorescence microscopical observation of cortical microtubule arrangement by gibberellin in d5 mutant of Zea mays L. Plant Cell Physiol. 1991;32:409–417. [Google Scholar]
- 61.Lloyd C, Shaw P, Warn RM, Yuan M. Gibberellic-acid-induced reorientation of cortical microtubules in living plant cells. J Microsc. 1995;181:140–144. [Google Scholar]
- 62.Shibaoka H, Takesue K. Auxin-induced longitudinal-to-transverse reorientation of cortical microtubules in nonelongating epidermal cells of azuki bean epicotyls. Protoplasma. 1999;206:27–30. [Google Scholar]
- 63.Yang SH, Choi D. Characterization of genes encoding ABA 8′-hydroxylase in ethylene-induced stem growth of deepwater rice (Oryza sativa L.) Biochem Biophys Res Commun. 2006;350:685–690. doi: 10.1016/j.bbrc.2006.09.098. [DOI] [PubMed] [Google Scholar]
- 64.Slovin JP, Schmitt K, Folta KM. An inbred line of the diploid strawberry Fragaria vesca f. semperflorens for genomic and molecular genetic studies in the Rosaceae. Plant Methods. 2009;5:15. doi: 10.1186/1746-4811-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB. Virus-induced gene silencing in tomato fruit. Plant J. 2005;43:299–308. doi: 10.1111/j.1365-313X.2005.02441.x. [DOI] [PubMed] [Google Scholar]
- 66.Sun L, et al. Reciprocity between abscisic acid and ethylene at the onset of berry ripening and after harvest. BMC Plant Biol. 2010;10:257. doi: 10.1186/1471-2229-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.